The Antimicrobial Activity of Micron-Thin Sol–Gel Films Loaded with Linezolid and Cefoxitin for Local Prevention of Orthopedic Prosthesis-Related Infections
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Samples Preparation
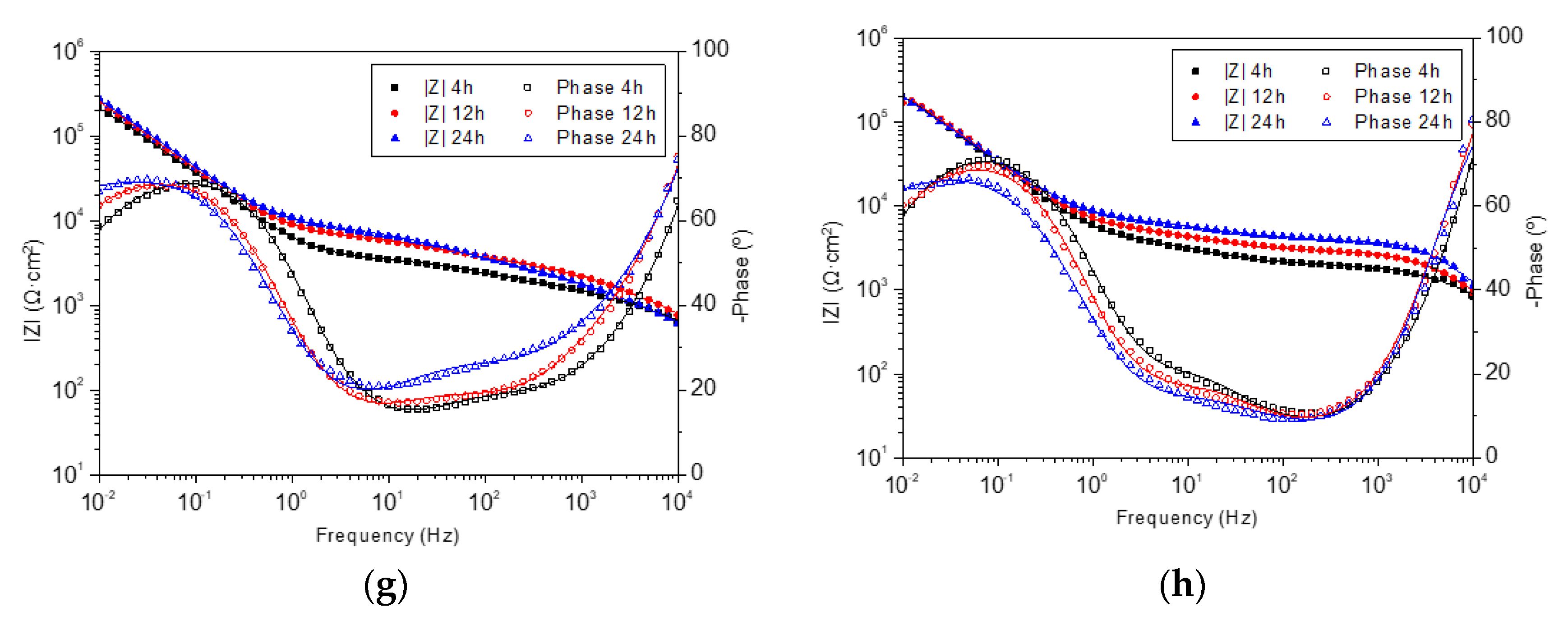
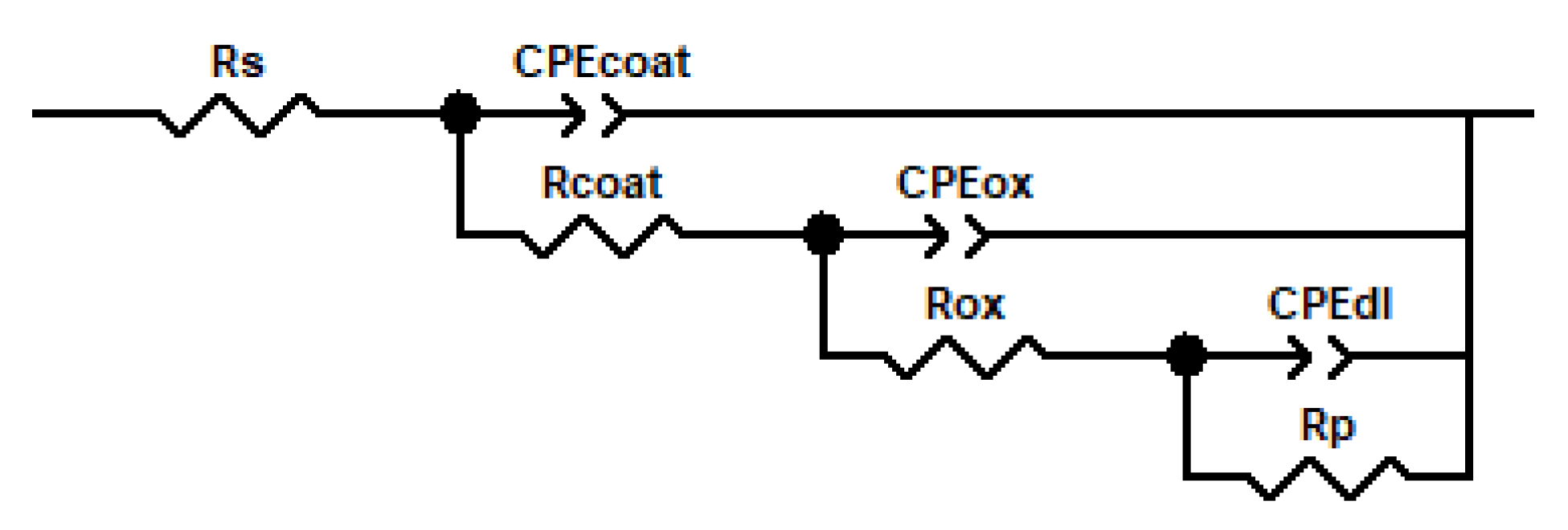
2.2. Coating Degradation Study
2.3. Antibiotics Release Study
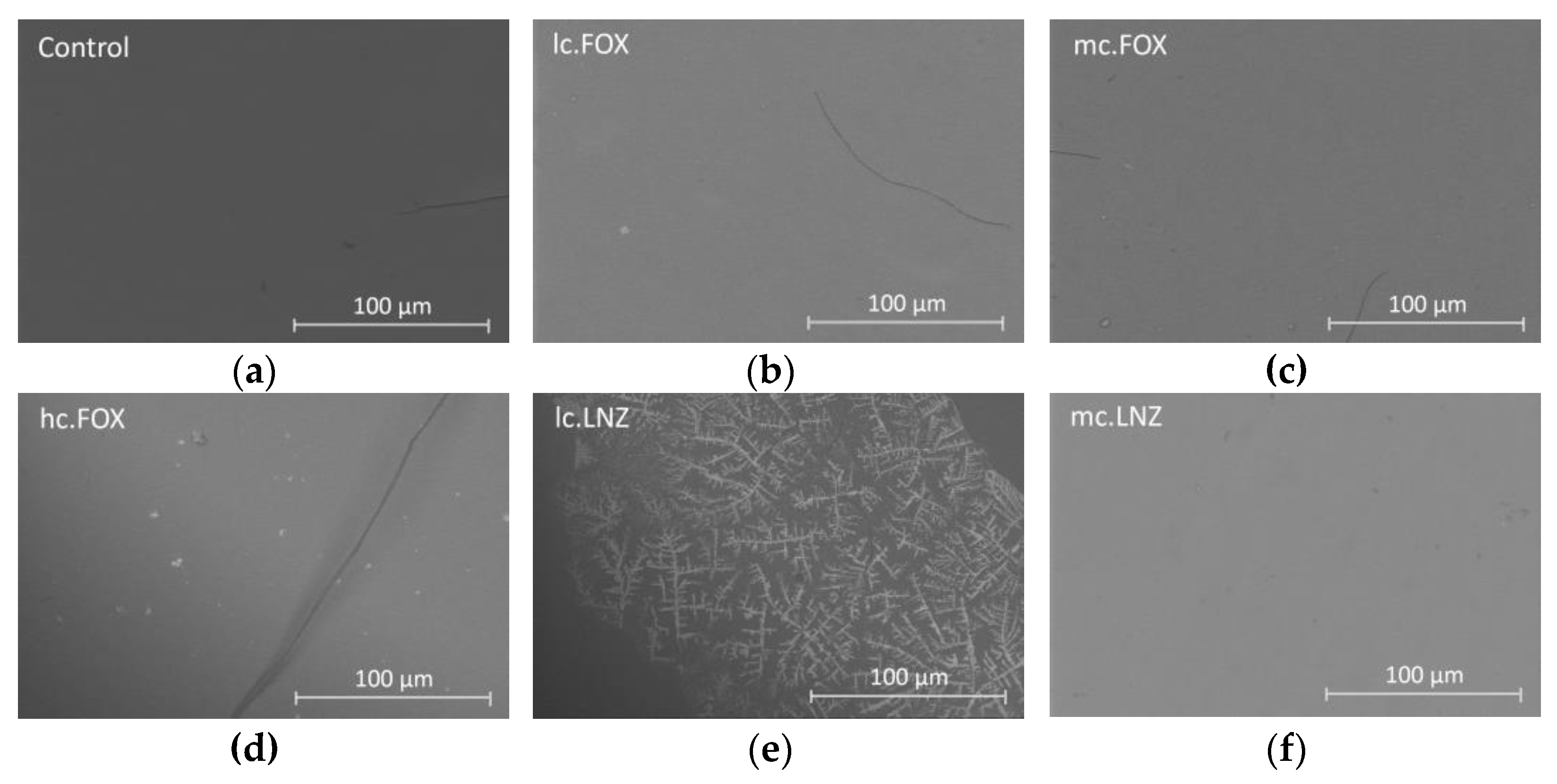
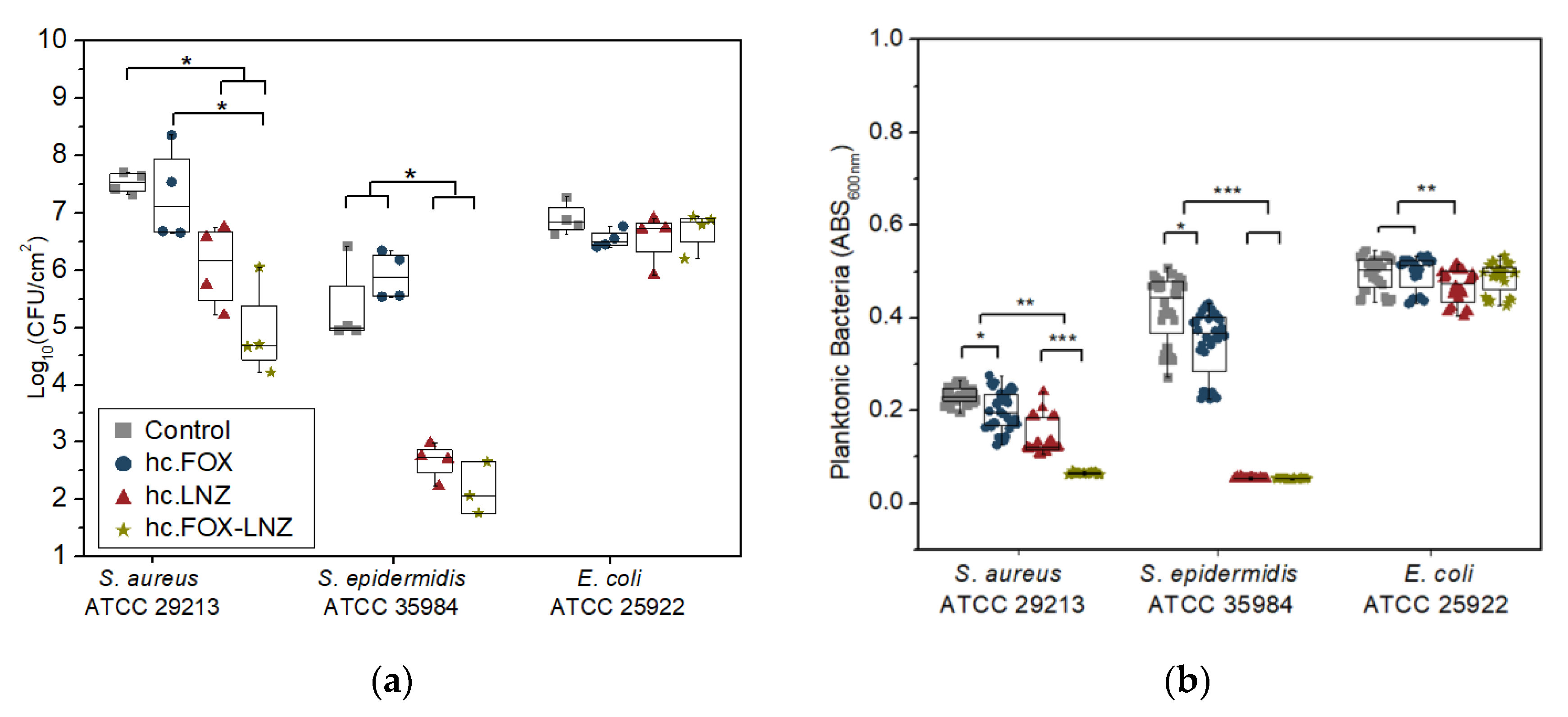
2.4. Microbiological Assays
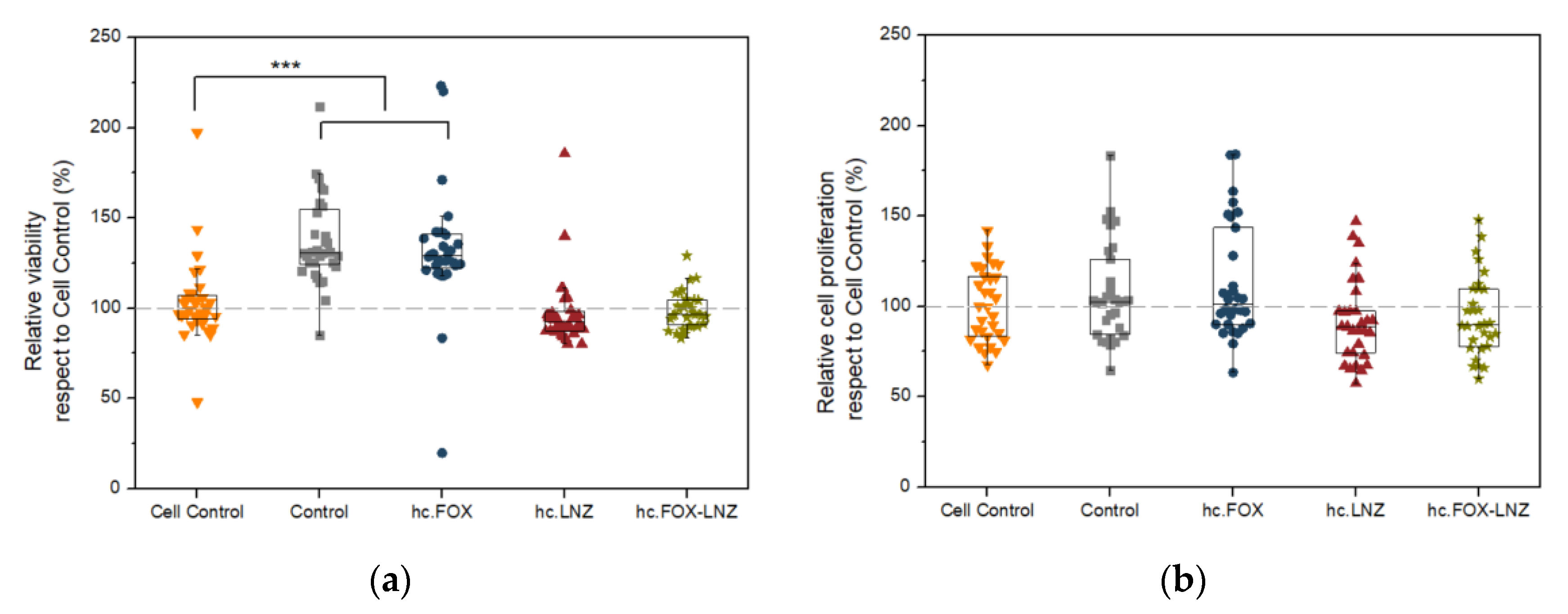
2.5. Cellular Studies
2.6. Research Limitations
3. Conclusions
4. Materials and Methods
4.1. Sol–Gel Synthesis and Coatings Preparation
4.2. Study of Sol–Gel Degradation
4.3. Kinetics Study of Antibiotics Release
4.4. Evaluation of Biofilm Formation Inhibition
4.5. Cell Study
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benito, N.; Franco, M.; Ribera, A.; Soriano, A.; Rodriguez-Pardo, D.; Sorlí, L.; Fresco, G.; Fernández-Sampedro, M.; Dolores del Toro, M.; Guío, L.; et al. Time trends in the aetiology of prosthetic joint infections: A multicentre cohort study. Clin. Microbiol. Infect. 2016, 22, 732.e1–732.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, N.; Mur, I.; Ribera, A.; Soriano, A.; Rodríguez-Pardo, D.; Sorlí, L.; Cobo, J.; Fernández-Sampedro, M.; del Toro, M.D.; Guío, L.; et al. The Different Microbial Etiology of Prosthetic Joint Infections according to Route of Acquisition and Time after Prosthesis Implantation, Including the Role of Multidrug-Resistant Organisms. J. Clin. Med. 2019, 8, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanellakopoulou, K.; Giamarellos-Bourboulis, E.J. Carrier systems for the local delivery of antibiotics in bone infections. Drugs 2000, 59, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.P.; Courtney, H.; Bumgardner, J.D.; Haggard, W.O. Chitosan Films: A Potential Local Drug Delivery System for Antibiotics. Clin. Orthop. Relat. Res. 2008, 466, 1377–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamret, F.; Colin, M.; Mongaret, C.; Gangloff, S.C.; Reffuveille, F. Antibiotic Tolerance of Staphylococcus aureus Biofilm in Periprosthetic Joint Infections and Antibiofilm Strategies. Antibiotics 2020, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Aslam, B.; Muzammil, S.; Aslam, N.; Shahid, M.; Almatroudi, A.; Allemailem, K.S.; Saqalein, M.; Nisar, M.A.; Rasool, M.H.; et al. The prospects of antimicrobial coated medical implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 1–21. [Google Scholar] [CrossRef]
- Brooks, B.D.; Brooks, A.E. Therapeutic strategies to combat antibiotic resistance. Adv. Drug Deliv. Rev. 2014, 78, 14–27. [Google Scholar] [CrossRef]
- Cao, G.; Yan, J.; Ning, X.; Zhang, Q.; Wu, Q.; Bi, L.; Zhang, Y.; Han, Y.; Guo, J. Antibacterial and antibiofilm properties of graphene and its derivatives. Colloids Surf. B Biointerfaces 2021, 200, 111588. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Zeng, Y.-R. Garlic extract in prosthesis-related infections: A literature review. J. Int. Med. Res. 2020, 48, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zapata, M.; Tovar, C.; Hernandez, J. The Role of Chitosan and Graphene Oxide in Bioactive and Antibacterial Properties of Acrylic Bone Cements. Biomolecules 2020, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Lisoń, J.; Taratuta, A.; Paszenda, Z.; Szindler, M.; Basiaga, M. Perspectives in Prevention of Biofilm for Medical Applications. Coatings 2022, 12, 197. [Google Scholar] [CrossRef]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azadani, R.N.; Sabbagh, M.; Salehi, H.; Cheshmi, A.; Kumari, A.R.B.; Erabi, G. Sol-gel: Uncomplicated, routine and affordable synthesis procedure for utilization of composites in drug delivery: Review. J. Compos. Compd. 2021, 2, 57–70. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef] [Green Version]
- Figueira, R.M.B.B.M.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coatings 2016, 6, 12. [Google Scholar] [CrossRef] [Green Version]
- Catauro, M.; Ciprioti, S. Characterization of Hybrid Materials Prepared by Sol-Gel Method for Biomedical Implementations. A Critical Review. Materials 2021, 14, 1788. [Google Scholar] [CrossRef]
- Saginur, R.; StDenis, M.; Ferris, W.; Aaron, S.; Chan, F.; Lee, C.; Ramotar, K. Multiple Combination Bactericidal Testing of Staphylococcal Biofilms from Implant-Associated Infections. Antimicrob. Agents Chemother. 2006, 50, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Greimel, F.; Scheuerer, C.; Gessner, A.; Simon, M.; Kalteis, T.; Grifka, J.; Benditz, A.; Springorum, H.-R.; Schaumburger, J. Efficacy of antibiotic treatment of implant-associated Staphylococcus aureus infections with moxifloxacin, flucloxacillin, rifampin, and combination therapy: An animal study. Drug Des. Dev. Ther. 2017, 11, 1729–1736. [Google Scholar] [CrossRef] [Green Version]
- Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol–gel films on titanium alloy fracture plate material. Biomaterials 2007, 28, 1721–1729. [Google Scholar] [CrossRef]
- Adams, C.S.; Antoci, V.; Harrison, G.; Patal, P.; Freeman, T.A.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J.; Radin, S.; Ducheyne, P. Controlled release of vancomycin from thin sol-gel films on implant surfaces successfully controls osteomyelitis. J. Orthop. Res. 2009, 27, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Agrawal, A.; Knabe, C.; Ducheyne, P. Sol–gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. Biomaterials 2014, 35, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Knabe, C.; Burke, M.; Radin, S.; Garino, J.; Schaer, T.; Ducheyne, P. Bactericidal Micron-Thin Sol–Gel Films Prevent Pin Tract and Periprosthetic Infection. Mil. Med. 2014, 179, 29–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, H.; Knabe, C.; Radin, S.; Garino, J.; Ducheyne, P. Percutaneous external fixator pins with bactericidal micron-thin sol–gel films for the prevention of pin tract infection. Biomaterials 2015, 62, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Hatton, P.V.; Le Maitre, C.; Smith, T.J.; Akid, R. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated titanium. Bone Jt. J. 2021, 103, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Toirac, B.; Garcia-Casas, A.; Cifuentes, S.C.; Aguilera-Correa, J.J.; Esteban, J.; Mediero, A.; Jiménez-Morales, A. Electrochemical characterization of coatings for local prevention of Candida infections on titanium-based biomaterials. Prog. Org. Coat. 2020, 146, 105681. [Google Scholar] [CrossRef]
- Romera, D.; Toirac, B.; Aguilera-Correa, J.-J.; García-Casas, A.; Mediero, A.; Jiménez-Morales, A.; Esteban, J. A Biodegradable Antifungal-Loaded Sol–Gel Coating for the Prevention and Local Treatment of Yeast Prosthetic-Joint Infections. Materials 2020, 13, 3144. [Google Scholar] [CrossRef]
- Garlito-Díaz, H.; Esteban, J.; Mediero, A.; Carias-Cálix, R.; Toirac, B.; Mulero, F.; Faus-Rodrigo, V.; Jiménez-Morales, A.; Calvo, E.; Aguilera-Correa, J. A New Antifungal-Loaded Sol-Gel Can Prevent Candida albicans Prosthetic Joint Infection. Antibiotics 2021, 10, 711. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Garcia-Casas, A.; Mediero, A.; Romera, D.; Mulero, F.; Cuevas-López, I.; Jiménez-Morales, A.; Esteban, J. A New Antibiotic-Loaded Sol-Gel Can Prevent Bacterial Prosthetic Joint Infection: From in vitro Studies to an in vivo Model. Front. Microbiol. 2020, 10, 2935. [Google Scholar] [CrossRef] [Green Version]
- Aguilera-Correa, J.J.; Vidal-Laso, R.; Carias-Cálix, R.A.; Toirac, B.; García-Casas, A.; Velasco-Rodríguez, D.; Llamas-Sillero, P.; Jiménez-Morales, A.; Esteban, J. A New Antibiotic-Loaded Sol-Gel can Prevent Bacterial Intravenous Catheter-Related Infections. Materials 2020, 13, 2946. [Google Scholar] [CrossRef]
- Bouza, E.; Muñoz, P. Monotherapy versus combination therapy for bacterial infections. Med. Clin. N. Am. 2000, 84, 1357–1389. [Google Scholar] [CrossRef] [PubMed]
- Band, V.I.; Hufnagel, D.A.; Jaggavarapu, S.; Sherman, E.X.; Wozniak, J.E.; Satola, S.W.; Farley, M.M.; Jacob, J.T.; Burd, E.M.; Weiss, D.S. Antibiotic combinations that exploit heteroresistance to multiple drugs effectively control infection. Nat. Microbiol. 2019, 4, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Flurin, L.; Greenwood-Quaintance, K.E.; Patel, R. Microbiology of polymicrobial prosthetic joint infection. Diagn. Microbiol. Infect. Dis. 2019, 94, 255–259. [Google Scholar] [CrossRef]
- Samuel, J.R.; Gould, F.K. Prosthetic joint infections: Single versus combination therapy. J. Antimicrob. Chemother. 2009, 65, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Sendi, P.; Zimmerli, W. The use of rifampin in staphylococcal orthopaedic-device-related infections. Clin. Microbiol. Infect. 2017, 23, 349–350. [Google Scholar] [CrossRef] [Green Version]
- Sendi, P.; Zimmerli, W. Antimicrobial treatment concepts for orthopaedic device-related infection. Clin. Microbiol. Infect. 2012, 18, 1176–1184. [Google Scholar] [CrossRef] [Green Version]
- Darley, E.S.R.; MacGowan, A.P. Antibiotic treatment of Gram-positive bone and joint infections. J. Antimicrob. Chemother. 2004, 53, 928–935. [Google Scholar] [CrossRef]
- Rosenfeld, M.B.; Campos, J.; Ratzan, K.R.; Uredo, I. Chemoprophylaxis with cefoxitin and cephalothin in orthopedic surgery: A comparison. Antimicrob. Agents Chemother. 1981, 19, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Schurman, D.J.; Dillingham, M. Clinical Evaluation of Cefoxitin in Treatment of Infections in 47 Orthopedic Patients. Clin. Infect. Dis. 1979, 1, 206–209. [Google Scholar] [CrossRef]
- Perkins, R.L. Surgical considerations in skin and soft-tissue infections and osteomyelitis treated with cefoxitin sodium. J. Antimicrob. Chemother. 1978, 4, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Kato, F.; Miyazaki, T.; Itoh, K.; Okamura, H.; Tohga, S. Clinical experience with cefoxitin used for the prevention of postoperative infections. Jpn. J. Antibiot. 1982, 35, 1993–1997. [Google Scholar] [PubMed]
- Toirac, B.; Garcia-Casas, A.; Monclús, M.A.; Aguilera-Correa, J.J.; Esteban, J.; Jiménez-Morales, A. Influence of Addition of Antibiotics on Chemical and Surface Properties of Sol-Gel Coatings. Materials 2022, 15, 4752. [Google Scholar] [CrossRef] [PubMed]
- Jianguo, L.; Gaoping, G.; Chuanwei, Y. EIS study of corrosion behaviour of organic coating/Dacromet composite systems. Electrochim. Acta 2005, 50, 3320–3332. [Google Scholar] [CrossRef]
- Romero-Gavilán, F.; Barros-Silva, S.; García-Cañadas, J.; Palla, B.; Izquierdo, R.; Gurruchaga, M.; Goñi, I.; Suay, J. Control of the degradation of silica sol-gel hybrid coatings for metal implants prepared by the triple combination of alkoxysilanes. J. Non-Cryst. Solids 2016, 453, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Pérez, M.J.G.; Castellanos, J.M.; Escrig, R.I.; Brugal, M.P.; García-García, C.; Zapiraín, J.J.G.; Antón, J.J.S. Evaluation of the role of surface pretreatments on the corrosion process. Correlation between conventional and electrochemical tests. Mater. Corros. 2017, 68, 1302–1313. [Google Scholar] [CrossRef]
- Taylor, R.; Sunderland, B.; Luna, G.; Czarniak, P. Evaluation of the stability of linezolid in aqueous solution and commonly used intravenous fluids. Drug Des. Dev. Ther. 2017, 11, 2087–2097. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.E.; Robins, R.H.; Bowman, P.B.; Duholke, W.K.; Farley, K.A.; Kaluzny, B.D.; Guido, J.E.; Sims, S.M.; Thamann, T.J.; Thompson, B.E.; et al. Susceptibility of morpholine substituents to photo-oxidative decomposition-identification of photo-oxidative degradants of linezolid (PNU-100766). J. Heterocycl. Chem. 1999, 36, 265–270. [Google Scholar] [CrossRef]
- Kim, K.; Luu, Y.K.; Chang, C.; Fang, D.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Incorporation and controlled release of a hydrophilic antibiotic using poly(lactide-co-glycolide)-based electrospun nanofibrous scaffolds. J. Control. Release 2004, 98, 47–56. [Google Scholar] [CrossRef]
- Back, D.A.; Bormann, N.; Calafi, A.; Zech, J.; Garbe, L.A.; Müller, M.; Willy, C.; Schmidmaier, G.; Wildemann, B. Testing of antibiotic releasing implant coatings to fight bacteria in combat-associated osteomyelitis—An in-vitro study. Int. Orthop. 2016, 40, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Boncu, T.E.; Guclu, A.U.; Catma, M.F.; Savaser, A.; Gokce, A.; Ozdemir, N. In vitro and in vivo evaluation of linezolid loaded electrospun PLGA and PLGA/PCL fiber mats for prophylaxis and treatment of MRSA induced prosthetic infections. Int. J. Pharm. 2020, 573, 118758. [Google Scholar] [CrossRef]
- Ghataty, D.S.; Amer, R.I.; Wasfi, R.; Shamma, R.N. Novel linezolid loaded bio-composite films as dressings for effective wound healing: Experimental design, development, optimization, and antimicrobial activity. Drug Deliv. 2022, 29, 3168–3185. [Google Scholar] [CrossRef]
- Miller, R.J.; Thompson, J.M.; Zheng, J.; Marchitto, M.C.; Archer, N.K.; Pinsker, B.L.; Ortines, R.V.; Jiang, X.; Martin, R.A.; Brown, I.D.; et al. In Vivo Bioluminescence Imaging in a Rabbit Model of Orthopaedic Implant-Associated Infection to Monitor Efficacy of an Antibiotic-Releasing Coating. J. Bone Jt. Surg. 2019, 101, e12. [Google Scholar] [CrossRef]
- Carreño, G.; Marican, A.; Vijayakumar, S.; Valdés, O.; Cabrera-Barjas, G.; Castaño, J.; Durán-Lara, E.F. Sustained Release of Linezolid from Prepared Hydrogels with Polyvinyl Alcohol and Aliphatic Dicarboxylic Acids of Variable Chain Lengths. Pharmaceutics 2020, 12, 982. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzmán, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Durán-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohydr. Polym. 2020, 242, 116383. [Google Scholar] [CrossRef]
- Guo, P.; Buttaro, B.A.; Xue, H.Y.; Tran, N.T.; Wong, H.L. Lipid-polymer hybrid nanoparticles carrying linezolid improve treatment of methicillin-resistant Staphylococcus aureus (MRSA) harbored inside bone cells and biofilms. Eur. J. Pharm. Biopharm. 2020, 151, 189–198. [Google Scholar] [CrossRef]
- Amanatidou, E.; Matthews, A.C.; Kuhlicke, U.; Neu, T.R.; McEvoy, J.P.; Raymond, B. Biofilms facilitate cheating and social exploitation of β-lactam resistance in Escherichia coli. Npj Biofilms Microbiomes 2019, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.P.; Poole, J.W. β-Lactam Antibiotics: Their Physicochemical Properties and Biological Activities in Relation to Structure. J. Pharm. Sci. 1971, 60, 503–532. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, R.; Vass, H.; Dawson, A.; Squires, T.; Tavaddod, S.; Allen, R.J. Stability of β-lactam antibiotics in bacterial growth media. PLoS ONE 2020, 15, e0236198. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Trittler, R.; Bohnert, J.A.; Kümmerer, K.; Pagès, J.-M.; Kern, W.V. Intracellular accumulation of linezolid in Escherichia coli, Citrobacter freundii and Enterobacter aerogenes: Role of enhanced efflux pump activity and inactivation. J. Antimicrob. Chemother. 2006, 59, 1261–1264. [Google Scholar] [CrossRef]
- Gottenbos, B.; van der Mei, H.C.; Busscher, H.J. Initial adhesion and surface growth of Staphylococcus epidermidis andPseudomonas aeruginosa on biomedical polymers. J. Biomed. Mater. Res. 2000, 50, 208–214. [Google Scholar] [CrossRef]
- Missiakas, D.M.; Schneewind, O. Growth and Laboratory Maintenance of Staphylococcus aureus. Curr. Protoc. Microbiol. 2013, 28, 9C.1.1–9C.1.9. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.; Wilson, D.J.; Feil, E.; Eyre-Walker, A. The distribution of bacterial doubling times in the wild. Proc. R. Soc. B Boil. Sci. 2018, 285, 20180789. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casas, A.; Aguilera-Correa, J.; Mediero, A.; Esteban, J.; Jimenez-Morales, A. Functionalization of sol-gel coatings with organophosphorus compounds for prosthetic devices. Colloids Surf. B Biointerfaces 2019, 181, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Holinka, M.; Moucha, C.S. Antibacterial Surface Treatment for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 13849–13880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinzi, V.; Gallusi, G.; Carli, E.; Pepe, F.; Rastelli, E.; Tecco, S. Elastodontic Devices in Orthodontics: An In-Vitro Study on Mechanical Deformation under Loading. Bioengineering 2022, 9, 282. [Google Scholar] [CrossRef]
- Pagano, S.; Lombardo, G.; Caponi, S.; Costanzi, E.; Di Michele, A.; Bruscoli, S.; Xhimitiku, I.; Coniglio, M.; Valenti, C.; Mattarelli, M.; et al. Bio-mechanical characterization of a CAD/CAM PMMA resin for digital removable prostheses. Dent. Mater. 2021, 37, e118–e130. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An overview on the environmental occurrence, toxicity, degradation, and removal methods. Bioengineered 2021, 12, 7376–7416. [Google Scholar] [CrossRef]
- Fux, C.; Shirtliff, M.; Stoodley, P.; Costerton, J. Can laboratory reference strains mirror ‘real-world’ pathogenesis? Trends Microbiol. 2005, 13, 58–63. [Google Scholar] [CrossRef]
- Li, G.; Liu, B.-S.; Zhang, Q.; Han, R. Investigation on the effect of fluorescence quenching of bovine serum albumin by cefoxitin sodium using fluorescence spectroscopy and synchronous fluorescence spectroscopy. Luminescence 2016, 31, 1054–1062. [Google Scholar] [CrossRef]
- Kumar, R.; Barakat, M.; Al-Mur, B.A.; Alseroury, F.A.; Eniola, J.O. Photocatalytic degradation of cefoxitin sodium antibiotic using novel BN/CdAl2O4 composite. J. Clean. Prod. 2020, 246, 119076. [Google Scholar] [CrossRef]
- Mali, R.R.; Gorle, A. Development and validation of Spectrophotometry methods for estimation of linezolid in bulk and in pharmaceutical Dosage formulation. J. Drug Deliv. Ther. 2019, 9, 60–65. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, A.; Ahmed, J.; Alsaiari, M.; Alkorbi, A.S.; Jalalah, M.; Alsareii, S.; Harraz, F.A. Rapid photodegradation of linezolid antibiotic and methylene blue dye over Pt nanoparticles/polypyrrole-carbon black/ZnO novel visible light photocatalyst. J. Environ. Chem. Eng. 2021, 9, 106773. [Google Scholar] [CrossRef]
- Faisal, M.; Rashed, A.; Ahmed, J.; Alsaiari, M.; Jalalah, M.; Alsareii, S.A.; Harraz, F.A. Ag nanoparticle-decorated chitosan/SrSnO3 nanocomposite for ultrafast elimination of antibiotic linezolid and methylene blue. Environ. Sci. Pollut. Res. 2022, 29, 52900–52914. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Gomez-Barrena, E.; Cordero-Ampuero, J.; Martín-De-Hijas, N.Z.; Kinnari, T.J.; Fernandez-Roblas, R. Evaluation of Quantitative Analysis of Cultures from Sonicated Retrieved Orthopedic Implants in Diagnosis of Orthopedic Infection. J. Clin. Microbiol. 2008, 46, 488–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef] [PubMed]






| Constant Release Rate up to 6 h (µg/h) | Concentration Released (µg) | |
|---|---|---|
| lc.FOX | 0.75 | 8.96 |
| mc.FOX | 1.47 | 21.51 |
| hc.FOX | 3.21 | 31.36 |
| lc.LNZ | 0.75 | 5.78 |
| mc.LNZ | 1.67 | 16.73 |
| hc.LNZ | 3.71 | 33.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toirac, B.; Aguilera-Correa, J.J.; Mediero, A.; Esteban, J.; Jiménez-Morales, A. The Antimicrobial Activity of Micron-Thin Sol–Gel Films Loaded with Linezolid and Cefoxitin for Local Prevention of Orthopedic Prosthesis-Related Infections. Gels 2023, 9, 176. https://doi.org/10.3390/gels9030176
Toirac B, Aguilera-Correa JJ, Mediero A, Esteban J, Jiménez-Morales A. The Antimicrobial Activity of Micron-Thin Sol–Gel Films Loaded with Linezolid and Cefoxitin for Local Prevention of Orthopedic Prosthesis-Related Infections. Gels. 2023; 9(3):176. https://doi.org/10.3390/gels9030176
Chicago/Turabian StyleToirac, Beatriz, John Jairo Aguilera-Correa, Aranzazu Mediero, Jaime Esteban, and Antonia Jiménez-Morales. 2023. "The Antimicrobial Activity of Micron-Thin Sol–Gel Films Loaded with Linezolid and Cefoxitin for Local Prevention of Orthopedic Prosthesis-Related Infections" Gels 9, no. 3: 176. https://doi.org/10.3390/gels9030176
APA StyleToirac, B., Aguilera-Correa, J. J., Mediero, A., Esteban, J., & Jiménez-Morales, A. (2023). The Antimicrobial Activity of Micron-Thin Sol–Gel Films Loaded with Linezolid and Cefoxitin for Local Prevention of Orthopedic Prosthesis-Related Infections. Gels, 9(3), 176. https://doi.org/10.3390/gels9030176








