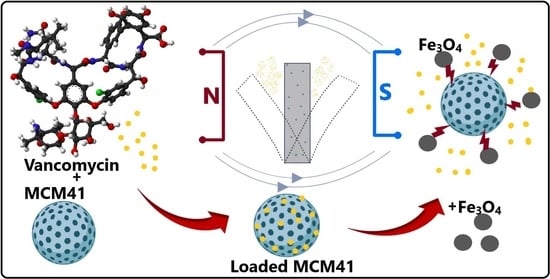
Synergistic Antimicrobial Activity of Magnetite and Vancomycin-Loaded Mesoporous Silica Embedded in Alginate Films
Abstract
:1. Introduction
2. Results and Discussion
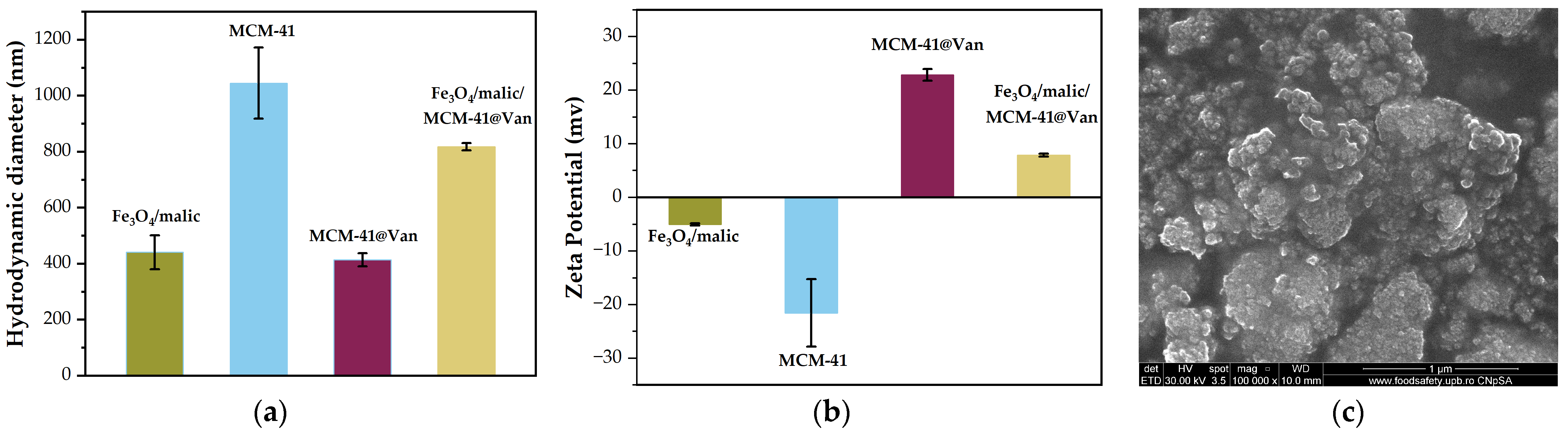
2.1. Nanoparticle Characterization
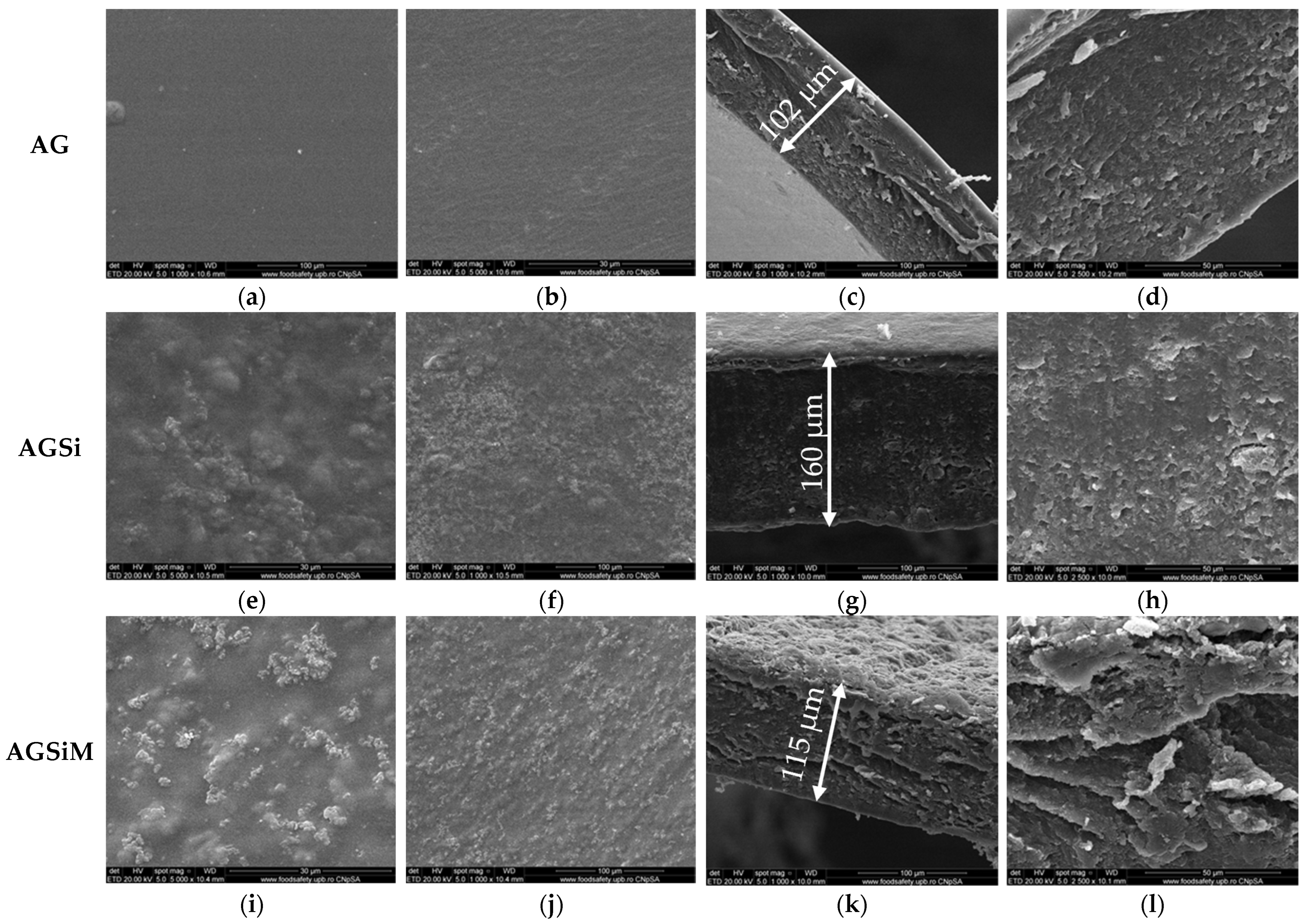
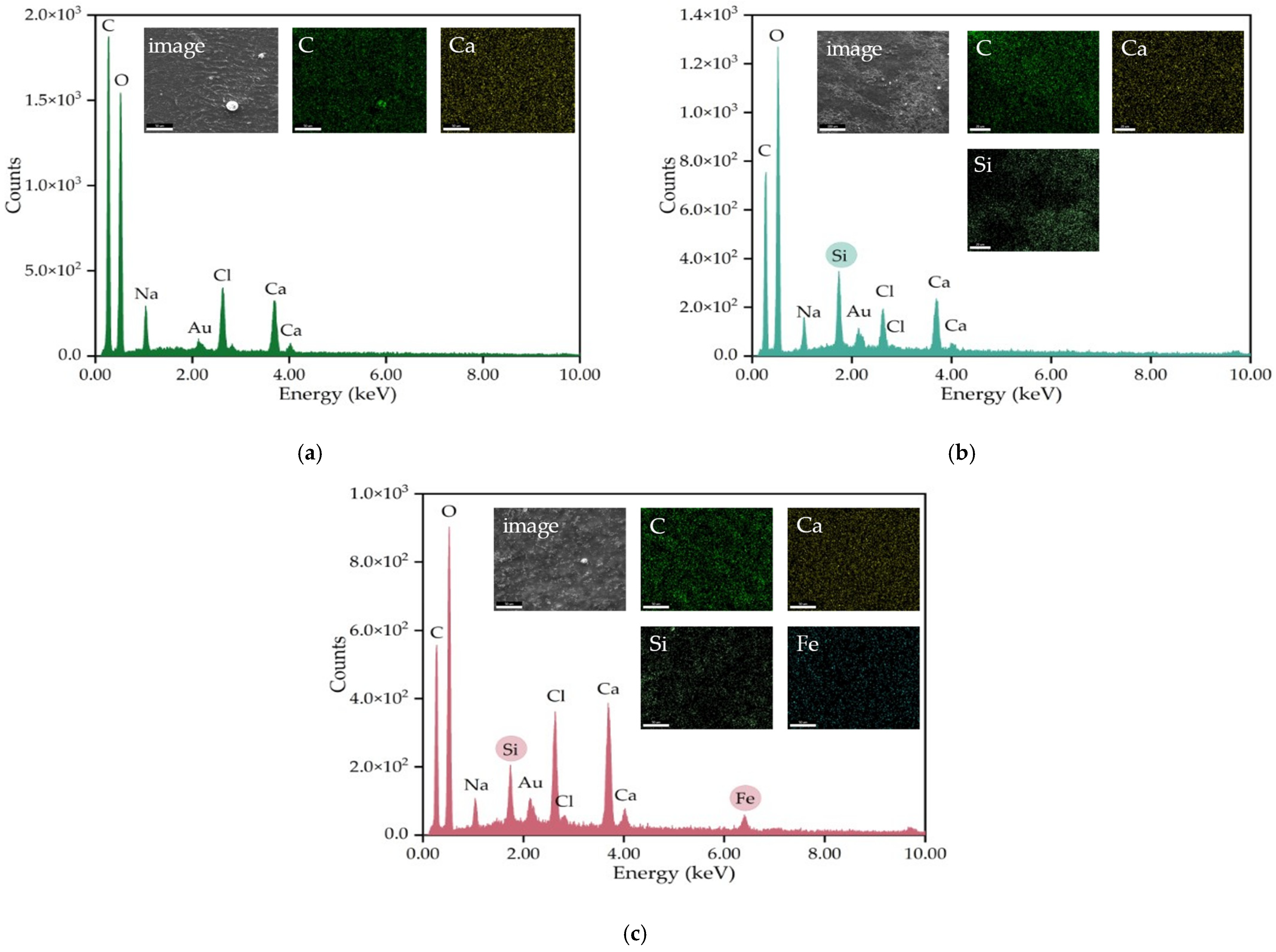
2.2. Characterization of the Vancomycin-Loaded Magnetic Alginate Film
2.3. Performance of Vancomycin-Loaded Magnetic Alginate Film
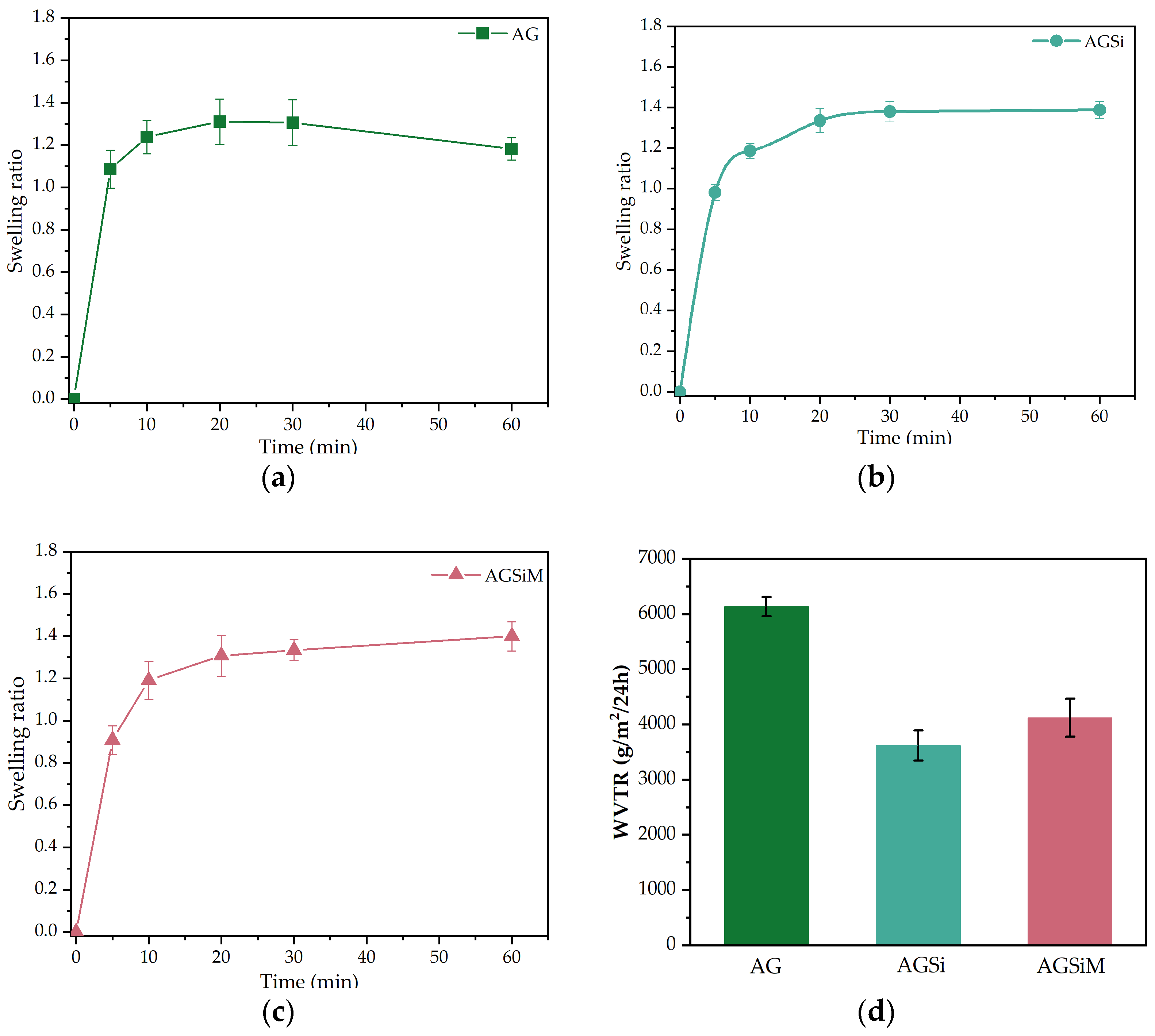
2.3.1. Wound Fluid Uptake and Water Vapor Transfer Rate
2.3.2. Vancomycin Release Behavior
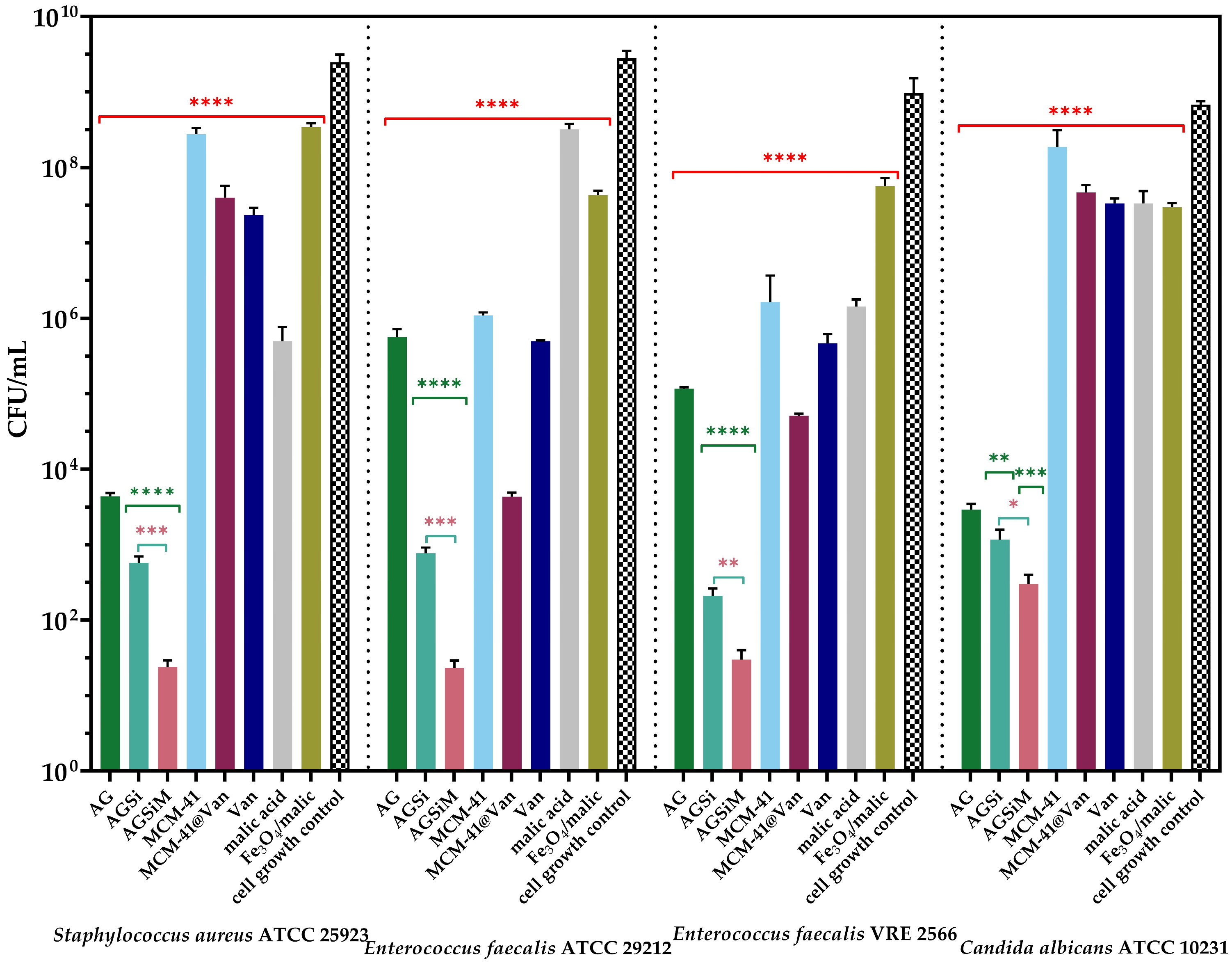
2.3.3. Antimicrobial Activity
Qualitative Evaluation of the Antimicrobial Activity
Quantitative Evaluation of the Anti-Adherence Capacity of the Alginate-Based Films
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization of Nanoparticles
4.3. Preparation and Characterization of the Vancomycin-Loaded Magnetic Alginate Film
4.4. Performance of the Obtained Films
4.4.1. Wound Fluid Uptake and Water Vapor Transfer Rate
4.4.2. Vancomycin Release Behavior
4.4.3. Antimicrobial Activity of Alginate-Based Films
Qualitative Evaluation of the Antimicrobial Activity
Quantitative Evaluation of the Anti-Adherence Capacity of the Alginate-Based Films
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catanzano, O.; Quaglia, F.; Boateng, J.S. Wound dressings as growth factor delivery platforms for chronic wound healing. Expert Opin. Drug Deliv. 2021, 18, 737–759. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Rani Raju, N.; Silina, E.; Stupin, V.; Manturova, N.; Chidambaram, S.B.; Achar, R.R. Multifunctional and Smart Wound Dressings—A Review on Recent Research Advancements in Skin Regenerative Medicine. Pharmaceutics 2022, 14, 1574. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial biomaterials for skin wound dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef]
- Radulescu, M.; Andronescu, E.; Dolete, G.; Popescu, R.C.; Fufă, O.; Chifiriuc, M.C.; Mogoantă, L.; Bălşeanu, T.-A.; Mogoşanu, G.D.; Grumezescu, A.M.; et al. Silver Nanocoatings for Reducing the Exogenous Microbial Colonization of Wound Dressings. Materials 2016, 9, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maneerung, T.; Tokura, S.; Rujiravanit, R. Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar] [CrossRef]
- Majumder, S.; Ranjan Dahiya, U.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C.M. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef]
- Cleetus, C.M.; Alvarez Primo, F.; Fregoso, G.; Lalitha Raveendran, N.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef]
- Motelica, L.; Vasile, B.S.; Ficai, A.; Surdu, A.V.; Ficai, D.; Oprea, O.C.; Andronescu, E.; Jinga, D.C.; Holban, A.M. Influence of the Alcohols on the ZnO Synthesis and Its Properties: The Photocatalytic and Antimicrobial Activities. Pharmaceutics 2022, 14, 2842. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Narain, R.; Zeng, H. Chapter 10—Hydrogels. In Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–244. [Google Scholar]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Namazi, H.; Rakhshaei, R.; Hamishehkar, H.; Kafil, H.S. Antibiotic loaded carboxymethylcellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int. J. Biol. Macromol. 2016, 85, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Koga, A.Y.; Felix, J.C.; Silvestre, R.G.M.; Lipinski, L.C.; Carletto, B.; Kawahara, F.A.; Pereira, A.V. Evaluation of wound healing effect of alginate film containing Aloe vera gel and cross-linked with zinc chloride. Acta Cirúrgica Bras. 2020, 35, e202000507. [Google Scholar] [CrossRef] [PubMed]
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study. Int. J. Bioprint. 2022, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zhao, W.; Zhang, L.; Zeng, X.; Sun, Z.; Zhang, D.; Shen, P.; Li, Z.; Han, Y.; Li, P.; et al. Bio-multifunctional alginate/chitosan/fucoidan sponges with enhanced angiogenesis and hair follicle regeneration for promoting full-thickness wound healing. Mater. Des. 2020, 193, 108863. [Google Scholar] [CrossRef]
- Mollah, M.Z.I.; Zahid, H.M.; Mahal, Z.; Faruque, M.R.I.; Khandaker, M.U. The Usages and Potential Uses of Alginate for Healthcare Applications. Front. Mol. Biosci. 2021, 8, 719972. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.P.; de Andrade, L.R.M.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2022, 12, 4701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raguvaran, R.; Manuja, B.K.; Chopra, M.; Thakur, R.; Anand, T.; Kalia, A.; Manuja, A. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int. J. Biol. Macromol. 2017, 96, 185–191. [Google Scholar] [CrossRef]
- Hu, L.; Sun, C.; Song, A.; Chang, D.; Zheng, X.; Gao, Y.; Jiang, T.; Wang, S. Alginate encapsulated mesoporous silica nanospheres as a sustained drug delivery system for the poorly water-soluble drug indomethacin. Asian J. Pharm. Sci. 2014, 9, 183–190. [Google Scholar] [CrossRef] [Green Version]
- de Lima, H.H.C.; Kupfer, V.L.; Moisés, M.P.; Guilherme, M.R.; Rinaldi, J.d.C.; Felisbino, S.L.; Rubira, A.F.; Rinaldi, A.W. Bionanocomposites based on mesoporous silica and alginate for enhanced drug delivery. Carbohydr. Polym. 2018, 196, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Domszy, R.C.; Hu, N.; Yang, A.J.; Yang, J.; David, A.E. Synthesis of silica-alginate nanoparticles and their potential application as pH-responsive drug carriers. J. Solgel Sci. Technol. 2019, 91, 11–20. [Google Scholar] [CrossRef]
- Savoldi, A.; Azzini, A.; Baur, D.; Tacconelli, E. Is there still a role for vancomycin in skin and soft-tissue infections? Curr. Opin. Infect. Dis. 2018, 31, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Cheng, L.; Xu, Z.; Chen, L.; Liu, Y.; Xu, Y.; Zhou, D.; Zhang, X.; Zhou, Q.; Sun, J. Preparation and Characterization of Vancomycin Hydrochloride-Loaded Mesoporous Silica Composite Hydrogels. Front. Bioeng. Biotechnol. 2022, 10, 826971. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, C.T.; Boakye-Agyeman, F.; Brinkman, C.L.; Reid, J.M.; Patel, R.; Bajzer, Z.; Dadsetan, M.; Yaszemski, M.J. Controlled Delivery of Vancomycin via Charged Hydrogels. PLoS ONE 2016, 11, e0146401. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-H.; Chen, C.S.; Chen, Y.-C.; Jiang, N.-E.; Farn, C.J.; Shen, Y.-S.; Hsu, M.-L.; Chang, C.-H. Vancomycin-loaded oxidized hyaluronic acid and adipic acid dihydrazide hydrogel: Bio-compatibility, drug release, antimicrobial activity, and biofilm model. J. Microbiol. Immunol. Infect. 2020, 53, 525–531. [Google Scholar] [CrossRef]
- Stein, G.E.; Wells, E.M. The importance of tissue penetration in achieving successful antimicrobial treatment of nosocomial pneumonia and complicated skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus: Vancomycin and linezolid. Curr. Med. Res. Opin. 2010, 26, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Richardson, I.P.; Sturtevant, R.; Heung, M.; Solomon, M.J.; Younger, J.G.; VanEpps, J.S. Hemodialysis Catheter Heat Transfer for Biofilm Prevention and Treatment. ASAIO J. 2016, 62, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, A.; Harris, M.A.; LeVine, D.; Ghimire, M.; Jennings, J.A.; Morshed, B.I.; Haggard, W.O.; Bumgardner, J.D.; Mishra, S.R.; Fujiwara, T. Magnetic stimulus responsive vancomycin drug delivery system based on chitosan microbeads embedded with magnetic nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Dolete, G.; Chircov, C.; Motelica, L.; Ficai, D.; Oprea, O.-C.; Gheorghe, M.; Ficai, A.; Andronescu, E. Magneto-Mechanically Triggered Thick Films for Drug Delivery Micropumps. Nanomaterials 2022, 12, 3598. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.; Lam, T.; Manh, D.H.; Tran, H.; Hong, L.; Phuc, N. Facile and solvent-free routes for the synthesis of size-controllable Fe3O4 nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 035001. [Google Scholar] [CrossRef]
- Shagholani, H.; Ghoreishi, s.m.; Mousazadeh, M. Improvement of interaction between PVA and chitosan via magnetite nanoparticles for drug delivery application. Int. J. Biol. Macromol. 2015, 78, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Dutta, B.; Checker, S.; Barick, K.C.; Salunke, H.G.; Gota, V.; Hassan, P.A. Malic acid grafted Fe3O4 nanoparticles for controlled drug delivery and efficient heating source for hyperthermia therapy. J. Alloys Compd. 2021, 883, 160950. [Google Scholar] [CrossRef]
- Alzoubi, F.Y.; Jabaly, A.; Noqta, A.; Al-Khateeb, H.M.; Alqadi, M.K.; Bououdina, M. Citric Acid Coated Iron Oxide Nanoparticles as Contrast Agent for Magnetic Resonance Imaging. 2022; preprint. [Google Scholar] [CrossRef]
- Chircov, C.; Matei, M.-F.; Neacșu, I.A.; Vasile, B.S.; Oprea, O.-C.; Croitoru, A.-M.; Trușcă, R.-D.; Andronescu, E.; Sorescu, I.; Bărbuceanu, F. Iron Oxide-Silica Core-Shell Nanoparticles Functionalized with Essential Oils for Antimicrobial Therapies. Antibiotics 2021, 10, 1138. [Google Scholar] [CrossRef]
- Istrati, D.; Moroșan, A.; Stan, R.; Vasile, B.Ș.; Vasilievici, G.; Oprea, O.; Dolete, G.; Purcăreanu, B.; Mihaiescu, D.E. Microwave-Assisted Sol-Gel Preparation of the Nanostructured Magnetic System for Solid-Phase Synthesis. Nanomaterials 2021, 11, 3176. [Google Scholar] [CrossRef]
- Fadhilah, H.R.; Saepudin, E.; Khalil, M. On the role of carboxylates-based capping agents for colloidal stability of Fe3O4 magnetic nanoparticles. AIP Conf. Proc. 2020, 2242, 040003. [Google Scholar] [CrossRef]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Tóth, J.; Kövér, L.; Mohai, M.; Borowicz, P. Surface Study of Fe3O4 Nanoparticles Functionalized with Biocompatible Adsorbed Molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef] [Green Version]
- Katsiotis, C.S.; Åhlén, M.; Strømme, M.; Welch, K. 3D-Printed Mesoporous Carrier System for Delivery of Poorly Soluble Drugs. Pharmaceutics 2021, 13, 1096. [Google Scholar] [CrossRef]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.-C.; Grumezescu, A.M.; Ditu, L.-M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef] [PubMed]
- Caciandone, M.; Niculescu, A.-G.; Roșu, A.R.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.; Vasile, B.Ș.; Bîrcă, A.C.; Grumezescu, A.M.; et al. PEG-Functionalized Magnetite Nanoparticles for Modulation of Microbial Biofilms on Voice Prosthesis. Antibiotics 2022, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Dolete, G.; Purcăreanu, B.; Mihaiescu, D.E.; Ficai, D.; Oprea, O.-C.; Bîrcă, A.C.; Chircov, C.; Vasile, B.Ș.; Vasilievici, G.; Ficai, A.; et al. A Comparative Loading and Release Study of Vancomycin from a Green Mesoporous Silica. Molecules 2022, 27, 5589. [Google Scholar] [CrossRef] [PubMed]
- Petrisor, G.; Motelica, L.; Ficai, D.; Trusca, R.D.; Surdu, V.-A.; Voicu, G.; Oprea, O.C.; Ficai, A.; Andronescu, E. New Mesoporous Silica Materials Loaded with Polyphenols: Caffeic Acid, Ferulic Acid and p-Coumaric Acid as Dietary Supplements for Oral Administration. Materials 2022, 15, 7982. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.; Freeman, R.; Percival, S.L. In vitro study of sustained antimicrobial activity of a new silver alginate dressing. J. Am. Coll. Certif. Wound Spec. 2009, 1, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.; Mohd Amin, M.C.I.; Pandey, M.; Ahmad, N.; Rajab, N.F. Bacterial cellulose/acrylic acid hydrogel synthesized via electron beam irradiation: Accelerated burn wound healing in an animal model. Carbohydr. Polym. 2014, 114, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Rezvanian, M.; Ahmad, N.; Mohd Amin, M.C.I.; Ng, S.-F. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017, 97, 131–140. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Chang, J.-J.; Yang, M.-C.; Chien, C.-T.; Lai, W.-F. Acceleration of wound healing in diabetic rats by layered hydrogel dressing. Carbohydr. Polym. 2012, 88, 809–819. [Google Scholar] [CrossRef]
- Aslani, Z.; Nazemi, N.; Rajabi, N.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Kasiri-Asgarani, M.; Najafinezhad, A.; Ismail, A.F.; Sharif, S.; Berto, F. Antibacterial Activity and Cell Responses of Vancomycin-Loaded Alginate Coating on ZSM-5 Scaffold for Bone Tissue Engineering Applications. Materials 2022, 15, 4786. [Google Scholar] [CrossRef]
- Kurczewska, J.; Pecyna, P.; Ratajczak, M.; Gajęcka, M.; Schroeder, G. Halloysite nanotubes as carriers of vancomycin in alginate-based wound dressing. Saudi Pharm. J. 2017, 25, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.M.; Hefnawy, A.; Anter, A.; Abdellatif, M.M.; Khalil, M.A.F.; Khalil, I.A. Silica-Coated Magnetic Nanoparticles for Vancomycin Conjugation. ACS Omega 2022, 7, 30161–30170. [Google Scholar] [CrossRef] [PubMed]
- Gounani, Z.; Asadollahi, M.A.; Pedersen, J.N.; Lyngsø, J.; Skov Pedersen, J.; Arpanaei, A.; Meyer, R.L. Mesoporous silica nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf. B Biointerfaces 2019, 175, 498–508. [Google Scholar] [CrossRef]
- Esmaeili, A.; Ghobadianpour, S. Vancomycin loaded superparamagnetic MnFe2O4 nanoparticles coated with PEGylated chitosan to enhance antibacterial activity. Int. J. Pharm. 2016, 501, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Demirci, F.; Baser, K.H.C. Chapter 16—Antimicrobial Strategies in Novel Drug Delivery Systems: Applications in the Treatment of Skin and Soft Tissue Infections. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Kon, K., Rai, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 2, pp. 271–286. [Google Scholar]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, M.; Niranjan, R.; Thangam, R.; Madhan, B.; Pandiyarasan, V.; Ramachandran, C.; Oh, D.-H.; Venkatasubbu, G.D. Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Appl. Surf. Sci. 2019, 479, 1169–1177. [Google Scholar] [CrossRef]
- Ndayishimiye, J.; Cao, Y.; Kumeria, T.; Blaskovich, M.; Falconer, J.; Popat, A. Engineering Mesoporous Silica Nanoparticles for Improved Oral Delivery of Vancomycin. J. Mater. Chem. B 2021, 9, 7145–7166. [Google Scholar] [CrossRef]
- Qi, G.; Li, L.; Yu, F.; Wang, H. Vancomycin-Modified Mesoporous Silica Nanoparticles for Selective Recognition and Killing of Pathogenic Gram-Positive Bacteria over Macrophage-Like Cells. ACS Appl. Mater. Interfaces 2013, 5, 10874–10881. [Google Scholar] [CrossRef]
- Marques, C.; Sotiles, A.R.; Farias, F.O.; Oliveira, G.; Mitterer-Daltoé, M.L.; Masson, M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arab. J. Chem. 2020, 13, 9118–9129. [Google Scholar] [CrossRef]
- Aissani, N. Dicarboxylic acids from Caper leaves enhance antibiotic susceptibility of Pseudomonas aeruginosa to vancomycin. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 54–64. [Google Scholar]
- Petrisor, G.; Ficai, D.; Motelica, L.; Trusca, R.D.; Bîrcă, A.C.; Vasile, B.S.; Voicu, G.; Oprea, O.C.; Semenescu, A.; Ficai, A.; et al. Mesoporous Silica Materials Loaded with Gallic Acid with Antimicrobial Potential. Nanomaterials 2022, 12, 1648. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Huang, Y.; Li, D.; Chen, X. Improving surface and mechanical properties of alginate films by using ethanol as a co-solvent during external gelation. Carbohydr. Polym. 2015, 123, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Spoială, A.; Ilie, C.-I.; Dolete, G.; Croitoru, A.-M.; Surdu, V.-A.; Trușcă, R.-D.; Motelica, L.; Oprea, O.-C.; Ficai, D.; Ficai, A.; et al. Preparation and Characterization of Chitosan/TiO2 Composite Membranes as Adsorbent Materials for Water Purification. Membranes 2022, 12, 804. [Google Scholar] [CrossRef] [PubMed]
- Spoială, A.; Ilie, C.-I.; Trușcă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, B.Ș.; Ficai, A.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef] [PubMed]
- Lemnaru, G.-M.; Truşcă, R.D.; Ilie, C.-I.; Țiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Dițu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100, 31st ed.; Clinical and Laboratory Standards Institute: San Antonio, TX, USA, 2021; p. 352. [Google Scholar]


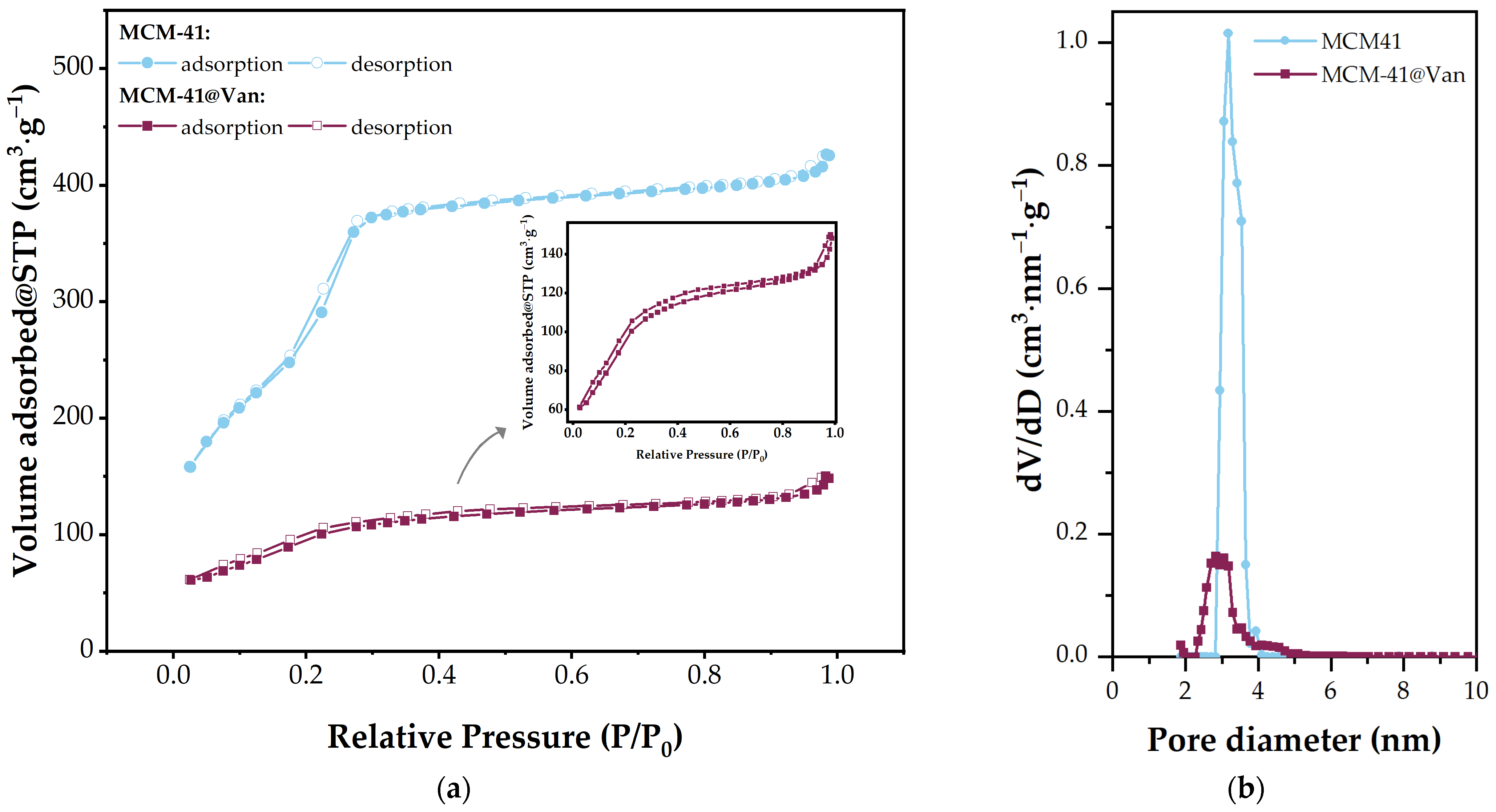





| Sample | Surface Area (m2·g−1) | Total Pore Volume (cm3·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| MCM-41 | 1233 | 0.6381 | 3.18 |
| MCM-41@Van | 353 | 0.2147 | 2.82 |
| Sample | Mass Loss (%) | Thermal Effects (°C) | |||
|---|---|---|---|---|---|
| RT-200 (°C) | 200–900 (°C) | Residual Mass | Endothermic | Exothermic | |
| Fe3O4/malic | 9.19 | 7.35 | 83.46 | 93.5 | 215.0 |
| 257.0 | |||||
| 486.9 | |||||
| MCM-41 | 1.35 | 6.57 | 92.17 | 55.1 | - |
| MCM-41@Van | 9.02 | 30.24 | 60.74 | 84.0 | 352.0 |
| 540.3 | |||||
| Strains | GIZD (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| AG | AGSi | AGSiM | MCM-41 | MCM-41@Van | Van | Malic Acid | Fe3O4@ Malic | |
| Staphylococcus aureus ATCC 10231 | 18.00 ± 1.00 | 27.50 ± 1.32 | 29.83 ± 0.29 | 2.00 ± 0.05 | 29.73 ± 0.64 | 20.10 ± 0.36 | 16.20 ± 0.52 | 9.93 ± 0.70 |
| Enterococcus faecalis ATCC 29212 | 4.83 ± 0.76 | 29.60 ± 0.52 | 34.00 ± 1.00 | 1.13 ± 0.23 | 39.90 ± 0.85 | 20.23 ± 0.32 | 9.00 ± 1.00 | 10.03 ± 0.45 |
| Enterococcus faecalis VRE 2566 | 14.93 ± 0.30 | 23.80 ± 0.72 | 32.67 ± 0.57 | 2.13 ± 0.23 | 35.67 ± 0.57 | 19.50 ± 0.50 | 9.50 ± 0.50 | 10.87 ± 0.23 |
| Candida albicans ATTC 10231 | 1.07 ± 0.11 | 10.23 ± 0.2 | 11.97 ± 0.45 | 2.10 ± 0.17 | 2.76 ± 0.66 | 1.56 ± 0.32 | 1.16 ± 0.11 | 14.27 ± 1.27 |
| Component | AG | AGSi | AGSiM |
|---|---|---|---|
| Sodium alginate/glycerol | 1.0 | 0.75 | 0.50 |
| MCM-41@Van | - | 0.25 | 0.25 |
| Fe3O4/malic acid | - | - | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolete, G.; Ilie, C.-I.; Chircov, C.; Purcăreanu, B.; Motelica, L.; Moroșan, A.; Oprea, O.C.; Ficai, D.; Andronescu, E.; Dițu, L.-M. Synergistic Antimicrobial Activity of Magnetite and Vancomycin-Loaded Mesoporous Silica Embedded in Alginate Films. Gels 2023, 9, 295. https://doi.org/10.3390/gels9040295
Dolete G, Ilie C-I, Chircov C, Purcăreanu B, Motelica L, Moroșan A, Oprea OC, Ficai D, Andronescu E, Dițu L-M. Synergistic Antimicrobial Activity of Magnetite and Vancomycin-Loaded Mesoporous Silica Embedded in Alginate Films. Gels. 2023; 9(4):295. https://doi.org/10.3390/gels9040295
Chicago/Turabian StyleDolete, Georgiana, Cornelia-Ioana Ilie, Cristina Chircov, Bogdan Purcăreanu, Ludmila Motelica, Alina Moroșan, Ovidiu Cristian Oprea, Denisa Ficai, Ecaterina Andronescu, and Lia-Mara Dițu. 2023. "Synergistic Antimicrobial Activity of Magnetite and Vancomycin-Loaded Mesoporous Silica Embedded in Alginate Films" Gels 9, no. 4: 295. https://doi.org/10.3390/gels9040295
APA StyleDolete, G., Ilie, C.-I., Chircov, C., Purcăreanu, B., Motelica, L., Moroșan, A., Oprea, O. C., Ficai, D., Andronescu, E., & Dițu, L.-M. (2023). Synergistic Antimicrobial Activity of Magnetite and Vancomycin-Loaded Mesoporous Silica Embedded in Alginate Films. Gels, 9(4), 295. https://doi.org/10.3390/gels9040295