Antibacterial and Anti-Acne Activity of Benzoyl Peroxide Nanoparticles Incorporated in Lemongrass Oil Nanoemulgel
Abstract
:1. Introduction
2. Results
2.1. Determination of the Optimum Wavelength and Calibration Curve of Benzoyl Peroxide
2.2. Solubility Testing of Benzoyl Peroxide in Different Oils and Surfactants
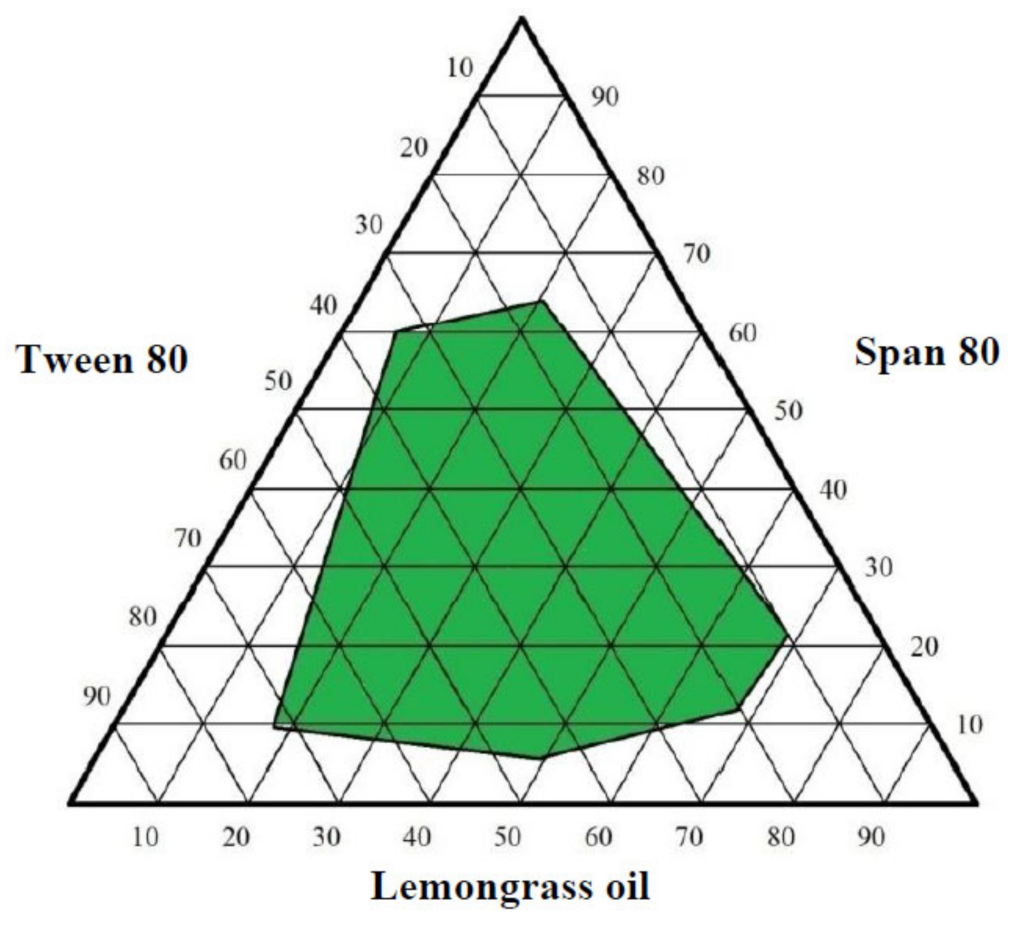
2.3. Optimization of Lemongrass Oil Nanoemulsion Formulation
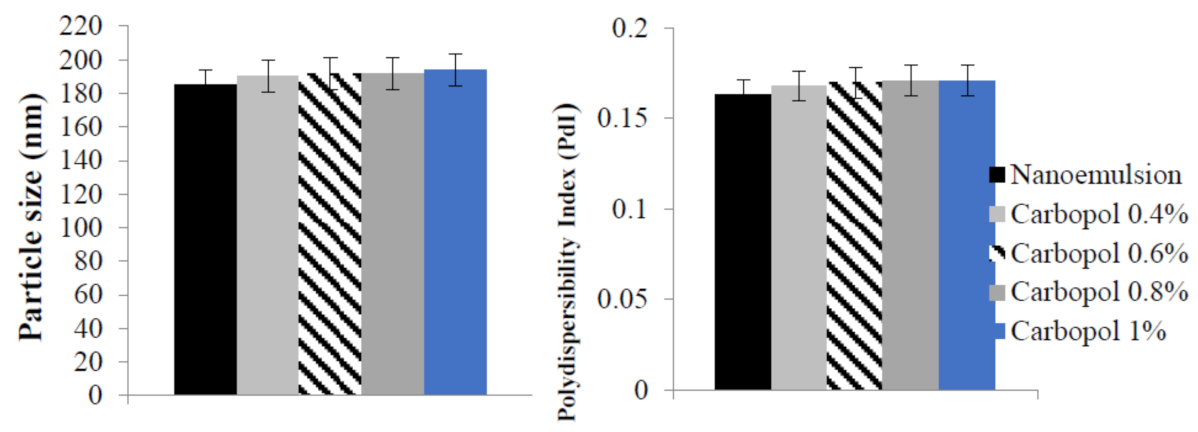
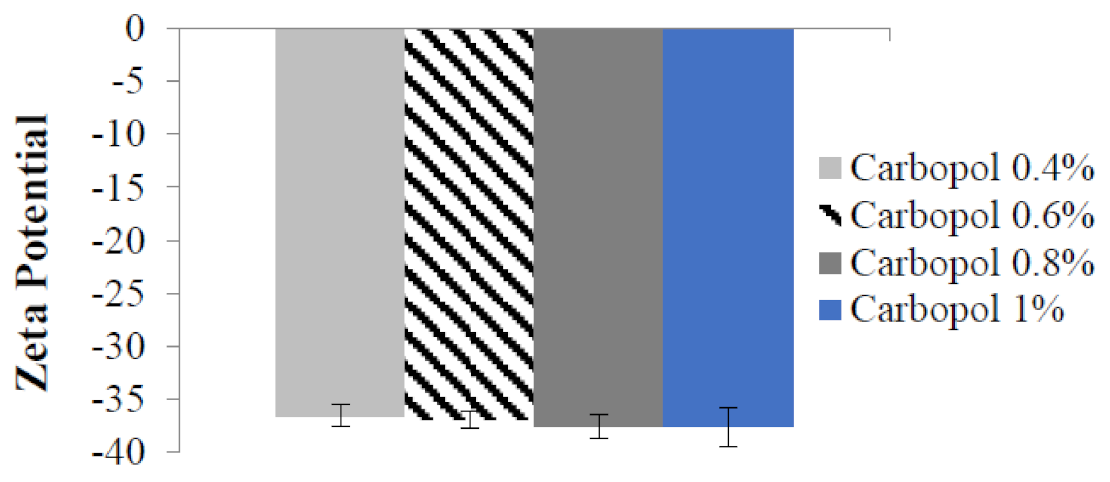
2.4. Particle Size, Polydispersity Index, and Zeta Potential of Benzoyl Peroxide Nanoemulgel
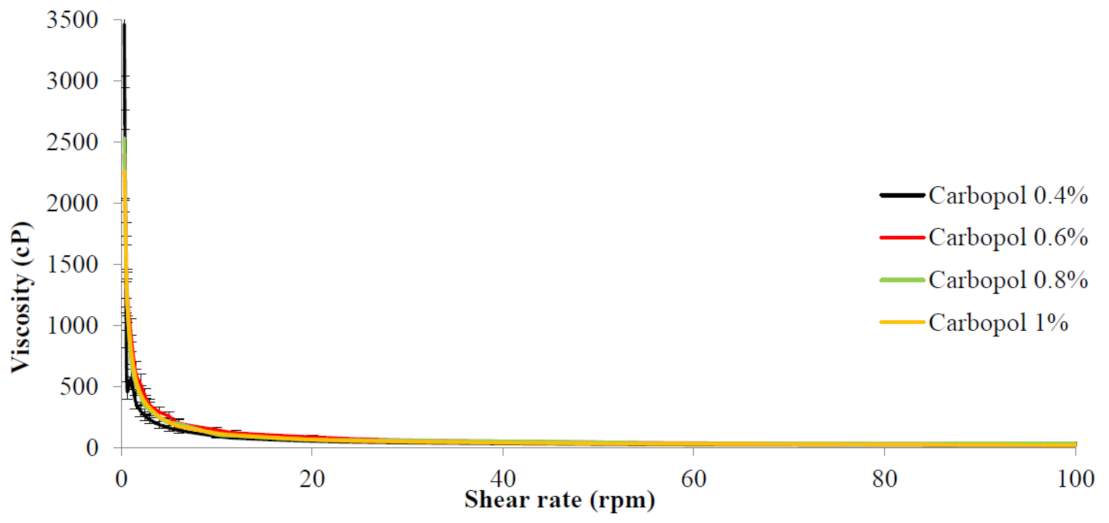
2.5. Rheological Behavior of Benzoyl Peroxide Nanoemulgel
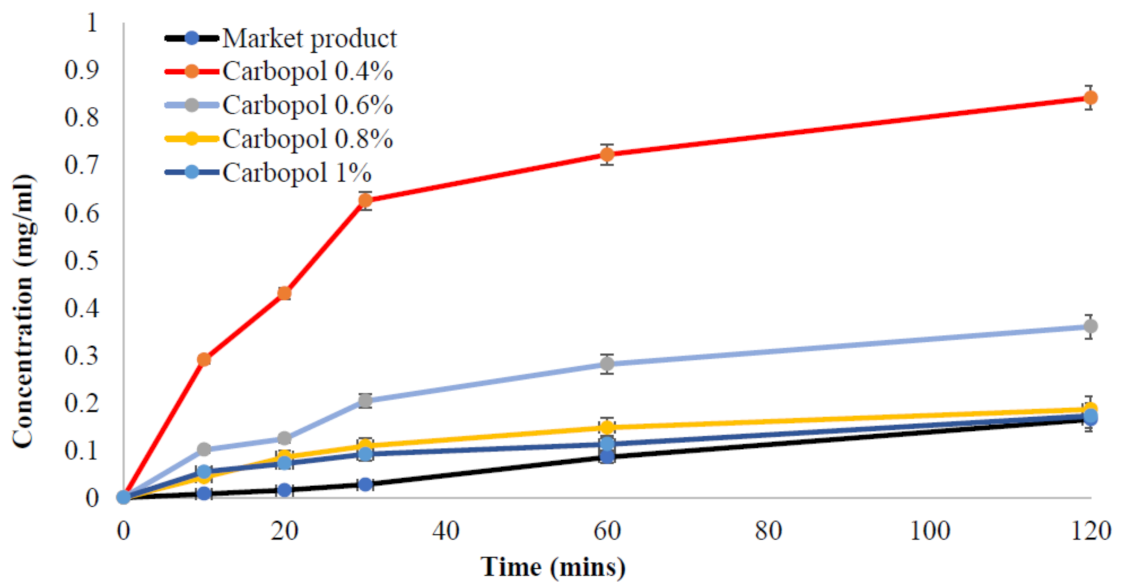
2.6. Release Test of Benzoyl Peroxide from the Nanoemulgel Formulation
2.7. Antibacterial and Anti-Acne Evaluation of Benzoyl Peroxide Nanoemulgel
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Determination of Wavelength and Calibration Curve of Benzoyl Peroxide
5.3. Solubility Analysis of Benzoyl Peroxide in Different Oils and Surfactants
5.4. Preparation of Lemongrass Oil Nanoemulsion
5.5. Loading Benzoyl Peroxide into the Nanoemulsion Formulation
5.6. Hydrogel Preparation
5.7. Nanoemulgel Preparation of Benzoyl Peroxide
5.8. Rheological Measurement for Benzoyl Peroxide Nanoemulgel
5.9. Release Test of Benzoyl Peroxide from Different Nanoemulgel Formulations
5.10. Antibacterial Test
5.10.1. Microorganisms
5.10.2. Culture Media
5.11. Anti-Acne Test
5.11.1. Microorganism
5.11.2. Culture Media
5.12. Statistical Assessment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeLouise, L.A. Applications of nanotechnology in dermatology. J. Investig. Dermatol. 2012, 132, 964–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.D.; Sahoo, A.; Shrivastav, O.P.; Charmode, N.; Kamat, R.; Kajave, N.; Chauhan, J.; Banga, S.; Tamboli, U.; Atigre, R. A Review on Aspects of Nanotechnology in Food Science and Animal Nutrition. ES Food Agrofor. 2022, 8, 12–46. [Google Scholar]
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The future medicine. J. Cutan. Aesthet. Surg. 2010, 3, 32–33. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Arianto, A.; Cindy, C. Preparation and Evaluation of Sunflower Oil Nanoemulsion as a Sunscreen. Open Access Maced. J. Med. Sci. 2019, 7, 3757–3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eid, A.M.; Istateyeh, I.; Salhi, N.; Istateyeh, T. Antibacterial Activity of Fusidic Acid and Sodium Fusidate Nanoparticles Incorporated in Pine Oil Nanoemulgel. Int. J. Nanomed. 2019, 14, 9411–9421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The antimicrobial resistance crisis: Causes, consequences, and management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, Q.-W.; Chen, Q.; Cui, C.; Duan, S.; Kang, Y.; Liu, Y.; Liu, Y.; Muhammad, W.; Shao, S. Biomedical polymers: Synthesis, properties, and applications. Sci. China Chem. 2022, 65, 1010–1075. [Google Scholar] [CrossRef]
- Jurairattanaporn, N.; Chalermchai, T.; Ophaswongse, S.; Udompataikul, M. Comparative trial of silver nanoparticle gel and 1% clindamycin gel when use in combination with 2.5% benzoyl peroxide in patients with moderate acne vulgaris. J. Med. Assoc. Thail. Chotmaihet Thangphaet 2017, 100, 78–85. [Google Scholar]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roller, L.; Gowan, J. Disease state management: Acne: Advice for your patients. Aust. J. Pharm. 2017, 98, 52–59. [Google Scholar]
- Stein Gold, L.; Baldwin, H.; Kircik, L.H.; Weiss, J.S.; Pariser, D.M.; Callender, V.; Lain, E.; Gold, M.; Beer, K.; Draelos, Z. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: A randomized phase II study of the first triple-combination drug. Am. J. Clin. Dermatol. 2022, 23, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Hauk, L. Acne Vulgaris: Treatment Guidelines from the AAD. Am. Fam. Physician 2017, 95, 740–741. [Google Scholar] [PubMed]
- Kircik, L.H. The role of benzoyl peroxide in the new treatment paradigm for acne. J. Drugs Dermatol. 2013, 12, s73–s76. [Google Scholar]
- Otlewska, A.; Baran, W.; Batycka-Baran, A. Adverse events related to topical drug treatments for acne vulgaris. Expert Opin. Drug Saf. 2020, 19, 513–521. [Google Scholar] [CrossRef]
- Sagransky, M.; Yentzer, B.A.; Feldman, S.R. Benzoyl peroxide: A review of its current use in the treatment of acne vulgaris. Expert Opin. Pharmacother. 2009, 10, 2555–2562. [Google Scholar] [CrossRef]
- Tanghetti, E. The evolution of benzoyl peroxide therapy. Cutis 2008, 82, 5–11. [Google Scholar]
- Bandyopadhyay, D. Topical antibacterials in dermatology. Indian J. Dermatol. 2021, 66, 117–125. [Google Scholar] [CrossRef]
- Tanghetti, E.A.; Popp, K.F. A current review of topical benzoyl peroxide: New perspectives on formulation and utilization. Dermatol. Clin. 2009, 27, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Brammann, C.; Müller-Goymann, C.C. An update on formulation strategies of benzoyl peroxide in efficient acne therapy with special focus on minimizing undesired effects. Int. J. Pharm. 2020, 578, 119074. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, D.; Yuan, Y.; Wu, H.; Hou, J.; Kang, W.; Bai, B. Advances of spontaneous emulsification and its important applications in enhanced oil recovery process. Adv. Colloid Interface Sci. 2020, 277, 102119. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.H.; Chow, P.S.; Macbeath, C. Microemulsion composed of combination of skin beneficial oils as vehicle: Development of resveratrol-loaded microemulsion based formulations for skin care applications. Colloids Surf. B Biointerfaces 2020, 194, 111161. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Paramasivam, S.; Arulkumar, A. Evaluation of the lemongrass plant (Cymbopogon citratus) extracted in different solvents for antioxidant and antibacterial activity against human pathogens. Asian Pac. J. Trop. Dis. 2014, 4, S134–S139. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Economakis, C.D. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innov. Food Sci. Emerg. Technol. 2007, 8, 253–258. [Google Scholar] [CrossRef]
- Soliman, W.S.; Salaheldin, S.; Amer, H.M. Chemical composition evaluation of Egyptian lemongrass, Cymbopogon citratus, essential oil. Int. J. Sci. Eng. Res 2017, 8, 630–634. [Google Scholar]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion components screening and selection: A technical note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef]
- Kustov, A.V.; Morshnev, P.K.; Kukushkina, N.y.V.; Smirnova, N.L.; Berezin, D.B.; Karimov, D.R.; Shukhto, O.V.; Kustova, T.V.; Belykh, D.V.; Mal’shakova, M.V. Solvation, cancer cell photoinactivation and the interaction of chlorin photosensitizers with a potential passive carrier non-ionic surfactant Tween 80. Int. J. Mol. Sci. 2022, 23, 5294. [Google Scholar] [CrossRef]
- Nechaev, A.I.; Voronina, N.S.; Valtsifer, V.A.; Strelnikov, V.N. Stability of the dispersed system in inverse emulsion polymerization of ionic acrylate monomers. Colloid Polym. Sci. 2021, 299, 1127–1138. [Google Scholar] [CrossRef]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for poorly water-soluble talinolol: Preparation, in vitro and in vivo assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltonen, L.; Hirvonen, J. Drug nanocrystals–versatile option for formulation of poorly soluble materials. Int. J. Pharm. 2018, 537, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying technologies for the stability and bioavailability of biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef] [Green Version]
- Nepal, P.R.; Han, H.K.; Choi, H.K. Preparation and in vitro-in vivo evaluation of Witepsol H35 based self-nanoemulsifying drug delivery systems (SNEDDS) of coenzyme Q(10). Eur. J. Pharm. Sci. 2010, 39, 224–232. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Desavathu, M. SNEDDS in shell: A novel approach to enhance the solubility of rosuvastatin calcium. Asian J. Pharm. 2020, 14, 399–506. [Google Scholar]
- Arriaga, L.R.; Drenckhan, W.; Salonen, A.; Rodrigues, J.A.; Íñiguez-Palomares, R.; Rio, E.; Langevin, D. On the long-term stability of foams stabilised by mixtures of nano-particles and oppositely charged short chain surfactants. Soft Matter 2012, 8, 11085–11097. [Google Scholar] [CrossRef]
- Naser, D.K.; Abbas, A.K.; Aadim, K.A. Zeta potential of Ag, Cu, ZnO, CdO and Sn nanoparticles prepared by pulse laser ablation in liquid environment. Iraqi J. Sci. 2020, 61, 2570–2581. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, X.; Gan, K.; Chen, Y.; Xu, J.; Yang, J. Effect of zeta potential on properties of foamed colloidal suspension. J. Eur. Ceram. Soc. 2019, 39, 574–583. [Google Scholar] [CrossRef]
- Kulkarni, N.S.; Ranpise, N.S.; Rathore, D.S.; Dhole, S.N. Characterization of Self-Microemulsifying Dosage Form: Special Emphasis on Zeta Potential Measurement. Int. J. Pharm. Biol. Arch. 2019, 10, 172–179. [Google Scholar]
- Salim, N.; Basri, M.; Rahman, M.A.; Abdullah, D.; Basri, H.; Salleh, A. Phase behaviour, formation and characterization of palm-based esters nanoemulsion formulation containing ibuprofen. J. Nanomedic. Nanotechnol. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiwani, S.; Sharmaa, A.D.; Naseer, A.; Singh, R. Formulation and evaluation of self emulsifying drug delivery system of ibuprofen using castor oil. Int. J. Pharm. Pharm. Sci. 2011, 4, 299–302. [Google Scholar]
- Saritha, D.; Bose, P.; Nagaraju, R. Formulation and evaluation of self emulsifying drug delivery system (SEDDS) of Ibuprofen. Int. J. Pharm. Sci. Res. 2014, 5, 3511–3519. [Google Scholar]
- Das, M.K.; Ahmed, A.B. Formulation and ex vivo evaluation of rofecoxib gel for topical application. Acta Pol. Pharm. 2007, 64, 461–467. [Google Scholar]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of nanoparticles for antimicrobial drug delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, Q.-U.-A.; Kanwal, A.; Qaseem, S.; Naeem, M.; Ali, S.R.; Shaffique, M.; Maqbool, M. Size-dependent inhibition of bacterial growth by chemically engineered spherical ZnO nanoparticles. J. Biol. Phys. 2019, 45, 147–159. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef]
- Marslin, G.; Selvakesavan, R.K.; Franklin, G.; Sarmento, B.; Dias, A.C.P. Antimicrobial activity of cream incorporated with silver nanoparticles biosynthesized from Withania somnifera. Int. J. Nanomed. 2015, 10, 5955–5963. [Google Scholar] [CrossRef] [Green Version]
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538. [Google Scholar] [CrossRef] [Green Version]
- Faiyazuddin, M.; Baboota, S.; Ali, J.; Ahuja, A.; Ahmad, S. Characterization and in vitro bioactive studies of lemongrass oil phytonanoemulsion system in the treatment of acne vulgaris. Int. J. Essent. Oil Ther. 2009, 3, 13–21. [Google Scholar]
- Eid, A.M.; Hawash, M. Biological evaluation of Safrole oil and Safrole oil Nanoemulgel as antioxidant, antidiabetic, antibacterial, antifungal and anticancer. BMC Complement. Med. Ther. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Jaradat, N.; Issa, L.; Abu-Hasan, A.; Salah, N.; Dalal, M.; Mousa, A.; Zarour, A. Evaluation of anticancer, antimicrobial, and antioxidant activities of rosemary (Rosmarinus Officinalis) essential oil and its Nanoemulgel. Eur. J. Integr. Med. 2022, 55, 102175. [Google Scholar] [CrossRef]
- Battistin, M.; Durini, E.; Dissette, V.; Bonetto, A.; Marcomini, A.; Casagrande, E.; Brunetta, A.; Ziosi, P.; Molesini, S.; Gavioli, R. Synthesis and characterization of new multifunctional self-boosted filters for UV protection: ZnO complex with dihydroxyphenyl benzimidazole carboxylic acid. Molecules 2019, 24, 4546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Oils | Concentration (mg\mL) |
|---|---|
| Crystal oil | 2.3 |
| Propylene glycol | 3.2 |
| Castor oil | 6.02 |
| Corn oil | 7.76 |
| Lemongrass oil | 8.9 |
| Paraffin oil | 7.1 |
| Coriander oil | 8.6 |
| Olive oil | 7.6 |
| Rosemary oil | 7.04 |
| Tween 20 | 5.76 |
| Tween 80 | 6.27 |
| Span 20 | 3.71 |
| Span 80 | 6.2 |
| Bacteria | Market Product | BPO Nanoemulgel | Lemongrass Oil Nanoemulgel | Lemongrass Oil (Pure Oil) |
|---|---|---|---|---|
| MRSA | 16 ± 0.8 | 44 ± 1.5 | 30 ± 1.1 | 28 ± 0.4 |
| E. coli | R | R | R | R |
| Pseudomonas | R | 44 ± 1.3 | 35 ± 0.2 | 28 ± 0.7 |
| Klebsiella | R | R | R | R |
| Staphylococcus Aureus | 12 ± 0.6 | 23 ± 0.9 | 20 ± 0.8 | 19 ± 1.1 |
| Proteus | 12 ± 0.7 | 49 ± 1.2 | 42 ± 1.1 | 30 ± 0.9 |
| Candida | R | 36 ± 1.0 | 30 | 26 ± 1.0 |
| Bacteria | Market Product | BPO Nanoemulgel | Lemongrass Oil Nanoemulgel | Lemongrass Oil (Pure Oil) |
|---|---|---|---|---|
| Cutibacterium acne | 38 ± 0.8 | 50 ± 1.2 | 36 ± 1.3 | 27 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eid, A.M.; Naseef, H.; Jaradat, N.; Ghanim, L.; Moqadeh, R.; Yaseen, M. Antibacterial and Anti-Acne Activity of Benzoyl Peroxide Nanoparticles Incorporated in Lemongrass Oil Nanoemulgel. Gels 2023, 9, 186. https://doi.org/10.3390/gels9030186
Eid AM, Naseef H, Jaradat N, Ghanim L, Moqadeh R, Yaseen M. Antibacterial and Anti-Acne Activity of Benzoyl Peroxide Nanoparticles Incorporated in Lemongrass Oil Nanoemulgel. Gels. 2023; 9(3):186. https://doi.org/10.3390/gels9030186
Chicago/Turabian StyleEid, Ahmad M., Hani Naseef, Nidal Jaradat, Lina Ghanim, Roaa Moqadeh, and Miasar Yaseen. 2023. "Antibacterial and Anti-Acne Activity of Benzoyl Peroxide Nanoparticles Incorporated in Lemongrass Oil Nanoemulgel" Gels 9, no. 3: 186. https://doi.org/10.3390/gels9030186






