Single Hydrogel Particle Mechanics and Dynamics Studied by Combining Capillary Micromechanics with Osmotic Compression
Abstract
1. Introduction
2. Materials and Methods
2.1. Step Emulsification
2.2. Capillary Micromechanics Setup
3. Results and Discussion
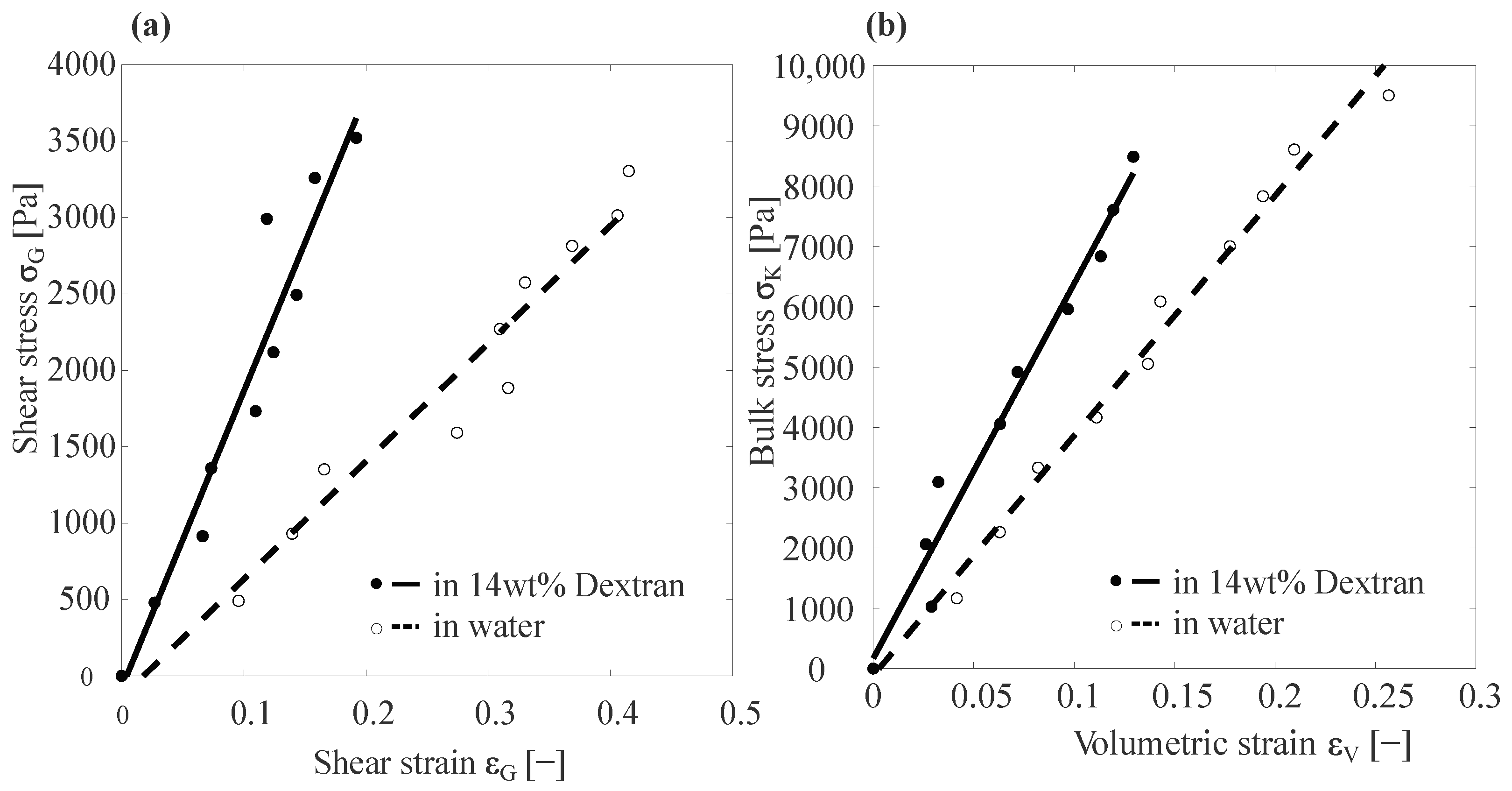
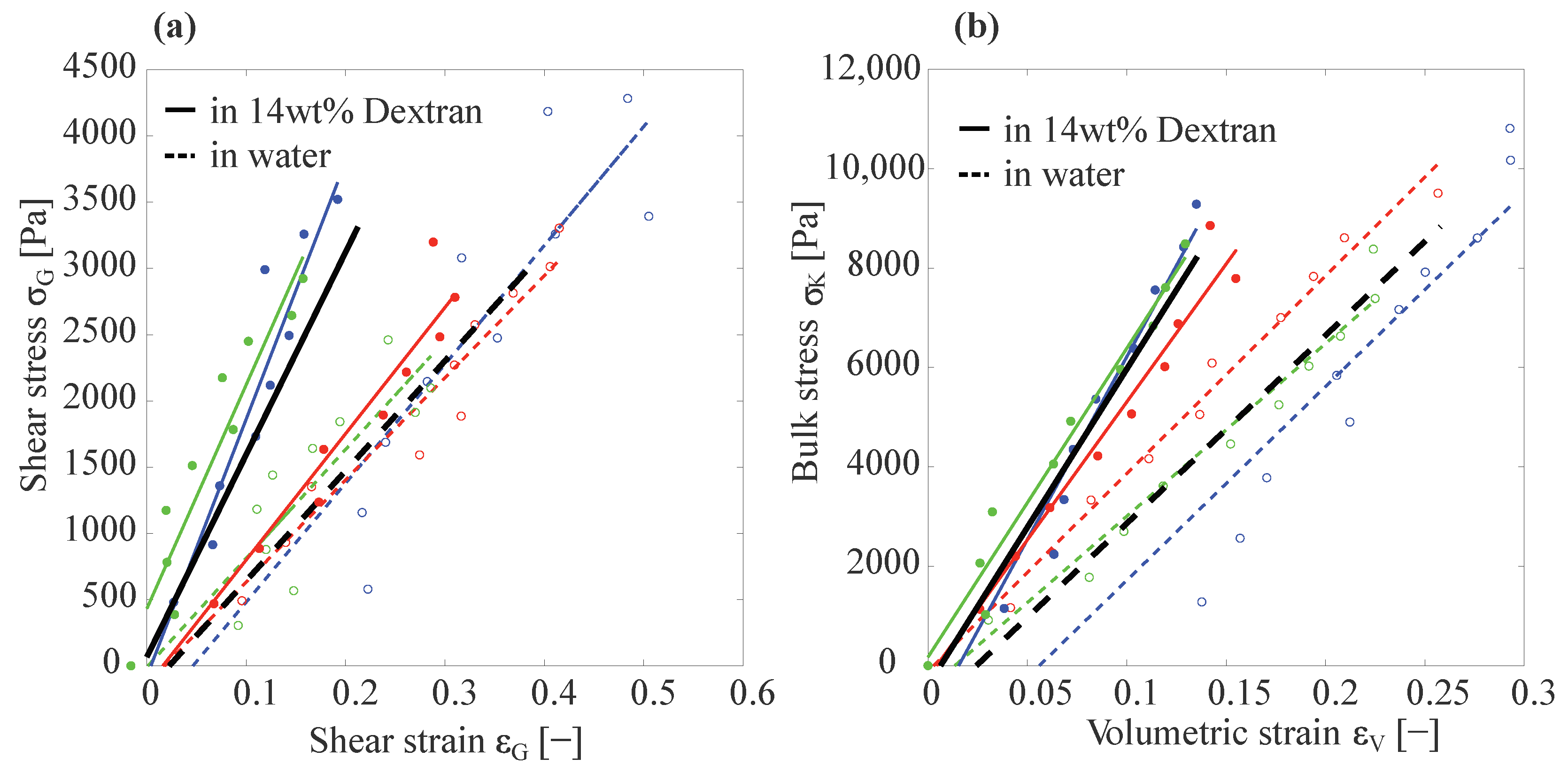
3.1. Basic Capillary Micromechanics Measurements
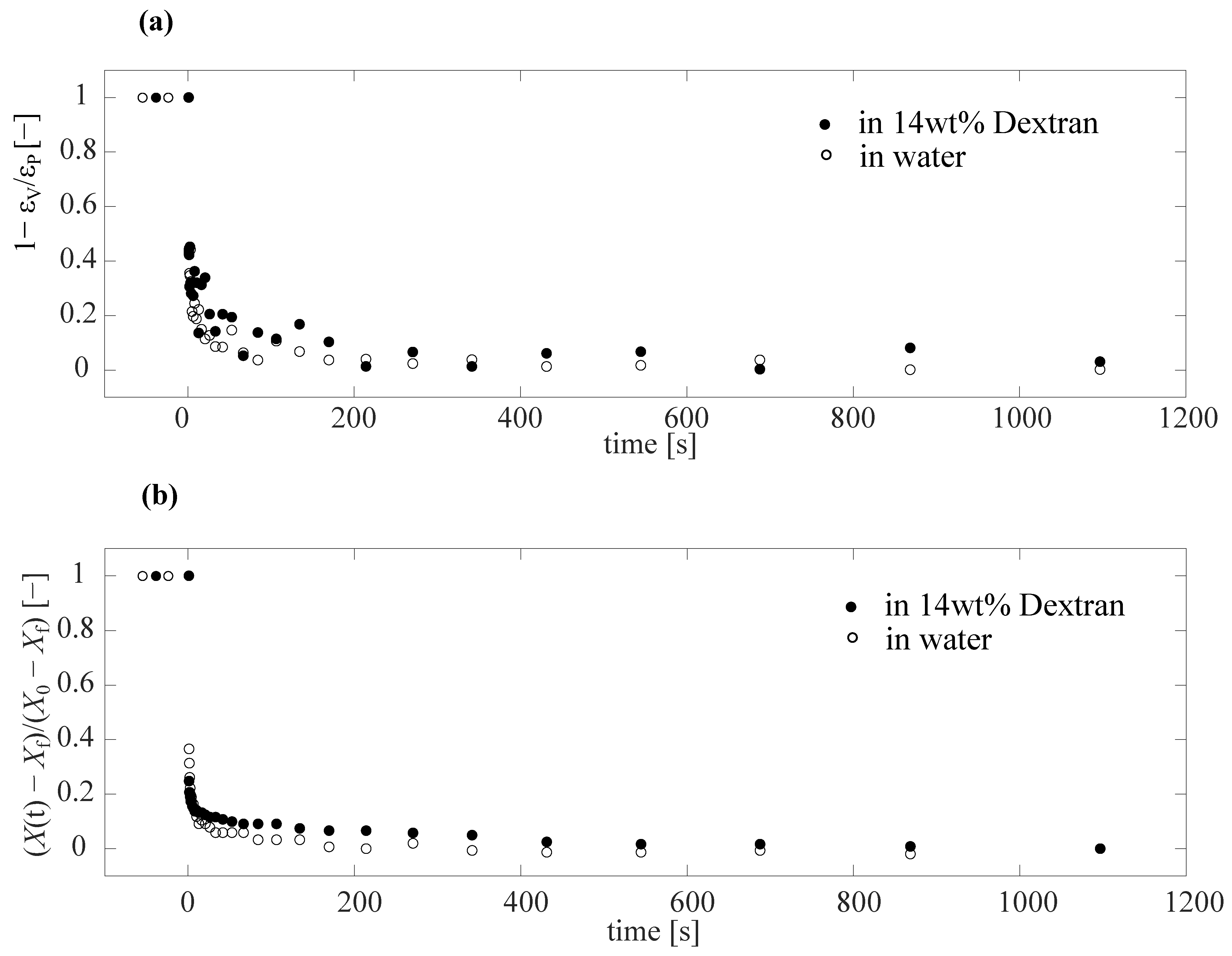
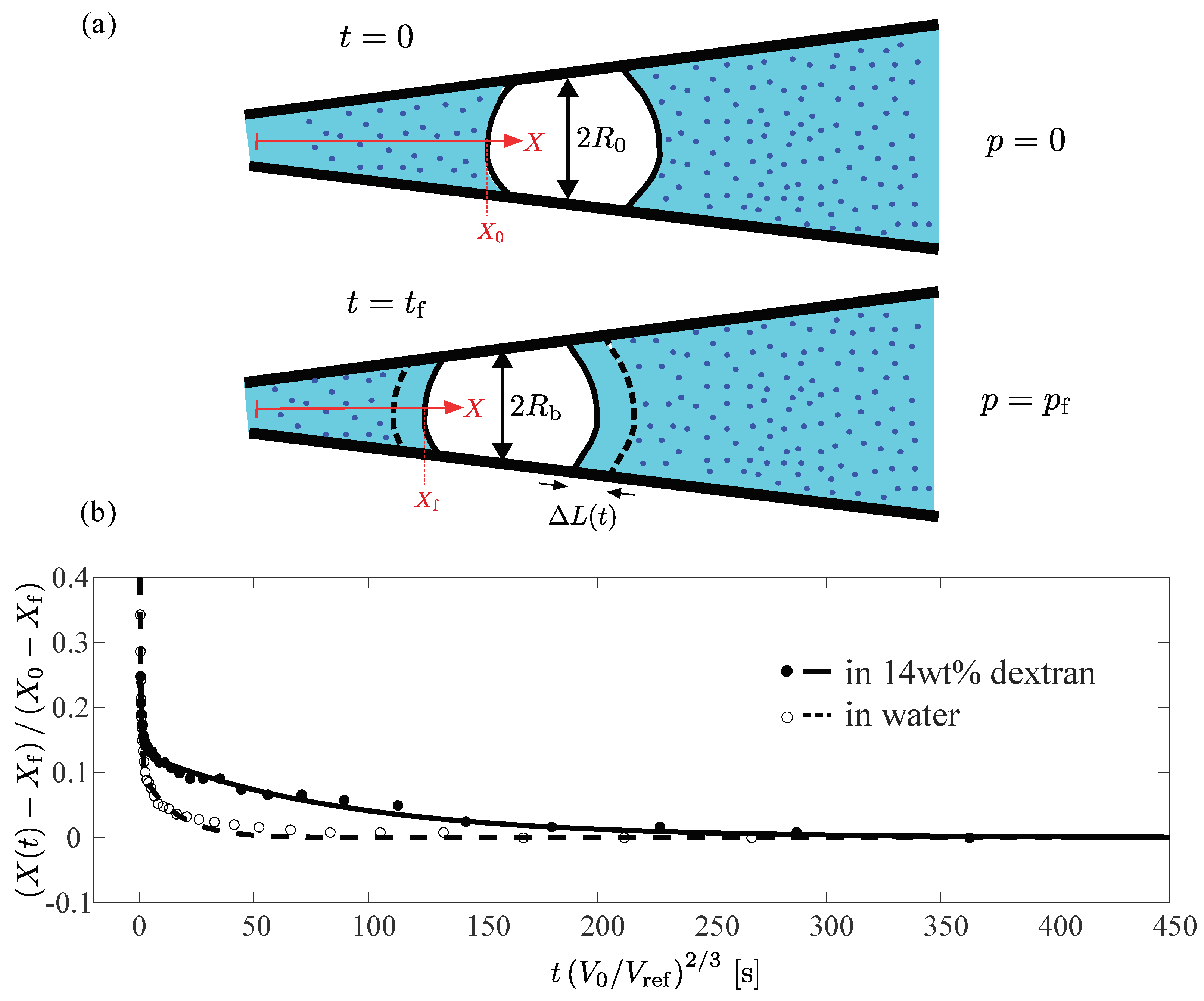
3.2. Creep Test Using Capillary Micromechanics
3.3. Simple Model to Account for Creep Test Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, P.; Zhang, J.; Dong, C.M. Photosensitive poly(o-nitrobenzyloxycarbonyl-L-lysine)-b-PEO polypeptide copolymers: Synthesis, multiple self-assembly behaviors, and the photo/pH-thermo-sensitive hydrogels. Polym. Chem. 2017, 8, 7033–7043. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, C.N.; Zeng, H.; Ji, X.; Xie, T.; Yan, X.; Wu, Z.L.; Huang, F. Reversible Ion-Conducting Switch in a Novel Single-Ion Supramolecular Hydrogel Enabled by Photoresponsive Host-Guest Molecular Recognition. Adv. Mater. 2019, 31, e1807328. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, C.; Zhou, W.; Luan, Q.; Li, W.; Deng, Q.; Dong, X.; Tang, H.; Huang, F. A pH-Responsive Gel Macrosphere Based on Sodium Alginate and Cellulose Nanofiber for Potential Intestinal Delivery of Probiotics. ACS Sustain. Chem. Eng. 2018, 6, 13924–13931. [Google Scholar] [CrossRef]
- Shang, J.; Theato, P. Smart composite hydrogel with pH-, ionic strength- and temperature-induced actuation. Soft Matter 2018, 14, 8401–8407. [Google Scholar] [CrossRef] [PubMed]
- Voudouris, P.; Florea, D.; Van Der Schoot, P.; Wyss, H.M. Micromechanics of temperature sensitive microgels: Dip in the Poisson ratio near the LCST. Soft Matter 2013, 9, 7158–7166. [Google Scholar] [CrossRef]
- Aangenendt, F.J.; Mattsson, J.; Ellenbroek, W.G.; Wyss, H.M. Mechanics from Calorimetry: Probing the Elasticity of Responsive Hydrogels. Phys. Rev. Appl. 2017, 8, 014003. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; Voudouris, P.; Punter, M.T.J.J.M.; Aangenendt, F.J.; Florea, D.; van der Schoot, P.; Wyss, H.M. Compression and Reswelling of Microgel Particles after an Osmotic Shock. Phys. Rev. Lett. 2017, 119, 098001. [Google Scholar] [CrossRef]
- Johnson, B.D.; Beebe, D.J.; Crone, W.C. Effects of swelling on the mechanical properties of a pH-sensitive hydrogel for use in microfluidic devices. Mater. Sci. Eng. C 2004, 24, 575–581. [Google Scholar] [CrossRef]
- Aangenendt, F.J.; Punter, M.T.J.J.M.; Mulder, B.M.; van der Schoot, P.; Wyss, H.M. Non-monotonic swelling and compression dynamics of hydrogels in polymer solutions. Phys. Rev. E 2020, 102, 062606. [Google Scholar] [CrossRef]
- Hsiue, G.H.; Chang, R.W.; Wang, C.H.; Lee, S.H. Development of in situ thermosensitive drug vehicles for glaucoma therapy. Biomaterials 2003, 24, 2423–2430. [Google Scholar] [CrossRef]
- Gordon, S.; Teichmann, E.; Young, K.; Finnie, K.; Rades, T.; Hook, S. In vitro and in vivo investigation of thermosensitive chitosan hydrogels containing silica nanoparticles for vaccine delivery. Eur. J. Pharm. Sci. 2010, 41, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Kono, K.; Ozawa, T.; Yoshida, T.; Ozaki, F.; Ishizaka, Y.; Maruyama, K.; Kojima, C.; Harada, A.; Aoshima, S. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials 2010, 31, 7096–7105. [Google Scholar] [CrossRef] [PubMed]
- Potta, T.; Chun, C.; Song, S.C. Chemically crosslinkable thermosensitive polyphosphazene gels as injectable materials for biomedical applications. Biomaterials 2009, 30, 6178–6192. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Convertine, A.J.; Reyes, C.R.; Stayton, P.S.; Porter, T.M. Thermosensitive liposomes modified with poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers for triggered release of doxorubicin. Biomacromolecules 2010, 11, 1915–1920. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, Y.; Wang, X.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R. Three-dimensional Gelatin and Gelatin/Hyaluronan Hydrogel Structures for Traumatic Brain Injury. J. Bioact. Compat. Polym. 2007, 22, 19–29. [Google Scholar] [CrossRef]
- Roos, A.; Klee, D.; Schuermann, K.; Höcker, H. Development of a temperature sensitive drug release system for polymeric implant devices. Biomaterials 2003, 24, 4417–4423. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Tian, F.; Wei, C.; Wang, C.C. Facile synthesis of triple-stimuli (photo/pH/thermo) responsive copolymers of 2-diazo-1,2-naphthoquinone-mediated poly(N-isopropylacrylamide-co-N-hydroxymethylacrylamide). J. Polym. Sci. A Polym. Chem. 2009, 47, 2763–2773. [Google Scholar] [CrossRef]
- Meyer, O.; Papahadjopoulos, D.; Leroux, J.C. Copolymers of N-isopropylacrylamide can trigger pH sensitivity to stable liposomes. FEBS Lett. 1998, 421, 61–64. [Google Scholar] [CrossRef]
- Shi, W.; Ji, Y.; Zhang, X.; Shu, S.; Wu, Z. Characterization of ph- and thermosensitive hydrogel as a vehicle for controlled protein delivery. J. Pharm. Sci. 2011, 100, 886–895. [Google Scholar] [CrossRef]
- Stickel, J.J.; Fotopoulos, A. Pressure-Flow Relationships for Packed Beds of Compressible Chromatography Media at Laboratory and Production Scale. Biotechnol. Prog. 2008, 17, 744–751. [Google Scholar] [CrossRef]
- Kong, D.Y.; Gerontas, S.; McCluckie, R.A.; Mewies, M.; Gruber, D.; Titchener-Hooker, N.J. Effects of bed compression on protein separation on gel filtration chromatography at bench and pilot scale. J. Chem. Technol. Biotechnol. 2018, 93, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- A-Hassan, E.; Heinz, W.F.; Antonik, M.D.; D’Costa, N.P.; Nageswaran, S.; Schoenenberger, C.A.; Hoh, J.H. Relative Microelastic Mapping of Living Cells by Atomic Force Microscopy. Biophys. J. 1998, 74, 1564–1578. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Nieves, A.; Fernández-Barbero, A.; Vincent, B.; de las Nieves, F.J. Osmotic de-swelling of ionic microgel particles. J. Chem. Phys. 2003, 119, 10383. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Frederick, J.A.; Laporte, Y.; Markou, G.; Lietor-Santos, J.J.; Fernandez-Nieves, A. Determination of the bulk modulus of microgel particles. Colloid Polym. Sci. 2011, 289, 721–728. [Google Scholar] [CrossRef]
- Mattsson, J.; Wyss, H.M.; Fernandez-Nieves, A.; Miyazaki, K.; Hu, Z.; Reichman, D.R.; Weitz, D.A. Soft colloids make strong glasses. Nature 2009, 462, 83–86. [Google Scholar] [CrossRef]
- Hochmuth, R.M. Micropipette aspiration of living cells. J. Biomech. 2000, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wyss, H.M.; Franke, T.; Mele, E.; Weitz, D.A. Capillary micromechanics: Measuring the elasticity of microscopic soft objects. Soft Matter 2010, 6, 4550–4555. [Google Scholar] [CrossRef]
- Guo, M.; Wyss, H.M. Micromechanics of Soft Particles. Macromol. Mater. Eng. 2011, 296, 223–229. [Google Scholar] [CrossRef]
- Kong, T.; Wang, L.; Wyss, H.M.; Shum, H.C. Capillary micromechanics for core-shell particles. Soft Matter 2014, 10, 3271–3276. [Google Scholar] [CrossRef]
- Wyss, H.M.; Henderson, J.M.; Byfield, F.J.; Bruggeman, L.A.; Ding, Y.; Huang, C.; Suh, J.H.; Franke, T.; Mele, E.; Pollak, M.R.; et al. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011, 300, 397–405. [Google Scholar] [CrossRef]
- Ravetto, A.; Wyss, H.M.; Anderson, P.D.; Den Toonder, J.M.J.; Bouten, C.V.C. Monocytic cells become less compressible but more deformable upon activation. PLoS ONE 2014, 9, e92814. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Hocker, L.O.; Benedek, G.B. Spectrum of light scattered from a viscoelastic gel. J. Chem. Phys. 1973, 59, 5151–5159. [Google Scholar] [CrossRef]
- Tokita, M.; Tanaka, T. Friction coefficient of polymer networks of gels. J. Chem. Phys. 1991, 95, 4613–4619. [Google Scholar] [CrossRef]
- Tong, J.; Anderson, J.L. Partitioning and diffusion of proteins and linear polymers in polyacrylamide gels. Biophys. J. 1996, 70, 1505–1513. [Google Scholar] [CrossRef]
- Williams, J.C.; Mark, L.A.; Eichholtz, S. Partition and Permeation of Dextran in Polyacrylamide Gel. Biophys. J. 1998, 75, 493–502. [Google Scholar] [CrossRef]
- Stolovicki, E.; Ziblat, R.; Weitz, D.A. Throughput enhancement of parallel step emulsifier devices by shear-free and efficient nozzle clearance. Lab Chip 2017, 18, 132–138. [Google Scholar] [CrossRef]
- Montessori, A.; Lauricella, M.; Succi, S.; Stolovicki, E.; Weitz, D. Elucidating the mechanism of step emulsification. Phys. Rev. Fluids 2018, 3, 072202. [Google Scholar] [CrossRef]
- Ofner, A.; Moore, D.G.; Rühs, P.A.; Schwendimann, P.; Eggersdorfer, M.; Amstad, E.; Weitz, D.A.; Studart, A.R. High-throughput step emulsification for the production of functional materials using a glass microfluidic device. Macromol. Chem. Phys. 2017, 218, 1600472. [Google Scholar] [CrossRef]
- Bonnet-Gonnet, C.; Belloni, L.; Cabane, B. Osmotic Pressure of Latex Dispersions. Langmuir 1994, 10, 4012–4021. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214. [Google Scholar] [CrossRef]
- Albro, M.B.; Rajan, V.; Li, R.; Hung, C.T.; Ateshian, G.A. Characterization of the Concentration-Dependence of Solute Diffusivity and Partitioning in a Model Dextran–Agarose Transport System. Cell. Mol. Bioeng. 2009, 2, 295–305. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakal, K.J.; Pollet, A.M.A.O.; den Toonder, J.M.J.; Wyss, H.M. Single Hydrogel Particle Mechanics and Dynamics Studied by Combining Capillary Micromechanics with Osmotic Compression. Gels 2023, 9, 194. https://doi.org/10.3390/gels9030194
Bakal KJ, Pollet AMAO, den Toonder JMJ, Wyss HM. Single Hydrogel Particle Mechanics and Dynamics Studied by Combining Capillary Micromechanics with Osmotic Compression. Gels. 2023; 9(3):194. https://doi.org/10.3390/gels9030194
Chicago/Turabian StyleBakal, Kalpit J., Andreas M. A. O. Pollet, Jaap M. J. den Toonder, and Hans M. Wyss. 2023. "Single Hydrogel Particle Mechanics and Dynamics Studied by Combining Capillary Micromechanics with Osmotic Compression" Gels 9, no. 3: 194. https://doi.org/10.3390/gels9030194
APA StyleBakal, K. J., Pollet, A. M. A. O., den Toonder, J. M. J., & Wyss, H. M. (2023). Single Hydrogel Particle Mechanics and Dynamics Studied by Combining Capillary Micromechanics with Osmotic Compression. Gels, 9(3), 194. https://doi.org/10.3390/gels9030194







