Nano-Gels: Recent Advancement in Fabrication Methods for Mitigation of Skin Cancer
Abstract
:1. Introduction
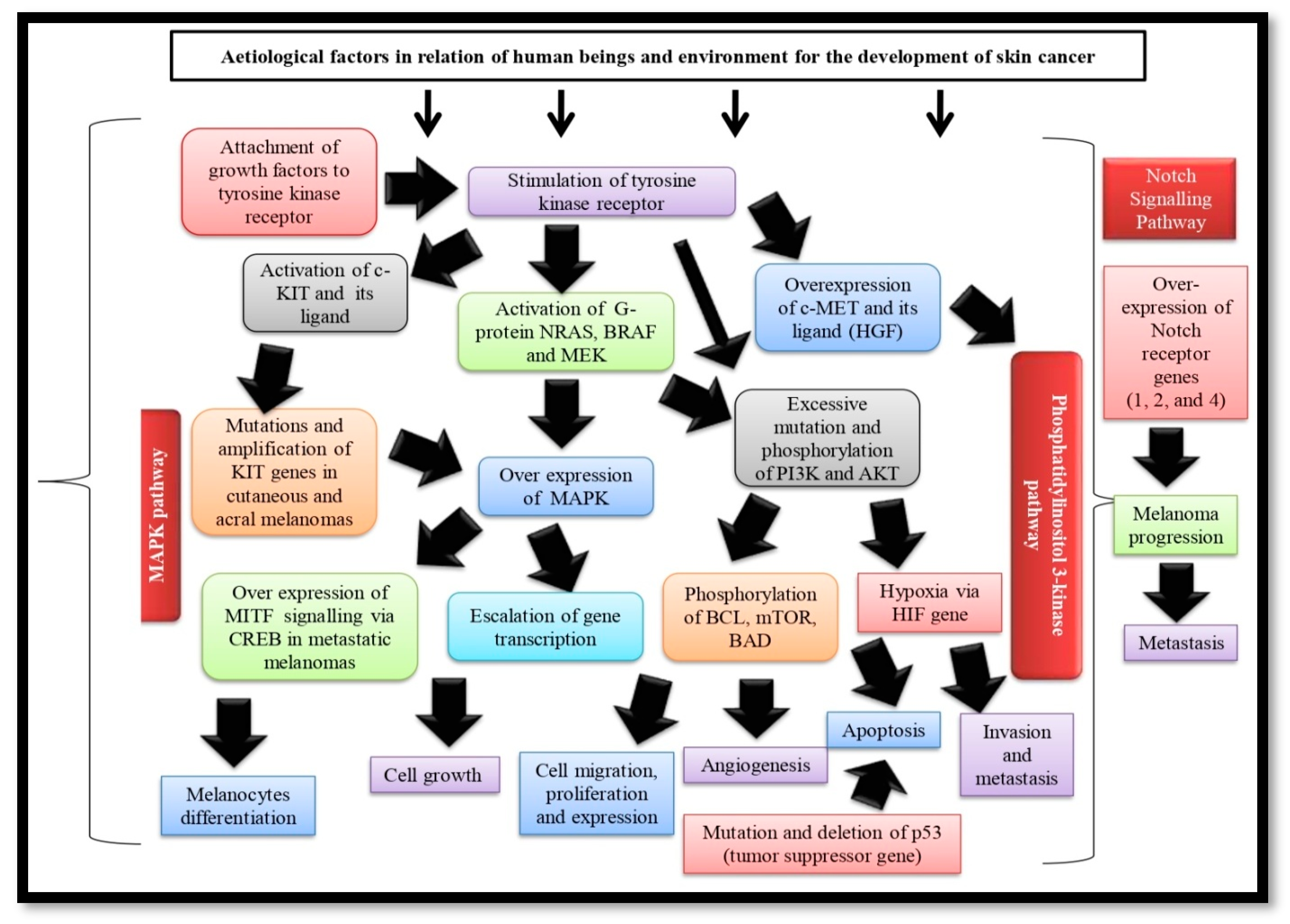
2. Skin Cancer Pathogenies
Mitogen-Activated Protein Kinase (MAPK) Pathway
3. Phosphatidylinositol 3-Kinase Pathway (PI3K) Pathway
4. Notch Signaling Pathway
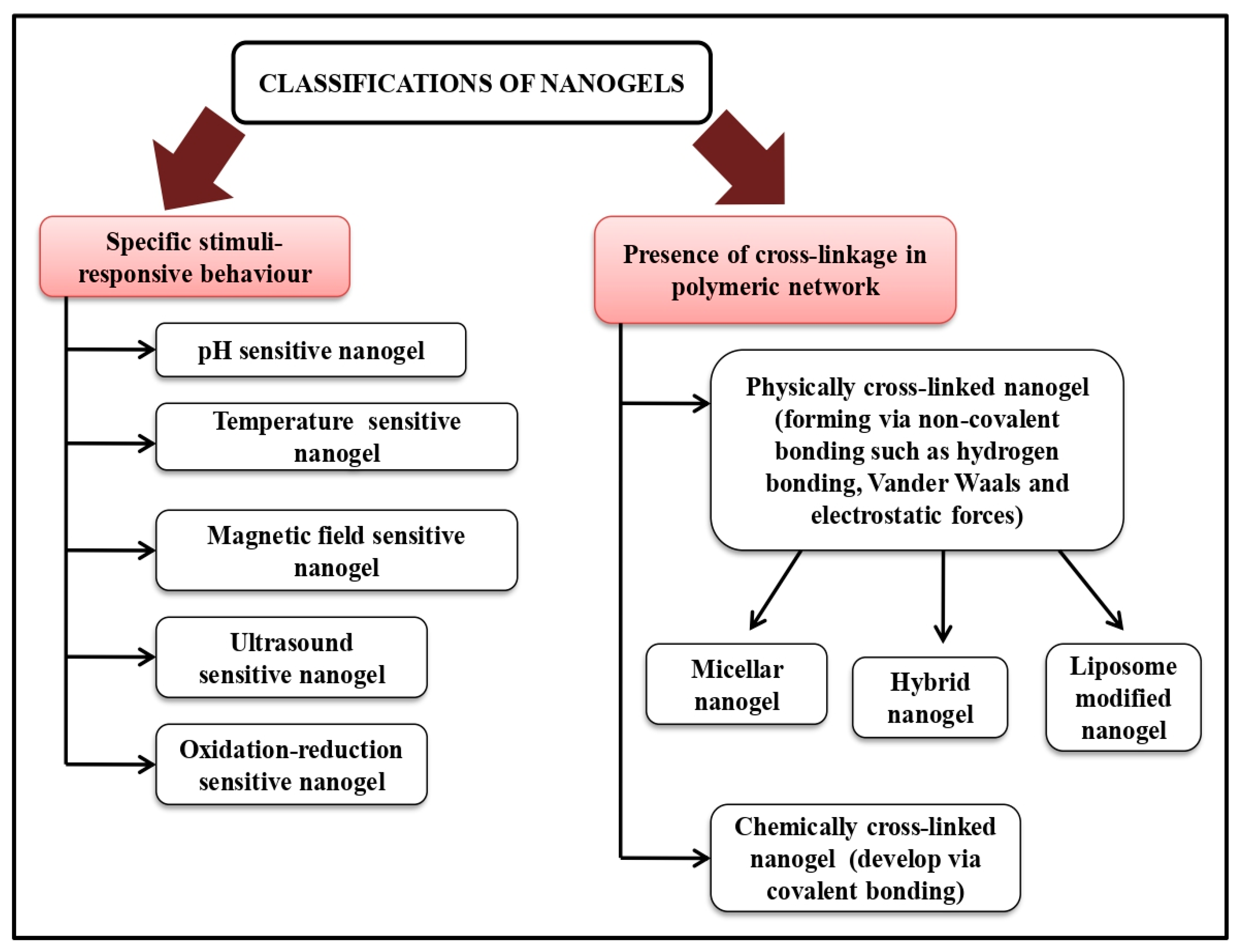
5. Method of Nano-Gel Preparations
5.1. Concurrent Polymerization and Cross-Linking
5.1.1. Precipitation Polymerization
5.1.2. Inverse Emulsion Polymerization
5.1.3. RAFT Polymerization
5.1.4. CCC Polymerization
5.1.5. PIC Polymerization
5.2. Separate Polymerization/Cross-Linking
6. Characterization of Nano-Gels for Skin Cancer Therapy
6.1. Physico-Chemical Characterizations
6.2. Entrapment Efficiency and Drug Content Study
6.3. Applicability Parameter
6.4. Swelling Study
6.5. In-Vitro Drug Release and Release Kinetics
6.6. In-Vitro Skin Permeation and Dermatokinetic Study
6.7. Cytotoxicity Study
| Nano-Gel | Particle Size (nm) | Zeta Potential | Comments | Reference |
|---|---|---|---|---|
| 5-Fluorouracil-loaded chitin nano-gel | 125–140 nm | +31.9 mV | The relaxing of keratin and the deposition of 5FU in the deeper layers of skin were eventually caused by the positive zeta potential of chitin nano-gel facilitating the establishment of a strong association with stratum corneum. | [88] |
| Nano-curcumin and sulforaphane loaded ethosomal nano-gel | 125.67 ± 10.43 nm | –17.1 ± 2.61 mV | The excellent cytotoxicity against the B16-F10 murine tumor cell line and the remarkable radical scavenging effect of optimized ethosomal nano-gel confirm effectiveness in the treatment of melanoma. | [78] |
| Curcumin loaded Carbopol nano-emulgel (containing Capmul: Tween20-PEG400 = 1:8) | 125.3 nm | −14.1 mV | The significant, sustained curcumin release from optimized nano-emulgel with enhanced permeability and less toxicity assures a promising candidate for the treatment of squamous cell carcinoma | [57] |
| Curcumin-chitin nano-gel | 70–80 nm | +49.34 mV | The satisfactory particle size, drug entrapment, release capacity, surface characterization, and excellent skin penetration property of curcumin-loaded chitin nano-gels became a potential candidate for the mitigation of skin cancer via transdermal route | [83] |
| Brucine-trans-liposomal nano-gel | 136.20 ± 2.87 nm | - | The optimized trans-liposomal nano-gel formed depots in the deeper layers of the skin via continuous release of brucine for a prolonged period of time, minimizing the dosage frequency | [79] |
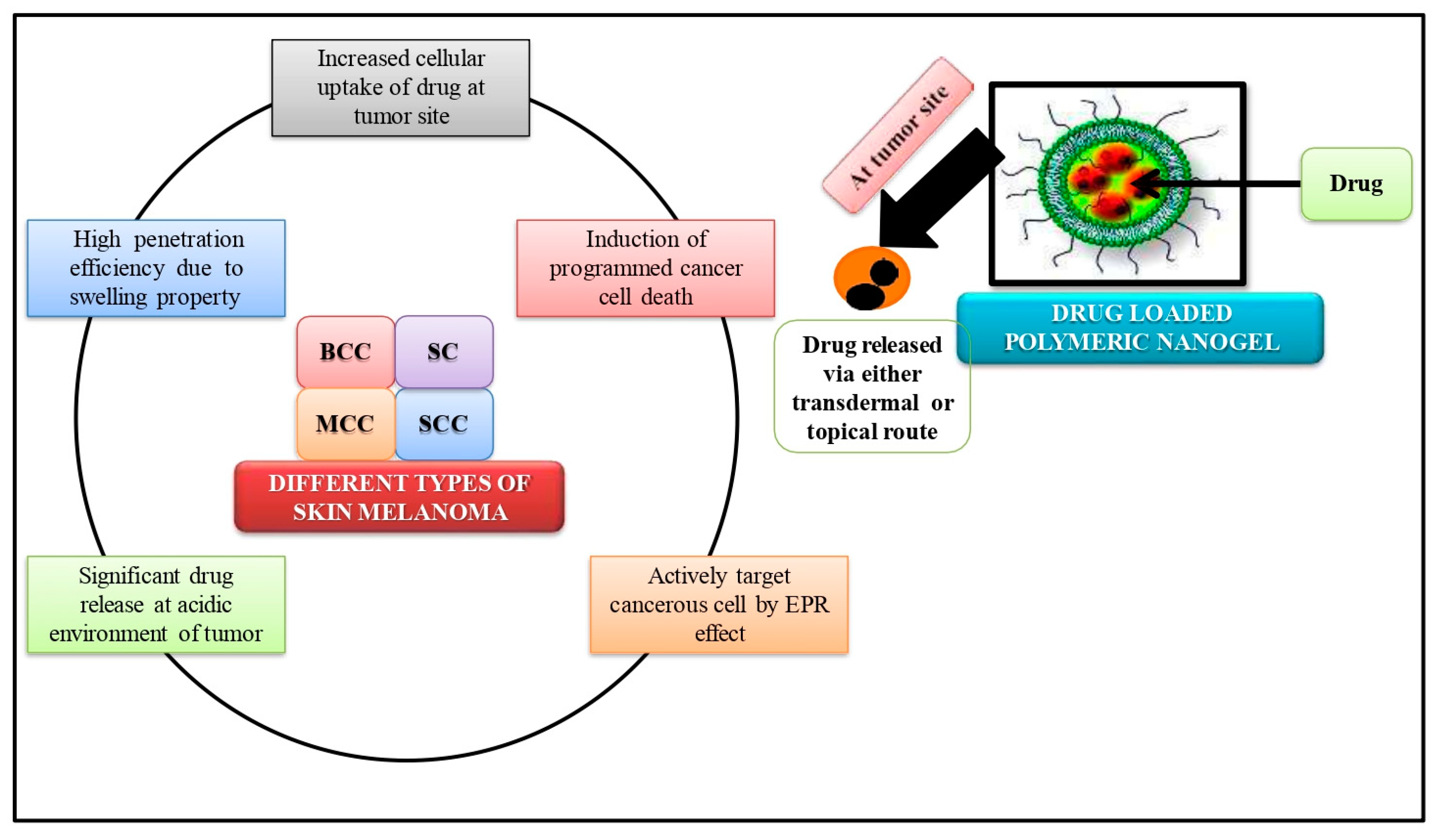
7. Nano-Gels in Skin Cancer
8. Overview of Patent Situation
9. Future Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhagwat, R.; Vaidhya, I. Novel drug delivery systems: An overview. Int. J. Pharm. Sci. Res. 2013, 4, 970. [Google Scholar]
- Hirai, T.; Ogiwara, T.; Fujii, K.; Ueki, T.; Kinoshita, K.; Takasaki, M. Electrically Active Artificial Pupil Showing Amoeba-Like Pseudopodial Deformation. Adv. Mater. 2009, 21, 2886–2888. [Google Scholar] [CrossRef]
- Kalaydina, R.-V.; Bajwa, K.; Qorri, B.; DeCarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anooj, E.; Charumathy, M.; Sharma, V.; Vibala, B.; Gopukumar, S.; Jainab, S.B.; Vallinayagam, S. Nanogels: An overview of properties, biomedical applications, future research trends and developments. J. Mol. Struct. 2021, 1239, 130446. [Google Scholar] [CrossRef]
- Vashist, A.; Atluri, V.; Raymond, A.; Kaushik, A.; Parira, T.; Huang, Z.; Durygin, A.; Tomitaka, A.; Nikkhah-Moshaie, R.; Vashist, A.; et al. Development of Multifunctional Biopolymeric Auto-Fluorescent Micro- and Nanogels as a Platform for Biomedical Applications. Front. Bioeng. Biotechnol. 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Karimi, M. Novel developments and trends of analytical methods for drug analysis in biological and environmental samples by molecularly imprinted polymers. TrAC Trends Anal. Chem. 2017, 89, 146–162. [Google Scholar] [CrossRef]
- Vashist, A.; Kaushik, A.; Vashist, A.; Bala, J.; Nikkhah-Moshaie, R.; Sagar, V.; Nair, M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov. Today 2018, 23, 1436–1443. [Google Scholar] [CrossRef]
- Attama, A.A.; Nnamani, P.O.; Onokala, O.B.; Ugwu, A.A.; Onugwu, A.L. Nanogels as target drug delivery systems in cancer therapy: A review of the last decade. Front. Pharmacol. 2022, 13, 874510. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Design and engineering of nanogels for cancer treatment. Drug Discov. Today 2011, 16, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaram, A.J.; Rajitha, P.; Maya, S.; Jayakumar, R.; Sabitha, M. Nanogels for delivery, imaging and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 509–533. [Google Scholar] [CrossRef]
- Ma, Y.; Ge, Y.; Li, L. Advancement of multifunctional hybrid nanogel systems: Construction and application in drug co-delivery and imaging technique. Mater. Sci. Eng. C 2017, 71, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoee, S.; Asadi, H. Nanogels: Chemical approaches to preparation. Encycl. Biomed. Polym. Polym. Biomater. 2016, 27, 5266–5293. [Google Scholar]
- Asadian-Birjand, M.; Sousa-Herves, A.; Steinhilber, D.; Cuggino, J.C.; Calderon, M. Functional nanogels for biomedical applications. Curr. Med. Chem. 2012, 19, 5029–5043. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, R.; Jin, H.; Lv, P.; Sun, Y.; Men, X.; Yang, S.; Qu, X.; Yang, Z.; Huang, Y. pH gradient difference around ischemic brain tissue can serve as a trigger for delivering polyethylene glycol-conjugated urokinase nanogels. J. Control. Release 2016, 225, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Maciel, D.; Rodrigues, J.; Shi, X.; Tomás, H. Biodegradable Polymer Nanogels for Drug/Nucleic Acid Delivery. Chem. Rev. 2015, 115, 8564–8608. [Google Scholar] [CrossRef] [PubMed]
- Engelman, D.; Fuller, L.C.; Solomon, A.W.; McCarthy, J.S.; Hay, R.J.; Lammie, P.J.; Steer, A.C. Opportunities for Integrated Control of Neglected Tropical Diseases That Affect the Skin. Trends Parasitol. 2016, 32, 843–854. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chittasupho, C.; Ditsri, S.; Singh, S.; Kanlayavattanakul, M.; Duangnin, N.; Ruksiriwanich, W.; Athikomkulchai, S. Ultraviolet Radiation Protective and Anti-Inflammatory Effects of Kaempferia galanga L. Rhizome Oil and Microemulsion: Formulation, Characterization, and Hydrogel Preparation. Gels 2022, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.C.; Annas, G.D. Epidemiology of melanoma and nonmelanoma skin cancer. Semin. Oncol. Nurs. 2003, 19, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Review: Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels For Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces 2018, 174, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.K.S.; Al Halabi, N.A.; Alsalloum, G.A. Nanogels as Novel Drug Delivery Systems-A Review. J. Pharm. Pharm. Res. 2017, 1, 1–8. [Google Scholar]
- Sarangarajan, R.; Apte, S.P. The polymerization of melanin: A poorly understood phenomenon with egregious biological implications. Melanoma Res. 2006, 16, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; Farmer, P.J.; Anton-Culver, H. Etiologic pathogenesis of melanoma: A unifying hypothesis for the missing attributable risk. Clin. Cancer Res. 2004, 10, 2581–2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, G.C.; Falzone, L.; Salemi, R.; Zanghì, A.; Spandidos, D.A.; McCubrey, J.A.; Candido, S.; Libra, M. Cutaneous melanoma: From pathogenesis to therapy (Review). Int. J. Oncol. 2018, 52, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, J.A.; Fisher, D.E. Melanoma pathogenesis. In BRAF Targets in Melanoma: Biological Mechanisms, Resistance, and Drug Discovery; Springer: New York, NY, USA, 2015; pp. 25–45. [Google Scholar]
- Davies, M.A. Molecular biology of cutaneous melanoma. In DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology; Wolters Kluwer Health Pharma Solutions (Europe) Ltd.: Alphen aan den Rijn, The Netherlands, 2018; pp. 1501–1509. [Google Scholar]
- Patel, H.; Yacoub, N.; Mishra, R.; White, A.; Yuan, L.; Alanazi, S.; Garrett, J.T. Current Advances in the Treatment of BRAF-Mutant Melanoma. Cancers 2020, 12, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdo, J.F.; Sharma, A.; Sharma, R. Role of heredity in melanoma susceptibility: A primer for the practicing surgeon. Surg. Clin. 2020, 100, 13–28. [Google Scholar]
- Kiuru, M.; Busam, K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017, 97, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Strashilov, S.; Yordanov, A. Aetiology and Pathogenesis of Cutaneous Melanoma: Current Concepts and Advances. Int. J. Mol. Sci. 2021, 22, 6395. [Google Scholar] [CrossRef]
- Schadendorf, D.; Fisher, D.E.; Garbe, C.; Gershenwald, J.E.; Grob, J.J.; Halpern, A.; Herlyn, M.; Marchetti, M.A.; McArthur, G.; Ribas, A.; et al. Melanoma. Nat. Rev. Dis. Prim. 2015, 1, 15003. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Álvarez, J.; Dilshat, R.; Fock, V.; Möller, K.; Karl, L.; LaRue, L.; Ögmundsdóttir, M.H.; Steingrímsson, E. MITF and TFEB cross-regulation in melanoma cells. PLoS ONE 2020, 15, e0238546. [Google Scholar] [CrossRef]
- Garraway, L.A.; Widlund, H.R.; Rubin, M.A.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, D.A.; Granter, S.R.; Du, J.; et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef]
- Du, J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Ross, K.; Huber, W.E.; Nishimura, E.K.; Golub, T.R.; Fisher, D.E. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 2004, 6, 565–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, D.; Carvajal, R.D. KIT as an Oncogenic Driver in Melanoma: An Update on Clinical Development. Am. J. Clin. Dermatol. 2019, 20, 315–323. [Google Scholar] [CrossRef]
- Czyz, M. HGF/c-MET Signaling in Melanocytes and Melanoma. Int. J. Mol. Sci. 2018, 19, 3844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avery-Kiejda, K.A.; Bowden, N.A.; Croft, A.J.; Scurr, L.L.; Kairupan, C.; Ashton, K.A.; Talseth-Palmer, B.A.; Rizos, H.; Zhang, X.D.; Scott, R.J.; et al. P53 in human melanoma fails to regulate target genes associated with apoptosis and the cell cycle and may contribute to proliferation. BMC Cancer 2011, 11, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Sirigu, P.; Piras, F.; Minerba, L.; Murtas, D.; Maxia, C.; Colombari, R.; Corbu, A.; Perra, M.T.; Ugalde, J. Prognostic prediction of the immunohistochemical expression of p16 and p53 in cutaneous melanoma: A comparison of two populations from different geographical regions. Eur. J. Histochem. 2006, 50, 191–198. [Google Scholar]
- Ragnarsson-Olding, B.; Platz, A.; Olding, L.; Ringborg, U. p53 protein expression and TP53 mutations in malignant melanomas of sun-sheltered mucosal membranes versus chronically sun-exposed skin. Melanoma Res. 2004, 14, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Onder, T.T.; Gupta, P.B.; Mani, S.A.; Yang, J.; Lander, E.S.; Weinberg, R.A. Loss of E-Cadherin Promotes Metastasis via Multiple Downstream Transcriptional Pathways. Cancer Res. 2008, 68, 3645–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wouters, B.G.; Koritzinsky, M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer 2008, 8, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Hurlbut, G.D.; Kankel, M.W.; Lake, R.J.; Artavanis-Tsakonas, S. Crossing paths with Notch in the hyper-network. Curr. Opin. Cell Biol. 2007, 19, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Lobry, C.; Oh, P.; Mansour, M.; Look, A.T.; Aifantis, I. Notch signaling: Switching an oncogene to a tumor suppressor. Blood 2014, 123, 2451–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balint, K.; Xiao, M.; Pinnix, C.C.; Soma, A.; Veres, I.; Juhasz, I.; Brown, E.J.; Capobianco, A.J.; Herlyn, M.; Liu, Z.J. Activation of Notch1 signaling is required for beta-catenin-mediated human primary melanoma progression. J. Clin. Investig. 2005, 115, 3166–3176. [Google Scholar] [CrossRef] [PubMed]
- Hoek, K.; Rimm, D.L.; Williams, K.R.; Zhao, H.; Ariyan, S.; Lin, A.; Kluger, H.M.; Berger, A.J.; Cheng, E.; Trombetta, E.S.; et al. Expression profiling reveals novel pathways in the transformation of melanocytes to melanomas. Cancer Res. 2004, 64, 5270–5282. [Google Scholar] [CrossRef] [PubMed]
- Pinnix, C.C.; Lee, J.T.; Liu, Z.-J.; McDaid, R.; Balint, K.; Beverly, L.J.; Brafford, P.A.; Xiao, M.; Himes, B.; Zabierowski, S.E.; et al. Active Notch1 Confers a Transformed Phenotype to Primary Human Melanocytes. Cancer Res 2009, 69, 5312–5320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedogni, B.; Warneke, J.A.; Nickoloff, B.J.; Giaccia, A.J.; Powell, M.B. Notch1 is an effector of Akt and hypoxia in melanoma development. J. Clin. Investig. 2008, 118, 3660–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aster, J.C.; Pear, W.S.; Blacklow, S.C. The varied roles of notch in cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 245–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Chittasupho, C.; Chaobankrang, K.; Sarawungkad, A.; Samee, W.; Singh, S.; Hemsuwimon, K.; Okonogi, S.; Kheawfu, K.; Kiattisin, K.; Chaiyana, W. Antioxidant, Anti-Inflammatory and Attenuating Intracellular Reactive Oxygen Species Activities of Nicotiana tabacum var. Virginia Leaf Extract Phytosomes and Shape Memory Gel Formulation. Gels 2023, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Hu, B.; Yuan, X.; Cai, L.; Gao, H.; Yang, Q. Nanogel: A Versatile Nano-Delivery System for Biomedical Applications. Pharmaceutics 2020, 12, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamenova, K.; Radeva, L.; Yoncheva, K.; Ublekov, F.; Ravutsov, M.A.; Marinova, M.K.; Simeonov, S.P.; Forys, A.; Trzebicka, B.; Petrov, P.D. Functional Nanogel from Natural Substances for Delivery of Doxorubicin. Polymers 2022, 14, 3694. [Google Scholar] [CrossRef]
- Ganesh, G.N.K.; Singh, M.K.; Datri, S.; Karri, V.V.S.R. Design and development of curcumin nanogel for squamous cell carcinoma. J. Pharm. Sci. Res. 2019, 11, 1683. [Google Scholar]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Peres, L.B.; dos Anjos, R.S.; Tappertzhofen, L.C.; Feuser, P.E.; de Araújo, P.H.H.; Landfester, K.; Sayer, C.; Muñoz-Espí, R. pH-responsive physically and chemically cross-linked glutamic-acid-based hydrogels and nanogels. Eur. Polym. J. 2018, 101, 341–349. [Google Scholar] [CrossRef]
- Peng, S.; Ouyang, B.; Xin, Y.; Zhao, W.; Shen, S.; Zhan, M.; Lu, L. Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm. Sin. B 2020, 11, 560–571. [Google Scholar] [CrossRef]
- Farmanbordar, H.; Amini-Fazl, M.S.; Mohammadi, R. pH-Sensitive silica-based core–shell nanogel prepared via RAFT polymerization: Investigation of the core size effect on the release profile of doxorubicin. New J. Chem. 2021, 45, 21824–21833. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, X.; Yan, G.; Fu, S.; Tang, R. pH-sensitive nanogels with ortho ester linkages prepared via thiol-ene click chemistry for efficient intracellular drug release. J. Colloid Interface Sci. 2017, 508, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Dispenza, C.; Spadaro, G.; Jonsson, M. Radiation engineering of multifunctional nanogels. In Applications of Radiation Chemistry in the Fields of Industry, Biotechnology and Environment; Springer: Cham, Switzerland, 2017; pp. 95–120. [Google Scholar]
- Denmark, D.J.; Hyde, R.H.; Gladney, C.; Phan, M.-H.; Bisht, K.S.; Srikanth, H.; Mukherjee, P.; Witanachchi, S. Photopolymerization-based synthesis of iron oxide nanoparticle embedded PNIPAM nanogels for biomedical applications. Drug Deliv. 2017, 24, 1317–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, E.A.; Majeti, B.K.; Mukthavaram, R.; Acevedo, L.M.; Barnes, L.A.; Cheresh, D.A. Targeted Nanogels: A Versatile Platform for Drug Delivery to Tumors. Mol. Cancer Ther. 2011, 10, 972–982. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Tang, S.; Lu, X.; Hu, Z. Formation and Volume Phase Transition of Hydroxypropyl Cellulose Microgels in Salt Solution. Macromolecules 2003, 36, 3695–3698. [Google Scholar] [CrossRef]
- Tong, X.F.; Zhao, F.Q.; Ren, Y.Z.; Zhang, Y.; Cui, Y.L.; Wang, Q.S. Injectable hydrogels based on glycyrrhizin, alginate, and calcium for three-dimensional cell culture in liver tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 3292–3302. [Google Scholar] [CrossRef]
- Soni, K.; Mujtaba, A.; Akhter, H.; Zafar, A.; Kohli, K. Optimisation of ethosomal nanogel for topical nano-CUR and sulphoraphane delivery in effective skin cancer therapy. J. Microencapsul. 2019, 37, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Mir, M.; Qian, L.; Baloch, M.; Khan, M.F.A.; Rehman, A.-U.; Ngowi, E.E.; Wu, D.-D.; Ji, X.-Y. Skin cancer biology and barriers to treatment: Recent applications of polymeric micro/nanostructures. J. Adv. Res. 2021, 36, 223–247. [Google Scholar] [CrossRef]
- Botella, P.; Abasolo, I.; Fernández, Y.; Muniesa, C.; Miranda, S.; Quesada, M.; Ruiz, J.; Schwartz, S.; Corma, A. Surface-modified silica nanoparticles for tumor-targeted delivery of camptothecin and its biological evaluation. J. Control. Release 2011, 156, 246–257. [Google Scholar] [CrossRef]
- Deaguero, I.G.; Huda, N.; Rodriguez, V.; Zicari, J.; Al-Hilal, T.A.; Badruddoza, A.Z. Nurunnabi Nano-Vesicle Based Anti-Fungal Formulation Shows Higher Stability, Skin Diffusion, Biosafety and Anti-Fungal Efficacy In Vitro. Pharmaceutics 2020, 12, 516. [Google Scholar] [CrossRef]
- Rizvi, S.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm. J. 2017, 26, 64–70. [Google Scholar] [CrossRef]
- Anand, P.; Nair, H.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. RETRACTED: Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharmacol. 2010, 79, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Fang, Y.-P.; Huang, Y.-B.; Wu, P.-C.; Tsai, Y.-H. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur. J. Pharm. Biopharm. 2009, 73, 391–398. [Google Scholar] [CrossRef]
- Paliwal, S.; Tilak, A.; Sharma, J.; Dave, V.; Sharma, S.; Yadav, R.; Patel, S.; Verma, K.; Tak, K. Flurbiprofen loaded ethosomes—Transdermal delivery of anti-inflammatory effect in rat model. Lipids Health Dis. 2019, 18, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhakamy, N.A.; Aldawsari, H.M.; Ali, J.; Gupta, D.K.; Warsi, M.H.; Bilgrami, A.L.; Asfour, H.Z.; Noor, A.O. Shadab Brucine-loaded transliposomes nanogel for topical delivery in skin cancer: Statistical optimization, in vitro and dermatokinetic evaluation. 3 Biotech 2021, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Mangalathillam, S.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.K.; Nair, S.V.; Jayakumar, R. Curcumin loaded chitin nanogels for skin cancer treatment via the transdermal route. Nanoscale 2012, 4, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sabitha, M.; Rejinold, N.S.; Nair, A.; Lakshmanan, V.-K.; Nair, S.V.; Jayakumar, R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr. Polym. 2013, 91, 48–57. [Google Scholar] [CrossRef]
- Danafar, H.; Sharafi, A.; Manjili, H.K.; Andalib, S. Sulforaphane delivery using mPEG–PCL co-polymer nanoparticles to breast cancer cells. Pharm. Dev. Technol. 2016, 22, 642–651. [Google Scholar] [CrossRef]
- Reeves, A.; Vinogradov, S.V.; Morrissey, P.; Chernin, M.; Ahmed, M.M. Curcumin-encapsulating Nanogels as an Effective Anticancer Formulation for Intracellular Uptake. Mol. Cell. Pharm. 2015, 7, 25–40. [Google Scholar] [CrossRef]
- Priya, P.; Raj, R.M.; Vasanthakumar, V.; Raj, V. Curcumin-loaded layer-by-layer folic acid and casein coated carboxymethyl cellulose/casein nanogels for treatment of skin cancer. Arab. J. Chem. 2020, 13, 694–708. [Google Scholar] [CrossRef]
- Bagde, A.; Patel, K.; Mondal, A.; Kutlehria, S.; Chowdhury, N.; Gebeyehu, A.; Patel, N.; Kumar, N.; Singh, M. Combination of UVB Absorbing Titanium Dioxide and Quercetin Nanogel for Skin Cancer Chemoprevention. AAPS PharmSciTech 2019, 20, 240. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.; Singh, B.; Sharma, G.; Beg, S.; Katare, O.P. Biocompatible lidocaine and prilocaine loaded-nanoemulsion system for enhanced percutaneous absorption: QbD-based optimisation, dermatokinetics and in vivo evaluation. J. Microencapsul. 2015, 32, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hu, W.; Qu, Y.-Q.; Dong, J.; Gu, W.; Gao, Y.; Fang, Y.; Fang, F.; Chen, Z.-P.; Cai, B.-C. Evaluation of the pharmacodynamics and pharmacokinetics of brucine following transdermal administration. Fitoterapia 2013, 86, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Mubharak, N.; Naseem, K.; Tabassum, H.; Rizwan, M.; Najda, A.; Kashif, M.; Bin-Jumah, M.; Hussain, A.; Shaheen, A.; et al. Recent advancement and development of chitin and chitosan-based nanocomposite for drug delivery: Critical approach to clinical research. Arab. J. Chem. 2020, 13, 8935–8964. [Google Scholar] [CrossRef]
- Lv, Q.; He, C.; Quan, F.; Yu, S.; Chen, X. DOX/IL-2/IFN-γ co-loaded thermo-sensitive polypeptide hydrogel for efficient melanoma treatment. Bioact. Mater. 2018, 3, 118–128. [Google Scholar] [CrossRef]
- Heit, J.A.; Silverstein, M.D.; Mohr, D.N.; Petterson, T.M.; O’Fallon, W.M.; Melton, L.J., 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch. Intern. Med. 2000, 160, 809–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, F.; Wen, J.; Yang, A.; Wang, Y.; Li, N.; Yu, P.; Wei, W.; Tang, J. The Influence of Hormone Therapy on secondary diabetes mellitus in Breast Cancer: A Meta-analysis. Clin. Breast Cancer 2022, 22, e48–e58. [Google Scholar] [CrossRef]
- Rancan, F.; Asadian-Birjand, M.; Dogan, S.; Graf, C.; Cuellar, L.; Lommatzsch, S.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Effects of thermoresponsivity and softness on skin penetration and cellular uptake of polyglycerol-based nanogels. J. Control. Release 2016, 228, 159–169. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Sau, S.; Kushwah, V.; Jain, S.; Agrawal, R.K.; Iyer, A.K. pH triggered and charge attracted nanogel for simultaneous evaluation of penetration and toxicity against skin cancer: In-vitro and ex-vivo study. Int. J. Biol. Macromol. 2019, 128, 740–751. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as Pharmaceutical Carriers: Finite Networks of Infinite Capabilities. Angew. Chem. Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Samah, N.H.; Williams, N.; Heard, C.M. Nanogel particulates located within diffusion cell receptor phases following topical application demonstrates uptake into and migration across skin. Int. J. Pharm. 2010, 401, 72–78. [Google Scholar] [CrossRef]
- Asadian-Birjand, M.; Bergueiro, J.; Rancan, F.; Cuggino, J.C.; Mutihac, R.-C.; Achazi, K.; Dernedde, J.; Blume-Peytayi, U.; Vogt, A.; Calderón, M. Engineering thermoresponsive polyether-based nanogels for temperature dependent skin penetration. Polym. Chem. 2015, 6, 5827–5831. [Google Scholar] [CrossRef] [Green Version]
- Witting, M.; Molina, M.; Obst, K.; Plank, R.; Eckl, K.M.; Hennies, H.C.; Calderon, M.; Frieß, W.; Hedtrich, S. Thermosensitive dendritic polyglycerol-based nanogels for cutaneous delivery of biomacromolecules. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1179–1187. [Google Scholar] [CrossRef]
- Sahle, F.F.; Giulbudagian, M.; Bergueiro, J.; Lademann, J.; Calderón, M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale 2016, 9, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Giulbudagian, M.; Yealland, G.; Hönzke, S.; Edlich, A.; Geisendörfer, B.; Kleuser, B.; Hedtrich, S.; Calderon, M. Breaking the Barrier-Potent Anti-Inflammatory Activity following Efficient Topical Delivery of Etanercept using Thermoresponsive Nanogels. Theranostics 2018, 8, 450–463. [Google Scholar] [CrossRef]
- Sahu, P.; Kashaw, S.K.; Kushwah, V.; Sau, S.; Jain, S.; Iyer, A.K. pH responsive biodegradable nanogels for sustained release of bleomycin. Bioorganic Med. Chem. 2017, 25, 4595–4613. [Google Scholar] [CrossRef]
- Divya, G.; Panonnummal, R.; Gupta, S.; Jayakumar, R.; Sabitha, M. Acitretin and aloe-emodin loaded chitin nanogel for the treatment of psoriasis. Eur. J. Pharm. Biopharm. 2016, 107, 97–109. [Google Scholar] [CrossRef]
- Jones, D. Immunotherapeutic Compositions and Use Thereof Torque Therapeutics Inc.. U.S. Patent US 2022/0195071 A1. 2022, 30 March 2020.
- Jones, D. Immunotherapeutic Compositions and Use Thereof Torque Therapeutics Inc.. WO Patent WO 2020/205808 A1. 2020, 30 March 2020.
- Dufrane, D. Cellular and/or Extracellular Extracts for Preventing and/or Treating Cancer and/or Inflammation Novadip Biosciences: EP Patent. EP 4005577 A1. 2022, 26 November 2021.
- Dufrane, D. Cellular and/or Extracellular Extracts for Preventing and/or Treating Cancer and/or Inflammation Novadip Biosciences: WO Patent. WO 2022/112528 A1. 2022, 26 November 2021.
- Dufrane, D. miRNA-Based Pharmaceutical Compositions and Uses Thereof for The Prevention and the Treatment of Tissue Disorders Novadip Biosciences: WO Patent. WO 2021/105407 A1. 2021, 27 November 2020.
- Dufrane, D.; Theys, N. Biomaterials for The Prevention and The Treatment of Tissue Disorders Novadip Biosciences: WO Patent. WO 2021/105404 A1. 2021, 27 November 2020.
- Vasiljeva, O.; Schaschke, N.; Mikhaylov, G.; Turk, B. Cathepsin-Binding Compounds Bound to A Carrier and Their Diagnostic Use J Stefan Inst: US Patent. US 2018/0085479 A1. 2018, 24 October 2017.
- Vasiljeva, O.; Schaschke, N.; Mikhaylov, G.; Turk, B. Cathepsin-Binding Compounds Bound To A Nanodevice And Their Diagnostic And Therapeutic Use Stefan Inst J Schaschke Norbert: EP Patent. EP 2537532 A1. 2012, 22 June 2011.
- Venkatesan, A.M.; Ma, K.; Chen, F.; Wu, F.; Turker, M.Z.; Gardinier, T.C.; Germano, G.J.; Adams, G.P.; Lee, F.Y. Carrier Particle-Drug Conjugates, Self-Immolative Linkers, And Uses Thereof ELUCIDA Oncology Inc.: WO Patent. WO 2022/093800 A2. 2022, 26 October 2021.
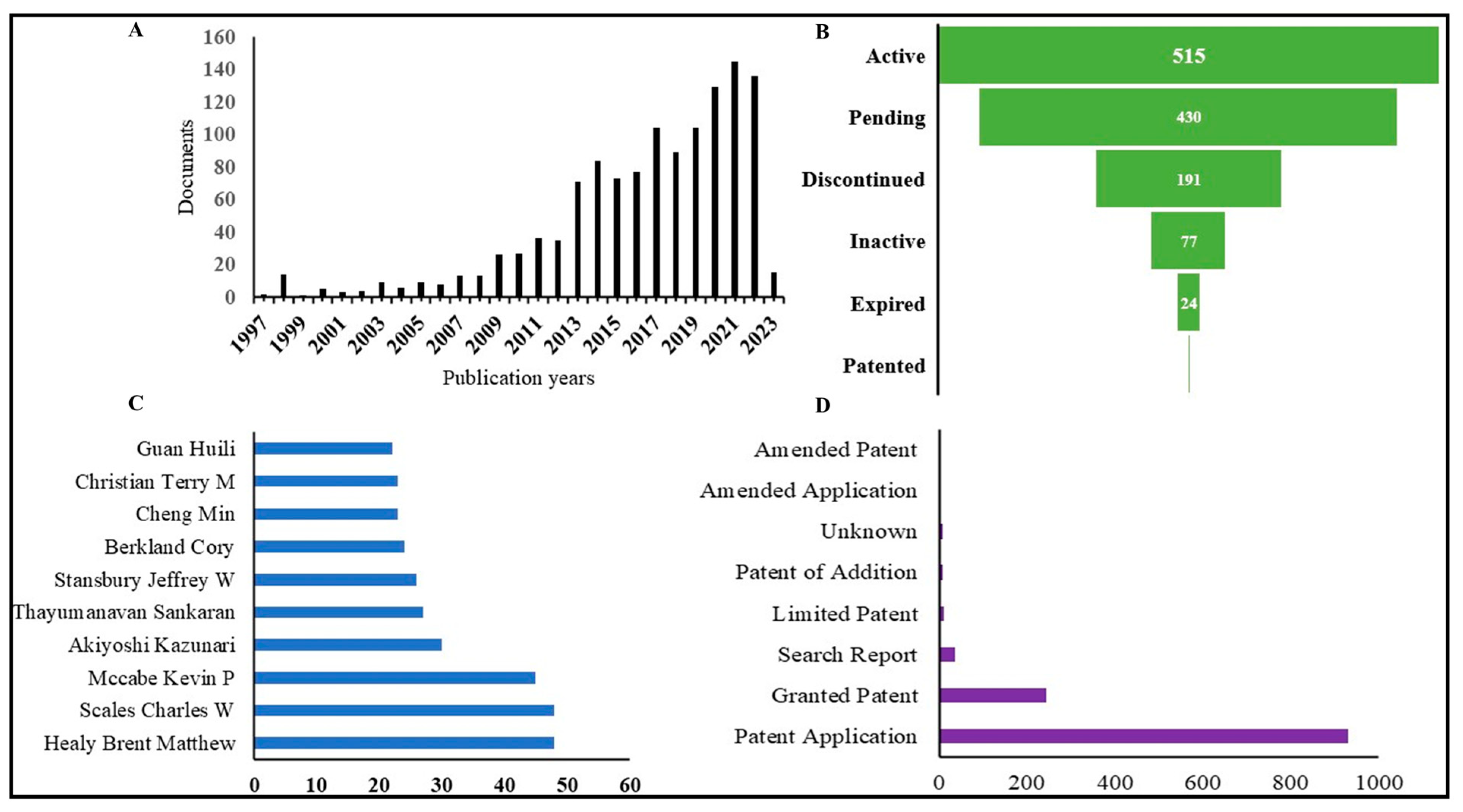
| Application Number/Display Key | Publication Date | Title | Legal Status | Reference |
|---|---|---|---|---|
| US 202017599948 A/ US 2022/0195071 A1 | 23 June 2022 | Immunotherapeutic compositions and use thereof | Pending | [102] |
| US 2020/0025844 W/ WO 2020/205808 A1 | 8 October 2020 | Pending | [103] | |
| EP 20210058 A/ EP 4005577 A1 | 1 June 2022 | Cellular and/or extracellular extracts for preventing and/or treating cancer and/or inflammation | Pending | [104] |
| EP 2021083238 W/WO 2022/112528 A1 | 2 June 2022 | Pending | [105] | |
| EP 2020083705 W/WO 2021/105407 A1 | 3 June 2021 | Mirna-based pharmaceutical compositions and uses thereof for the prevention and treatment of tissue disorders | Pending | [106] |
| EP 2020083702 W/WO 2021/105404 A1 | 3 June 2021 | Biomaterials for the prevention and treatment of tissue disorders | Pending | [107] |
| US 201715791999 A/US 2018/0085479 A1 | 29 March 2018 | Cathepsin-binding compounds bound to a carrier and their diagnostic use | Active | [108] |
| EP 11005110 A/ EP 2537532 A1 | 26 December 2012 | Cathepsin-binding compounds bound to a nanodevice and their diagnostic and therapeutic use | Discontinued | [109] |
| US 2021/0056621 W/ WO 2022/093800 A2 | 5 May 2022 | Carrier particle-drug conjugates, self-immolative linkers, and uses thereof | Pending | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, G.; Alharthi, S.; Basu, B.; Ash, D.; Dutta, S.; Singh, S.; Prajapati, B.G.; Bhattacharya, S.; Chidrawar, V.R.; Chitme, H. Nano-Gels: Recent Advancement in Fabrication Methods for Mitigation of Skin Cancer. Gels 2023, 9, 331. https://doi.org/10.3390/gels9040331
Alotaibi G, Alharthi S, Basu B, Ash D, Dutta S, Singh S, Prajapati BG, Bhattacharya S, Chidrawar VR, Chitme H. Nano-Gels: Recent Advancement in Fabrication Methods for Mitigation of Skin Cancer. Gels. 2023; 9(4):331. https://doi.org/10.3390/gels9040331
Chicago/Turabian StyleAlotaibi, Ghallab, Sitah Alharthi, Biswajit Basu, Dipanjana Ash, Swarnali Dutta, Sudarshan Singh, Bhupendra G. Prajapati, Sankha Bhattacharya, Vijay R. Chidrawar, and Havagiray Chitme. 2023. "Nano-Gels: Recent Advancement in Fabrication Methods for Mitigation of Skin Cancer" Gels 9, no. 4: 331. https://doi.org/10.3390/gels9040331











