Study of Hydroxypropyl β-Cyclodextrin and Puerarin Inclusion Complexes Encapsulated in Sodium Alginate-Grafted 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
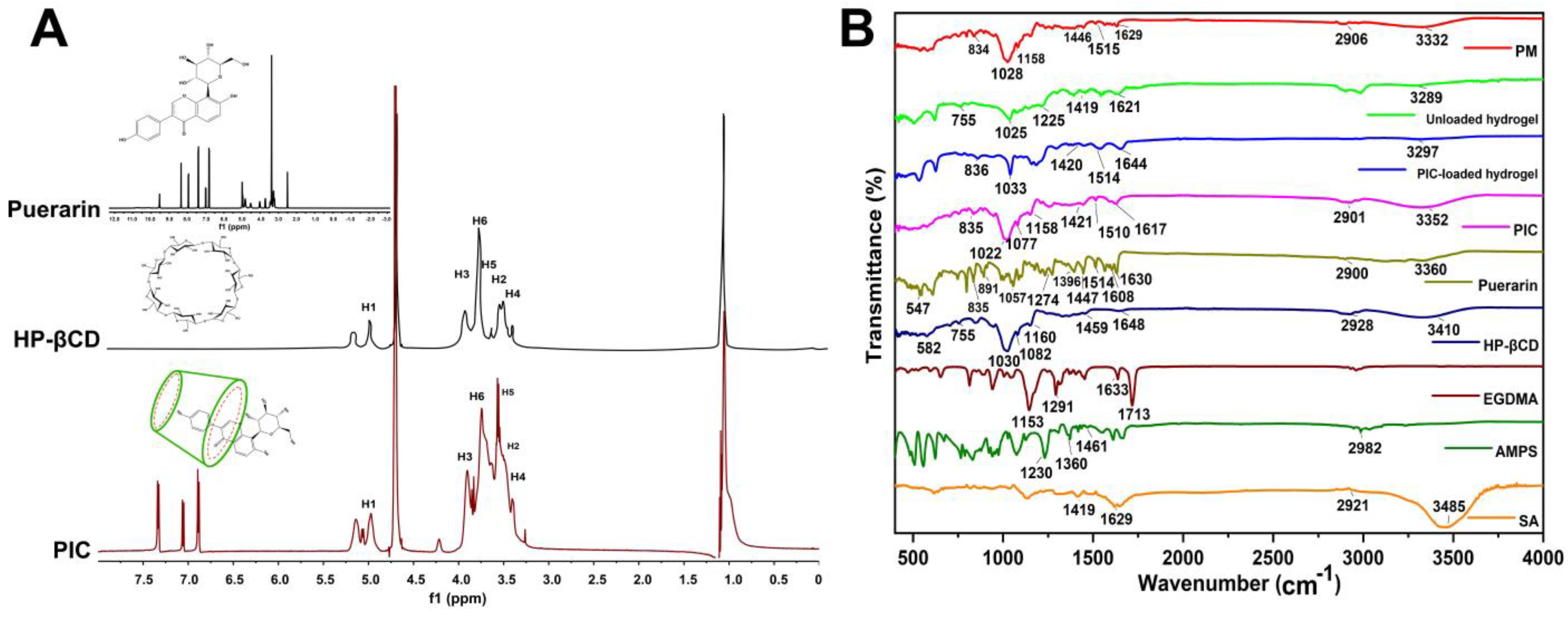
2.1. 1H NMR and FTIR Analysis
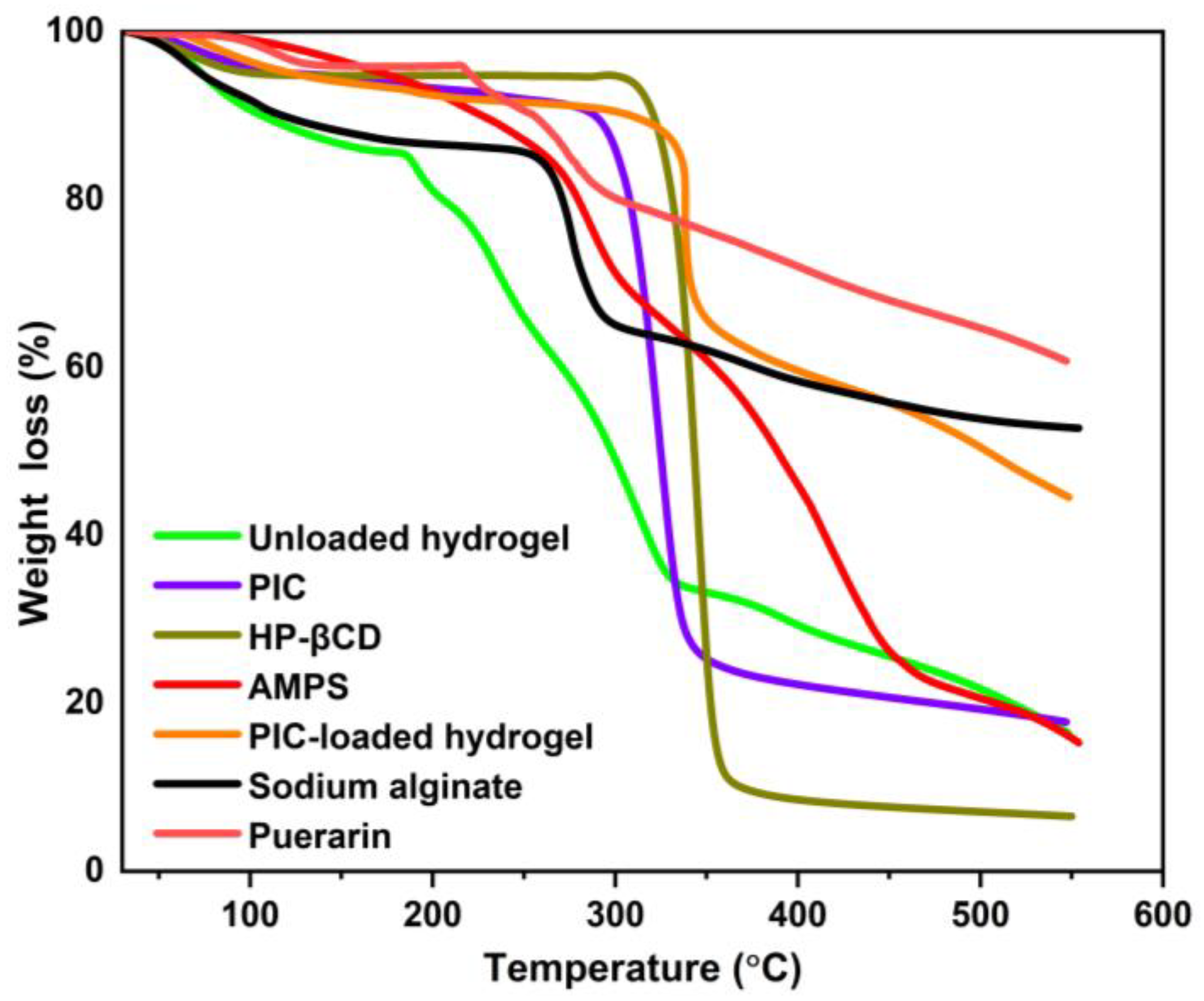
2.2. TGA Study
2.3. DSC Analysis
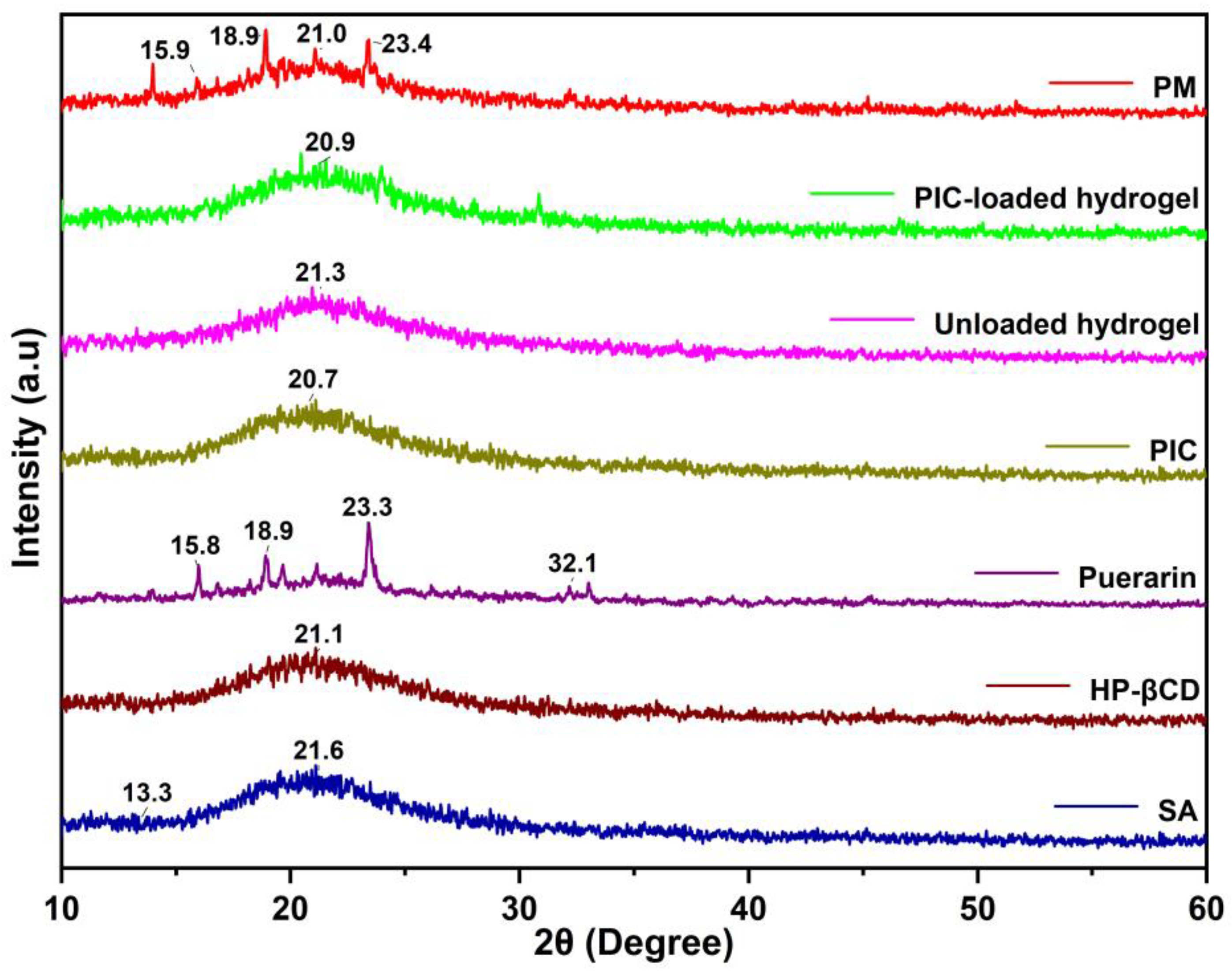
2.4. XRD Study
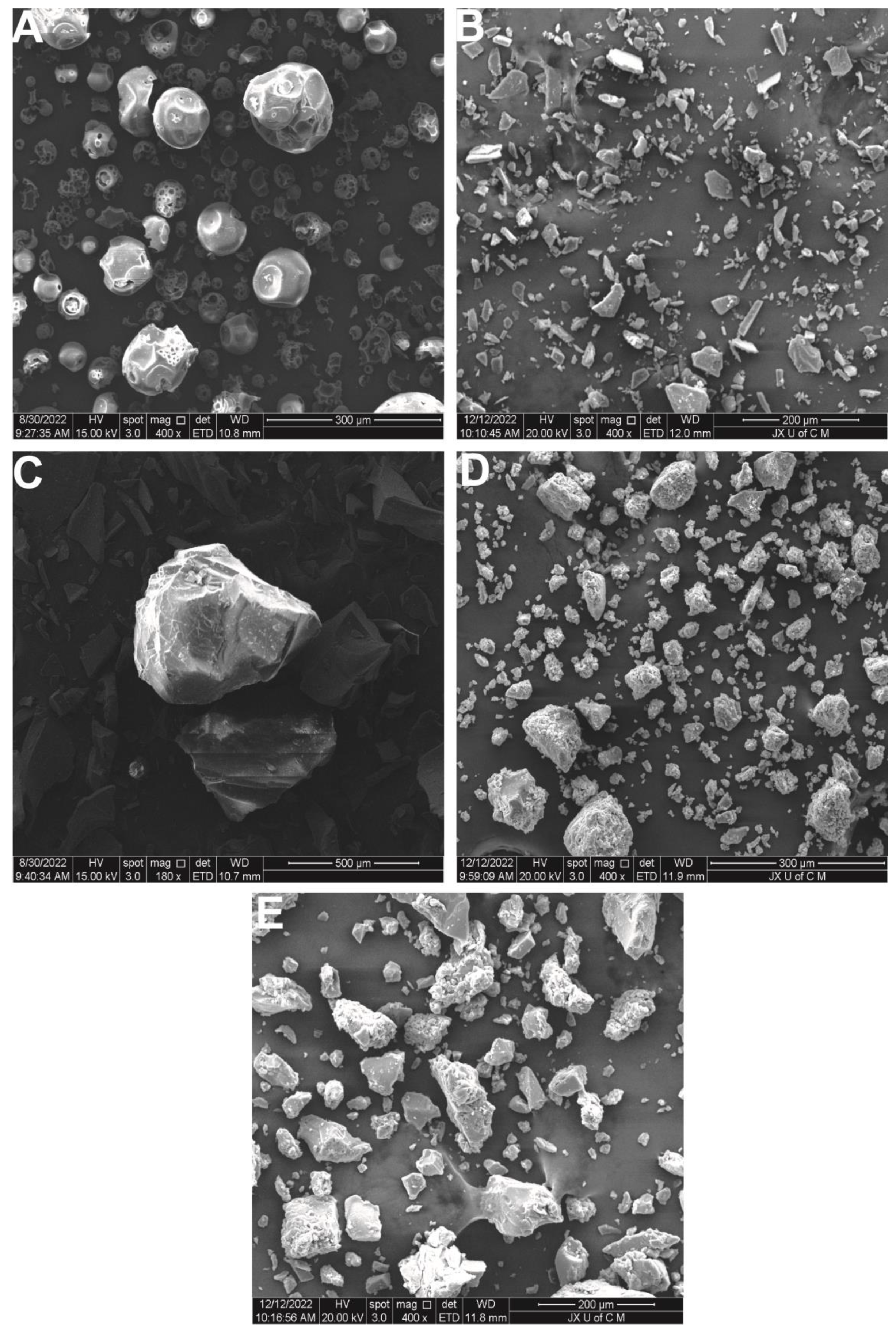
2.5. SEM
2.6. Mechanical Properties of Hydrogels
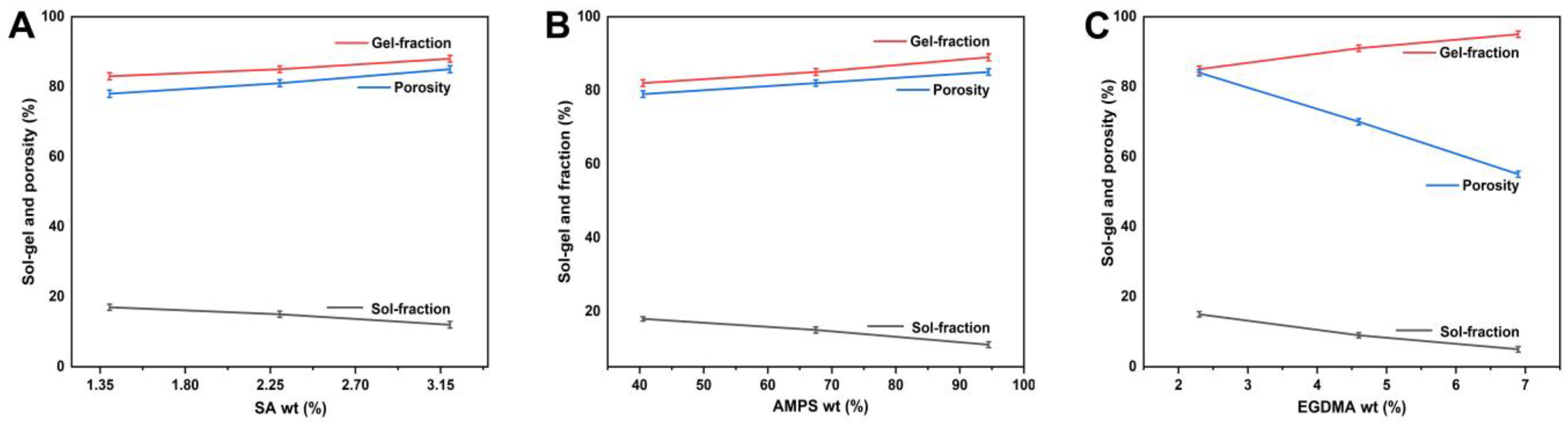
2.7. Sol–Gel Evaluation
2.8. Porosity Assessment
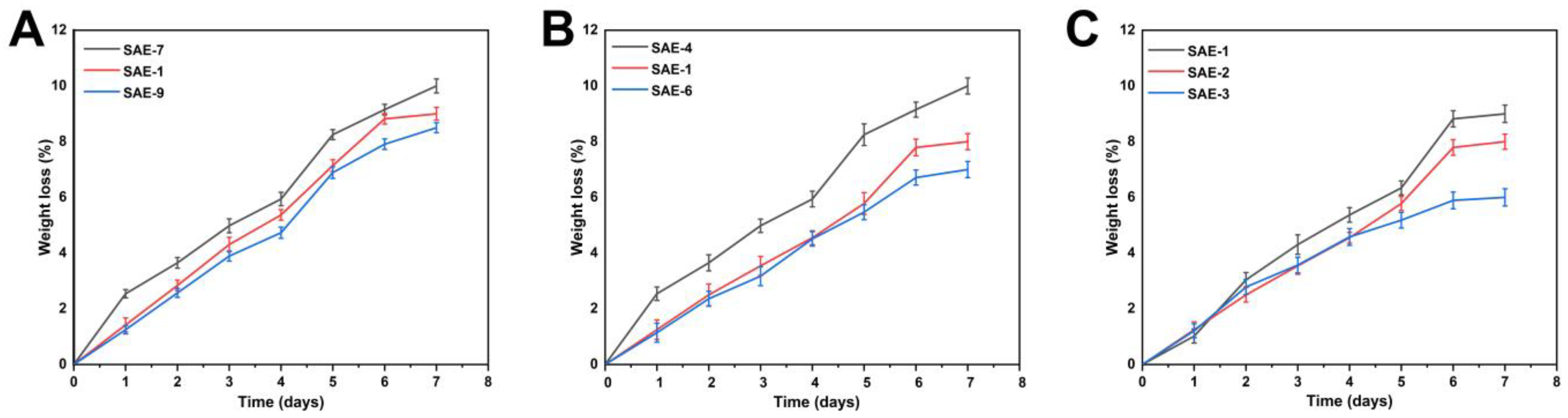
2.9. Biodegradation Determination
2.10. Structural Parameters Analysis of SA-g-AMPS Hydrogels
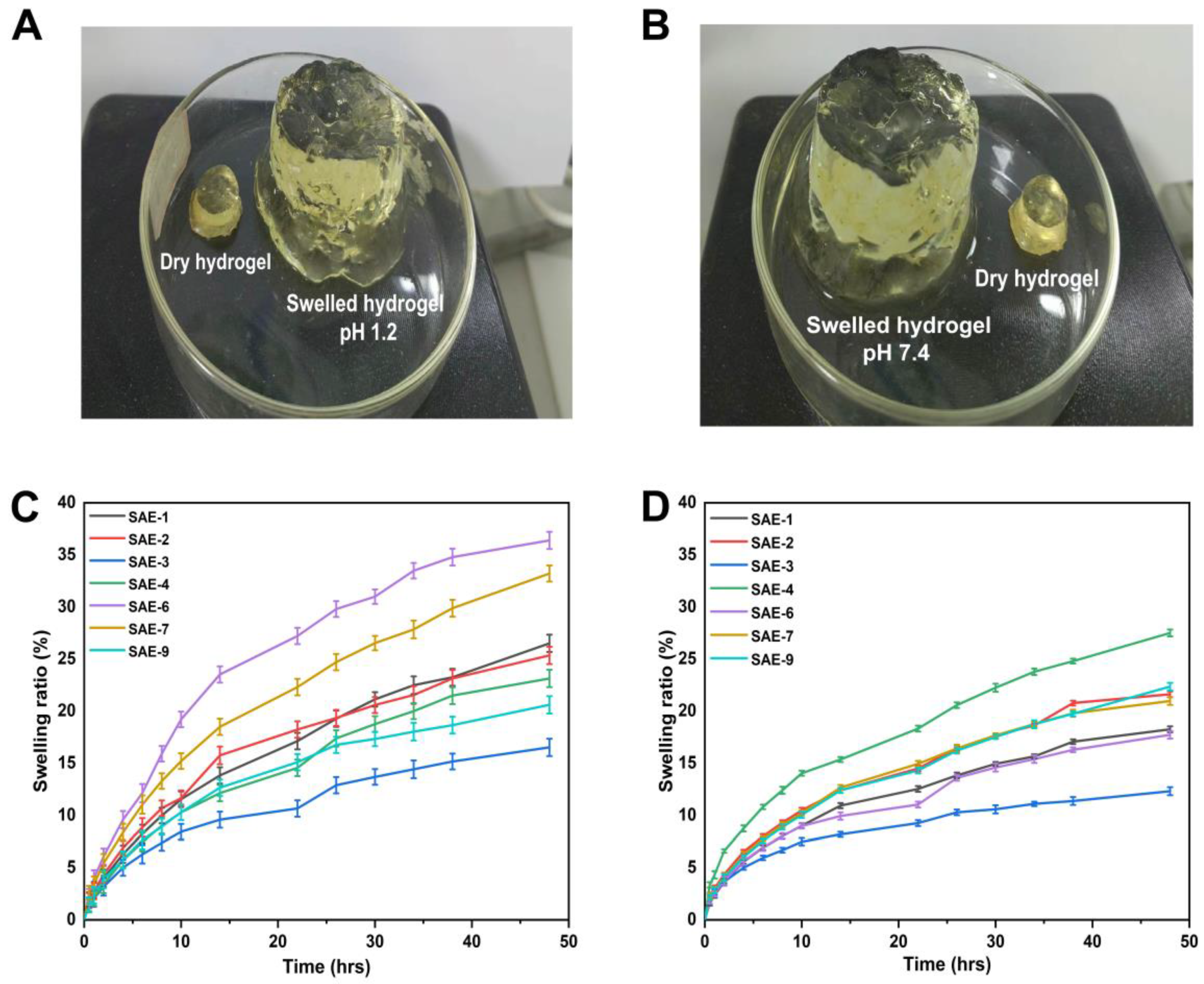
2.11. Swelling Study
2.12. Drug Release and Kinetic Data Modelling
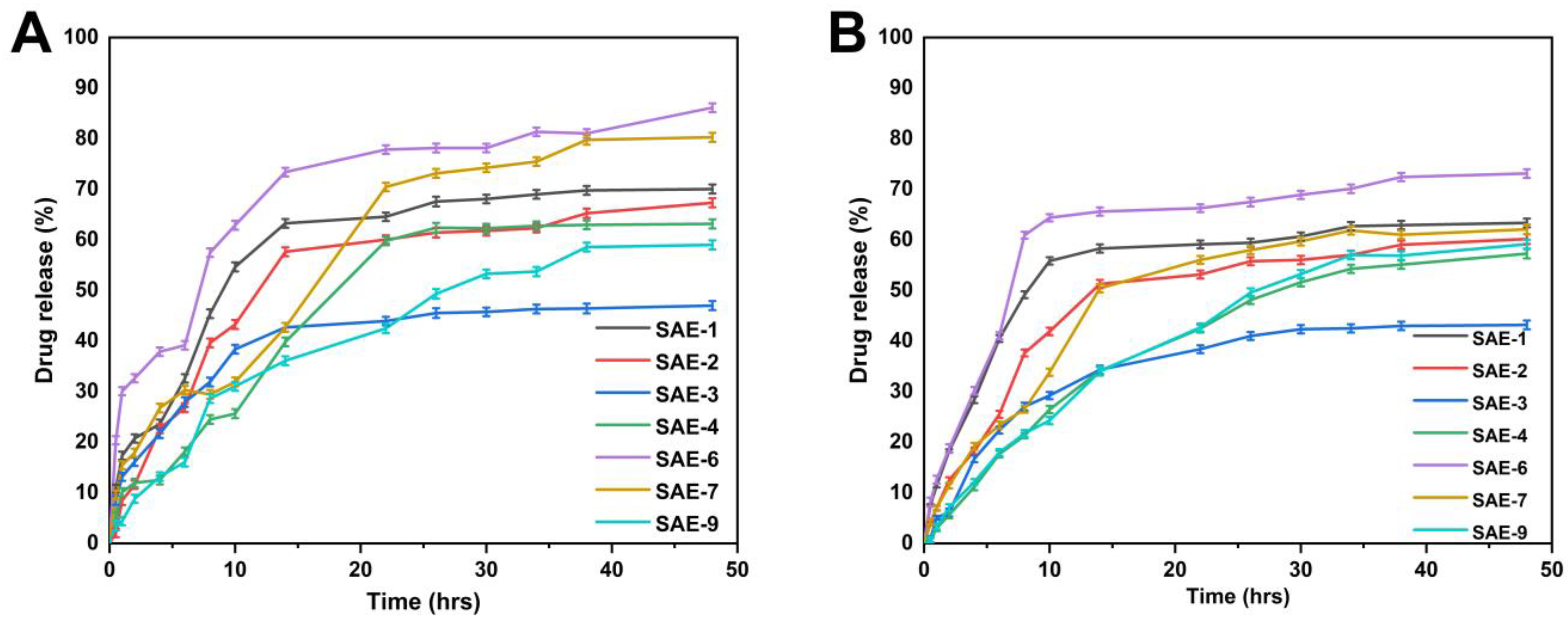
2.13. Antioxidation Effects
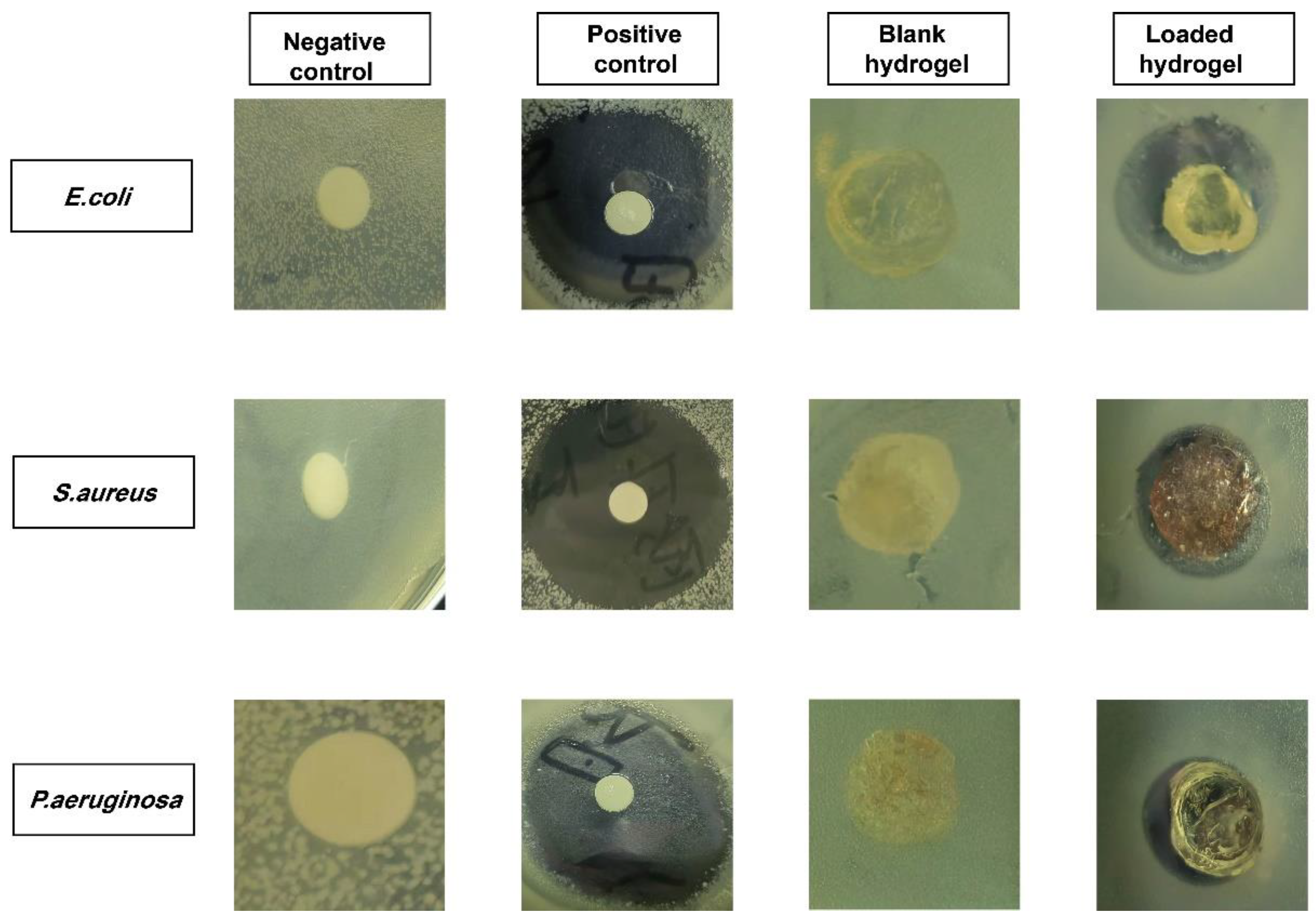
2.14. Antibacterial Evaluation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of HP-βCD-Puerarin Inclusion Complexes (PIC)
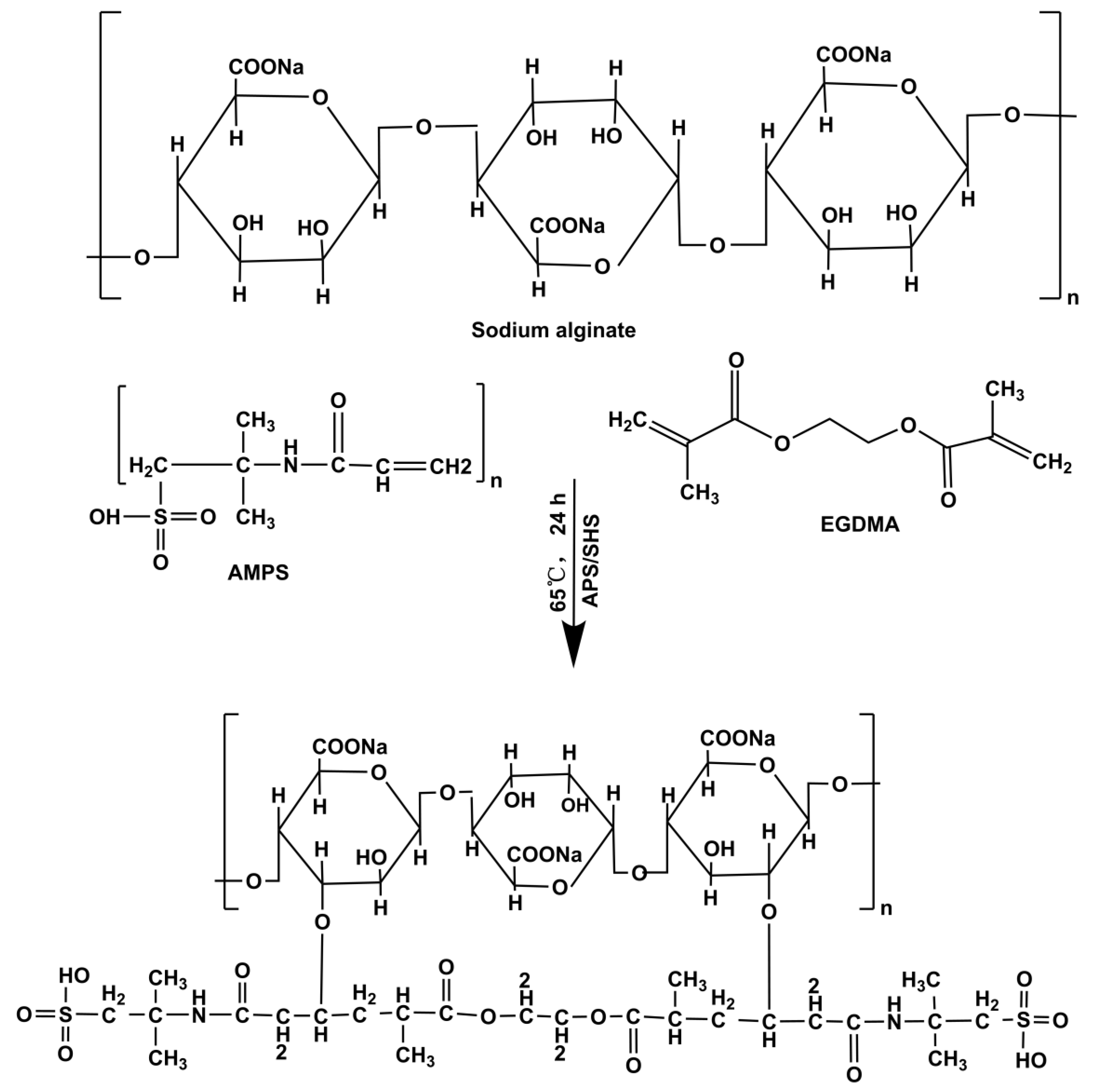
4.3. Fabrication of SA-g-AMPS Hydrogels
4.4. PIC Loading in Hydrogels
4.5. In Vitro Characterization
4.5.1. 1H NMR and Fourier Transform Infrared Spectroscopy (FTIR)
4.5.2. Thermal Study (TGA and DSC)
4.5.3. X-ray Diffraction (XRD)
4.5.4. Scanning Electron Microscopy (SEM)
4.5.5. Mechanical Properties Determination
4.5.6. Sol–Gel Study
4.5.7. Porosity Determination
4.5.8. Biodegradation Assessment
4.6. Evaluation of Polymer Network Parameters of Hydrogels
4.6.1. Diffusion Coefficient
4.6.2. Volume Fraction of Polymer (V2,s)
4.6.3. Average Molecular Weight between Crosslinks (Mc)
4.6.4. Solvent Interaction Parameters (χ)
4.6.5. Number of Repeating Units between Crosslinks (N)
4.7. Swelling Ratio
4.8. Drug Release and Kinetic Release Data Modeling
4.9. Antioxidant Assays
4.9.1. DPPH Activity
4.9.2. ABTS Activity
4.10. Antibacterial Effects
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naeem, A.; Ming, Y.; Pengyi, H.; Jie, K.Y.; Yali, L.; Haiyan, Z.; Shuai, X.; Wenjing, L.; Ling, W.; Xia, Z.M. The fate of flavonoids after oral administration: A comprehensive overview of its bioavailability. Crit. Rev. Food Sci. Nutr. 2022, 62, 6169–6186. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.; Nikitin, I.; Zhevlakova, A.; Selivanova, I. Insights into the Pharmacological Effects of Flavonoids: The Systematic Review of Computer Modeling. Int. J. Mol. Sci. 2022, 23, 6023. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Zhang, L. Pharmacokinetics and drug delivery systems for puerarin, a bioactive flavone from traditional Chinese medicine. Drug Deliv. 2019, 26, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.-Y.; Gao, L.-N.; Meng, F.-Y.; Cui, Y.-L. Mucoadhesive microparticles for gastroretentive delivery: Preparation, biodistribution and targeting evaluation. Mar. Drugs 2014, 12, 5764–5787. [Google Scholar] [CrossRef] [Green Version]
- Anukunwithaya, T.; Poo, P.; Hunsakunachai, N.; Rodsiri, R.; Malaivijitnond, S.; Khemawoot, P. Absolute oral bioavailability and disposition kinetics of puerarin in female rats. BMC Pharmacol. Toxicol. 2018, 19, 25. [Google Scholar] [CrossRef]
- Wang, D.; Bu, T.; Li, Y.; He, Y.; Yang, F.; Zou, L. Pharmacological Activity, Pharmacokinetics, and Clinical Research Progress of Puerarin. Antioxidants 2022, 11, 2121. [Google Scholar] [CrossRef]
- Haimhoffer, Á.; Rusznyák, Á.; Réti-Nagy, K.; Vasvári, G.; Váradi, J.; Vecsernyés, M.; Bácskay, I.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F. Cyclodextrins in drug delivery systems and their effects on biological barriers. Sci. Pharm. 2019, 87, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Lv, P.; Zhou, C.; Zhao, Y.; Liao, X.; Yang, B. Cyclodextrin-based delivery systems for cancer treatment. Mater. Sci. Eng. C 2019, 96, 872–886. [Google Scholar] [CrossRef]
- Haley, R.M.; Gottardi, R.; Langer, R.; Mitchell, M.J. Cyclodextrins in drug delivery: Applications in gene and combination therapy. Drug Deliv. Transl. Res. 2020, 10, 661–677. [Google Scholar] [CrossRef]
- Ding, X.; Zheng, M.; Lu, J.; Zhu, X. Preparation and evaluation of binary and ternary inclusion complexes of fenofibrate/hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 17–24. [Google Scholar] [CrossRef]
- Yu, C.; Chen, X.; Zhu, W.; Li, L.; Peng, M.; Zhong, Y.; Naeem, A.; Zang, Z.; Guan, Y. Synthesis of Gallic Acid-Loaded Chitosan-Grafted-2-Acrylamido-2-Methylpropane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery: In Vitro Biodegradation, Antioxidant, and Antibacterial Effects. Gels 2022, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural polymers-based light-induced hydrogels: Promising biomaterials for biomedical applications. Coord. Chem. Rev. 2020, 420, 213432. [Google Scholar] [CrossRef]
- Zang, Z.; Zhao, S.; Yang, M.; Yu, C.; Ouyang, H.; Chen, L.; Zhu, W.; Liao, Z.-g.; Naeem, A.; Guan, Y. Blood chemical components analysis of honeysuckle and formulation of xanthan gum/starch-based (PVA-co-AA) hydrogels for controlled release. Arab. J. Chem. 2022, 15, 104312. [Google Scholar] [CrossRef]
- Omer, A.M.; Ahmed, M.S.; El-Subruiti, G.M.; Khalifa, R.E.; Eltaweil, A.S. pH-sensitive alginate/carboxymethyl chitosan/aminated chitosan microcapsules for efficient encapsulation and delivery of diclofenac sodium. Pharmaceutics 2021, 13, 338. [Google Scholar] [CrossRef]
- İsmail, O.; Gökçe Kocabay, Ö. Absorption and adsorption studies of polyacrylamide/sodium alginate hydrogels. Colloid Polym. Sci. 2021, 299, 783–796. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- El-Hag Ali, A.; El-Rehiem, H.A.A.; Hegazy, E.S.A.; Ghobashy, M.M. Characterization and Potential Application of Electro-Active Acrylamido-2-methyl Propane Sulfonic Acid/Acrylic Acid Copolymer Prepared by Ionizing Radiation. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 91–98. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, J.; Shi, X.; Song, F.; Gao, W.; Liu, S. Stereocomplexation of Poly (lactic acid) and Chemical Crosslinking of Ethylene Glycol Dimethacrylate (EGDMA) Double-Crosslinked Temperature/pH Dual Responsive Hydrogels. Polymers 2020, 12, 2204. [Google Scholar] [CrossRef]
- Bulani, V.D.; Kothavade, P.S.; Nagmoti, D.M.; Kundaikar, H.S.; Degani, M.S.; Juvekar, A.R. Characterisation and anti-inflammatory evaluation of the inclusion complex of ellagic acid with hydroxypropyl-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2015, 82, 361–372. [Google Scholar] [CrossRef]
- Zhao, R.; Tan, T.; Sandström, C. NMR studies on puerarin and its interaction with beta-cyclodextrin. J. Biol. Phys. 2011, 37, 387–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Q.; Gu, X.; Tan, S. Drying process of sodium alginate films studied by two-dimensional correlation ATR-FTIR spectroscopy. Food Chem. 2014, 164, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Hosseinzadeh, H.; Mazidi, R. Modified carrageenan. 4. Synthesis and swelling behavior of crosslinked κC-g-AMPS superabsorbent hydrogel with antisalt and pH-responsiveness properties. J. Appl. Polym. Sci. 2005, 98, 255–263. [Google Scholar] [CrossRef]
- Duran, A.; Soylak, M.; Tuncel, S.A. Poly (vinyl pyridine-poly ethylene glycol methacrylate-ethylene glycol dimethacrylate) beads for heavy metal removal. J. Hazard. Mater. 2008, 155, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Cegłowski, M.; Kurczewska, J.; Lusina, A.; Nazim, T.; Ruszkowski, P. EGDMA-and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery. Polymers 2022, 14, 1027. [Google Scholar] [CrossRef] [PubMed]
- Misiuk, W.; Zalewska, M. Investigation of inclusion complex of trazodone hydrochloride with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2009, 77, 482–488. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, J.; Liu, Y.; Zhu, X.; Zeng, J. Physiochemical properties of the inclusion complex of puerarin and glucosyl-β-cyclodextrin. J. Agric. Food Chem. 2012, 60, 12501–12507. [Google Scholar] [CrossRef]
- Xie, J.; Yang, F.; Shi, X.; Zhu, X.; Su, W.; Wang, P. Improvement in solubility and bioavailability of puerarin by mechanochemical preparation. Drug Dev. Ind. Pharm. 2013, 39, 826–835. [Google Scholar] [CrossRef]
- Yang, L.-Q.; Lan, Y.-Q.; Guo, H.; Cheng, L.-Z.; Fan, J.-Z.; Cai, X.; Zhang, L.-M.; Chen, R.-F.; Zhou, H.-S. Ophthalmic drug-loaded N, O-carboxymethyl chitosan hydrogels: Synthesis, in vitro and in vivo evaluation. Acta Pharmacol. Sin. 2010, 31, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospun formulation of acyclovir/cyclodextrin nanofibers for fast-dissolving antiviral drug delivery. Mater. Sci. Eng. C 2021, 118, 111514. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Mishra, S.; Sen, G. Microwave based synthesis of polymethyl methacrylate grafted sodium alginate: Its application as flocculant. Carbohydr. Polym. 2013, 91, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.V.; Boppana, R.; Mohan, G.K.; Mutalik, S.; Kalyane, N.V. pH-responsive interpenetrating network hydrogel beads of poly (acrylamide)-g-carrageenan and sodium alginate for intestinal targeted drug delivery: Synthesis, in vitro and in vivo evaluation. J. Colloid Interface Sci. 2012, 367, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Ashames, A.; Ullah, K.; Al-Tabakha, M.; Khan, S.A.; Hassan, N.; Mannan, A.; Ikram, M.; Buabeid, M.; Murtaza, G. Development, characterization and In-vitro evaluation of guar gum based new polymeric matrices for controlled delivery using metformin HCl as model drug. PLoS ONE 2022, 17, e0271623. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S. Sodium alginate/alumina composite biomembrane preparation and performance in DMFC application. Polym. Test. 2020, 81, 106183. [Google Scholar] [CrossRef]
- Hassani, A.; Mahmood, S.; Enezei, H.H.; Hussain, S.A.; Hamad, H.A.; Aldoghachi, A.F.; Hagar, A.; Doolaanea, A.A.; Ibrahim, W.N. Formulation, characterization and biological activity screening of sodium alginate-gum arabic nanoparticles loaded with curcumin. Molecules 2020, 25, 2244. [Google Scholar] [CrossRef]
- Li, P.; Jia, H.; Zhang, S.; Yang, Y.; Sun, H.; Wang, H.; Pan, W.; Yin, F.; Yang, X. Thermal extrusion 3D printing for the fabrication of puerarin immediate-release tablets. AAPS PharmSciTech 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, H.; Hu, H.; Ruan, J.; Shi, C.; Cao, J.; Zhang, X. Preparation, characterization and antioxidant activity of inclusion complex loaded with puerarin and corn peptide. Food Biosci. 2022, 49, 101886. [Google Scholar] [CrossRef]
- Khushbu; Jindal, R. Comparative evaluation for controlled release of amoxicillin from RSM-CCD-optimized nanocomposites based on sodium alginate and chitosan-containing inclusion complexes. Mol. Pharm. 2021, 18, 3795–3810. [Google Scholar] [CrossRef]
- Cheng, M.; Yuan, F.; Liu, J.; Liu, W.; Feng, J.; Jin, Y.; Tu, L. Fabrication of fine puerarin nanocrystals by Box–Behnken Design to enhance intestinal absorption. Aaps Pharmscitech 2020, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Gao, X.; Fu, J.; Hu, L. Application of cyclodextrin in food industry. Crit. Rev. Food Sci. Nutr. 2022, 62, 2627–2640. [Google Scholar] [CrossRef]
- Khan, M.U.A.; Yaqoob, Z.; Ansari, M.N.M.; Razak, S.I.A.; Raza, M.A.; Sajjad, A.; Haider, S.; Busra, F.M. Chitosan/poly vinyl alcohol/graphene oxide based pH-responsive composite hydrogel films: Drug release, anti-microbial and cell viability studies. Polymers 2021, 13, 3124. [Google Scholar] [CrossRef] [PubMed]
- Park, D.W.; Haam, S.; Lee, T.G.; Kim, H.S.; Kim, W.S. Chemoenzymatic synthesis of sugar-containing biocompatible hydrogels: Crosslinked poly (β-methylglucoside acrylate) and poly (β-methylglucoside methacrylate). J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2004, 71, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Peng, Z.; Gu, J.; Peng, Y.; Huang, X.; Wu, L. Exploring environmentally friendly biopolymer material effect on soil tensile and compressive behavior. Int. J. Environ. Res. Public Health 2020, 17, 9032. [Google Scholar] [CrossRef]
- Khan, Z.; Minhas, M.U.; Ahmad, M.; Khan, K.U.; Sohail, M.; Khalid, I. Functionalized pectin hydrogels by cross-linking with monomer: Synthesis, characterization, drug release and pectinase degradation studies. Polym. Bull. 2020, 77, 339–356. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.-l. Review of applications and future prospects of stimuli-responsive hydrogel based on thermo-responsive biopolymers in drug delivery systems. Polymers 2021, 13, 2086. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Khan, S.A.; Azam, W.; Ashames, A.; Fahelelbom, K.M.; Ullah, K.; Mannan, A.; Murtaza, G. β-Cyclodextrin-based (IA-co-AMPS) Semi-IPNs as smart biomaterials for oral delivery of hydrophilic drugs: Synthesis, characterization, in-Vitro and in-Vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 101970. [Google Scholar] [CrossRef]
- Ghauri, Z.H.; Islam, A.; Qadir, M.A.; Gull, N.; Haider, B.; Khan, R.U.; Riaz, T. Development and evaluation of pH-sensitive biodegradable ternary blended hydrogel films (Chitosan/Guar gum/PVP) for drug delivery application. Sci. Rep. 2021, 11, 21255. [Google Scholar] [CrossRef]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Butt, A.; Jabeen, S.; Nisar, N.; Islam, A.; Gull, N.; Iqbal, S.S.; Khan, S.M.; Yameen, B. Controlled release of cephradine by biopolymers based target specific crosslinked hydrogels. Int. J. Biol. Macromol. 2019, 121, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Abbasi, A.R.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Munir, A. Bioinspired sodium alginate based thermosensitive hydrogel membranes for accelerated wound healing. Int. J. Biol. Macromol. 2020, 155, 751–765. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.; Hashem, M.; El-Awady, M.; Rabie, A. pH-sensitive sodium alginate hydrogels for riboflavin controlled release. Carbohydr. Polym. 2012, 89, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Khalid, I.; Ahmad, M.; Minhas, M.U.; Barkat, K. Synthesis and evaluation of chondroitin sulfate based hydrogels of loxoprofen with adjustable properties as controlled release carriers. Carbohydr. Polym. 2018, 181, 1169–1179. [Google Scholar] [CrossRef]
- Anwar, H.; Ahmad, M.; Minhas, M.U.; Rehmani, S. Alginate-polyvinyl alcohol based interpenetrating polymer network for prolonged drug therapy, optimization and in-vitro characterization. Carbohydr. Polym. 2017, 166, 183–194. [Google Scholar] [CrossRef]
- Hanna, D.H.; Lotfy, V.F.; Basta, A.H.; Saad, G.R. Comparative evaluation for controlling release of niacin from protein-and cellulose-chitosan based hydrogels. Int. J. Biol. Macromol. 2020, 150, 228–237. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.; Zhao, C.; Ren, L.; Wang, C.; Georgiev, M.I.; Xiao, J.; Zhang, T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.S.; Ahmad, M.; Minhas, M.U.; Tulain, R.; Barkat, K.; Khalid, I.; Khalid, Q. Chitosan/xanthan gum based hydrogels as potential carrier for an antiviral drug: Fabrication, characterization, and safety evaluation. Front. Chem. 2020, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Yu, C.; Zang, Z.; Wan, X.; Naeem, A.; Zhang, R.; Zhu, W. Chitosan/xanthan gum-based (Hydroxypropyl methylcellulose-co-2-Acrylamido-2-methylpropane sulfonic acid) interpenetrating hydrogels for controlled release of amorphous solid dispersion of bioactive constituents of Pueraria lobatae. Int. J. Biol. Macromol. 2022, 224, 380–395. [Google Scholar] [CrossRef]
- Naeem, A.; Yu, C.; Zhu, W.; Chen, X.; Wu, X.; Chen, L.; Zang, Z.; Guan, Y. Gallic Acid-Loaded Sodium Alginate-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Hydrogel Membranes for Cutaneous Wound Healing: Synthesis and Characterization. Molecules 2022, 27, 8397. [Google Scholar] [CrossRef] [PubMed]
- Karnakar, R.R.; Gite, V.V. Eco-friendly slow release of ZnSO4 as a micronutrient from poly (acrylic acid: Acrylamide) and guar gum based crosslinked biodegradable hydrogels. Polym.-Plast. Technol. Mater. 2022, 61, 691–708. [Google Scholar] [CrossRef]
- Ghadami, A.; Taheri, S.; Alinejad, Z.; Dinari, M. Preparation of acrylate-based double and triple interpenetrating polymer networks hydrogels: Rheological, thermal, and swelling behavior. Polym. Adv. Technol. 2022, 33, 4330–4340. [Google Scholar] [CrossRef]
- Sulaeman, A.S.; Putro, P.A.; Nikmatin, S. Thermal studies of hydrogels based on poly (acrylic acid) and its copolymers by differential scanning calorimetry: A systematic literature review. Polym. Polym. Compos. 2022, 30, 09673911221094022. [Google Scholar] [CrossRef]
- Abdalla, T.H.; Nasr, A.S.; Bassioni, G.; Harding, D.R.; Kandile, N.G. Fabrication of sustainable hydrogels-based chitosan Schiff base and their potential applications. Arab. J. Chem. 2022, 15, 103511. [Google Scholar] [CrossRef]
- Kocak, F.Z.; Yar, M.; Rehman, I.U. Hydroxyapatite-integrated, heparin-and glycerol-functionalized chitosan-based injectable hydrogels with improved mechanical and proangiogenic performance. Int. J. Mol. Sci. 2022, 23, 5370. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.; Zhao, Y.; Fang, Y. Agarose/konjac glucomannan double network hydrogels to mimic the texture of beef tripe. Food Hydrocoll. 2023, 135, 108173. [Google Scholar] [CrossRef]
- Siddiqua, A.; Ranjha, N.M.; Rehman, S.; Shoukat, H.; Ramzan, N.; Sultana, H. Preparation and characterization of methylene bisacrylamide crosslinked pectin/acrylamide hydrogels. Polym. Bull. 2022, 79, 7655–7677. [Google Scholar] [CrossRef]
- Araújo, D.; Rodrigues, T.; Alves, V.D.; Freitas, F. Chitin-glucan complex hydrogels: Optimization of gel formation and demonstration of drug loading and release ability. Polymers 2022, 14, 785. [Google Scholar] [CrossRef]
- Azeem, M.K.; Islam, A.; Rizwan, M.; Rasool, A.; Gul, N.; Khan, R.U.; Khan, S.M.; Rasheed, T. Sustainable and environment Friendlier carrageenan-based pH-responsive hydrogels: Swelling behavior and controlled release of fertilizers. Colloid Polym. Sci. 2023, 301, 209–219. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, V. Influence of polymer network parameters of tragacanth gum-based pH responsive hydrogels on drug delivery. Carbohydr. Polym. 2014, 101, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.A.; Abd Razak, S.I.; Haider, S.; Mannan, H.A.; Hussain, J.; Hasan, A. Sodium alginate-f-GO composite hydrogels for tissue regeneration and antitumor applications. Int. J. Biol. Macromol. 2022, 208, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ran, Y.; Ge, Y.; Raza, F.; Li, S.; Zafar, H.; Wu, Y.; Paiva-Santos, A.C.; Yu, C.; Sun, M. pH-sensitive peptide hydrogels as a combination drug delivery system for cancer treatment. Pharmaceutics 2022, 14, 652. [Google Scholar] [CrossRef]
- Muangsri, R.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Sukhavattanakul, P.; Ummartyotin, S. Release Characteristic and Antioxidant Activity of 4-Hydroxybenzoic Acid (4HB) from Sodium Alginate and Polyvinyl Alcohol-based Hydrogel. ChemistrySelect 2022, 7, e202202329. [Google Scholar] [CrossRef]
- Tunit, P.; Thammarat, P.; Okonogi, S.; Chittasupho, C. Hydrogel Containing Borassus flabellifer L. Male Flower Extract for Antioxidant, Antimicrobial, and Anti-Inflammatory Activity. Gels 2022, 8, 126. [Google Scholar] [CrossRef]
- Lorz, L.R.; Yoo, B.C.; Kim, M.-Y.; Cho, J.Y. Anti-wrinkling and anti-melanogenic effect of Pradosia mutisii methanol extract. Int. J. Mol. Sci. 2019, 20, 1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| F. Codes | Thickness (mm) | TS (N/mm) | EAB (%) | PIC- Loaded per 1 g Hydrogel (g) |
|---|---|---|---|---|
| SAE-1 | 1.24 | 0.424 | 34.7 | 0.513 |
| SAE-2 | 1.32 | 0.675 | 65.1 | 0.472 |
| SAE-3 | 1.23 | 1.207 | 76.4 | 0.279 |
| SAE-4 | 1.47 | 0.701 | 69.8 | 0.437 |
| SAE-5 | 1.32 | 0.675 | 65.1 | 0.472 |
| SAE-6 | 1.52 | 0.403 | 30.4 | 0.601 |
| SAE-7 | 1.27 | 0.418 | 36.4 | 0.570 |
| SAE-8 | 1.32 | 0.675 | 65.1 | 0.472 |
| SAE-9 | 1.36 | 0.601 | 61.7 | 0.345 |
| F. Codes | V2,s | χ | Mc | Mr | N | D × 10−5 (cm2 s−1) |
|---|---|---|---|---|---|---|
| SAE-1 | 0.048 | 0.516 | 2652.173 | 207.000 | 25.624 | 0.053 |
| SAE-2 | 0.049 | 0.518 | 1695.142 | 206.790 | 16.394 | 0.030 |
| SAE-3 | 0.060 | 0.520 | 1147.058 | 206.590 | 11.104 | 0.048 |
| SAE-4 | 0.027 | 0.509 | 2288.888 | 207.384 | 22.073 | 0.015 |
| SAE-5 | 0.049 | 0.518 | 1695.142 | 206.790 | 16.394 | 0.030 |
| SAE-6 | 0.043 | 0.514 | 2333.33 | 207.000 | 22.544 | 0.048 |
| SAE-7 | 0.030 | 0.510 | 1610.00 | 206.913 | 15.562 | 0.024 |
| SAE-8 | 0.049 | 0.518 | 1695.142 | 206.790 | 16.394 | 0.030 |
| SAE-9 | 0.037 | 0.512 | 2644.44 | 207.084 | 25.539 | 0.010 |
| Formulations | Sodium Alginate (g) | APS/SHS (g) | AMPS (g) | EGDMA (g) |
|---|---|---|---|---|
| SAE-1 | 0.5 | 0.3/0.3 | 20 | 0.5 |
| SAE-2 | 0.5 | 0.3/0.3 | 20 | 1 |
| SAE-3 | 0.5 | 0.3/0.3 | 20 | 1.5 |
| SAE-4 | 0.5 | 0.3/0.3 | 12 | 0.5 |
| SAE-5 | 0.5 | 0.3/0.3 | 20 | 0.5 |
| SAE-6 | 0.5 | 0.3/0.3 | 28 | 0.5 |
| SAE-7 | 0.3 | 0.3/0.3 | 20 | 0.5 |
| SAE-8 | 0.5 | 0.3/0.3 | 20 | 0.5 |
| SAE-9 | 0.7 | 0.3/0.3 | 20 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naeem, A.; Yu, C.; Zhu, W.; Zang, Z.; Guan, Y. Study of Hydroxypropyl β-Cyclodextrin and Puerarin Inclusion Complexes Encapsulated in Sodium Alginate-Grafted 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery. Gels 2023, 9, 246. https://doi.org/10.3390/gels9030246
Naeem A, Yu C, Zhu W, Zang Z, Guan Y. Study of Hydroxypropyl β-Cyclodextrin and Puerarin Inclusion Complexes Encapsulated in Sodium Alginate-Grafted 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery. Gels. 2023; 9(3):246. https://doi.org/10.3390/gels9030246
Chicago/Turabian StyleNaeem, Abid, Chengqun Yu, Weifeng Zhu, Zhenzhong Zang, and Yongmei Guan. 2023. "Study of Hydroxypropyl β-Cyclodextrin and Puerarin Inclusion Complexes Encapsulated in Sodium Alginate-Grafted 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery" Gels 9, no. 3: 246. https://doi.org/10.3390/gels9030246
APA StyleNaeem, A., Yu, C., Zhu, W., Zang, Z., & Guan, Y. (2023). Study of Hydroxypropyl β-Cyclodextrin and Puerarin Inclusion Complexes Encapsulated in Sodium Alginate-Grafted 2-Acrylamido-2-Methyl-1-Propane Sulfonic Acid Hydrogels for Oral Controlled Drug Delivery. Gels, 9(3), 246. https://doi.org/10.3390/gels9030246







