Injectable Chitosan-Based Hydrogels for Trans-Cinnamaldehyde Delivery in the Treatment of Diabetic Foot Ulcer Infections
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cinnamaldehyde—Cyclodextrin Complexation Study
2.1.1. Phase Solubility Diagram
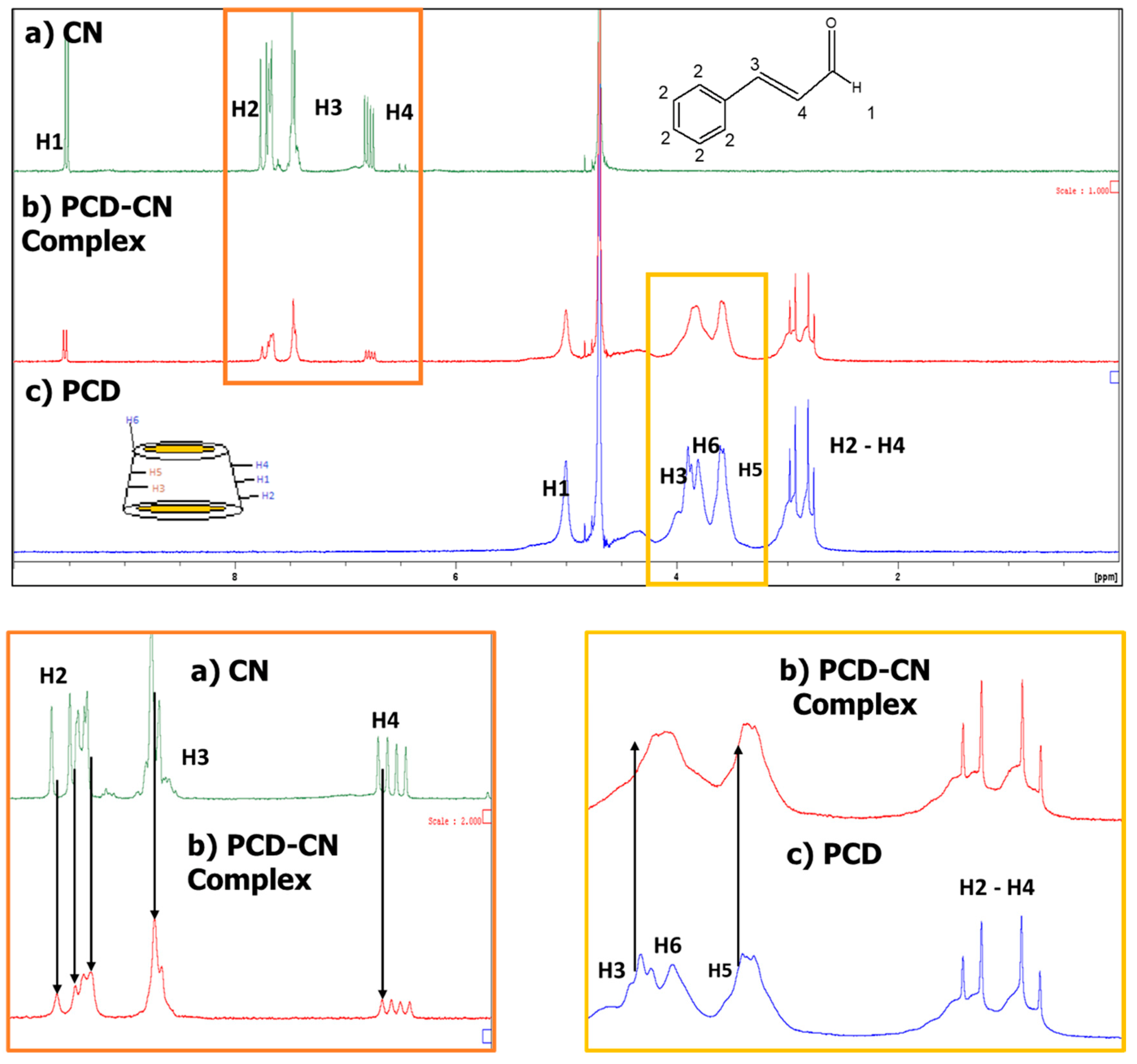
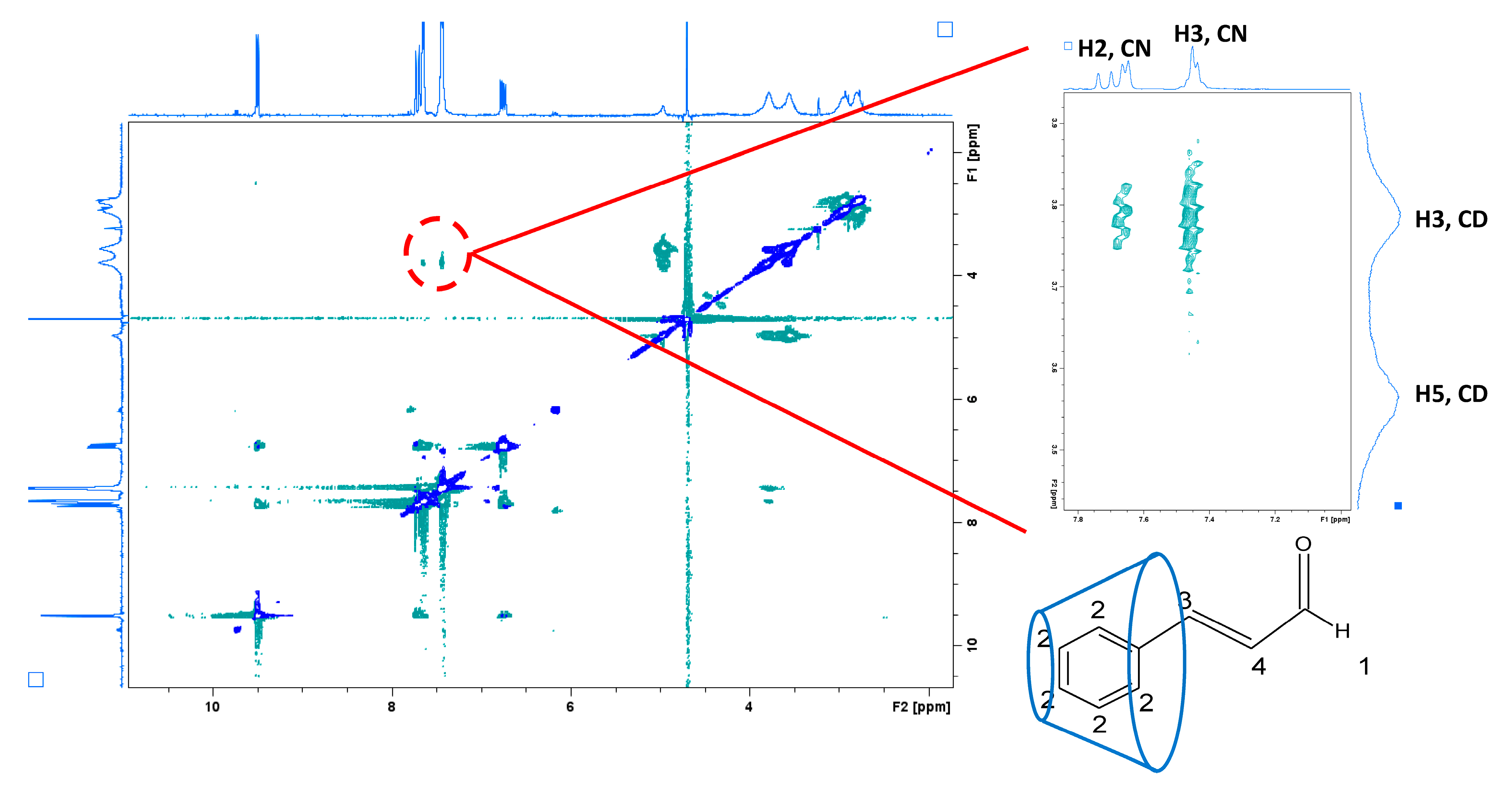
2.1.2. Nuclear Magnetic Resonance (NMR) Study
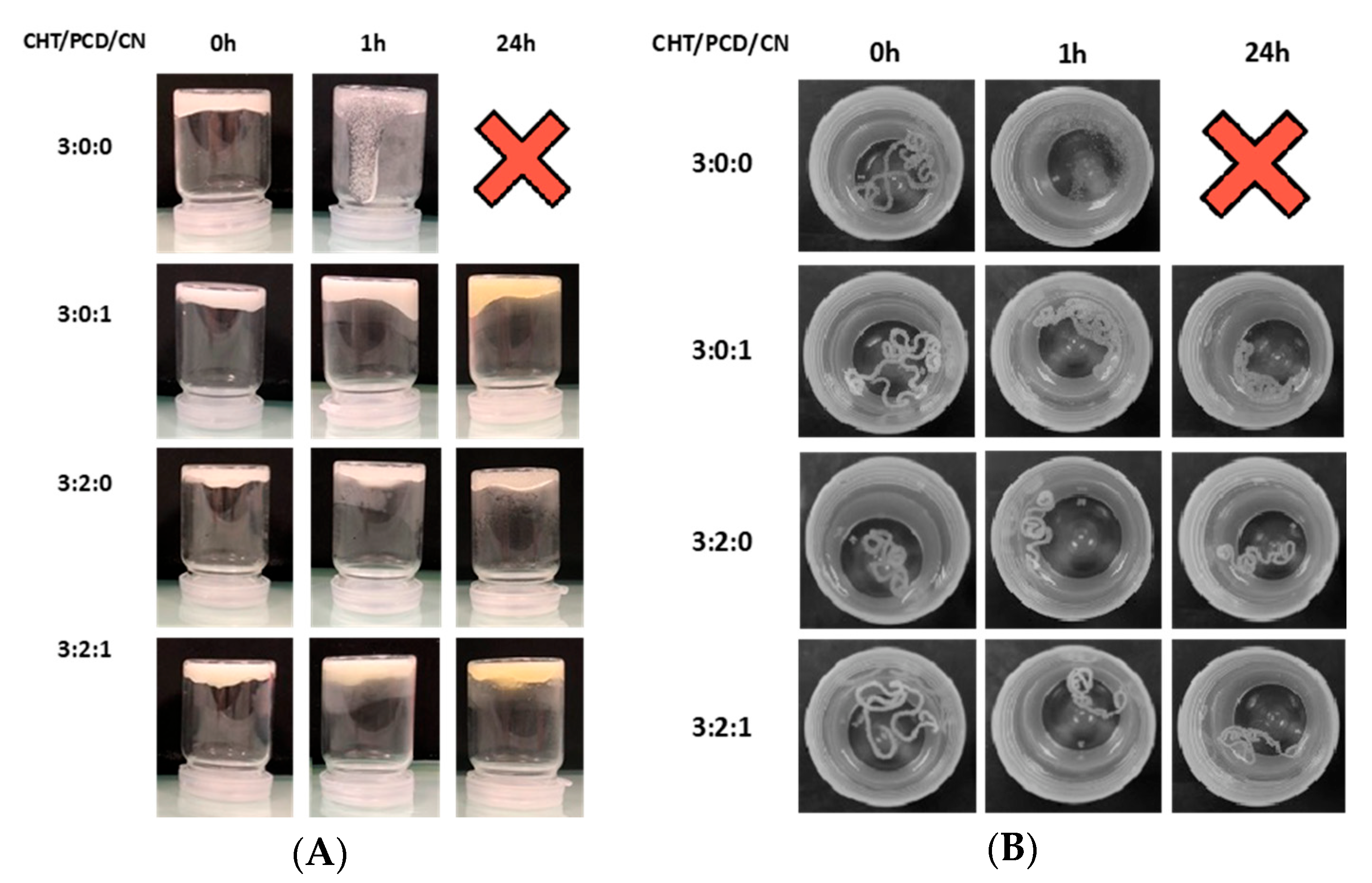
2.1.3. Hydrogel Formation: Vial Turnover Test and Hydrogel Stability
2.1.4. Rheological Analysis
2.1.5. CN Release Study and Modelling
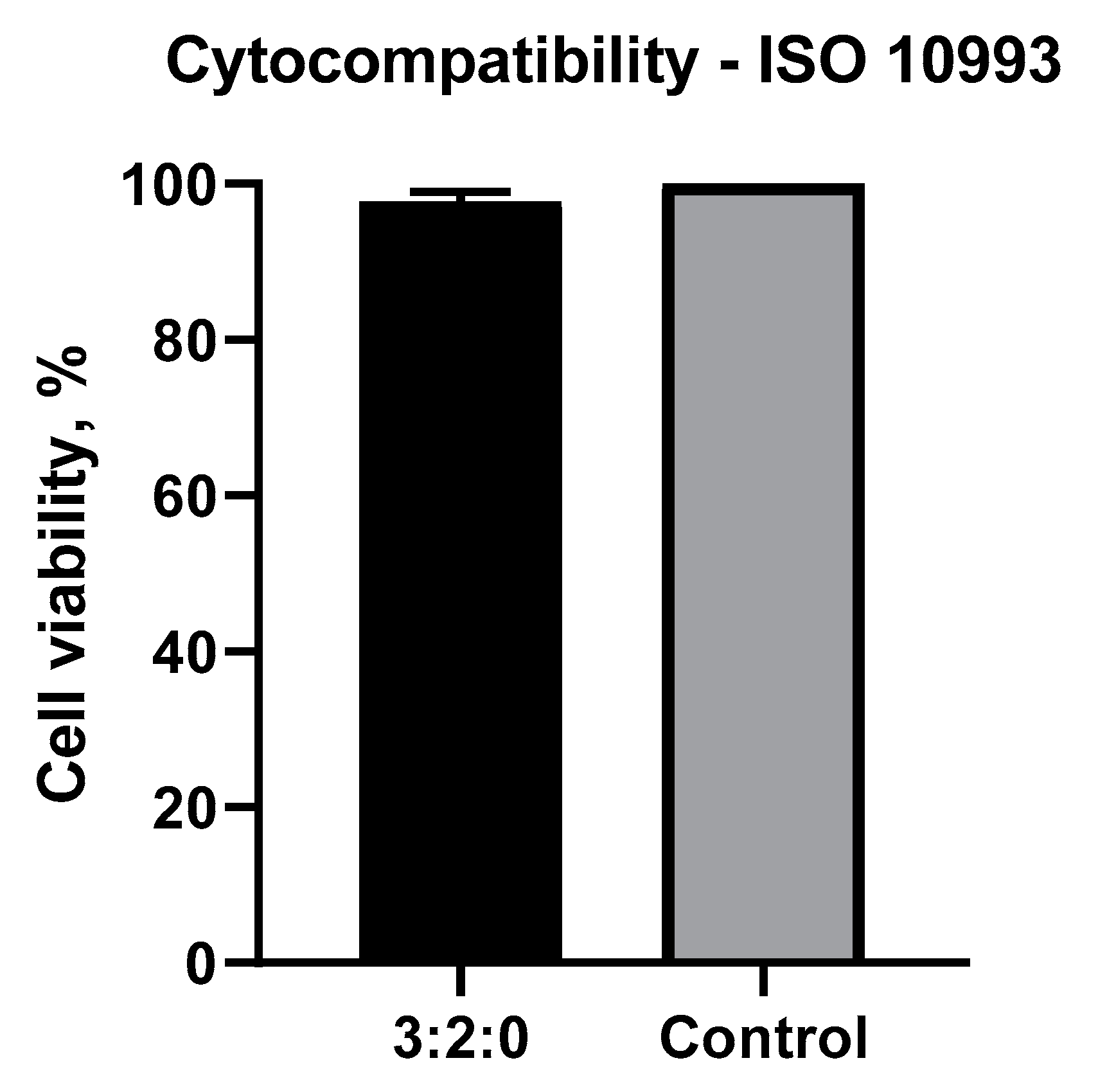
2.1.6. Cytotoxicity Test
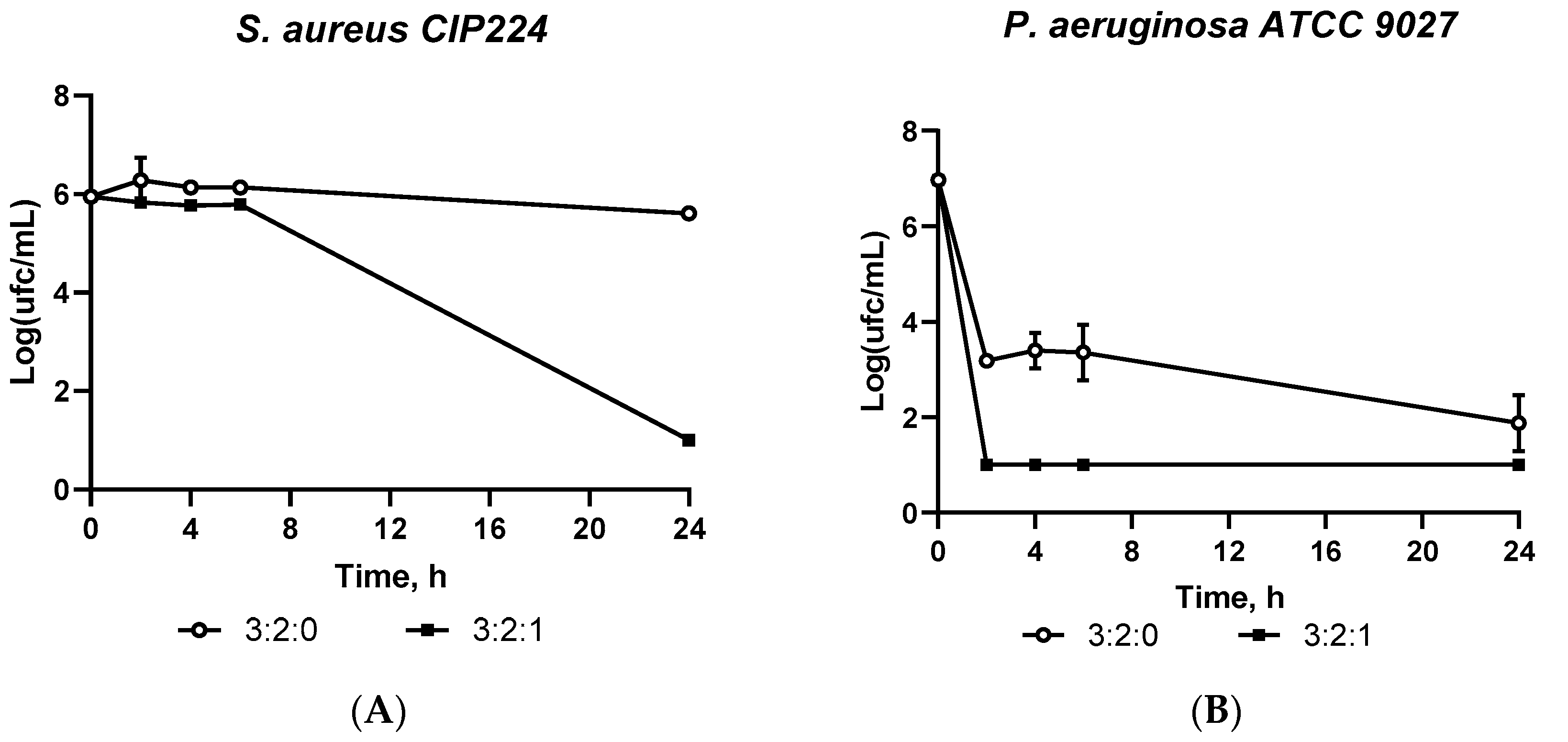
2.1.7. Antimicrobial Assessment: Kill Time
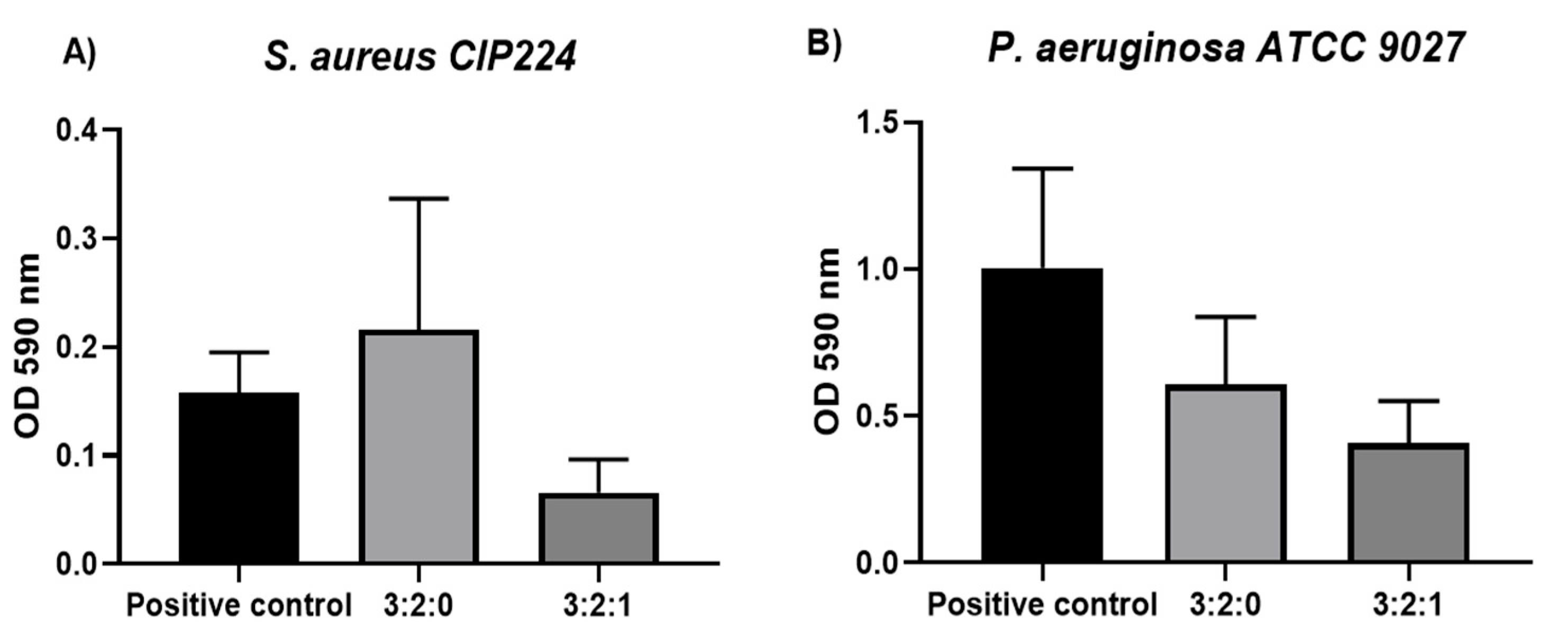
2.1.8. Antibiofilm Activity
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cinnamaldehyde—Cyclodextrin Complexation Study
Phase Solubility Diagram
Nuclear Magnetic Resonance (NMR) Study
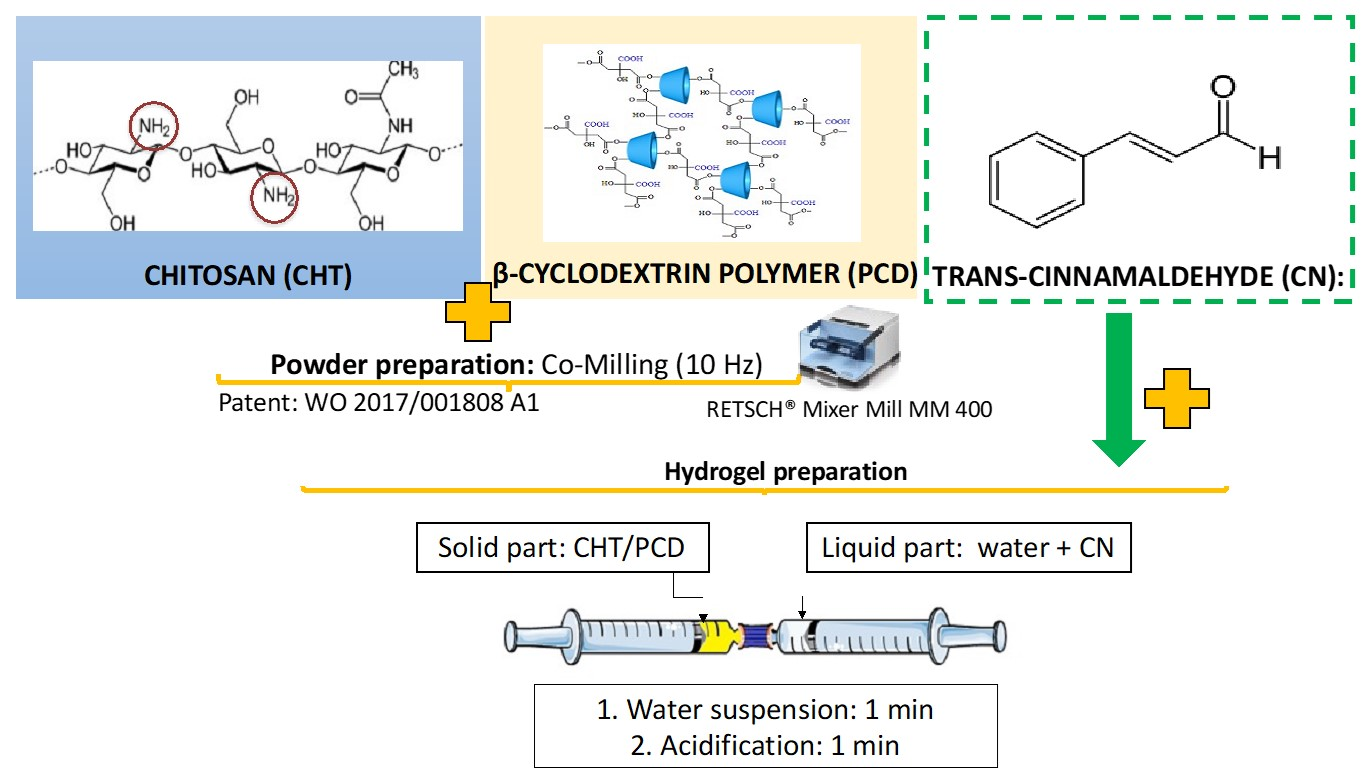
Mixed CHT/PCD Powder Preparation
Hydrogel Preparation
Hydrogel Formation: Vial Turnover Test and Hydrogel Injection in Phosphate-Buffered Saline (PBS)
Rheological Analysis
CN Release Kinetics Study and Modelling
Cytotoxicity Assay
Antimicrobial Assessment: Kill Time
Antibiofilm Study
Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.; Mathioudakis, N. Update on management of diabetic foot ulcers. Ann. N. Y. Acad. Sci. 2018, 1411, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. Available online: https://pubs.acs.org/doi/10.1021/acs.biomac.0c00760 (accessed on 9 March 2023). [CrossRef] [PubMed]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandal, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef] [Green Version]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A.; Larsson, A. Drug Delivery From Hydrogels: A General Framework for the Release Modeling. Curr. Drug Deliv. 2017, 14, 179–189. [Google Scholar] [CrossRef]
- Ji, J.-Y.; Ren, D.-Y.; Weng, Y.-Z. Efficiency of Multifunctional Antibacterial Hydrogels for Chronic Wound Healing in Diabetes: A Comprehensive Review. Int. J. Nanomed. 2022, 17, 3163–3176. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Khodadadi Yazdi, M.; Youssefi Azarfam, M.; Zare, M.; Ramsey, J.D.; Seidi, F.; Reza Saeb, M.; Ramakrishna, S.; Mozafari, M. Injectable Cell-Laden Hydrogels for Tissue Engineering: Recent Advances and Future Opportunities. Tissue Eng. Part A 2021, 27, 821–843. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Gołyńska, M.; Polkowska, I.; Szponder, T.; Nehrbass, D.; Osyczka, A.M. In vivo study on scaffolds based on chitosan, collagen, and hyaluronic acid with hydroxyapatite. Int. J. Biol. Macromol. 2018, 118, 938–944. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. Available online: https://pubs.acs.org/doi/10.1021/acs.biomac.1c00086 (accessed on 9 March 2023). [CrossRef]
- Zhong, Y.; Seidi, F.; Wang, Y.; Zheng, L.; Jin, Y.; Xiao, H. Injectable chitosan hydrogels tailored with antibacterial and antioxidant dual functions for regenerative wound healing. Carbohydr. Polym. 2022, 298, 120103. [Google Scholar] [CrossRef]
- Djekic, L.; Martinović, M.; Ćirić, A.; Fraj, J. Composite Chitosan Hydrogels as Advanced Wound Dressings with Sustained Ibuprofen Release and Suitable Application Characteristics. Pharm. Dev. Technol. 2020, 25, 332–339. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [Green Version]
- Kravanja, G.; Primožič, M.; Knez, Ž.; Leitgeb, M. Chitosan-Based (Nano)Materials for Novel Biomedical Applications. Molecules 2019, 24, 1960. [Google Scholar] [CrossRef] [Green Version]
- Andreica, B.-I.; Cheng, X.; Marin, L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization. Eur. Polym. J. 2020, 139, 110016. [Google Scholar] [CrossRef]
- Hemmingsen, L.M.; Škalko-Basnet, N.; Jøraholmen, M.W. The Expanded Role of Chitosan in Localized Antimicrobial Therapy. Mar. Drugs 2021, 19, 697. [Google Scholar] [CrossRef]
- Shao, J.; Yu, N.; Kolwijck, E.; Wang, B.; Tan, K.W.; Jansen, J.A.; Walboomers, X.F.; Yang, F. Biological evaluation of silver nanoparticles incorporated into chitosan-based membranes. Nanomedicine 2017, 12, 2771–2785. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, C.; Wei, T.; Zhang, L.; Shen, J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. Int. J. Mol. Sci. 2020, 21, 313. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu, R.P.; Profire, L.; Nita, L.E.; Dragostin, O.M.; Ghetu, N.; Pieptu, D.; Vasile, C. Sulfadiazine—Chitosan Conjugates and Their Polyelectrolyte Complexes with Hyaluronate Destined to the Management of Burn Wounds. Materials 2015, 8, 317–338. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Saokham, P.; Sá Couto, A.R. Self-association of cyclodextrins and cyclodextrin complexes in aqueous solutions. Int. J. Pharm. 2019, 560, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Kanjickal, D.; Lopina, S.; Evancho-Chapman, M.M.; Schmidt, S.; Donovan, D. Improving delivery of hydrophobic drugs from hydrogels through cyclodextrins. J. Biomed. Mater. Res. Part A 2005, 74A, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Rohner, N.A.; Schomisch, S.J.; Marks, J.M.; von Recum, H.A. Cyclodextrin Polymer Preserves Sirolimus Activity and Local Persistence for Antifibrotic Delivery over the Time Course of Wound Healing. Mol. Pharm. 2019, 16, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morcellet, M. Water-soluble polymers and gels from the polycondensation between cyclodextrins and poly(carboxylic acid)s: A study of the preparation parameters. J. Appl. Polym. Sci. 2005, 97, 433–442. [Google Scholar] [CrossRef]
- Palomino-Durand, C.; Lopez, M.; Cazaux, F.; Martel, B.; Blanchemain, N.; Chai, F. Influence of the Soluble–Insoluble Ratios of Cyclodextrins Polymers on the Viscoelastic Properties of Injectable Chitosan–Based Hydrogels for Biomedical Application. Polymers 2019, 11, 214. [Google Scholar] [CrossRef] [Green Version]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; de Carvalho, M.G.; Catunda, F.E.A., Jr. Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef] [Green Version]
- Flores, C.; Lopez, M.; Tabary, N.; Neut, C.; Chai, F.; Betbeder, D.; Herkt, C.; Cazaux, F.; Gaucher, V.; Martel, B.; et al. Preparation and characterization of novel chitosan and β-cyclodextrin polymer sponges for wound dressing applications. Carbohydr. Polym. 2017, 173, 535–546. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Costa Duarte, F.Í.; Heimfarth, L.; Siqueira Quintans, J.d.S.; Quintans-Júnior, L.J.; da Veiga Júnior, V.F.; Neves de Lima, Á.A. Cyclodextrin–Drug Inclusion Complexes: In Vivo and In Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [Green Version]
- Mogrovejo-Valdivia, A.; Rahmouni, O.; Tabary, N.; Maton, M.; Neut, C.; Martel, B.; Blanchemain, N. In vitro evaluation of drug release and antibacterial activity of a silver-loaded wound dressing coated with a multilayer system. Int. J. Pharm. 2019, 556, 301–310. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
- Yildiz, Z.I.; Kilic, M.E.; Durgun, E.; Uyar, T. Molecular Encapsulation of Cinnamaldehyde within Cyclodextrin Inclusion Complex Electrospun Nanofibers: Fast-Dissolution, Enhanced Water Solubility, High Temperature Stability, and Antibacterial Activity of Cinnamaldehyde. J. Agric. Food Chem. 2019, 67, 11066–11076. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Tang, P.; Zhao, L.; Pu, H.; Zhai, Y.; Li, H. Mechanism and structure studies of cinnamaldehyde/cyclodextrins inclusions by computer simulation and NMR technology. Carbohydr. Polym. 2018, 194, 294–302. [Google Scholar] [CrossRef]
- Rieger, K.A.; Schiffman, J.D. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Bai, J.; Zeng, Z.; Yang, X.; Wei, B.; Yang, Z. Cinnamaldehyde-modified chitosan hybrid nanoparticles for DOX delivering to produce synergistic anti-tumor effects. Front. Bioeng. Biotechnol. 2022, 10, 968065. [Google Scholar] [CrossRef]
- Malheiro, J.F.; Maillard, J.-Y.; Borges, F.; Simões, M. Evaluation of cinnamaldehyde and cinnamic acid derivatives in microbial growth control. Int. Biodeterior. Biodegrad. 2019, 141, 71–78. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef]
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus. Antibiotics 2022, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Advances in analytical chemistry and instrumentation. In Index to Reviews, Symposia Volumes and Monographs in Organic Chemistry; Phase Solubility Studies; Elsevier: Amsterdam, The Netherlands, 1965; pp. 117–212. [Google Scholar]
- Kfoury, M.; Landy, D.; Fourmentin, S. Characterization of Cyclodextrin/Volatile Inclusion Complexes: A Review. Molecules 2018, 23, 1204. [Google Scholar] [CrossRef] [Green Version]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Promising applications of cyclodextrins in food: Improvement of essential oils retention, controlled release and antiradical activity. Carbohydr. Polym. 2015, 131, 264–272. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the morphology of hydrophilic polymeric matrices on the diffusion and release of water soluble drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. Available online: https://pubmed.ncbi.nlm.nih.gov/14088963/ (accessed on 2 November 2022). [CrossRef] [PubMed]


| Slope | S0 (M) | Kf (M−1) | CE | |
|---|---|---|---|---|
| βCD | 0.27 ± 0.05 | 0.016 | 24.07 ± 6 | 0.38 ± 0.1 |
| PCD | 0.62 ± 0.02 | 0.016 | 104.25 ± 12 | 1.67 ± 0.2 |
| Formulation | Viscosity (s−1) | Viscoelastic Modulus at 10 min | ||
|---|---|---|---|---|
| 0.01 | 1000 | Storage Moduli (G′) | Loss Modulus (G″) | |
| 3:0:0 | 280 | 0.4 | 184 | 128 |
| 3:0:1 | 397 | 1.0 | 300 | 167 |
| 3:2:0 | 783 | 1.2 | 489 | 145 |
| 3:2:1 | 1200 | 1.4 | 796 | 190 |
| mmol/g of Compound | µmol/g of Hydrogel | |
|---|---|---|
| CHT | 5.0 | 151 (3% w/w) |
| CN | 7.6 | 76 (1% w/w) |
| CHT/PCD/CN | 3:0:1 | 3:2:1 |
|---|---|---|
| First Order | y = −0.0075x + 1.856 | y = −0.0042x + 1.7076 |
| r² = 0.7547 | r² = 0.4444 | |
| Zero Order | y = 1.0827x + 27.997 | y = 0.4834x + 48.484 |
| r² = 0.6958 | r² = 0.3984 | |
| Higuchi | y = 7.1598x + 17.715 | y = 3.4115x + 43.195 |
| r² = 0.8467 | r² = 0.5521 | |
| Korsmeyer-Peppas | y = 0.4633x + 1.2689 | y = 0.2911x + 1.6071 |
| r² = 0.9572 | r² = 0.9503 |
| Formulation CHT/PCD/CN | CHT | PCD | CN | W | LA | |
|---|---|---|---|---|---|---|
| Control | 3:0:0 | 3 | 0 | 0 | 96 | 1 |
| 3:0:1 | 3 | 0 | 1 | 95 | 1 | |
| Sample | 3:2:0 | 3 | 2 | 0 | 94 | 1 |
| 3:2:1 | 3 | 2 | 1 | 93 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chijcheapaza-Flores, H.; Tabary, N.; Chai, F.; Maton, M.; Staelens, J.-N.; Cazaux, F.; Neut, C.; Martel, B.; Blanchemain, N.; Garcia-Fernandez, M.J. Injectable Chitosan-Based Hydrogels for Trans-Cinnamaldehyde Delivery in the Treatment of Diabetic Foot Ulcer Infections. Gels 2023, 9, 262. https://doi.org/10.3390/gels9030262
Chijcheapaza-Flores H, Tabary N, Chai F, Maton M, Staelens J-N, Cazaux F, Neut C, Martel B, Blanchemain N, Garcia-Fernandez MJ. Injectable Chitosan-Based Hydrogels for Trans-Cinnamaldehyde Delivery in the Treatment of Diabetic Foot Ulcer Infections. Gels. 2023; 9(3):262. https://doi.org/10.3390/gels9030262
Chicago/Turabian StyleChijcheapaza-Flores, Henry, Nicolas Tabary, Feng Chai, Mickaël Maton, Jean-Noel Staelens, Frédéric Cazaux, Christel Neut, Bernard Martel, Nicolas Blanchemain, and Maria José Garcia-Fernandez. 2023. "Injectable Chitosan-Based Hydrogels for Trans-Cinnamaldehyde Delivery in the Treatment of Diabetic Foot Ulcer Infections" Gels 9, no. 3: 262. https://doi.org/10.3390/gels9030262
APA StyleChijcheapaza-Flores, H., Tabary, N., Chai, F., Maton, M., Staelens, J.-N., Cazaux, F., Neut, C., Martel, B., Blanchemain, N., & Garcia-Fernandez, M. J. (2023). Injectable Chitosan-Based Hydrogels for Trans-Cinnamaldehyde Delivery in the Treatment of Diabetic Foot Ulcer Infections. Gels, 9(3), 262. https://doi.org/10.3390/gels9030262







