Walnut Protein Isolate-κ-Carrageenan Composite Gels Improved with Synergetic Ultrasound-Transglutaminase: Gelation Properties and Structure
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Ultrasonic Power on WHC, Swelling Ratio, Freeze–Thaw Stability and Moisture Distribution of WNPI-KC Composite Gels
2.2. Effects of Ultrasonic Power on Texture Properties of WNPI-KC Composite Gels
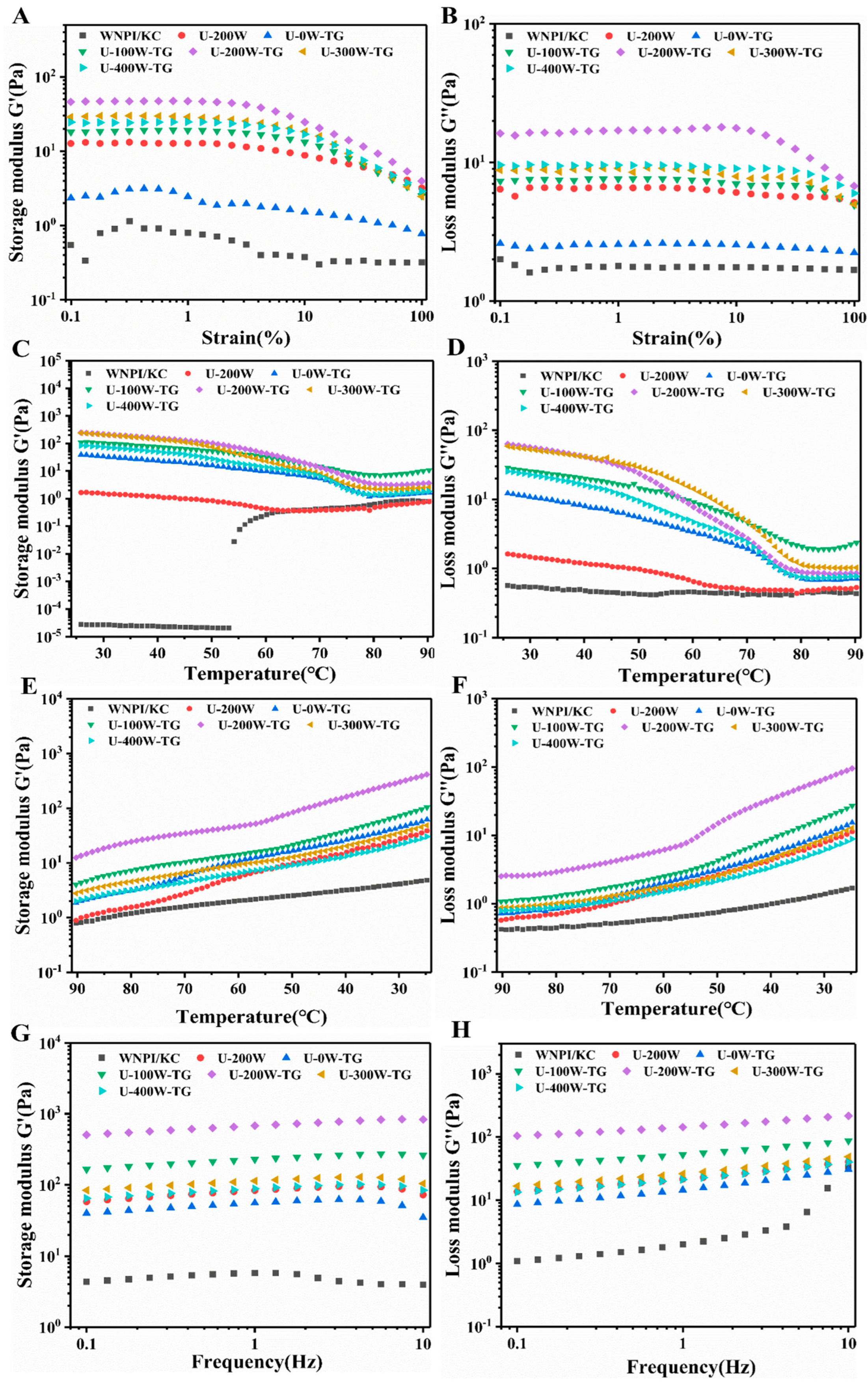
2.3. Effects of Ultrasonic Power on Rheological Behavior of WNPI-KC Composite Gels
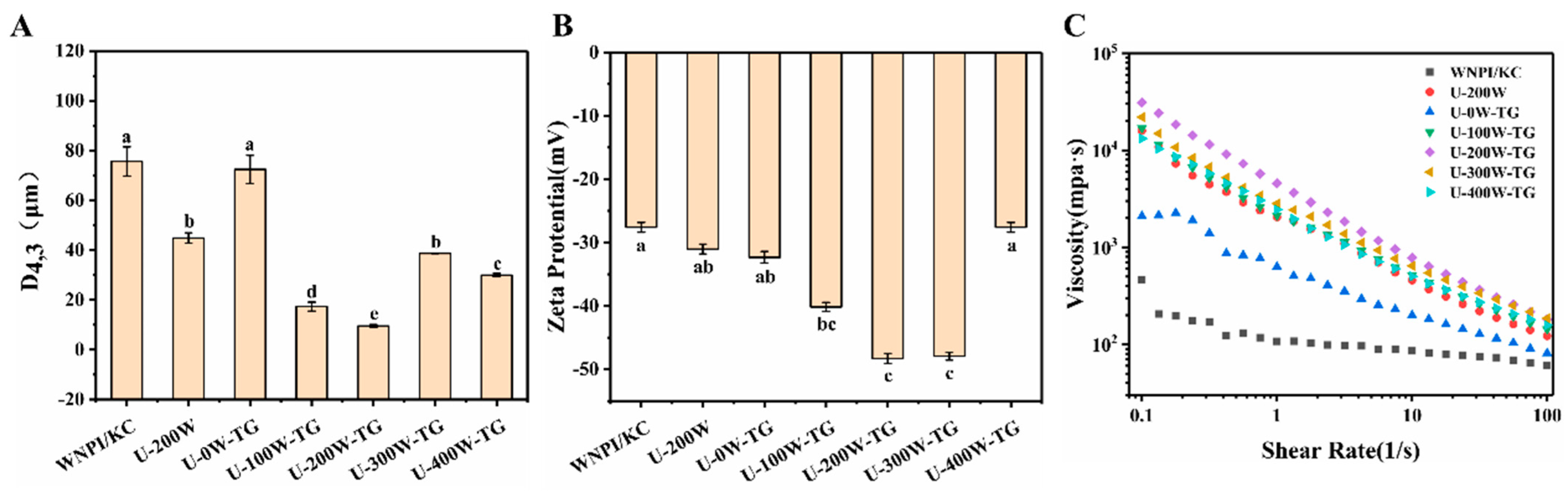
2.4. Effects of Ultrasonic Power on D [4,3], Zeta Potential and Apparent Viscosity of WNPI-KC Composite Gels
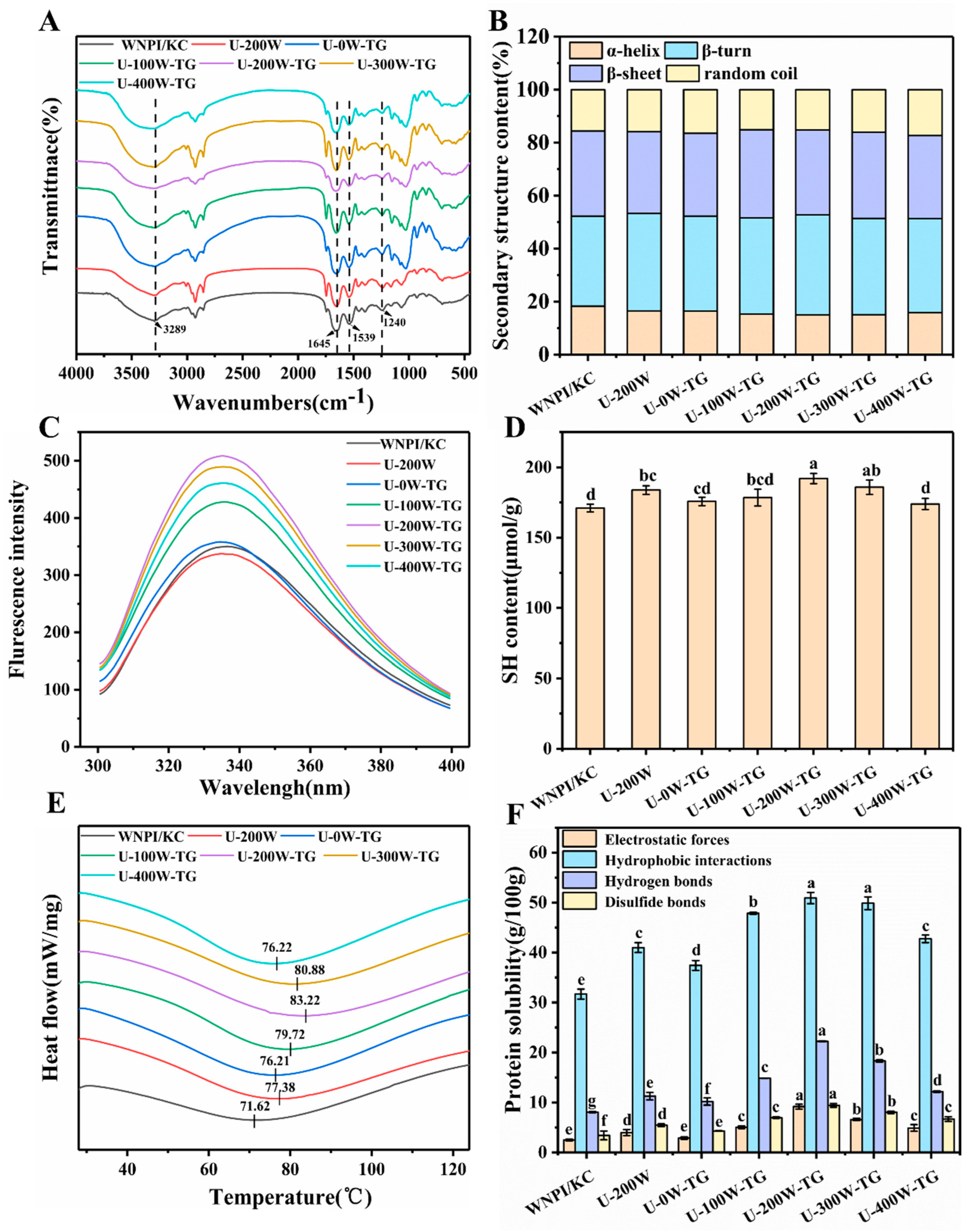
2.5. Effects of Ultrasonic Power on the Structural Properties of WNPI-KC Composite Gels
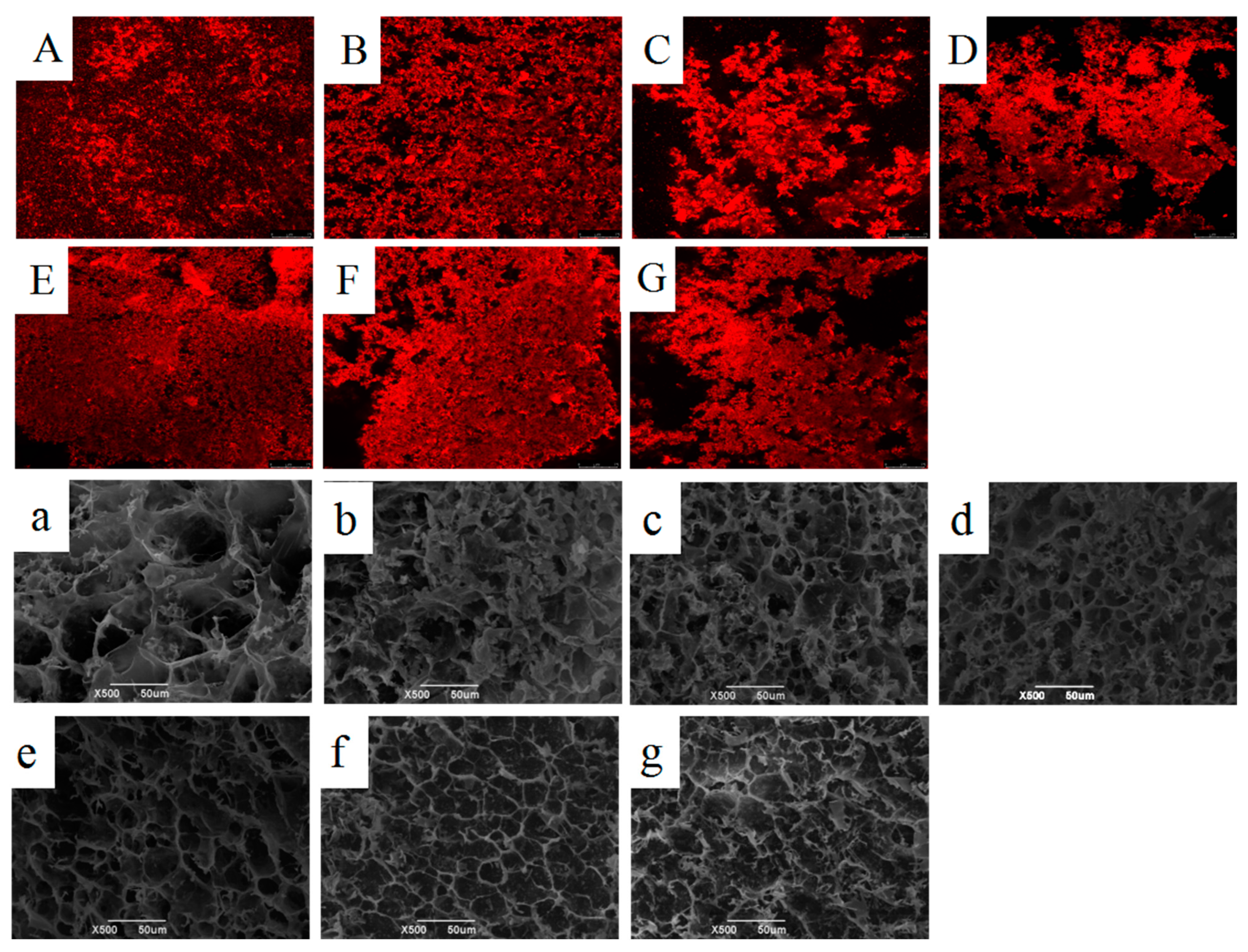
2.5.1. Microstructure
2.5.2. FTIR
2.5.3. Intrinsic Fluorescence Spectroscopy
2.5.4. Free Sulfhydryl Content
2.5.5. Thermal Stability
2.5.6. Intermolecular Forces
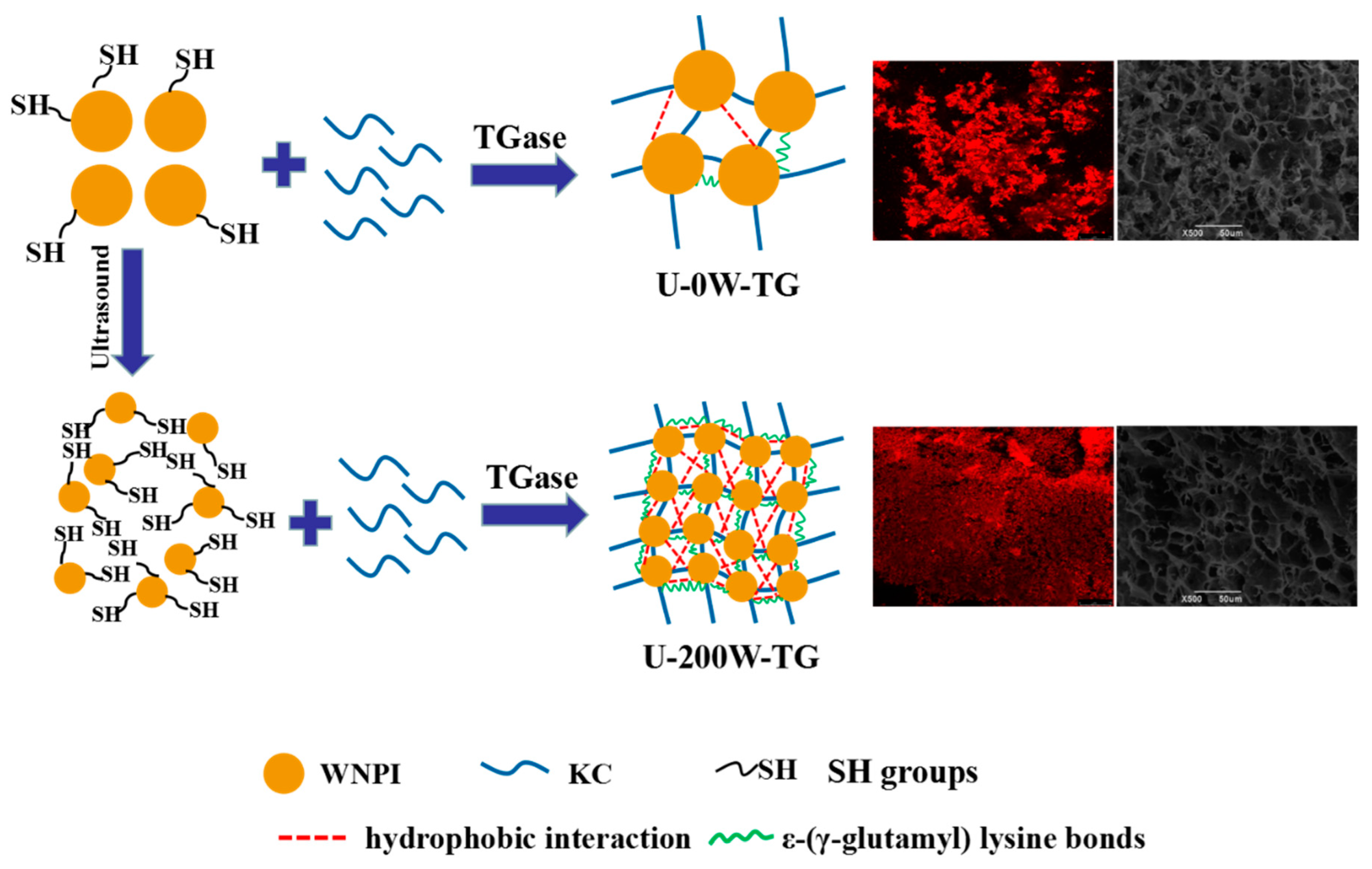
2.6. Schematic Mechanism
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of WNPI/KC Composite Gels
4.3. Water-Holding Capacity (WHC)
4.4. Swelling Capacity
4.5. Freeze–Thaw Stability
4.6. Moisture Distribution
4.7. Texture Properties
4.8. Rheological Properties
4.9. Zeta Potential and Particle Size
4.10. Microstructure
4.11. Fourier Transform Infrared Spectroscopy (FTIR)
4.12. Intrinsic Fluorescence Spectroscopy
4.13. Free Sulfhydryl Content
4.14. Thermal Stability
4.15. Intermolecular Forces
4.16. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Z.; Zhu, W.; Yi, J.; Liu, N.; Cao, Y.; Lu, J.; Decker, E.A.; McClements, D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018, 106, 853–861. [Google Scholar] [CrossRef]
- Shi, A.; Jiao, B.; Liu, H.; Zhu, S.; Shen, M.; Feng, X.; Hu, H.; Liu, L.; Faisal, S.; Wang, Q.; et al. Effects of proteolysis and transglutaminase crosslinking on physicochemical characteristics of walnut protein isolate. LWT-Food Sci. Technol. 2018, 97, 662–667. [Google Scholar] [CrossRef]
- Chen, Y.; Pei, H.; Dai, Q.; Zhang, C.; Hong, X.; Hua, Y. Raw walnut kernel: A natural source for dietary proteases and bioactive proteins. Food Chem. 2021, 369, 130961. [Google Scholar] [CrossRef]
- Szemilao, K.W.C.; Sathe, S.K. Walnuts (Juglans regia L): Proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J. Sci. Food Agr. 2000, 80, 1393–1401. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Li, W.; Qin, F.; Chen, J. Effects of Oligosaccharides and Soy Soluble Polysaccharide on the Rheological and Textural Properties of Calcium Sulfate-Induced Soy Protein Gels. Food Bioprocess Tech. 2017, 10, 556–567. [Google Scholar] [CrossRef]
- Wang, W.; Shen, M.; Liu, S.; Jiang, L.; Song, Q.; Xie, J. Gel properties and interactions of Mesona blumes polysaccharide-soy protein isolates mixed gel: The effect of salt addition. Carbohydr. Polym. 2018, 192, 193–201. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, Y.; Wang, Y.; Wang, R.; Zeng, M. Effect of κ-carrageenan on the gelation properties of oyster protein. Food Chem. 2022, 382, 132329. [Google Scholar] [CrossRef]
- Walayat, N.; Wang, X.; Liu, J.; Nawaz, A.; Zhang, Z.; Khalifa, I.; Rincón Cervera, M.Á.; Pateiro, M.; Lorenzo, J.M.; Nikoo, M.; et al. Kappa-carrageenan as an effective cryoprotectant on water mobility and functional properties of grass carp myofibrillar protein gel during frozen storage. LWT-Food Sci. Technol. 2022, 154, 112675. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci. 2013, 93, 469–476. [Google Scholar] [CrossRef]
- Huang, W.; Li, L.; Wang, F.; Wan, J.; Tilley, M.; Ren, C.; Wu, S. Effects of transglutaminase on the rheological and Mixolab thermomechanical characteristics of oat dough. Food Chem. 2010, 121, 934–939. [Google Scholar] [CrossRef]
- Jiang, S.; Zhao, X. Transglutaminase-induced cross-linking and glucosamine conjugation of casein and some functional properties of the modified product. Int. Dairy J. 2011, 21, 198–205. [Google Scholar] [CrossRef]
- Gauche, C.; Barreto, P.L.M.; Bordignon-Luiz, M.T. Effect of thermal treatment on whey protein polymerization by transglutaminase: Implications for functionality in processed dairy foods. LWT-Food Sci. Technol. 2010, 43, 214–219. [Google Scholar] [CrossRef]
- Gaspar, A.L.C.; de Góes-Favoni, S.P. Action of microbial transglutaminase (MTGase) in the modification of food proteins: A review. Food Chem. 2015, 171, 315–322. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, Q.; Xiao, L.; Huang, Y.; Yang, Z.; Wu, Z.; Tu, Z.; Qin, W.; Chen, H.; Wu, D. Effects of ultrasound on functional properties, structure and glycation properties of proteins: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2471–2481. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles-An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, X.; Hu, T.; Cheung, I.W.Y.; Pan, S.; Li-Chan, E.C.Y. Effect of ultrasound pre-treatment on formation of transglutaminase-catalysed soy protein hydrogel as a riboflavin vehicle for functional foods. J. Funct. Foods 2015, 19, 182–193. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Tian, X.; Liu, J.; Ye, H.; Shen, X. Effect of ultrasound pretreatment on structural, physicochemical, rheological and gelation properties of transglutaminase cross-linked whey protein soluble aggregates. Ultrason. Sonochem. 2021, 74, 105553. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, T.; Feng, S.; Xu, Q.; Zheng, T.; Zhou, M.; Chu, X.; Huang, X.; Lu, X.; Pan, S.; et al. Effect of high intensity ultrasound on transglutaminase-catalyzed soy protein isolate cold set gel. Ultrason. Sonochem. 2016, 29, 380–387. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Fan, Z.; Li, Y.; Teng, F. Preparation of okara cellulose hydrogels using ionic liquids: Structure, properties, and performance. J. Mol. Liq. 2021, 331, 115744. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Han, K.; Cai, Y.; Ma, M.; Tong, Q.; Sheng, L. Effect of nano-TiO2 on the physical, mechanical and optical properties of pullulan film. Carbohydr. Polym. 2019, 218, 95–102. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Gong, Y.; Li, Z.; Guo, Y.; Yu, D.; Pan, M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochem. 2021, 79, 105756. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Li, D.; Wang, L.; Adhikari, B. Viscoelastic properties and fractal analysis of acid-induced SPI gels at different ionic strength. Carbohydr. Polym. 2013, 92, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Arntfield, S.D. Gelation properties of salt-extracted pea protein isolate catalyzed by microbial transglutaminase cross-linking. Food Hydrocoll. 2011, 25, 25–31. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Xiao, W.; van Boekel, M.; Minor, M.; Stieger, M. Effect of extraction pH on heat-induced aggregation, gelation and microstructure of protein isolate from quinoa (Chenopodium quinoa Willd). Food Chem. 2016, 209, 203–210. [Google Scholar] [CrossRef]
- Sheng, L.; Tang, G.; Wang, Q.; Zou, J.; Ma, M.; Huang, X. Molecular characteristics and foaming properties of ovalbumin-pullulan conjugates through the Maillard reaction. Food Hydrocoll. 2020, 100, 105384. [Google Scholar] [CrossRef]
- Lizarraga, M.S.; Piante Vicin, D.D.; González, R.; Rubiolo, A.; Santiago, L.G. Rheological behaviour of whey protein concentrate and λ-carrageenan aqueous mixtures. Food Hydrocoll. 2006, 20, 740–748. [Google Scholar] [CrossRef]
- Gauche, C.; Vieira, J.T.C.; Ogliari, P.J.; Bordignon-Luiz, M.T. Crosslinking of milk whey proteins by transglutaminase. Process Biochem. 2008, 43, 788–794. [Google Scholar] [CrossRef]
- Taha, A.; Hu, T.; Zhang, Z.; Bakry, A.M.; Khalifa, I.; Pan, S.; Hu, H. Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason. Sonochem. 2018, 49, 283–293. [Google Scholar] [CrossRef]
- Zhao, C.; Chu, Z.; Miao, Z.; Liu, J.; Liu, J.; Xu, X.; Wu, Y.; Qi, B.; Yan, J. Ultrasound heat treatment effects on structure and acid-induced cold set gel properties of soybean protein isolate. Food Biosci. 2021, 39, 100827. [Google Scholar] [CrossRef]
- Chu, L.; Yang, L.; Li, J.; Lin, L.; Zheng, G. Effect of Smilax china L. starch on the gel properties and interactions of calcium sulfate-induced soy protein isolate gel. Int. J. Biol. Macromol. 2019, 135, 127–132. [Google Scholar] [CrossRef]
- Aït Kaddour, A.; Mondet, M.; Cuq, B. Application of two-dimensional cross-correlation spectroscopy to analyse infrared (MIR and NIR) spectra recorded during bread dough mixing. J. Cereal Sci. 2008, 48, 678–685. [Google Scholar] [CrossRef]
- Qin, X.; Sun, Q.; Zhao, Y.; Zhong, X.; Mu, D.; Jiang, S.; Luo, S.; Zheng, Z. Transglutaminase-set colloidal properties of wheat gluten with ultrasound pretreatments. Ultrason. Sonochem. 2017, 39, 137–143. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhou, G.; Zhang, W. Effects of regenerated cellulose fiber on the characteristics of myofibrillar protein gels. Carbohydr. Polym. 2019, 209, 276–281. [Google Scholar] [CrossRef]
- Chen, H.; Xu, Z.; Mo, J.; Lyu, Y.; Tang, X.; Shen, X. Effects of guar gum on adhesion properties of soybean protein isolate onto porcine bones. Int. J. Adhes. Adhes. 2017, 75, 124–131. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Li, C.P.; Enomoto, H.; Ohki, S.; Ohtomo, H.; Aoki, T. Improvement of Functional Properties of Whey Protein Isolate Through Glycation and Phosphorylation by Dry Heating. J. Dairy Sci. 2005, 88, 4137–4145. [Google Scholar] [CrossRef] [Green Version]
- Cui, C.; Zhao, M.; Yuan, B.; Zhang, Y.; Ren, J. Effect of pH and Pepsin Limited Hydrolysis on the Structure and Functional Properties of Soybean Protein Hydrolysates. J. Food Sci. 2013, 78, C1871–C1877. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Effects of microwave combined with conduction heating on surimi quality and morphology. J. Food Eng. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Jiang, L.; Ren, Y.; Xiao, Y.; Liu, S.; Zhang, J.; Yu, Q.; Chen, Y.; Xie, J. Effects of Mesona chinensis polysaccharide on the thermostability, gelling properties, and molecular forces of whey protein isolate gels. Carbohydr. Polym. 2020, 242, 116424. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Gao, S.; Xiang, X.; Li, X.; Yu, X.; Li, S. Physicochemical, structural and adhesion properties of walnut protein isolate-xanthan gum composite adhesives using walnut protein modified by ethanol. Int. J. Biol. Macromol. 2021, 192, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Chen, L.; Foegeding, E.A. Mechanical and Water-Holding Properties and Microstructures of Soy Protein Isolate Emulsion Gels Induced by CaCl2, Glucono-δ-lactone (GDL), and Transglutaminase: Influence of Thermal Treatments before and/or after Emulsification. J. Agric. Food Chem. 2011, 59, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Jia, X.; Zhu, Q.; Liu, Y.; Li, J.; Yin, L. Investigation of the mechanical, rheological and microstructural properties of sugar beet pectin/soy protein isolate-based emulsion-filled gels. Food Hydrocoll. 2019, 89, 813–820. [Google Scholar] [CrossRef]
- Liang, X.; Ma, C.; Yan, X.; Zeng, H.; McClements, D.J.; Liu, X.; Liu, F. Structure, rheology and functionality of whey protein emulsion gels: Effects of double cross-linking with transglutaminase and calcium ions. Food Hydrocoll. 2020, 102, 105569. [Google Scholar] [CrossRef]
- Shi, L.; Yin, T.; Huang, Q.; You, J.; Hu, Y.; Jia, D.; Xiong, S. Effects of filleting methods on composition, gelling properties and aroma profile of grass carp surimi. Food Sci. Hum. Well. 2021, 10, 308–315. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Yarmand, M.S.; Salami, M.; Momen, S.; Moosavi-Movahedi, A.A. Cold gelation of curcumin loaded whey protein aggregates mixed with k-carrageenan: Impact of gel microstructure on the gastrointestinal fate of curcumin. Food Hydrocoll. 2018, 85, 267–280. [Google Scholar] [CrossRef]
- Arzeni, C.; Pérez, O.E.; Pilosof, A.M.R. Functionality of egg white proteins as affected by high intensity ultrasound. Food Hydrocoll. 2012, 29, 308–316. [Google Scholar] [CrossRef]
- Lei, Y.; Ouyang, H.; Peng, W.; Yu, X.; Jin, L.; Li, S. Effect of NaCl on the Rheological, Structural, and Gelling Properties of Walnut Protein Isolate-kappa-Carrageenan Composite Gels. Gels 2022, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
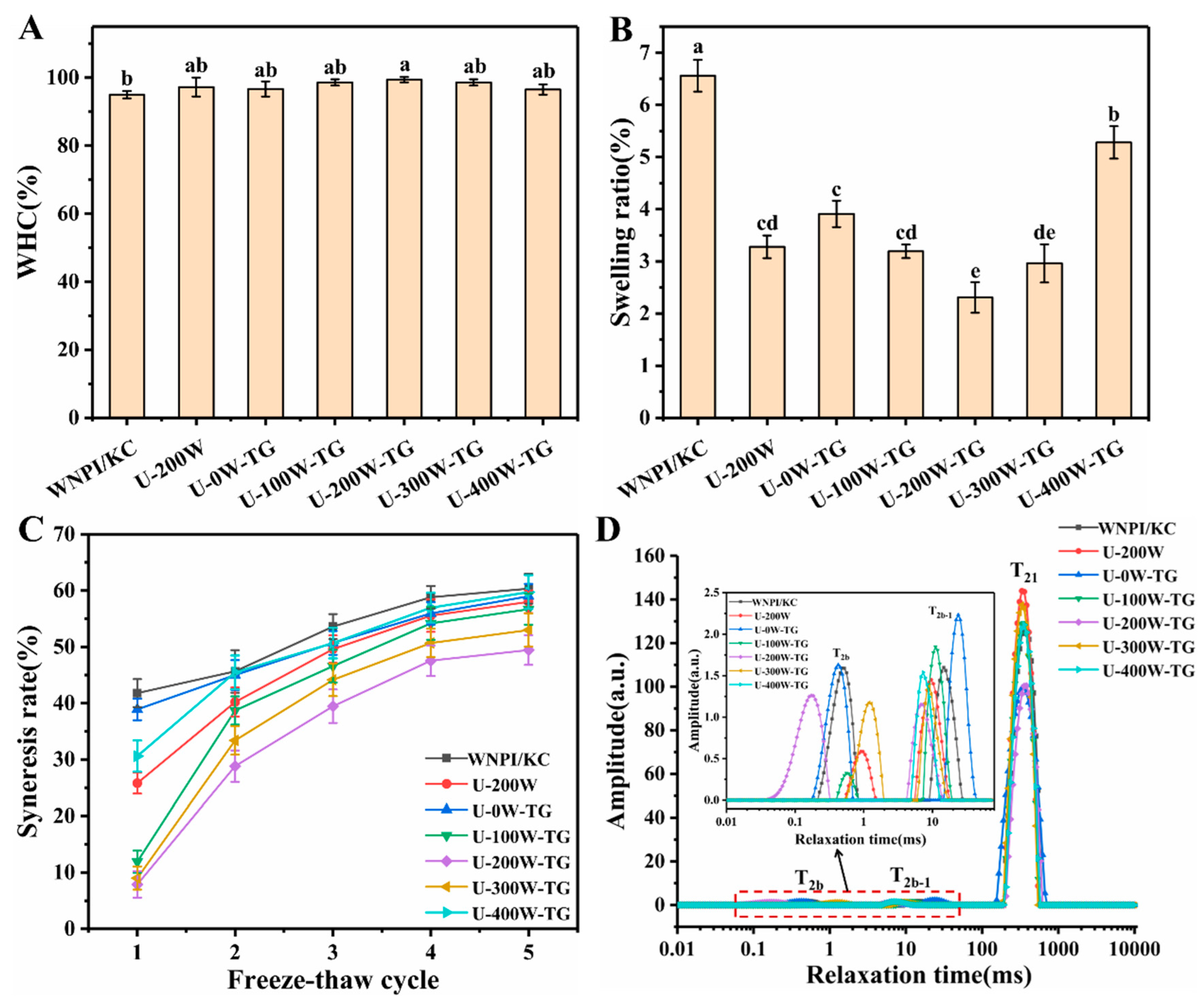

| T2b (ms) | T2b−1 (ms) | T21 (ms) | P2b (%) | P2b−1 (%) | P21 (%) | |
|---|---|---|---|---|---|---|
| WNPI/KC | 2.42 ± 0.65 ab | 23.28 ± 0.92 a | 420.10 ± 17.03 a | 0.45 ± 0.23 ab | 1.66 ± 0.19 a | 97.89 ± 0.12 ab |
| U-200 W | 0.55 ± 0.32 c | 8.82 ± 0.72 bc | 333.13 ± 0.00 bc | 0.25 ± 0.20 b | 1.06 ± 0.08 bc | 98.69 ± 0.13 a |
| U-0 W-TG | 2.84 ± 1.86 a | 21.26 ± 1.66 a | 357.08 ± 0.00 b | 0.39 ± 0.13 ab | 1.23 ± 0.13 b | 98.38 ± 0.04 ab |
| U-100 W-TG | 0.47 ± 0.08 c | 10.60 ± 0.43 bc | 357.08 ± 0.00 b | 0.65 ± 0.50 ab | 1.10 ± 0.11 bc | 98.25 ± 0.47 ab |
| U-200 W-TG | 0.31 ± 0.13 c | 10.86 ± 3.92 b | 357.65 ± 24.81 b | 1.63 ± 1.29 a | 0.93 ± 0.13 c | 97.44 ± 1.32 b |
| U-300 W-TG | 0.94 ± 0.28 bc | 9.51 ± 1.41 bc | 318.23 ± 12.90 c | 0.67 ± 0.20 ab | 1.02 ± 0.19 bc | 98.31 ± 0.04 ab |
| U-400 W-TG | -- | 7.15 ± 0.28 c | 349.10 ± 13.83 b | -- | 1.10 ± 0.08 bc | 98.90 ± 0.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lei, Y.; Kang, X.; Ouyang, H.; Li, X.; Yu, X.; Gu, Q.; Li, S. Walnut Protein Isolate-κ-Carrageenan Composite Gels Improved with Synergetic Ultrasound-Transglutaminase: Gelation Properties and Structure. Gels 2023, 9, 91. https://doi.org/10.3390/gels9020091
Liu Y, Lei Y, Kang X, Ouyang H, Li X, Yu X, Gu Q, Li S. Walnut Protein Isolate-κ-Carrageenan Composite Gels Improved with Synergetic Ultrasound-Transglutaminase: Gelation Properties and Structure. Gels. 2023; 9(2):91. https://doi.org/10.3390/gels9020091
Chicago/Turabian StyleLiu, Yanlong, Yuqing Lei, Xu Kang, Hui Ouyang, Xiuting Li, Xiongwei Yu, Qianhui Gu, and Shugang Li. 2023. "Walnut Protein Isolate-κ-Carrageenan Composite Gels Improved with Synergetic Ultrasound-Transglutaminase: Gelation Properties and Structure" Gels 9, no. 2: 91. https://doi.org/10.3390/gels9020091






