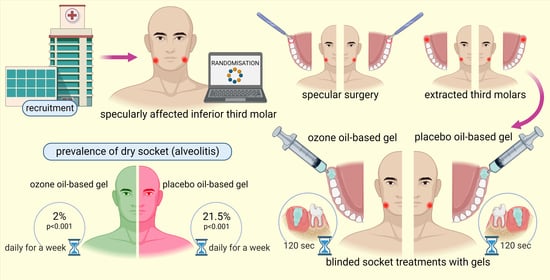
Prevention of Dry Socket with Ozone Oil-Based Gel after Inferior Third Molar Extraction: A Double-Blind Split-Mouth Randomized Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
2. Results
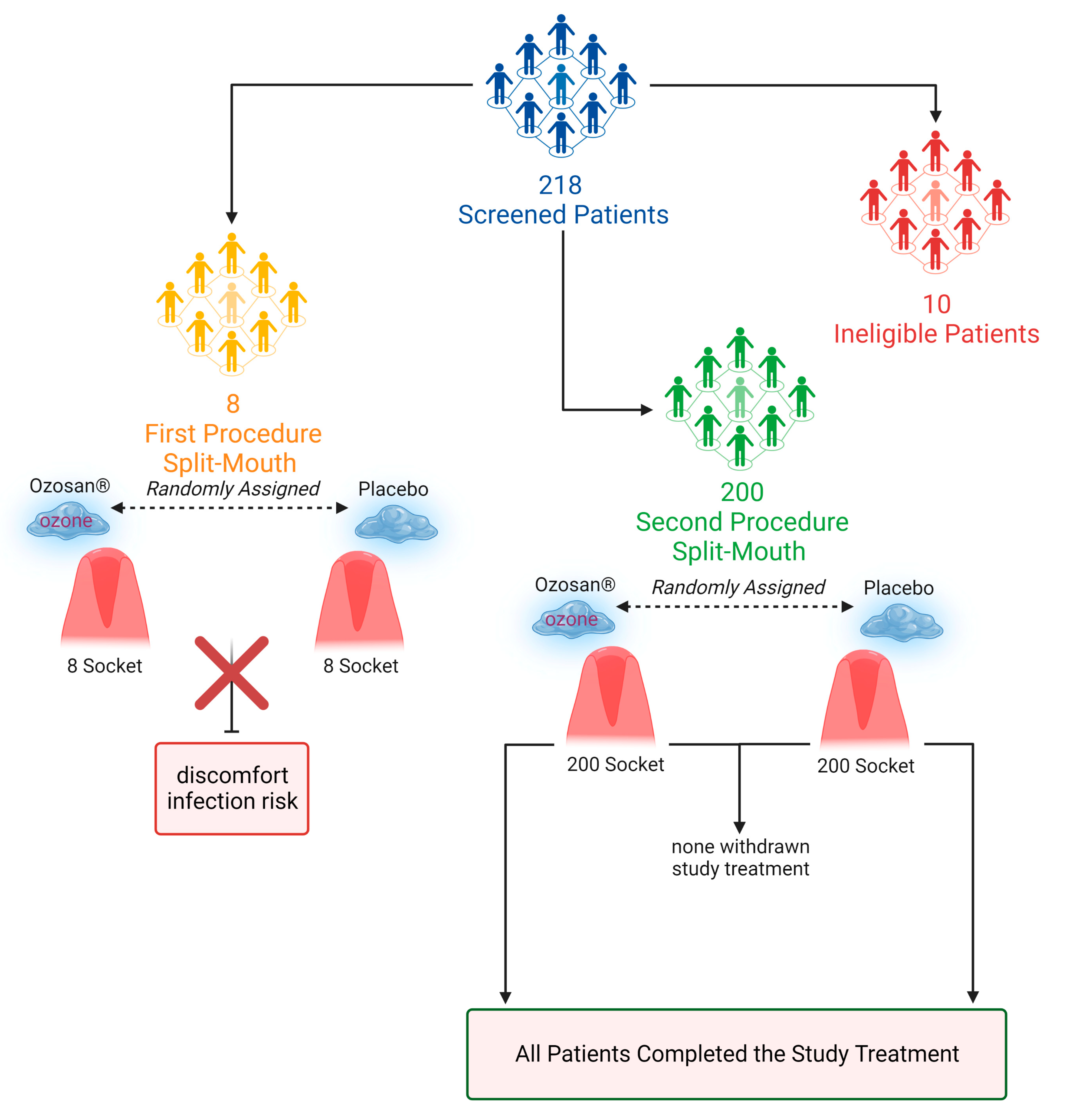
2.1. Patient and Baseline Characteristics
2.2. Effect of Ozonated Gel on Dry Socket Incidence
2.3. Amount of Peroxide in Ozosan® Gel and Release
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design and Participants
5.2. Randomization and Masking
5.3. Operative Technique
5.4. Ozone Gel or Placebo Treatment
5.5. Outcomes
5.6. Determination of Peroxide Values in Ozosan® Gel
5.7. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, C.L.; Rodrigues, M.T.V.; Ferreira, O.; Garlet, G.P.; de Carvalho, P.S.P. Clinical Concepts of Dry Socket. J. Oral Maxillofac. Surg. 2010, 68, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Chow, O.; Wang, R.; Ku, D.; Huang, W. Alveolar Osteitis: A Review of Current Concepts. J. Oral Maxillofac. Surg. 2020, 78, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Blum, I.R. Contemporary Views on Dry Socket (Alveolar Osteitis): A Clinical Appraisal of Standardization, Aetiopathogenesis and Management: A Critical Review. Int J. Oral Maxillofac. Surg. 2002, 31, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhshan, V. Common Risk Factors of Dry Socket (Alveolitis Osteitis) Following Dental Extraction: A Brief Narrative Review. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 407–411. [Google Scholar] [CrossRef]
- Taberner-Vallverdú, M.; Nazir, M.; Sánchez-Garcés, M.Á.; Gay-Escoda, C. Efficacy of Different Methods Used for Dry Socket Management: A Systematic Review. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e633–e639. [Google Scholar] [CrossRef]
- Taberner-Vallverdú, M.; Camps-Font, O.; Gay-Escoda, C.; Sánchez-Garcés, M.A. Previous Dry Socket as a Risk Factor for Alveolar Osteitis: A Nested Case-Control Study in Primary Healthcare Services. J. Clin. Exp. Dent. 2022, 14, 479–485. [Google Scholar] [CrossRef]
- Bowe House Officer, D.C.; Rogers, S.; Stassen, L.F. The Management of Dry Socket Alveolar Osteitis The Management of Dry Socket/ Alveolar Osteitis Introduction/Review of Literature. J. Ir. Dent. Assoc. 2011, 57, 305–310. [Google Scholar]
- Mamoun, J. Dry Socket Etiology, Diagnosis, and Clinical Treatment Techniques. J. Korean Assoc. Oral Maxillofac. Surg. 2018, 44, 52. [Google Scholar] [CrossRef] [Green Version]
- Leon, B.R.; Romary, D.J.; Landsberger, S.A.; Bradner, K.N.; Ramirez, M.; Lubitz, R.M. Risks of Ozonated Oil and Ozonated Water on Human Skin: A Systematic Review. Int. Wound J. 2022, 19, 1901–1910. [Google Scholar] [CrossRef]
- Scribante, A.; Gallo, S.; Pascadopoli, M.; Soleo, R.; Di Fonso, F.; Politi, L.; Venugopal, A.; Marya, A.; Butera, A. Management of Periodontal Disease with Adjunctive Therapy with Ozone and Photobiomodulation (PBM): A Randomized Clinical Trial. Photonics 2022, 9, 138. [Google Scholar] [CrossRef]
- Pattanaik, B.; Jetwa, D.; Pattanaik, S.; Manglekar, S.; Naitam, D.N.; Dani, A. Ozone Therapy in Dentistry: A Literature Review. J. Interdiscip. Dent. 2011, 1, 87. [Google Scholar] [CrossRef]
- Saini, R. Ozone Therapy in Dentistry: A Strategic Review. J. Nat. Sci. Biol. Med. 2011, 2, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scribante, A.; Butera, A.; Alovisi, M. Customized Minimally Invasive Protocols for the Clinical and Microbiological Management of the Oral Microbiota. Microorganisms 2022, 10, 675. [Google Scholar] [CrossRef] [PubMed]
- Re, L. Ozone in Medicine: A Few Points of Reflections. Front. Physiol. 2022, 13, 842229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; He, H. Recent Advances in Catalytic Decomposition of Ozone. J. Environ. Sci. 2020, 94, 14–31. [Google Scholar] [CrossRef]
- Batakliev, T.; Georgiev, V.; Anachkov, M.; Rakovsky, S.; Zaikov, G.E. Ozone Decomposition. Interdiscip. Toxicol. 2014, 7, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Bianco, E.; Maddalone, M.; Porcaro, G.; Amosso, E.; Baldoni, M. Treatment of Osteoradionecrosis of the Jaw with Ozone in the Form of Oil-Based Gel: 1-Year Follow-Up. J. Contemp. Dent. Pract. 2019, 20, 270–276. [Google Scholar] [CrossRef]
- Abstracts of the 10th Virtual Conseuro 2021 Congress. Clin. Oral Investig. 2021, 25, 4185–4238. [CrossRef]
- Houston, J.P.; McCollum, J.; Pietz, D.; Schneck, D. Alveolar Osteitis: A Review of Its Etiology, Prevention, and Treatment Modalities. Gen. Dent. 2002, 50, 457–463; quiz 464. [Google Scholar]
- Kaur, J.; Raval, R.; Bansal, A.; Kumawat, V. Repercussions of Intraalveolar Placement of Combination of 0.2% Chlorhexidine & 10 Mg Metronidazole Gel on the Occurrence of Dry Sockets—A Randomized Control Trial. J. Clin. Exp. Dent. 2017, 9, e284–e288. [Google Scholar] [CrossRef]
- Hermesch, C.B.; Hilton, T.J.; Biesbrock, A.R.; Baker, R.A.; Cain-Hamlin, J.; McClanahan, S.F.; Gerlach, R.W. Perioperative Use of 0.12% Chlorhexidine Gluconate for the Prevention of Alveolar Osteitis: Efficacy and Risk Factor Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, K.; Smith, A.; Chandu, A. Factors Affecting Incidence of Dry Socket: A Prospective Community-Based Study. J. Oral Maxillofac. Surg. 2011, 69, 1880–1884. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lagares, D.; Serrera-Figallo, M.; Romero-Ruiz, M.-M.; Infante-Cossio, P.; García-Calderón, M.; Gutierrez-Perez, J. Update on Dry Socket: A Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2005, 10, 81–85. [Google Scholar]
- Haraji, A.; Rakhshan, V.; Khamverdi, N.; Alishahi, H. Effects of Intra-Alveolar Placement of 0.2% Chlorhexidine Bioadhesive Gel on Dry Socket Incidence and Postsurgical Pain: A Double-Blind Split-Mouth Randomized Controlled Clinical Trial. J. Orofac. Pain 2013, 27, 256–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huth, K.C.; Quirling, M.; Lenzke, S.; Paschos, E.; Kamereck, K.; Brand, K.; Hickel, R.; Ilie, N. Effectiveness of Ozone against Periodontal Pathogenic Microorganisms. Eur. J. Oral Sci. 2011, 119, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, C.; Kallenberger, A. Influence of Chlorhexidine Rinsing on the Healing of Oral Mucosa and Osseous Lesions. J. Clin. Periodontol. 1980, 7, 443–456. [Google Scholar] [CrossRef]
- Küçük, F.; Yıldırım, S.; Çetiner, S. Cytotoxicity Assessment of Different Doses of Ozonated Water on Dental Pulp Cells. BMC Oral Health 2021, 21, 32. [Google Scholar] [CrossRef]
- Srikanth, A.; Sathish, M.; Harsha, A.V.S. Application of Ozone in the Treatment of Periodontal Disease. J. Pharm. Bioallied. Sci. 2013, 5, S89. [Google Scholar] [CrossRef]
- Rizk, M.; Witte, M.B.; Barbul, A. Nitric Oxide and Wound Healing. World J. Surg. 2004, 28, 301–306. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, X.; Guo, H.; Huang, H.; Huang, S.; Huang, S.; Xue, W.; Zhu, P.; Guo, R. Nitric Oxide Released Injectable Hydrogel Combined with Synergistic Photothermal Therapy for Antibacterial and Accelerated Wound Healing. Appl. Mater. Today 2020, 20, 100781. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef] [PubMed]
- Viebahn-Haensler, R.; León Fernández, O.S.; Malatesta, M.; Cisterna, B.; Costanzo, M. Ozone in Medicine. The Low-Dose Ozone Concept and Its Basic Biochemical Mechanisms of Action in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7890. [Google Scholar] [CrossRef] [PubMed]
- Azarpazhooh, A.; Limeback, H. The Application of Ozone in Dentistry: A Systematic Review of Literature. J. Dent. 2008, 36, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Tallón, F.J.; Torres-Morera, L.M.; Baeza-Noci, J.; Carrillo-Izquierdo, M.D.; Pinto-Bonilla, R. Updated Review on Ozone Therapy in Pain Medicine. Front. Physiol. 2022, 13, 840623. [Google Scholar] [CrossRef]
- Ghanem, W.M.A.; Al-Moudallal, Y.; Droubi, M.; Al-Nerabieah, Z. Effect of Intrasocket Application of Ozonized Olive Oil Gel on Postsurgical Pain and Soft Tissue Healing of Impacted Mandibular Third Molars Surgery: Split-Mouth Randomized Controlled Trial. Int. J. Dent. Oral Sci. 2021, 8, 1408–1414. [Google Scholar] [CrossRef]
- Cores, Á.; Piquero, M.; Villacampa, M.; León, R.; Menéndez, J.C. NRF2 Regulation Processes as a Source of Potential Drug Targets against Neurodegenerative Diseases. Biomolecules 2020, 10, 904. [Google Scholar] [CrossRef]
- Materni, A.; de Angelis, N.; di Tullio, N.; Colombo, E.; Benedicenti, S.; Amaroli, A. Flapless Surgical Approach to Extract Impacted Inferior Third Molars: A Retrospective Clinical Study. J. Clin. Med. 2021, 10, 593. [Google Scholar] [CrossRef]
- Dolanmaz, D.; Esen, A.; Isik, K.; Candirli, C. Effect of 2 Flap Designs on Postoperative Pain and Swelling after Impacted Third Molar Surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e244–e246. [Google Scholar] [CrossRef]
- Materni, A.; Pasquale, C.; Signore, A.; Benedicenti, S.; Amaroli, A. Comparison between the Flapless Surgical Approach and a Novel Single Incision Access in Terms of Recovery Time and Comfort after Extraction of Impacted Inferior Third Molars: A Randomised, Blinded, Split-Mouth Controlled Clinical Trial. J Clin Med. 2023, 2, 1995. [Google Scholar] [CrossRef]
- Ghohestani, E.; Tashkhourian, J.; Hemmateenejad, B. Colorimetric Determination of Peroxide Value in Vegetable Oils Using a Paper Based Analytical Device. Food Chem. 2023, 403, 134345. [Google Scholar] [CrossRef] [PubMed]
- Radzimierska-Kazmierczak, M.; Smigielski, K.; Sikora, M.; Nowak, A.; Plucinska, A.; Kunicka-Styczynska, A.; Czarnecka-Chrebelska, K.H. Olive Oil with Ozone-Modified Properties and Its Application. Molecules 2021, 26, 3074. [Google Scholar] [CrossRef] [PubMed]
- Rosner, B. Bernard Rosner Fundamentals of Biostatistics, 8th ed.; Cengage Learning: Boston, MA, USA, 2015; p. 927. ISBN 978-1305268920. [Google Scholar]

| Patients’ Age | (Mean ± Standard Deviation (Min-Max)) | |||
|---|---|---|---|---|
| 33.1 ± 12.4 (18–64) | ||||
| Patients’ gender | Male | Female | ||
| 87 | 113 | |||
| Smoker | Yes | No | ||
| 36.60% | 63.40% | |||
| Winter classification of the third molar | Vertical | Mesioangular | Distoangular | Horizontal |
| 45% | 42% | 13% | 0% | |
| Dry Sockets Diagnosis | Ozosan | Placebo |
|---|---|---|
| Presence of dry socket (number) | 4 | 43 |
| Absence of dry socket (number) | 196 | 157 |
| % of diagnosed dry sockets | 2% * | 21.5% * |
| Treatment with Ozone in the Form of Sunflower Oil-Based Gel (Ozosan) | ||
|---|---|---|
| Males | Females | |
| Presence of dry socket (number) | 2 | 2 |
| Absence of dry socket (number) | 85 | 111 |
| % of diagnosed dry sockets | 2.30% | 1.80% |
| Treatment with placebo gel | ||
| Males | Females | |
| Presence of dry socket (number) | 17 | 26 |
| Absence of dry socket (number) | 70 | 87 |
| % of diagnosed dry sockets | 19.50% | 23.00% |
| Treatment with Ozone in the Form of Sunflower Oil-Based Gel (Ozosan) | |||
|---|---|---|---|
| Mesioangular | Vertical | Distoangular | |
| Presence of dry socket (number) | 2 | 1 | 1 |
| Absence of dry socket (number) | 82 | 87 | 27 |
| % of diagnosed dry sockets | 2.40% | 1.10% | 3.60% |
| Treatment with placebo gel | |||
| Mesioangular | Vertical | Distoangular | |
| Presence of dry socket (number) | 21 | 14 | 8 |
| Absence of dry socket (number) | 63 | 74 | 20 |
| % of diagnosed dry sockets | 25.00% | 15.90% | 28.60% |
| Treatment with Ozone in the Form of Sunflower-Oil-Based Gel (Ozosan) | ||
|---|---|---|
| Smokers | Non-Smokers | |
| Presence of dry socket (number) | 2 | 2 |
| Absence of dry socket (number) | 71 | 125 |
| % of diagnosed dry sockets | 2.70% | 1.60% |
| Treatment with placebo gel | ||
| Males | Females | |
| Presence of dry socket (number) | 20 | 23 |
| Absence of dry socket (number) | 53 | 104 |
| % of diagnosed dry sockets | 27.40% | 18.10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Materni, A.; Pasquale, C.; Longo, E.; Frosecchi, M.; Benedicenti, S.; Bozzo, M.; Amaroli, A. Prevention of Dry Socket with Ozone Oil-Based Gel after Inferior Third Molar Extraction: A Double-Blind Split-Mouth Randomized Placebo-Controlled Clinical Trial. Gels 2023, 9, 289. https://doi.org/10.3390/gels9040289
Materni A, Pasquale C, Longo E, Frosecchi M, Benedicenti S, Bozzo M, Amaroli A. Prevention of Dry Socket with Ozone Oil-Based Gel after Inferior Third Molar Extraction: A Double-Blind Split-Mouth Randomized Placebo-Controlled Clinical Trial. Gels. 2023; 9(4):289. https://doi.org/10.3390/gels9040289
Chicago/Turabian StyleMaterni, Alberto, Claudio Pasquale, Eugenio Longo, Massimo Frosecchi, Stefano Benedicenti, Matteo Bozzo, and Andrea Amaroli. 2023. "Prevention of Dry Socket with Ozone Oil-Based Gel after Inferior Third Molar Extraction: A Double-Blind Split-Mouth Randomized Placebo-Controlled Clinical Trial" Gels 9, no. 4: 289. https://doi.org/10.3390/gels9040289