Controlling the LCST-Phase Transition in Azobenzene-Functionalized Poly (N-Isopropylacrlyamide) Hydrogels by Light
Abstract
:1. Introduction
2. Results
2.1. Synthesis: Azo-co-NIPAAm Hydrogels
2.2. UV-vis and 1H NMR Spectroscopy Study: Quantification of the Photoresponsiveness of Azoacrylate monomer Azo (1), Azo-co-NIPAAm Hydrogels and Polymers
- Monomer:
- Azo-co-NIPAAm Hydrogels:
- Azo-co-NIPAAm Polymers:
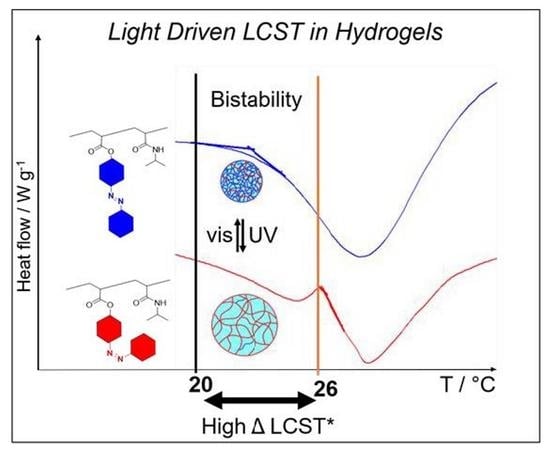
2.3. Photo Tunable LCST of Azo-co-PNIPAAm Hydrogels via DSC
2.4. Light Driven Structural Modulation in Azo-co-NIPAAm Hydrogels (H2.5%)
3. Discussion
4. Conclusions
5. Experimental Section/Methods
5.1. Materials
5.2. Methods
- Sample preparation and method for DSC measurement
- Photographs for volume change in UV-switched hydrogel
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. Engl. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Imran, A.B.; Seki, T.; Takeoka, Y. Recent advances in hydrogels in terms of fast stimuli responsiveness and superior mechanical performance. Polym. J. 2010, 42, 839–851. [Google Scholar] [CrossRef] [Green Version]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Meeussen, F.; Nies, E.; Berghmans, H.; Verbrugghe, S.; Goethals, E.; Du Prez, F. Phase behavior of poly(N-vinyl caprolactam) in water. Polymer 2000, 41, 8597–8602. [Google Scholar] [CrossRef]
- Shibayama, M.; Morimoto, M.; Nomura, S. Phase Separation Induced Mechanical Transition of Poly(N-isopropylacrylamide)/Water Isochore Gels. Macromolecules 1994, 27, 5060–5066. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X. Globule-to-Coil Transition of a Single Homopolymer Chain in Solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, C.D.L.H.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef]
- Hiruta, Y. Poly(N-isopropylacrylamide)-based temperature- and pH-responsive polymer materials for application in biomedical fields. Polym. J. 2022, 54, 1419–1430. [Google Scholar] [CrossRef]
- Jelken, J.; Jung, S.H.; Lomadze, N.; Gordievskaya, Y.D.; Kramarenko, E.Y.; Pich, A.; Santer, S. Tuning the Volume Phase Transition Temperature of Microgels by Light. Adv. Funct. Mater. 2022, 32, 2107946. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, 1807333. [Google Scholar] [CrossRef] [Green Version]
- Le, X.; Lu, W.; Zhang, J.; Chen, T. Recent Progress in Biomimetic Anisotropic Hydrogel Actuators. Adv. Sci. 2019, 6, 1801584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasban, S.; Raissi, H. PNIPAM/Hexakis as a thermosensitive drug delivery system for biomedical and pharmaceutical applications. Sci. Rep. 2022, 12, 14363. [Google Scholar] [CrossRef] [PubMed]
- Spratte, T.; Arndt, C.; Wacker, I.; Hauck, M.; Adelung, R.; Schröder, R.R.; Schütt, F.; Selhuber-Unkel, C. Thermoresponsive Hydrogels with Improved Actuation Function by Interconnected Microchannels. Adv. Intell. Syst. 2022, 4, 2100081. [Google Scholar] [CrossRef]
- Lee, B.P.; Konst, S. Novel Hydrogel Actuator Inspired by Reversible Mussel Adhesive Protein Chemistry. Adv. Mater. 2014, 26, 3415–3419. [Google Scholar] [CrossRef] [PubMed]
- Le, X.; Lu, W.; Zheng, J.; Tong, D.; Zhao, N.; Ma, C.; Xiao, H.; Zhang, J.; Huang, Y.; Chen, T. Stretchable supramolecular hydrogels with triple shape memory effect. Chem. Sci. 2016, 7, 6715–6720. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Zhong, H.; Gu, B.; Wang, Y.; Li, X.; Cheng, Z.; Zhang, L.; Yao, C. Thermosensitive phase behavior and drug release of in situ N-isopropylacrylamide copolymer. Mater. Sci. Eng. C 2012, 32, 2199–2204. [Google Scholar] [CrossRef]
- Rana, M.M.; De la Hoz Siegler, H. Tuning the Properties of PNIPAm-Based Hydrogel Scaffolds for Cartilage Tissue Engineering. Polymers 2021, 13, 3154. [Google Scholar] [CrossRef] [PubMed]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Wang, T.; Sun, W.; Tong, Z. Polyampholyte Hydrogels with pH Modulated Shape Memory and Spontaneous Actuation. Adv. Funct. Mater. 2018, 28, 1707245. [Google Scholar] [CrossRef]
- Ohnsorg, M.L.; Ting, J.M.; Jones, S.D.; Jung, S.; Bates, F.S.; Reineke, T.M. Tuning PNIPAm self-assembly and thermoresponse: Roles of hydrophobic end-groups and hydrophilic comonomer. Polym. Chem. 2019, 10, 3469–3479. [Google Scholar] [CrossRef]
- Kostyurina, E.; De Mel, J.U.; Vasilyeva, A.; Kruteva, M.; Frielinghaus, H.; Dulle, M.; Barnsley, L.; Förster, S.; Schneider, G.J.; Biehl, R.; et al. Controlled LCST Behavior and Structure Formation of Alternating Amphiphilic Copolymers in Water. Macromolecules 2022, 55, 1552–1565. [Google Scholar] [CrossRef]
- Ida, S.; Kawahara, T.; Fujita, Y.; Tanimoto, S.; Hirokawa, Y. Thermoresponsive Properties of Copolymer Gels Induced by Appropriate Hydrophilic/Hydrophobic Balance of Monomer Combination. Macromol. Symp. 2015, 350, 14–21. [Google Scholar] [CrossRef]
- Kungwatchakun, D.; Irie, M. Photoresponsive polymers. Photocontrol of the phase separation temperature of aqueous solutions of poly[N-isopropylacrylamide-co-N-(4-phenylazophenyl)acrylamide. Die Makromol. Chem. Rapid Commun. 1988, 9, 243–246. [Google Scholar] [CrossRef]
- Mamada, A.; Tanaka, T.; Kungwatchakun, D.; Irie, M. Photoinduced phase transition of gels. Macromolecules 1990, 23, 1517–15179. [Google Scholar] [CrossRef]
- Weis, P.; Hess, A.; Kircher, G.; Huang, S.; Auernhammer, G.K.; Koynov, K.; Butt, H.J.; Wu, S. Effects of Spacers on Photoinduced Reversible Solid-to-Liquid Transitions of Azobenzene-Containing Polymers. Chemistry 2019, 25, 10946–10953. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Chang, A.C.; Yamamoto, S.; Tamate, R.; Ueki, T.; Nakanishi, J. Design of azobenzene-bearing hydrogel with photoswitchable mechanics driven by photo-induced phase transition for in vitro disease modeling. Acta Biomater. 2021, 132, 103–113. [Google Scholar] [CrossRef]
- Rosales, A.M.; Mabry, K.M.; Nehls, E.M.; Anseth, K.S. Photoresponsive elastic properties of azobenzene-containing poly(ethylene-glycol)-based hydrogels. Biomacromolecules 2015, 16, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.L.; Stoddart, J.F. Azobenzene-based light-responsive hydrogel system. Langmuir 2009, 25, 8442–8446. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive polymers with lower critical solution temperature: From fundamental aspects and measuring techniques to recommended turbidimetry conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- He, J.; Tremblay, L.; Lacelle, S.; Zhao, Y. How can photoisomerization of azobenzene induce a large cloud point temperature shift of PNIPAM? Polym. Chem. 2014, 5, 5403–5411. [Google Scholar] [CrossRef]
- Akiyama, H.; Tamaoki, N. Synthesis and Photoinduced Phase Transitions of Poly(N-isopropylacrylamide) Derivative Functionalized with Terminal Azobenzene Units. Macromolecules 2007, 40, 5129–5132. [Google Scholar] [CrossRef]
- Xia, Y.; Burke, N.A.D.; Stöver, H.D.H. End Group Effect on the Thermal Response of Narrow-Disperse Poly(N-isopropylacrylamide) Prepared by Atom Transfer Radical Polymerization. Macromolecules 2006, 39, 2275–2283. [Google Scholar] [CrossRef]
- Li, L.; Xing, X.; Liu, Z. Triply-responsive (thermo/light/pH) copolymeric hydrogel of N-isopropylacrylamide with an azobenzene-containing monomer. J. Appl. Polym. Sci. 2012, 124, 1128–1136. [Google Scholar] [CrossRef]
- Sumaru, K.; Ohi, K.; Takagi, T.; Kanamori, T.; Shinbo, T. Photoresponsive Properties of Poly(N-isopropylacrylamide) Hydrogel Partly Modified with Spirobenzopyran. Langmuir 2006, 22, 4353–4356. [Google Scholar] [CrossRef] [PubMed]
- Meeks, A.; Lerch, M.M.; Schroeder, T.B.H.; Shastri, A.; Aizenberg, J. Spiropyran Photoisomerization Dynamics in Multiresponsive Hydrogels. J. Am. Chem. Soc. 2022, 144, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Francis, W.; Dunne, A.; Delaney, C.; Florea, L.; Diamond, D. Spiropyran based hydrogels actuators—Walking in the light. Sens. Actuators B Chem. 2017, 250, 608–616. [Google Scholar] [CrossRef]
- Li, C.; Iscen, A.; Palmer, L.C.; Schatz, G.C.; Stupp, S.I. Light-Driven Expansion of Spiropyran Hydrogels. J. Am. Chem. Soc. 2020, 142, 8447–8453. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Dubovik, A.S.; Kuznetsov, D.V.; Grinberg, N.V.; Grosberg, A.Y.; Tanaka, T. Studies of the Thermal Volume Transition of Poly(N-isopropylacrylamide) Hydrogels by High-Sensitivity Differential Scanning Microcalorimetry. 2. Thermodynamic Functions. Macromolecules 2000, 33, 8685–8692. [Google Scholar] [CrossRef]
- Cho, E.; Jaeyoung, L.; Cho, K. Role of Bound Water and Hydrophobic Interaction in Phase Transition of Poly(N-isopropylacrylamide) Aqueous Solution. Macromolecules 2003, 36, 9929–9934. [Google Scholar] [CrossRef]
- Ren, H.; Qiu, X.-P.; Shi, Y.; Yang, P.; Winnik, F.M. The Two Phase Transitions of Hydrophobically End-Capped Poly(N-isopropylacrylamide)s in Water. Macromolecules 2020, 53, 5105–5115. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, B.; Zhang, Y.; Xing, X.; Wu, X.; Liu, Z. Multi-sensitive copolymer hydrogels of N-isopropylacrylamide with several polymerizable azobenzene-containing monomers. J. Polym. Res. 2015, 22, 176. [Google Scholar] [CrossRef]
- Dong, Q.; Luo, C.; Hu, H.; Zhang, Q.; Fan, S. Synthesis and photo-controllable thermosensitivity of poly(N-isopropylacrylamide) terminated with dimethylaminochalcone unit. J. Polym. Res. 2017, 24, 229. [Google Scholar] [CrossRef]
- Hartley, G.S.; Le Fèvre, R.J.W. 119. The dipole moments of cis- and trans-azobenzenes and of some related compounds. J. Chem. Soc. (Resumed) 1939, 531–535. [Google Scholar] [CrossRef]







| Sample | Azo /Mol% | Azo /mg | NIPAAM /mg | AIBN /mg | BIS /mg | Comments |
|---|---|---|---|---|---|---|
| H0% | - | - | 102.5 | 10.0 | 9.0 | Control gel |
| H1% | 1.0% | 2.5 | 101.5 | 10.0 | 9.0 | Gel |
| H2.5% | 2.5% | 6.0 | 100 | 10.0 | 9.0 | Gel |
| H5% | 5.0% | 12.0 | 97.5 | 10.0 | 9.0 | Gel |
| Hydrogel | H0% | H1% | H2.5% | H5% |
|---|---|---|---|---|
| LCSTnon-treated | 32°C | 26 °C | 20 °C | 9 °C |
| LCST*(Z) | N/A | 28 °C | 26 °C | - |
| LCST*(E) | N/A | 26 °C | 20 °C | - |
| ΔLCST* | N/A | 2 °C | 6 °C | No LCST shift |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colaco, R.; Appiah, C.; Staubitz, A. Controlling the LCST-Phase Transition in Azobenzene-Functionalized Poly (N-Isopropylacrlyamide) Hydrogels by Light. Gels 2023, 9, 75. https://doi.org/10.3390/gels9020075
Colaco R, Appiah C, Staubitz A. Controlling the LCST-Phase Transition in Azobenzene-Functionalized Poly (N-Isopropylacrlyamide) Hydrogels by Light. Gels. 2023; 9(2):75. https://doi.org/10.3390/gels9020075
Chicago/Turabian StyleColaco, Ruchira, Clement Appiah, and Anne Staubitz. 2023. "Controlling the LCST-Phase Transition in Azobenzene-Functionalized Poly (N-Isopropylacrlyamide) Hydrogels by Light" Gels 9, no. 2: 75. https://doi.org/10.3390/gels9020075
APA StyleColaco, R., Appiah, C., & Staubitz, A. (2023). Controlling the LCST-Phase Transition in Azobenzene-Functionalized Poly (N-Isopropylacrlyamide) Hydrogels by Light. Gels, 9(2), 75. https://doi.org/10.3390/gels9020075