Bioprinted Schwann and Mesenchymal Stem Cell Co-Cultures for Enhanced Spatial Control of Neurite Outgrowth
Abstract
:1. Introduction
2. Results
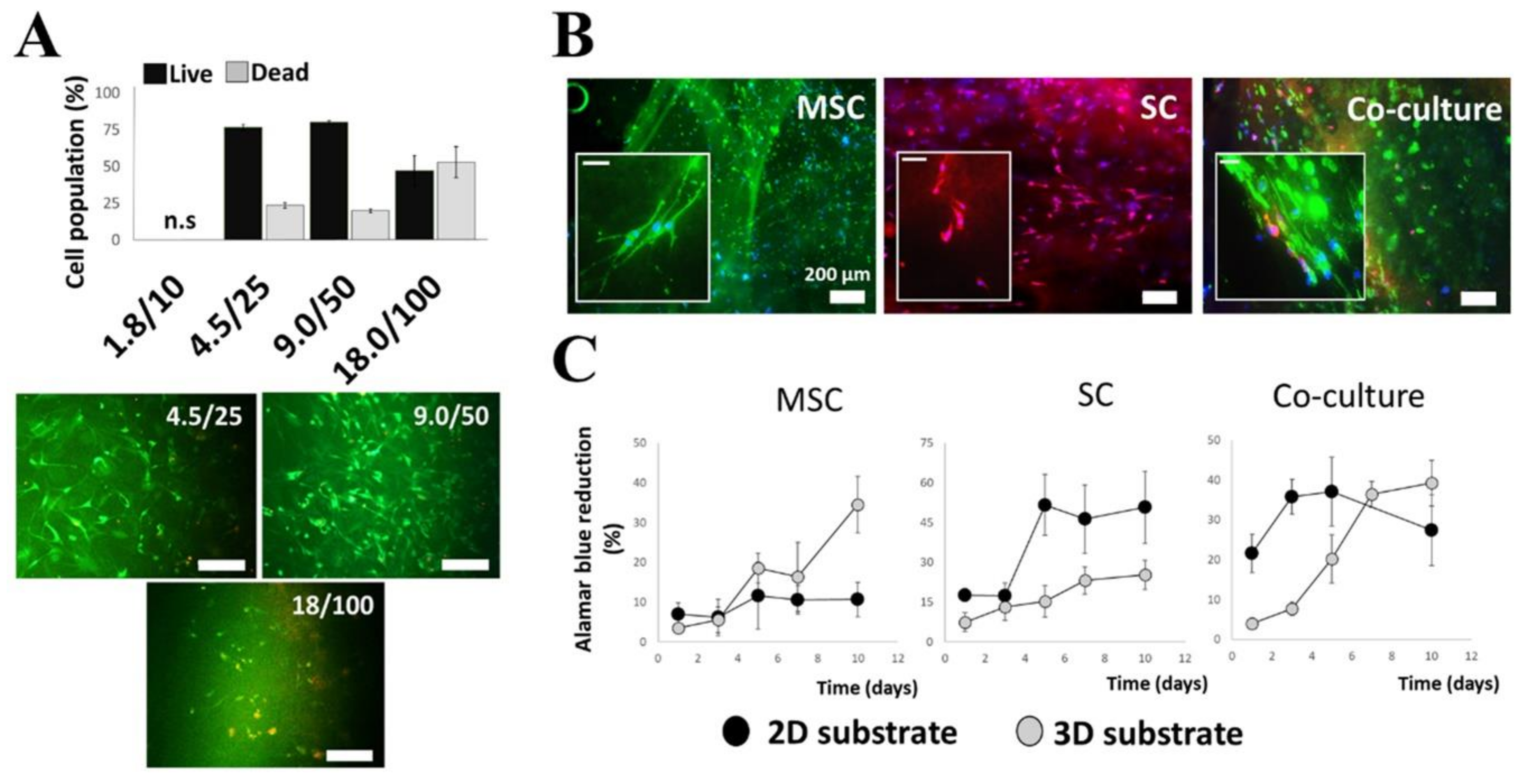
2.1. Selection and Optimization of Hydrogel Matrices
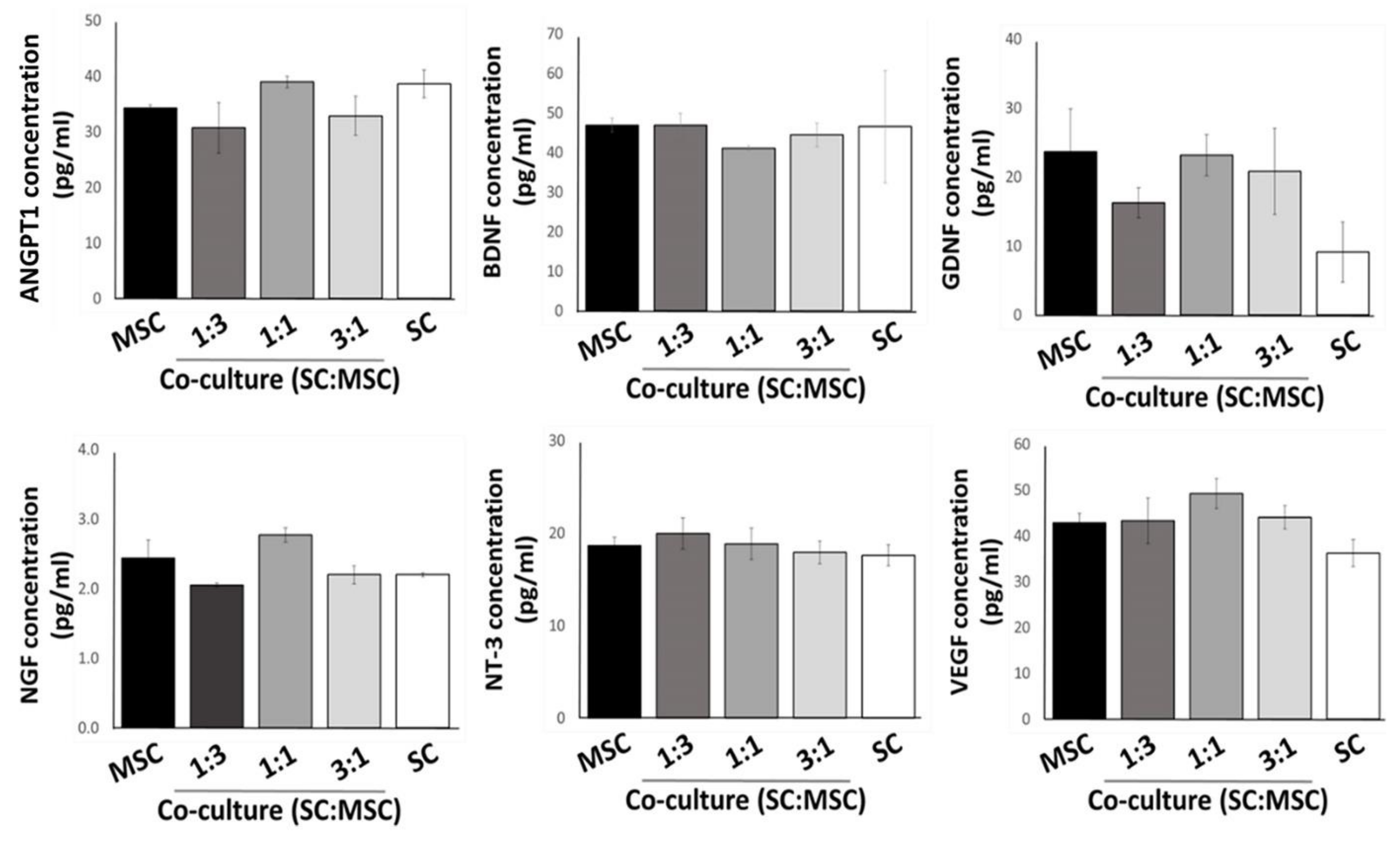
2.2. Expression of Growth Factors by Cultured MSCs and Schwann Cells in 3D Substrates
2.3. Effects of 3D-Printed Cells in Fibrin Hydrogel on Neurite Outgrowth
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Preparation of Hydrogels
5.2. Cell Cultures
5.3. Immunocytochemistry
5.4. Live/Dead Cell Assay
5.5. Cell Proliferation Assay
5.6. RT-PCR
5.7. Enzyme-Linked Immunosorbent Assays
5.8. Preparation of Hydrogels and Cells for 3D Bioprinting
5.8.1. Numerical Simulations
5.8.2. Experimental—Initial Testing Parameters
5.8.3. Experimental—Final Parameters for Fibrin Hydrogel
5.9. PCL Substrates for 3D Printing of Hydrogels
5.10. Neurite Outgrowth Assays
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dahlin, L.B.; Wiberg, M. Nerve injuries of the upper extremity and hand. EFORT Open Rev. 2017, 2, 158–170. [Google Scholar] [CrossRef]
- Palispis, W.A.; Gupta, R. Surgical repair in humans after traumatic nerve injury provides limited functional neural regeneration in adults. Exp. Neurol. 2017, 290, 106–114. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.V.L.; De Faria, J.C.M.; Isaac, C.; Nepomuceno, A.C.; Teixeira, N.H.; Gemperli, R. Comparisons of the results of peripheral nerve defect repair with fibrin conduit and autologous nerve graft: An experimental study in rats. Microsurgery 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Armstrong, S.J.; Wiberg, M.; Terenghi, G.; Kingham, P.J. ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng. 2007, 13, 2863–2870. [Google Scholar] [CrossRef]
- Maturana, L.G.; Pierucci, A.; Simoes, G.F.; Vidigal, M.; Duek, E.A.R.; Vidal, B.C.; Oliveira, A.L.R. Enhanced peripheral nerve regeneration by the combination of a polycaprolactone tubular prosthesis and a scaffold of collagen with supramolecular organization. Brain Behav. 2013, 3, 417–430. [Google Scholar] [CrossRef]
- Miller, C.; Jeftinija, S.; Mallapragada, S. Micropatterned Schwann cell-seeded biodegradable polymer substrates significantly enhance neurite alignment and outgrowth. Tissue Eng. 2001, 7, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.S.; Ding, F.; Williams, D.F. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef]
- Yeh, C.W.; Wang, L.W.; Wu, H.C.; Hsieh, Y.K.; Wang, J.; Chen, M.H.; Wang, T.W. Development of biomimetic micro-patterned device incorporated with neurotrophic gradient and supportive Schwann cells for the applications in neural tissue engineering. Biofabrication 2017, 9, 015024. [Google Scholar] [CrossRef]
- McGrath, A.M.; Brohlin, M.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Novikova, L.N. Fibrin conduit supplemented with human mesenchymal stem cells and immunosuppressive treatment enhances regeneration after peripheral nerve injury. Neurosci. Lett. 2012, 516, 171–176. [Google Scholar] [CrossRef]
- Schaakxs, D.; Kalbermatten, D.F.; Pralong, E.; Raffoul, W.; Wiberg, M.; Kingham, P.J. Poly-3-hydroxybutyrate strips seeded with regenerative cells are effective promoters of peripheral nerve repair. J. Tissue Eng. Regen. Med. 2017, 11, 812–821. [Google Scholar] [CrossRef]
- Mosahebi, A.; Fuller, P.; Wiberg, M.; Terenghi, G. Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp. Neurol. 2002, 173, 213–223. [Google Scholar] [CrossRef]
- Walsh, S.; Midha, R. Use of stem cells to augment nerve injury repair. Neurosurgery 2009, 65, A80–A86. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kolar, M.K.; Novikova, L.N.; Novikov, L.N.; Wiberg, M. Stimulating the Neurotrophic and Angiogenic Properties of Human Adipose-Derived Stem Cells Enhances Nerve Repair. Stem Cells Dev. 2014, 23, 741–754. [Google Scholar] [CrossRef]
- Wang, J.; Ding, F.; Gu, Y.; Liu, J.; Gu, X.S. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009, 1262, 7–15. [Google Scholar] [CrossRef]
- Kolar, M.K.; Itte, V.N.; Kingham, P.J.; Novikov, L.N.; Wiberg, M.; Kelk, P. The neurotrophic effects of different human dental mesenchymal stem cells. Sci. Rep. 2017, 7, 12605. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Kitada, M.; Ishikawa, H.; Itokazu, Y.; Wakao, S.; Dezawa, M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem. Biophys. Res. Commun. 2007, 359, 915–920. [Google Scholar] [CrossRef]
- Wakao, S.; Hayashi, T.; Kitada, M.; Kohama, M.; Matsue, D.; Teramoto, N.; Ose, T.; Itokazu, Y.; Koshino, K.; Watabe, H.; et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp. Neurol. 2010, 223, 537–547. [Google Scholar] [CrossRef]
- Sethi, R.; Redmond, A.; Lavik, E. Olfactory Ensheathing Cells Promote Differentiation of Neural Stem Cells and Robust Neurite Extension. Stem Cell Rev. Rep. 2014, 10, 772–785. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Z.; Sakiyama-Elbert, S.E. Matrices, scaffolds & carriers for cell delivery in nerve regeneration. Exp. Neurol. 2018, 319, 112837. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Decante, G.; Costa, J.B.; Silva-Correia, J.; Collins, M.N.; Reis, R.L.; Oliveira, J.M. Engineering bioinks for 3D bioprinting. Biofabrication 2021, 13, 032001. [Google Scholar] [CrossRef]
- Berg, J.; Hiller, T.; Kissner, M.S.; Qazi, T.H.; Duda, G.N.; Hocke, A.C.; Hippenstiel, S.; Elomaa, L.; Weinhart, M.; Fahrenson, C.; et al. Optimization of cell-laden bioinks for 3D bioprinting and efficient infection with influenza A virus. Sci. Rep. 2018, 8, 13877. [Google Scholar] [CrossRef] [Green Version]
- Gohl, J.; Markstedt, K.; Mark, A.; Hakansson, K.; Gatenholm, P.; Edelvik, F. Simulations of 3D bioprinting: Predicting bioprintability of nanofibrillar inks. Biofabrication 2018, 10, 034105. [Google Scholar] [CrossRef]
- Petcu, E.B.; Midha, R.; McColl, E.; Popa-Wagner, A.; Chirila, T.V.; Dalton, P.D. 3D printing strategies for peripheral nerve regeneration. Biofabrication 2018, 10, 032001. [Google Scholar] [CrossRef]
- Ouyang, L.; Yao, R.; Zhao, Y.; Sun, W. Effect of bioink properties on printability and cell viability for 3D bioplotting of embryonic stem cells. Biofabrication 2016, 8, 035020. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Liu, J.; Yao, S.; Mao, H.; Peng, J.; Sun, X.; Cao, Z.; Yang, Y.; Xiao, B.; Wang, Y.; et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017, 55, 296–309. [Google Scholar] [CrossRef]
- Bensaid, W.; Triffitt, J.T.; Blanchat, C.; Oudina, K.; Sedel, L.; Petite, H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials 2003, 24, 2497–2502. [Google Scholar] [CrossRef]
- Kalbermatten, D.F.; Kingham, P.J.; Mahay, D.; Mantovani, C.; Pettersson, J.; Raffoul, W.; Balcin, H.; Pierer, G.; Terenghi, G. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 669–675. [Google Scholar] [CrossRef]
- Jones, I.; Novikova, L.N.; Wiberg, M.; Carlsson, L.; Novikov, L.N. Human Embryonic Stem Cell-derived Neural Crest Cells Promote Sprouting and Motor Recovery Following Spinal Cord Injury in Adult Rats. Cell Transpl. 2021, 30, 963689720988245. [Google Scholar] [CrossRef]
- Chang, R.; Nam, Y.; Sun, W. Direct cell writing of 3D microorgan for in vitro pharmacokinetic model. Tissue Eng. Part C-Methods 2008, 14, 157–166. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of hydrogels for bio-printing applications. J. Biomed. Mater. Res. Part A 2013, 101, 272–284. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.J.; Chung, S.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Cell-laden 3D bioprinting hydrogel matrix depending on different compositions for soft tissue engineering: Characterization and evaluation. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 71, 678–684. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Schreyer, D.J.; Chen, X. Effect of needle geometry on flow rate and cell damage in the dispensing-based biofabrication process. Biotechnol. Prog. 2011, 27, 1777–1784. [Google Scholar] [CrossRef]
- Li, M.; Tian, X.; Zhu, N.; Schreyer, D.J.; Chen, X. Modeling Process-Induced Cell Damage in the Biodispensing Process. Tissue Eng. Part C-Methods 2010, 16, 533–542. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Jahnen-Dechent, W.; Labude, N.; Bovi, M.; Hieronymus, T.; Zenke, M.; Schneider, R.K.; Neuss, S. Cord blood-hematopoietic stem cell expansion in 3D fibrin scaffolds with stromal support. Biomaterials 2012, 33, 6987–6997. [Google Scholar] [CrossRef]
- Catelas, I.; Sese, N.; Wu, B.M.; Dunn, J.C.; Helgerson, S.; Tawil, B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006, 12, 2385–2396. [Google Scholar] [CrossRef]
- Murphy, K.C.; Fang, S.Y.; Leach, J.K. Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing. Cell Tissue Res. 2014, 357, 91–99. [Google Scholar] [CrossRef]
- Bauernfeind, A.L.; Babbitt, C.C. The predictive nature of transcript expression levels on protein expression in adult human brain. BMC Genom. 2017, 18, 322. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Beyer, A.; Aebersold, R. On the Dependency of Cellular Protein Levels on mRNA Abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef] [Green Version]
- Alakpa, E.V.; Jayawarna, V.; Lampel, A.; Burgess, K.V.; West, C.C.; Bakker, S.C.J.; Roy, S.; Javid, N.; Fleming, S.; Lamprou, D.A.; et al. Tunable Supramolecular Hydrogels for Selection of Lineage-Guiding Metabolites in Stem Cell Cultures. Chem 2016, 1, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, A.; Ling, Q.-D.; Chang, Y.; Hsu, S.-T.; Umezawa, A. Physical Cues of Biomaterials Guide Stem Cell Differentiation Fate. Chem. Rev. 2013, 113, 3297–3328. [Google Scholar] [CrossRef]
- Krampera, M.; Marconi, S.; Pasini, A.; Galie, M.; Rigotti, G.; Mosna, F.; Tinelli, M.; Lovato, L.; Anghileri, E.; Andreini, A.; et al. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone 2007, 40, 382–390. [Google Scholar] [CrossRef]
- Ladak, A.; Olson, J.; Tredget, E.E.; Gordon, T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat model. Exp. Neurol. 2011, 228, 242–252. [Google Scholar] [CrossRef]
- Mareschi, K.; Novara, M.; Rustichelli, D.; Ferrero, I.; Guido, D.; Carbone, E.; Medico, E.; Madon, E.; Vercelli, A.; Fagioli, F. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp. Hematol. 2006, 34, 1563–1572. [Google Scholar] [CrossRef]
- Ching, R.C.; Wiberg, M.; Kingham, P.J. Schwann cell-like differentiated adipose stem cells promote neurite outgrowth via secreted exosomes and RNA transfer. Stem Cell Res. Ther. 2018, 9, 266. [Google Scholar] [CrossRef]
- Xu, X.Y.; Geremia, N.; Bao, F.; Pniak, A.; Rossoni, M.; Brown, A. Schwann Cell Coculiture Improves the Therapeutic Effect of Bone Marrow Stromal Cells on Recovery in Spinal Cord-Injured Mice. Cell Transplant. 2011, 20, 1065–1086. [Google Scholar] [CrossRef] [Green Version]
- Jones, I.; Novikova, L.N.; Novikov, L.N.; Renardy, M.; Ullrich, A.; Wiberg, M.; Carlsson, L.; Kingham, P.J. Regenerative effects of human embryonic stem cell-derived neural crest cells for treatment of peripheral nerve injury. J. Tissue Eng. Regen. Med. 2018, 12, E2099–E2109. [Google Scholar] [CrossRef] [Green Version]
- Ning, L.; Sun, H.; Lelong, T.; Guilloteau, R.; Zhu, N.; Schreyer, D.J.; Chen, X. 3D bioprinting of scaffolds with living Schwann cells for potential nerve tissue engineering applications. Biofabrication 2018, 10, 035014. [Google Scholar] [CrossRef]
- Oraee-Yazdani, S.; Akhlaghpasand, M.; Golmohammadi, M.; Hafizi, M.; Zomorrod, M.S.; Kabir, N.M.; Oraee-Yazdani, M.; Ashrafi, F.; Zali, A.; Soleimani, M. Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: Safety considerations and possible outcomes. Stem Cell Res. Ther. 2021, 12, 445. [Google Scholar] [CrossRef]
- Farrukh, A.; Zhao, S.; del Campo, A. Microenvironments Designed to Support Growth and Function of Neuronal Cells. Front. Mater. 2018, 5, 62. [Google Scholar] [CrossRef]
- Ortiz, A.C.; Fideles, S.O.M.; Pomini, K.T.; Bellini, M.Z.; Pereira, E.; Reis, C.H.B.; Pilon, J.P.G.; de Marchi, M.A.; Trazzi, B.F.M.; da Silva, W.S.; et al. Potential of Fibrin Glue and Mesenchymal Stem Cells (MSCs) to Regenerate Nerve Injuries: A Systematic Review. Cells 2022, 11, 221. [Google Scholar] [CrossRef]
- Kolar, M.K.; Kingham, P.J.; Novikova, L.N.; Wiberg, M.; Novikov, L.N. The Therapeutic Effects of Human Adipose-Derived Stem Cells in a Rat Cervical Spinal Cord Injury Model. Stem Cells Dev. 2014, 23, 1659–1674. [Google Scholar] [CrossRef]
- Novikova, L.N.; Brohlin, M.; Kingham, P.J.; Novikov, L.N.; Wiberg, M. Neuroprotective and growth-promoting effects of bone marrow stromal cells after cervical spinal cord injury in adult rats. Cytotherapy 2011, 13, 873–887. [Google Scholar] [CrossRef]
- Novikova, L.N.; Pettersson, J.; Brohlin, M.; Wiberg, M.; Novikov, L.N. Biodegradable poly-beta-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials 2008, 29, 1198–1206. [Google Scholar] [CrossRef]
- Caddick, J.; Kingham, P.J.; Gardiner, N.J.; Wiberg, M.; Terenghi, G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 2006, 54, 840–849. [Google Scholar] [CrossRef]
- Pettersson, L.F.; Kingham, P.J.; Wiberg, M.; Kelk, P. In Vitro Osteogenic Differentiation of Human Mesenchymal Stem Cells from Jawbone Compared with Dental Tissue. Tissue Eng. Regen. Med. 2017, 14, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s C-T difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Blackman, A.V.; Oyrer, J.; Jayabal, S.; Chung, A.J.; Watt, A.J.; Sjostrom, P.J.; van Meyel, D.J. Neuronal morphometry directly from bitmap images. Nat. Methods 2014, 11, 982–984. [Google Scholar] [CrossRef] [Green Version]


| Gene Name | Sequence: | 5′ → 3′ | Annealing Temperatures (°C) |
|---|---|---|---|
| ANGPT1 | Forward Reverse | GAA AAT TAT ACT CAG TGG CTG GAA AA TTC TAG GAT TTT ATG CTC TAA TAA ACT | 58.4 |
| BDNF | Forward Reverse | ATG GGA CTC TGG AGA GCG TGA A CGC CAG CCA ATT CTC TTT TTG C | 66.9 |
| GDNF | Forward Reverse | GGG TTT AGC TTT CAA GGG CTG A CGGAACAGACAGACAAATGG | 61.8 |
| NGF | Forward Reverse | AAG GAT CCT GGA CCC AAG CTC ACC TCA GAG TGA CGT GGA TGA GCG CTT GCT CCT | 74.1 |
| NTF3 | Forward Reverse | CTT ATC TCC GTG GCA TCC AAG G TCT GAA GTC AGT GCT CGG ACG T | 65.6 |
| VEGFA | Forward Reverse | TGC ACC CAC GAC AGA AGG GGA TCA CCG CCT TGG CTT GTC ACA | 70.9 |
| GAPDH | Forward Reverse | CCC CCA ATG TAT CCG TTG TG TAG CCC AGG ATG CCC TTT A | 63.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alakpa, E.V.; Bahrd, A.; Wiklund, K.; Andersson, M.; Novikov, L.N.; Ljungberg, C.; Kelk, P. Bioprinted Schwann and Mesenchymal Stem Cell Co-Cultures for Enhanced Spatial Control of Neurite Outgrowth. Gels 2023, 9, 172. https://doi.org/10.3390/gels9030172
Alakpa EV, Bahrd A, Wiklund K, Andersson M, Novikov LN, Ljungberg C, Kelk P. Bioprinted Schwann and Mesenchymal Stem Cell Co-Cultures for Enhanced Spatial Control of Neurite Outgrowth. Gels. 2023; 9(3):172. https://doi.org/10.3390/gels9030172
Chicago/Turabian StyleAlakpa, Enateri V., Anton Bahrd, Krister Wiklund, Magnus Andersson, Lev N. Novikov, Christina Ljungberg, and Peyman Kelk. 2023. "Bioprinted Schwann and Mesenchymal Stem Cell Co-Cultures for Enhanced Spatial Control of Neurite Outgrowth" Gels 9, no. 3: 172. https://doi.org/10.3390/gels9030172
APA StyleAlakpa, E. V., Bahrd, A., Wiklund, K., Andersson, M., Novikov, L. N., Ljungberg, C., & Kelk, P. (2023). Bioprinted Schwann and Mesenchymal Stem Cell Co-Cultures for Enhanced Spatial Control of Neurite Outgrowth. Gels, 9(3), 172. https://doi.org/10.3390/gels9030172






