Nanofluorapatite Hydrogels in the Treatment of Dentin Hypersensitivity: A Study of Physiochemical Properties and Fluoride Release
Abstract
1. Introduction
2. Results and Discussion
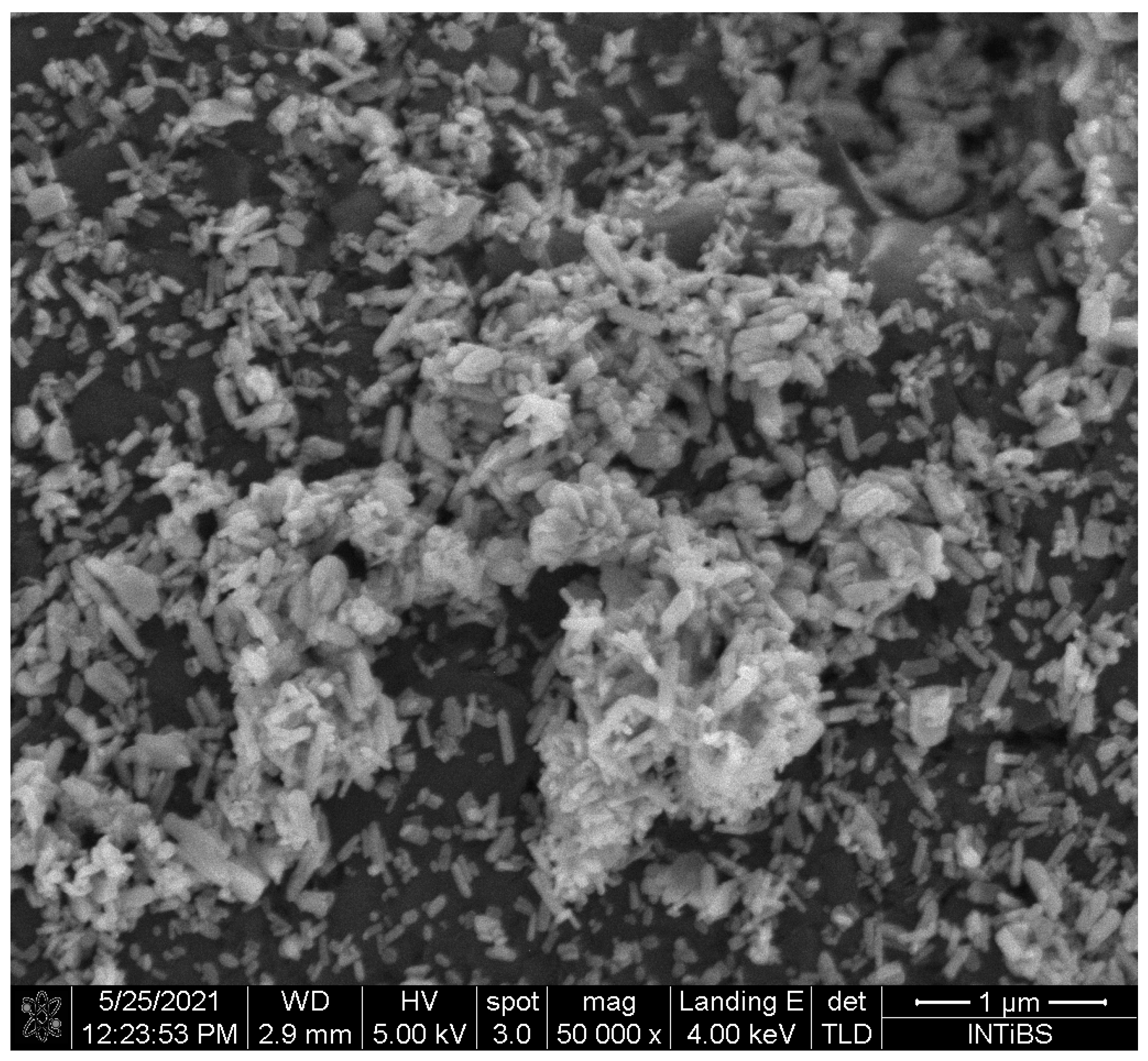
2.1. Physicochemical Analysis of the Fluorapatite Compound
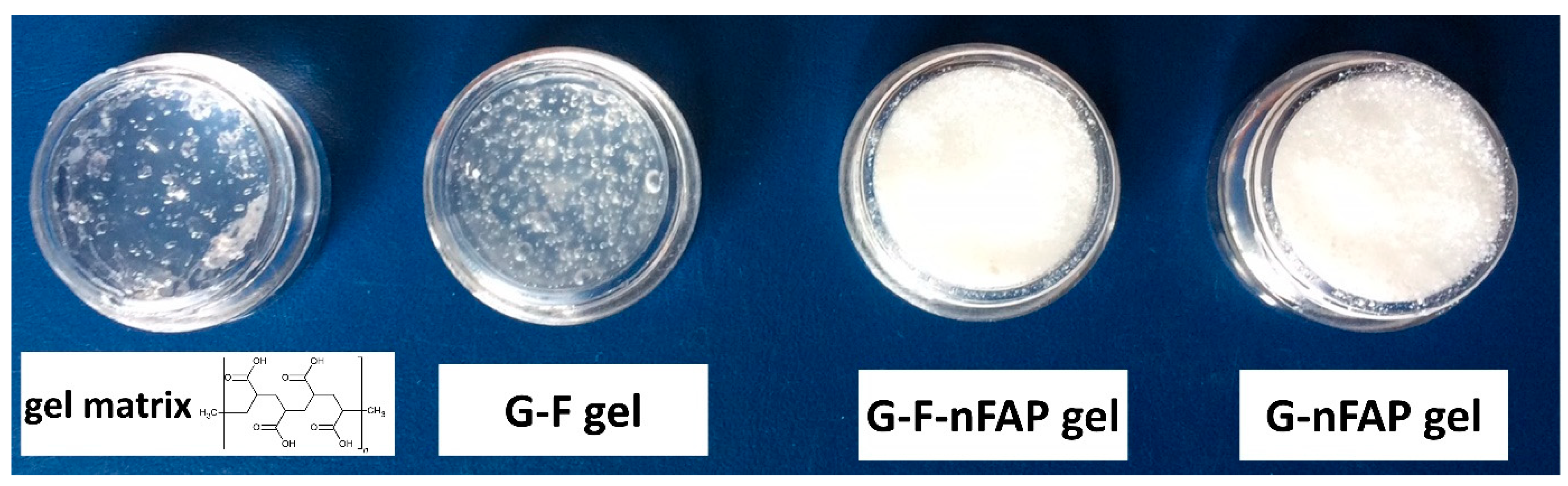
2.2. The Physicochemical Characteristics of the Gels
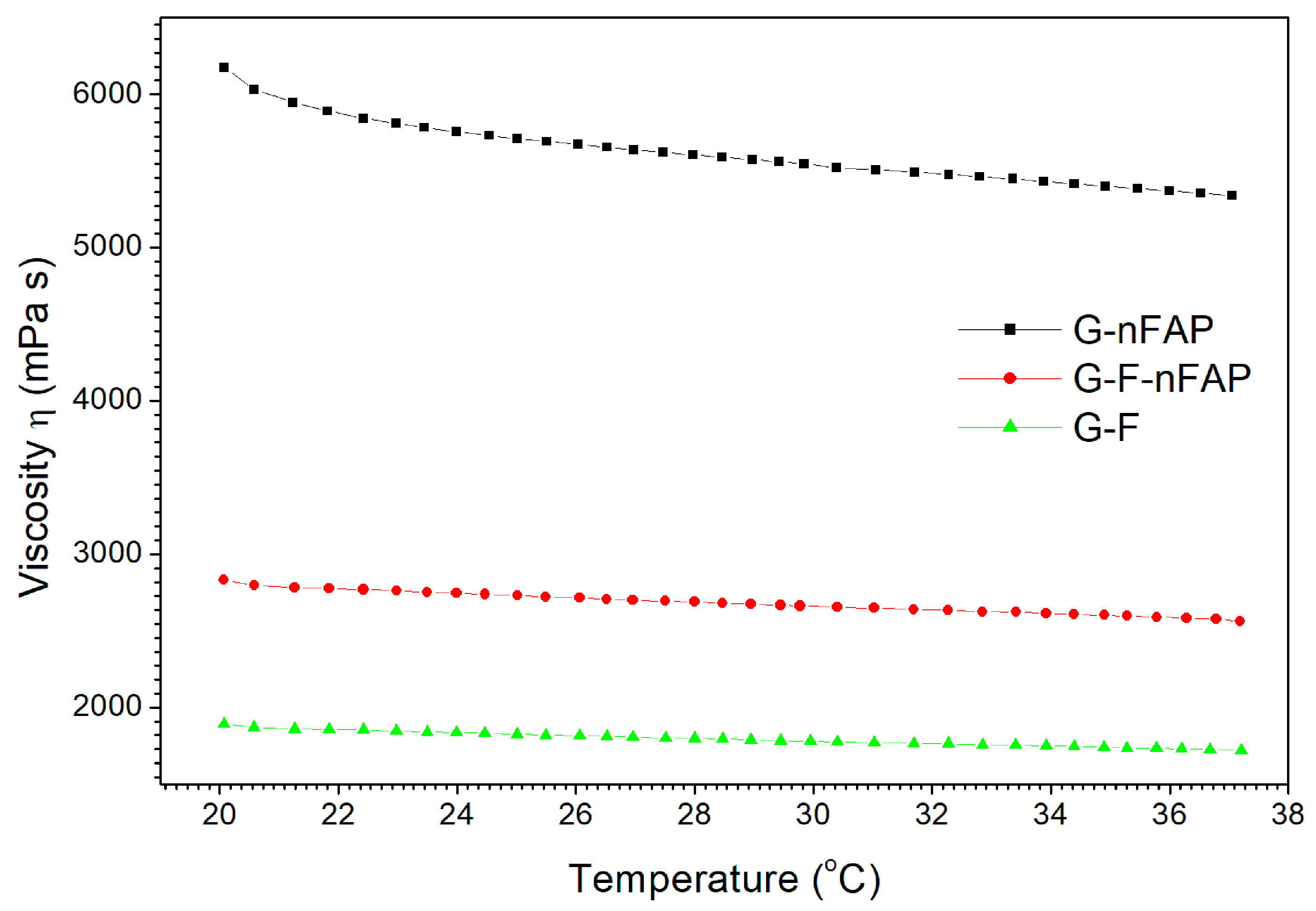
2.2.1. The Rheological Test
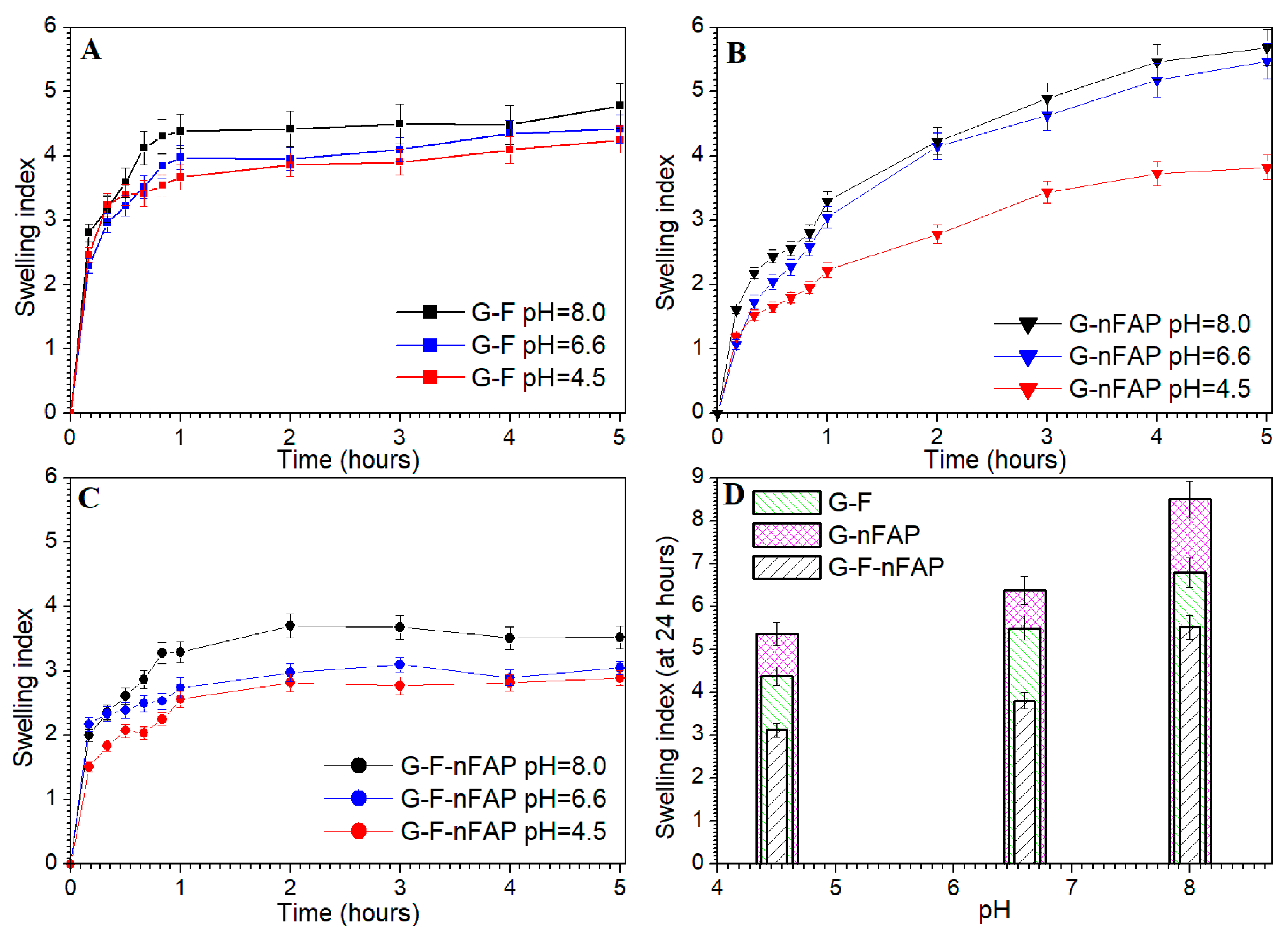
2.2.2. The Swelling Study
2.2.3. Thermogravimetric Analysis
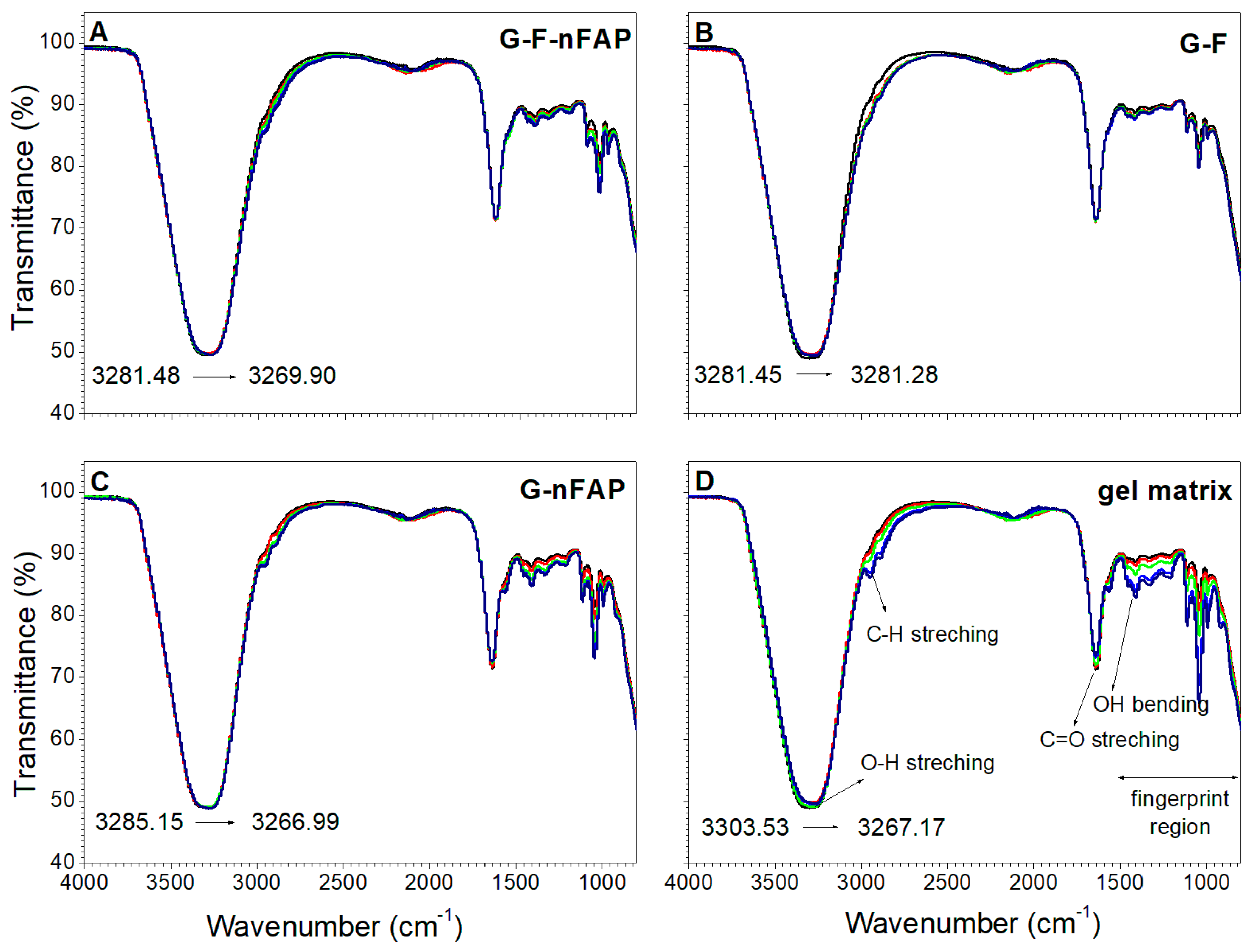
2.2.4. The Control of the Gels’ Network
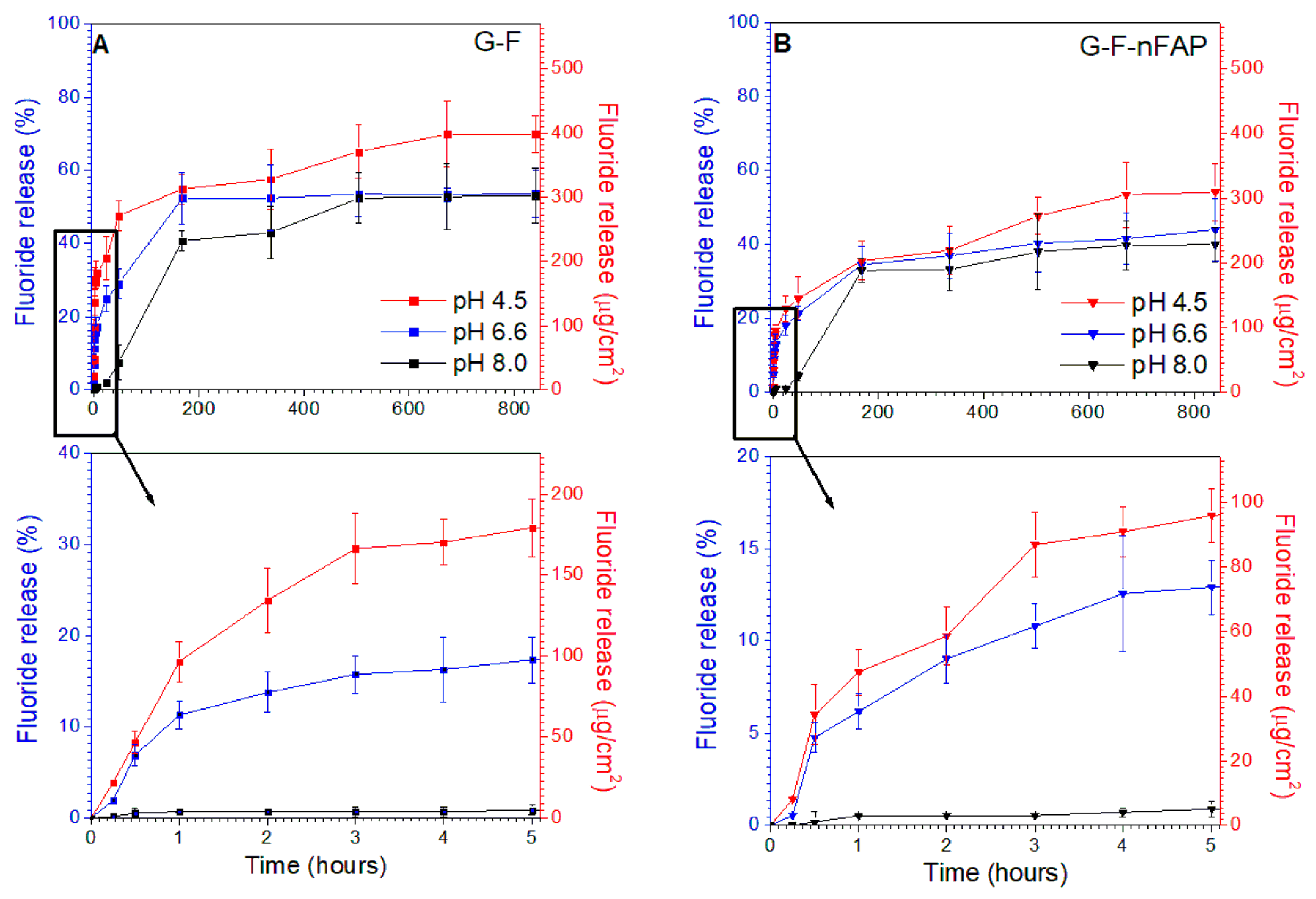
2.3. Release Experiments
3. Conclusions
4. Materials and Methods
4.1. Materials
4.1.1. Nanosized Fluorapatite Synthesis
4.1.2. Procedure for the Preparation of Hydrogels
4.2. Methods
4.2.1. Physicochemical Analysis of the Fluorapatite Compound
4.2.2. Rheological Measurements
4.2.3. FT-IR Spectroscopy Measurement
4.2.4. Swelling Test
4.2.5. Thermogravimetric Analysis
4.2.6. In Vitro Study of Fluoride Release from Hydrogels
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Addy, M. Dentine hypersensitivity: New perspectives on an old problem. Int. Dent. J. 2002, 52, 367–375. [Google Scholar] [CrossRef]
- Addy, M. Etiology and Clinical Implications of Dentine Hypersensitivity. Dent. Clin. N. Am. 1990, 34, 503–514. [Google Scholar] [CrossRef]
- Absi, E.G.; Addy, M.; Adams, D. Dentine hypersensitivity. J. Clin. Periodontol. 1987, 14, 280–284. [Google Scholar] [CrossRef]
- Zero, D.T.; Lussi, A. Erosion—Chemical and biological factors of importance to the dental practitioner. Int. Dent. J. 2005, 55, 285–290. [Google Scholar] [CrossRef]
- Bartold, P. Dentinal hypersensitivity: A review. Aust. Dent. J. 2006, 51, 212–218. [Google Scholar] [CrossRef]
- Absi, E.G.; Addy, M.; Adams, D. Dentine hypersensitivity—The effect of toothbrushing and dietary compounds on dentine in vitro: An SEM study. J. Oral Rehabil. 1992, 19, 101–110. [Google Scholar] [CrossRef]
- Pesevska, S.; Nakova, M.; Ivanovski, K.; Angelov, N.; Kesic, L.; Obradovic, R.; Mindova, S.; Nares, S. Dentinal hypersensitivity following scaling and root planing: Comparison of low-level laser and topical fluoride treatment. Lasers Med. Sci. 2010, 25, 647–650. [Google Scholar] [CrossRef]
- Ganss, C. Definition of Erosion and Links to Tooth Wear. In Monographs in Oral Science; Lussi, A., Ed.; KARGER: Basel, Switzerland, 2006; Volume 20, pp. 9–16. ISBN 978-3-8055-8097-7. [Google Scholar]
- Magloire, H.; Maurin, J.C.; Couble, M.L.; Shibukawa, Y.; Tsumura, M.; Thivichon-Prince, B.; Bleicher, F. Topical review. Dental pain and odontoblasts: Facts and hypotheses. J. Orofac. Pain 2010, 24, 335–349. [Google Scholar] [PubMed]
- Orchardson, R.; Gillam, D.G. Managing dentin hypersensitivity. J. Am. Dent. Assoc. 2006, 137, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I. The relationship between dentine sensitivity and movements in the contents of dentinal tubules. Br. Dent. J. 1955, 98, 391–392. [Google Scholar]
- Brännström, M. [Dentin sensitivity]. Arsb. Goteb. Tandlak. -Sallsk. 1964, 15–35. [Google Scholar]
- Camilotti, V.; Zilly, J.; do Monte Ribeiro Busato, P.; Nassar, C.A.; Nassar, P.O. Desensitizing treatments for dentin hypersensitivity: A randomized, split-mouth clinical trial. Braz. Oral Res. 2012, 26, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Cruz, J.; Stout, J.R.; Heaton, L.J.; Wataha, J.C.; for Northwest PRECEDENT. Dentin Hypersensitivity and Oxalates: A Systematic Review. J. Dent. Res. 2011, 90, 304–310. [Google Scholar] [CrossRef]
- Banfield, N.; Addy, M. Dentine hypersensitivity: Development and evaluation ofamodel in situ to study tubulepatency. J. Clin. Periodontol. 2004, 31, 325–335. [Google Scholar] [CrossRef]
- Kim, S. Hypersensitive teeth: Desensitization of pulpal sensory nerves. J. Endod. 1986, 12, 482–485. [Google Scholar] [CrossRef]
- Farooq, I.; Moheet, I.A.; AlShwaimi, E. In vitro dentin tubule occlusion and remineralization competence of various toothpastes. Arch. Oral Biol. 2015, 60, 1246–1253. [Google Scholar] [CrossRef]
- Hill, R.G.; Chen, X.; Gillam, D.G. In Vitro Ability of a Novel Nanohydroxyapatite Oral Rinse to Occlude Dentine Tubules. Int. J. Dent. 2015, 2015, 153284. [Google Scholar] [CrossRef]
- Lawson, K.; Gross, K.B.; Overman, P.R.; Anderson, D. Effectiveness of chlorhexidine and sodium fluoride in reducing dentin hypersensitivity. J. Dent. Hyg. JDH 1991, 65, 340–344. [Google Scholar]
- Jacobsen, P.L.; Bruce, G. Clinical dentin hypersensitivity: Understanding the causes and prescribing a treatment. J. Contemp. Dent. Pract. 2001, 2, 1–12. [Google Scholar] [CrossRef]
- Lee, B.-S.; Kang, S.-H.; Wang, Y.-L.; Lin, F.-H.; Lin, C.-P. In Vitro Study of Dentinal Tubule Occlusion with Sol-gel DP-bioglass for Treatment of Dentin Hypersensitivity. Dent. Mater. J. 2007, 26, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Tredwin, C.J.; Young, A.M.; Abou Neel, E.A.; Georgiou, G.; Knowles, J.C. Hydroxyapatite, fluor-hydroxyapatite and fluorapatite produced via the sol–gel method: Dissolution behaviour and biological properties after crystallisation. J. Mater. Sci. Mater. Med. 2014, 25, 47–53. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Gao, X.; Wei, Y.; Deng, X.; Chen, H.; Zhang, X. Remineralizing Efficacy of Fluorohydroxyapatite Gel on Artificial Dentinal Caries Lesion. J. Nanomater. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Bhadang, K.A.; Holding, C.A.; Thissen, H.; McLean, K.M.; Forsythe, J.S.; Haynes, D.R. Biological responses of human osteoblasts and osteoclasts to flame-sprayed coatings of hydroxyapatite and fluorapatite blends. Acta Biomater. 2010, 6, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Barandehfard, F.; Kianpour Rad, M.; Hosseinnia, A.; Khoshroo, K.; Tahriri, M.; Jazayeri, H.E.; Moharamzadeh, K.; Tayebi, L. The addition of synthesized hydroxyapatite and fluorapatite nanoparticles to a glass-ionomer cement for dental restoration and its effects on mechanical properties. Ceram. Int. 2016, 42, 17866–17875. [Google Scholar] [CrossRef]
- Taha, S.T.; Han, H.; Chang, S.-R.; Sovadinova, I.; Kuroda, K.; Langford, R.M.; Clarkson, B.H. Nano/micro fluorhydroxyapatite crystal pastes in the treatment of dentin hypersensitivity: An in vitro study. Clin. Oral Investig. 2015, 19, 1921–1930. [Google Scholar] [CrossRef]
- Fu, J. Study on of bioadhesive property of carbomer934 by a gamma camera in vivo. World J. Gastroenterol. 2002, 8, 176. [Google Scholar] [CrossRef]
- Kumar, K.; Dhawan, N.; Sharma, H.; Vaidya, S.; Vaidya, B. Bioadhesive polymers: Novel tool for drug delivery. Artif. Cells Nanomed. Biotechnol. 2014, 42, 274–283. [Google Scholar] [CrossRef]
- Varshosaz, J.; Tavakoli, N.; Saidian, S. Development and Physical Characterization of a Periodontal Bioadhesive Gel of Metronidazole. Drug Deliv. 2002, 9, 127–133. [Google Scholar] [CrossRef]
- Kulkarni, V.; Shaw, C. Essential Chemistry for Formulators of Semisolid and Liquid Dosages; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 978-0-12-801024-2. [Google Scholar]
- Lodish, H.; Berk, A.; Kaiser, C.; Krieger, M.; Bretscher, A.; Ploegh, H.; Martin, K.C.; Yaffe, M.B.; Amon, A. Molecular Cell Biology, 9th ed.; Macmillan International Higher Education: New York, NY, USA, 2021; ISBN 978-1-319-36548-6. [Google Scholar]
- Younes, M.; Aquilina, G.; Engel, K.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gürtler, R.; Gundert-Remy, U.; Husøy, T.; Manco, M.; et al. Safety evaluation of crosslinked polyacrylic acid polymers (carbomer) as a new food additive. EFSA J. 2021, 19, e06693. [Google Scholar] [CrossRef]
- Pałka, Ł.; Rybak, Z.; Kuropka, P.; Szymonowicz, M.; Kiryk, J.; Marycz, K.; Dobrzyński, M. In vitro SEM analysis of desensitizing agents and experimental hydroxyapatite-based composition effectiveness in occluding dentin tubules. Adv. Clin. Exp. Med. 2020, 29, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Herman, K.; Wujczyk, M.; Dobrzynski, M.; Diakowska, D.; Wiglusz, K.; Wiglusz, R.J. In Vitro Assessment of Long-Term Fluoride Ion Release from Nanofluorapatite. Materials 2021, 14, 3747. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Lutterotti, L.; Bortolotti, M. Object-oriented programming and fast computation techniques in Maud, a program for powder diffraction analysis written in JavaTM. IUCr Compcomm Newslett 2003, 1, 43–50. [Google Scholar]
- Ghica, M.; Hîrjău, M.; Lupuleasa, D.; Dinu-Pîrvu, C.-E. Flow and Thixotropic Parameters for Rheological Characterization of Hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 5th revised ed.; European coatings library; Vincentz: Hannover, Germany, 2020; ISBN 978-3-86630-532-8. [Google Scholar]
- Barnes, H.A. A Handbook of Elementary Rheology; University of Wales Institute of Non-Newtonian Fluid Mechanics: Aberystwyth, UK, 2000; ISBN 978-0-9538032-0-0. [Google Scholar]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From controlled release to pH-responsive drug delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Schwarz, T.W.; Levy, G. A Report on the Oxidative Degradation of Neutralized Carbopol *. University of California School of Pharmacy, San Francisco 22. J. Am. Pharm. Assoc. Sci. Ed. 1958, 47, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Kanis, L.A.; Viel, F.C.; Crespo, J.S.; Bertolino, J.R.; Pires, A.T.N.; Soldi, V. Study of poly(ethylene oxide)/Carbopol blends through thermal analysis and infrared spectroscopy. Polymer 2000, 41, 3303–3309. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef] [PubMed]
- Malamed, S. Medical Emergencies in the Dental Office, 8th ed.; Elsevier, Inc.: Philadelphia, PA, USA, 2022; ISBN 978-0-323-77615-8. [Google Scholar]
- Nahum, S.-V.E.; Rogelio José, S.-V.; Edith, L.-C.; Hugo, T.-R.V.; Ulises, V.-E.; Alejandra, M.-V.A. Fluoride Release and Recharge in Conventional Varnishes, Compared to a Giomer and a Resin Modified Glass Ionomer. J. Dent. Mater. Tech. 2021, 10, 63–70. [Google Scholar] [CrossRef]
- McCann, H.G. The solubility of fluorapatite and its relationship to that of calcium fluoride. Arch. Oral Biol. 1968, 13, 987–1001. [Google Scholar] [CrossRef]
- Tressaud, A.; Haufe, G. Fluorine and Health: Molecular Imaging, Biomedical Materials and Pharmaceuticals; Elsevier Science: Amsterdam, The Netherlands; London, UK, 2008; ISBN 978-0-444-53086-8. [Google Scholar]
- Taqa, A.A.; Abdal, A.; Dawood, A.I. The Effect of pH on Fluoride Release of Glass Ionomer Based Restorative Materials. Int. J. Dent. Sci. Res. 2016, 4, 52–57. [Google Scholar] [CrossRef]
- Bahadure, R.; Pandey, R.; Kumar, R.; Gopal, K.; Singh, R. An estimation of fluoride release from various dental restorative materials at different pH: In vitro study. J. Indian Soc. Pedod. Prev. Dent. 2012, 30, 122. [Google Scholar] [CrossRef] [PubMed]
- Miller, C. The Stokes-Einstein law for diffusion in solution. Proc. R. Soc. Lond. Ser. Contain. Pap. Math. Phys. Character 1924, 106, 724–749. [Google Scholar] [CrossRef]
- Turkalj, M.; Šutej, I.; Peroš, K. Comparison of Fluoride Ion Release from Fluoride Gel in Various Solvents. Acta Stomatol. Croat. 2020, 54, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Milburn, J.L.; Henrichs, L.E. Substantive Fluoride Release from a New Fluoride Varnish Containing CXP. Dentistry 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P. Rheological Characterization of Carbopol® Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Guo, M.; Wu, Y.; Xue, S.; Xia, Y.; Yang, X.; Dzenis, Y.; Li, Z.; Lei, W.; Smith, A.T.; Sun, L. A highly stretchable, ultra-tough, remarkably tolerant, and robust self-healing glycerol-hydrogel for a dual-responsive soft actuator. J. Mater. Chem. A 2019, 7, 25969–25977. [Google Scholar] [CrossRef]
- Chafran, L.; Carfagno, A.; Altalhi, A.; Bishop, B. Green Hydrogel Synthesis: Emphasis on Proteomics and Polymer Particle-Protein Interaction. Polymers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Di Giuseppe, E.; Corbi, F.; Funiciello, F.; Massmeyer, A.; Santimano, T.N.; Rosenau, M.; Davaille, A. Characterization of Carbopol® hydrogel rheology for experimental tectonics and geodynamics. Tectonophysics 2015, 642, 29–45. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Song, J.-Y.; Lee, E.-J.; Park, S.-K. Rheological properties and microstructures of Carbopol gel network system. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, W.; Jin, C. Protocol efficiently measuring the swelling rate of hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef] [PubMed]
- Heravi, F.; Moayed, M.H.; Mokhber, N. Effect of fluoride on nickel-titanium and stainless steel orthodontic archwires: An in-vitro study. J. Dent. Tehran Iran 2015, 12, 49–59. [Google Scholar]
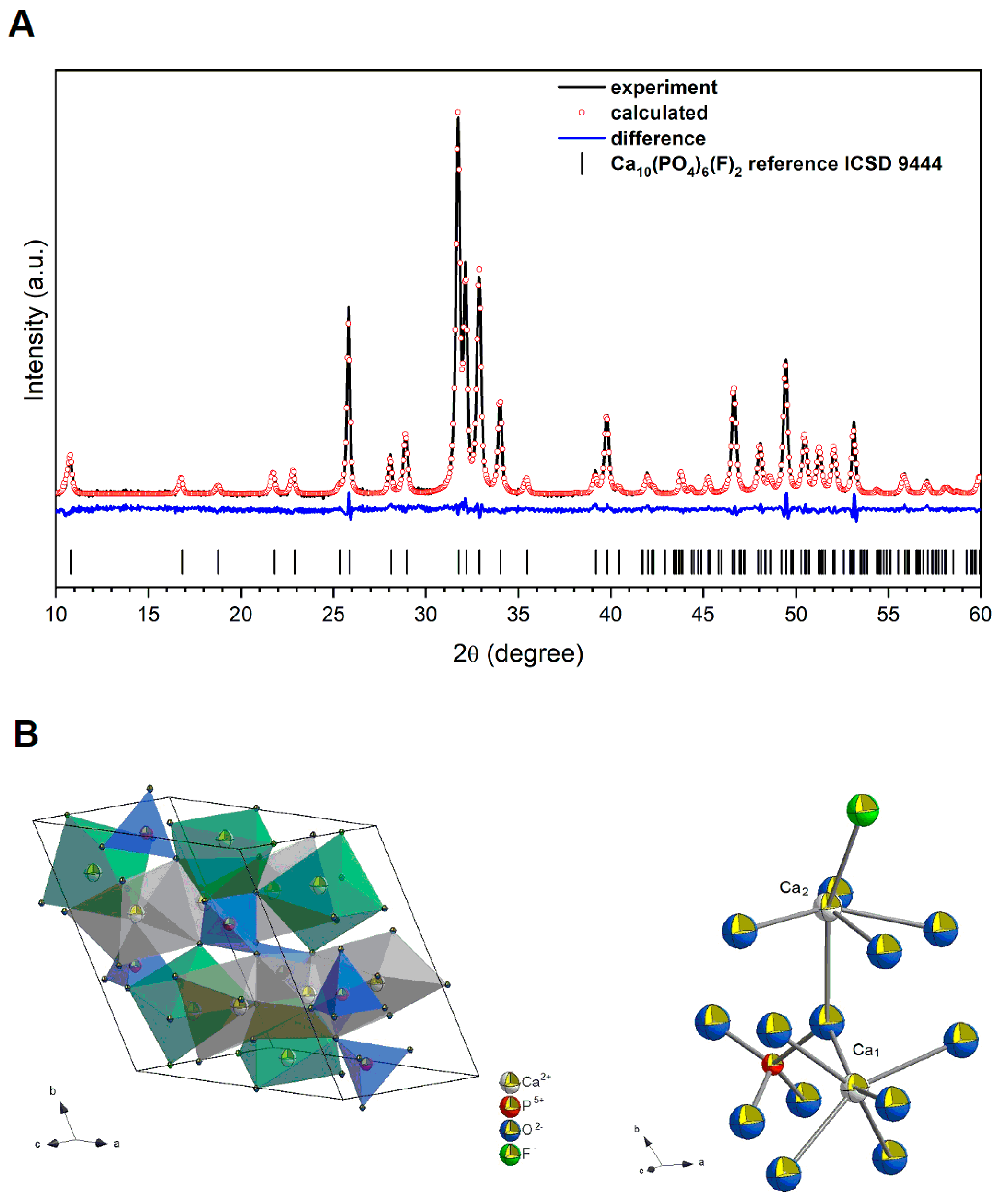
| Sample | Ca10(PO4)6F2; Z = 2 | |||||
|---|---|---|---|---|---|---|
| Space group | Hexagonal P63/m (No. 176) | |||||
| Calculated cell parameters | a = 9.382(0) Å c = 6.892(1) Å V = 525.37(8) Å3 | |||||
| Rw Rwnb Rall Rnb σ | 2.87% 2.35% 2.22% 2.08% 1.32% | |||||
| Selected shortest contacts | ||||||
| Ca–Ca Ca–O Ca–P P–O Ca–O–Ca | 4.0049(33) Å 2.3562(64) Å 3.2043(73) Å 1.5294(86) Å 111.823(276)° | |||||
| Atom | Wyckoff positions | x | y | z | Biso | Occ. (<1) |
| Ca1 | 4 f | 0.3333 | 0.6665 | 0.00064 | 0.690871 | 0.989 |
| Ca2 | 6 h | 0.2408 | 0.9822 | 0.2498 | 0.412068 | 0.986 |
| P1 | 6 h | 0.3981 | 0.3682 | 0.25 | 0.709863 | 1.007 |
| O1 | 6 h | 0.3224 | 0.4796 | 0.2499 | 0.318567 | 987 |
| O2 | 6 h | 0.5904 | 0.4687 | 0.2498 | 0.218500 | 987 |
| O3 | 12 i | 0.3339 | 0.2510 | 0.0644 | 0.254603 | 988 |
| F1 | 2 a | 0 | 0 | 0.2499 | 0.030193 | 0.906 |
| Hydrogel | Yield Point (Pa) at 25 °C | Yield Point (Pa) at 37 °C |
|---|---|---|
| G-F | 142.55 ± 10.09 | 139.21 ± 9.05 |
| G-F-nFAP | 178.01 ± 9.13 | 172.15 ± 10.03 |
| G-nFAP | 182.34 ± 12.20 | 178.48 ± 8.15 |
| Hydrogels | Carbopol 974 (g) | 86% Glycerol (g) | Sodium Fluoride (g) | nFAP (g) | 10% Sodium Hydroxide (g) * | Water (g) |
|---|---|---|---|---|---|---|
| G-F | 1.5 | 10 | 4 | - | 6.4 | 78.1 |
| G-F-nFAP | 1.5 | 10 | 4 | 10 | 2.8 | 71.7 |
| G-nFAP | 1.5 | 10 | −- | 10 | 6.2 | 72.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiglusz, K.; Dobrzynski, M.; Gutbier, M.; Wiglusz, R.J. Nanofluorapatite Hydrogels in the Treatment of Dentin Hypersensitivity: A Study of Physiochemical Properties and Fluoride Release. Gels 2023, 9, 271. https://doi.org/10.3390/gels9040271
Wiglusz K, Dobrzynski M, Gutbier M, Wiglusz RJ. Nanofluorapatite Hydrogels in the Treatment of Dentin Hypersensitivity: A Study of Physiochemical Properties and Fluoride Release. Gels. 2023; 9(4):271. https://doi.org/10.3390/gels9040271
Chicago/Turabian StyleWiglusz, Katarzyna, Maciej Dobrzynski, Martina Gutbier, and Rafal J. Wiglusz. 2023. "Nanofluorapatite Hydrogels in the Treatment of Dentin Hypersensitivity: A Study of Physiochemical Properties and Fluoride Release" Gels 9, no. 4: 271. https://doi.org/10.3390/gels9040271
APA StyleWiglusz, K., Dobrzynski, M., Gutbier, M., & Wiglusz, R. J. (2023). Nanofluorapatite Hydrogels in the Treatment of Dentin Hypersensitivity: A Study of Physiochemical Properties and Fluoride Release. Gels, 9(4), 271. https://doi.org/10.3390/gels9040271








