Facile Enhancement of Electrochemical Performance of Solid-State Supercapacitor via Atmospheric Plasma Treatment on PVA-Based Gel-Polymer Electrolyte
Abstract
:1. Introduction
2. Results and Discussion
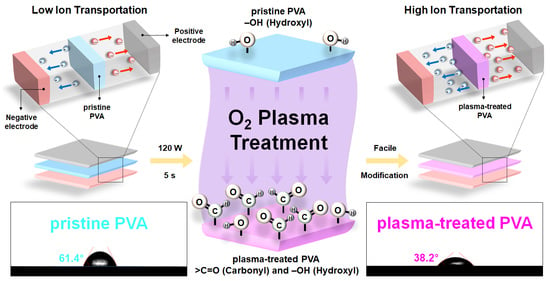
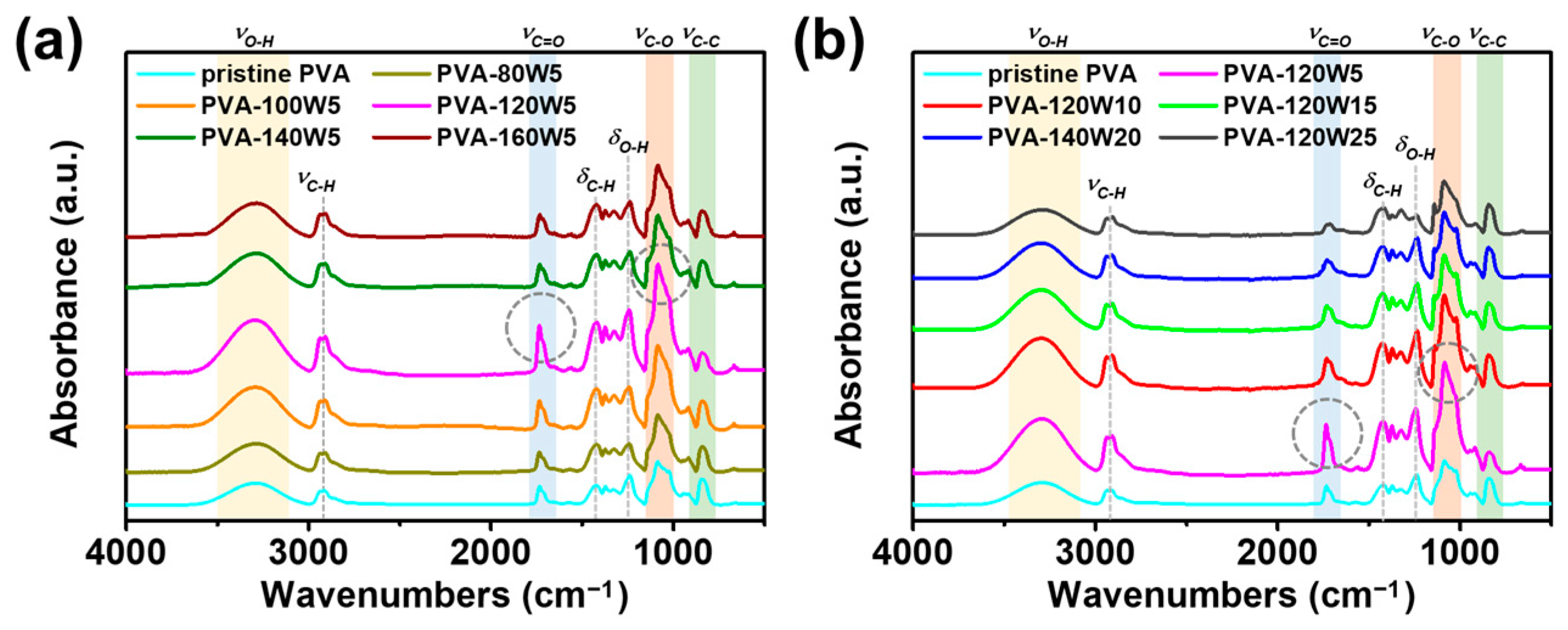
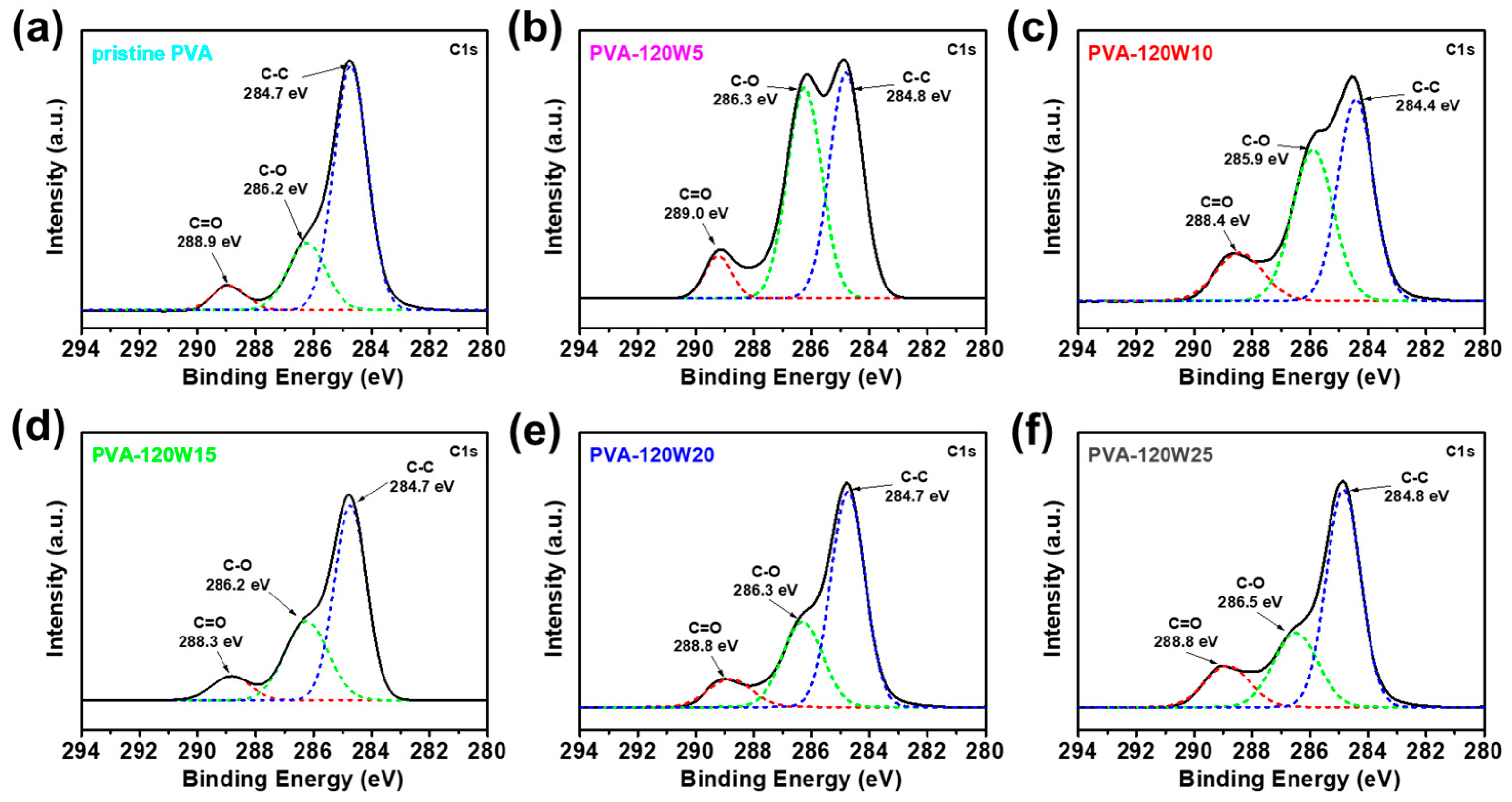
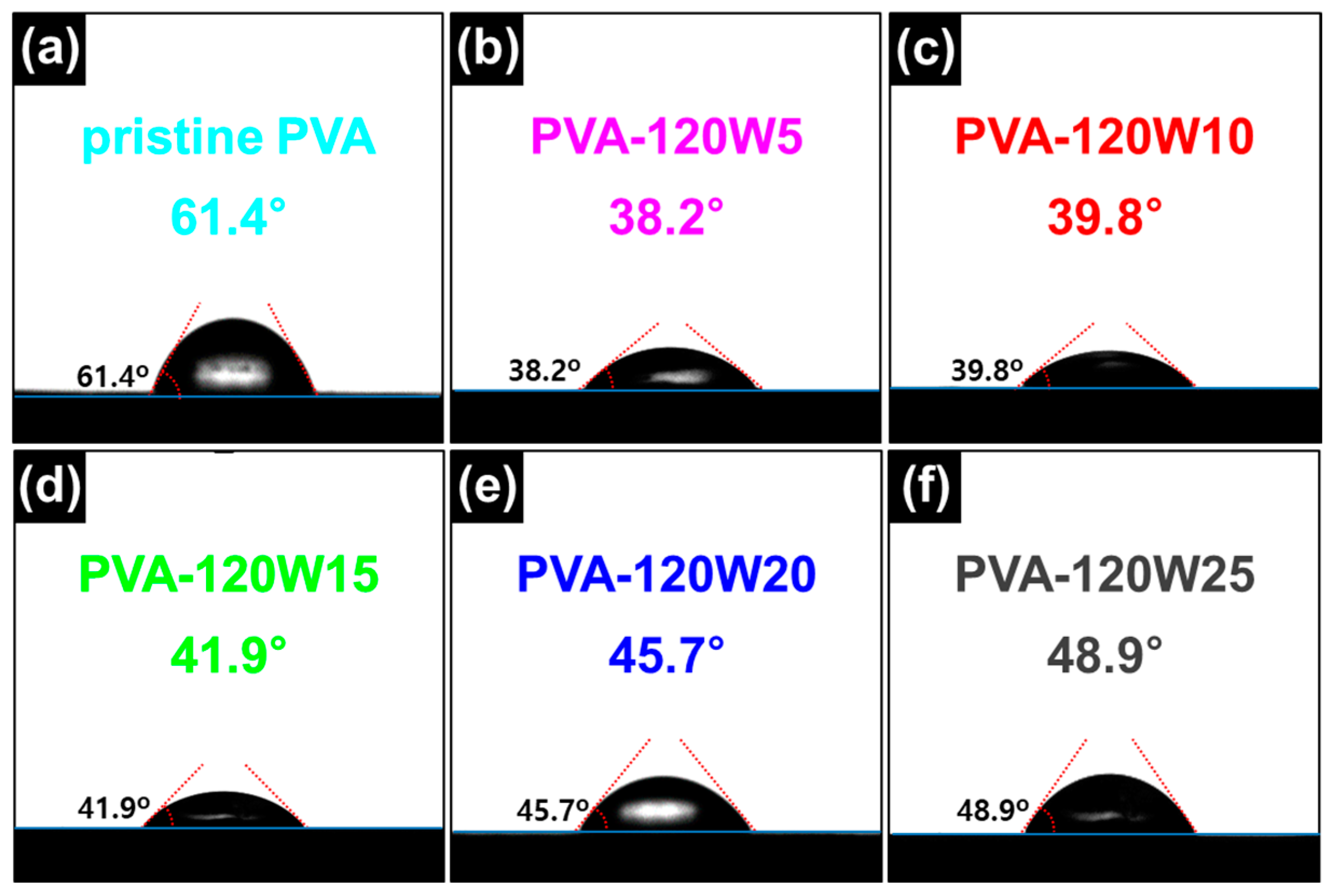
2.1. Fabrication of Plasma-Treated PVA-Based Gel-Polymer Electrolyte (Plasma-Treated PVA)
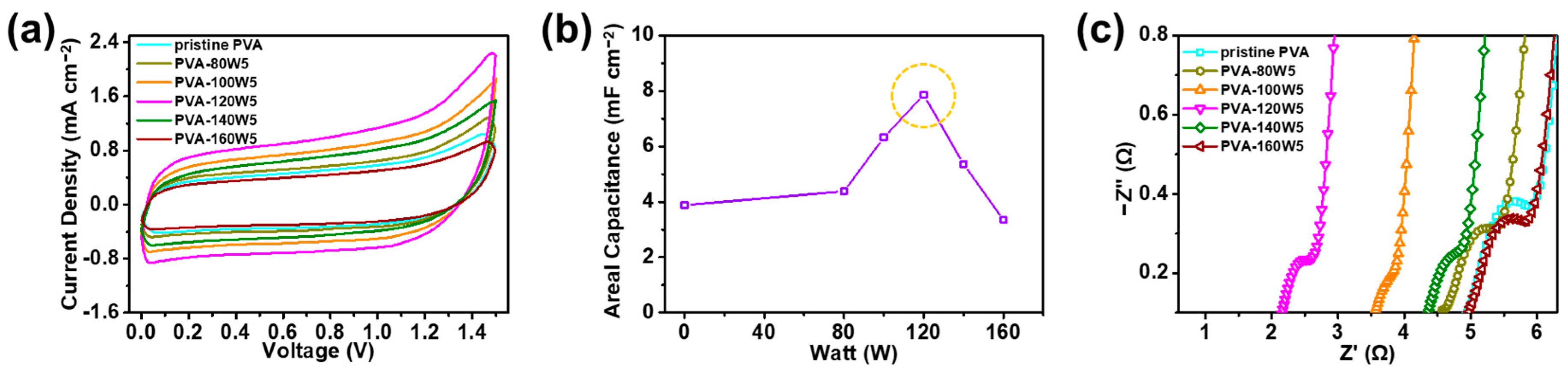
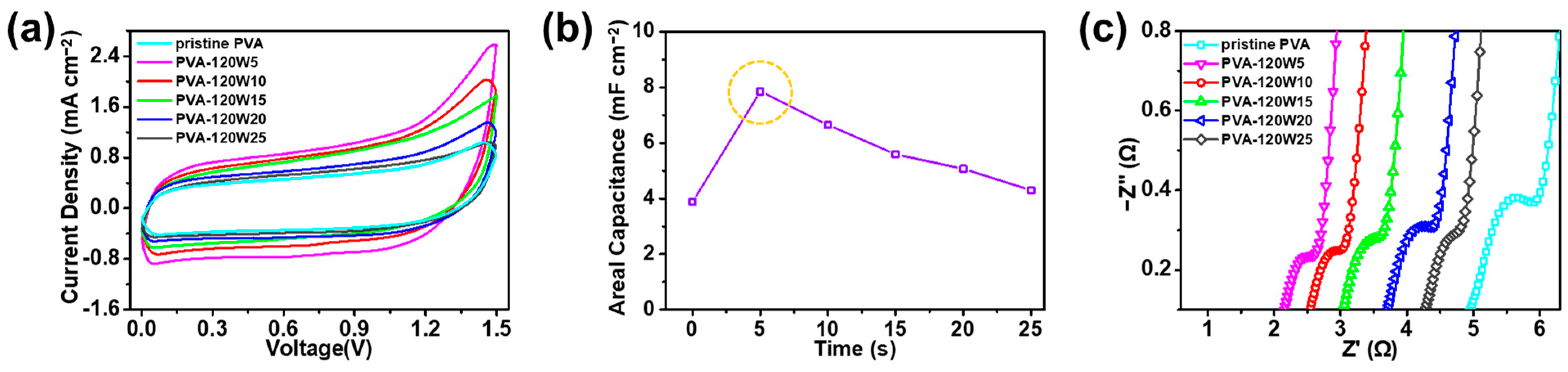
2.2. Electrochemical Properties of Plasma-Treated PVA-Based SSC
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of PVA-Based Gel-Polymer Electrolyte (Pristine PVA)
4.3. Surface Modification of PVA-Based Gel-Polymer Electrolyte Using O2 Atmospheric Plasma Treatment
4.4. Characterization
4.5. Assembly of SSC
4.6. Electrochemical Measurement
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rizoug, N.; Mesbahi, T.; Sadoun, R.; Bartholomeüs, P.; Le Moigne, P. Development of new improved energy management strategies for electric vehicle battery/supercapacitor hybrid energy storage system. Energy Effic. 2018, 11, 823–843. [Google Scholar] [CrossRef]
- Gao, D.; Luo, Z.; Liu, C.; Fan, S. A survey of hybrid energy devices based on supercapacitors. Green Energy Environ. 2022, in press. [Google Scholar] [CrossRef]
- Na, W.; Jun, J.; Park, J.W.; Lee, G.; Jang, J. Highly porous carbon nanofibers co-doped with fluorine and nitrogen for outstanding supercapacitor performance. J. Mater. Chem. A 2017, 5, 17379–17387. [Google Scholar] [CrossRef]
- Asl, M.S.; Hadi, R.; Salehghadimi, L.; Tabrizi, A.G.; Farhoudian, S.; Babapoor, A.; Pahlevani, M. Flexible all-solid-state supercapacitors with high capacitance, long cycle life, and wide operational potential window: Recent progress and future perspectives. J. Energy Storage 2022, 50, 104223. [Google Scholar] [CrossRef]
- Shi, S.; Xu, C.; Yang, C.; Li, J.; Du, H.; Li, B.; Kang, F. Flexible supercapacitors. Particuology 2013, 11, 371–377. [Google Scholar] [CrossRef]
- Guan, M.; Wang, Q.; Zhang, X.; Bao, J.; Gong, X.; Liu, Y. Two-Dimensional Transition Metal Oxide and Hydroxide-Based Hierarchical Architectures for Advanced Supercapacitor Materials. Front. Chem. 2020, 8, 390. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, X.; Liu, X.; Xie, X.; Duan, L.; Wei, L.Ü.; Gao, G. Nucleotide-Tackified Organohydrogel Electrolyte for Environmentally Self-Adaptive Flexible Supercapacitor with Robust Electrolyte/Electrode Interface. Small 2021, 17, 2103091. [Google Scholar] [CrossRef]
- Alipoori, S.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Review of PVA-based gel polymer electrolytes in flexible solid-state supercapacitors: Opportunities and challenges. J. Energy Storage 2020, 27, 101072. [Google Scholar] [CrossRef]
- Redda, H.G.; Nikodimos, Y.; Su, W.-N.; Chen, R.-S.; Jiang, S.-K.; Abrha, L.H.; Hagos, T.M.; Bezabh, H.K.; Weldeyohannes, H.H.; Hwang, B.J. Enhancing the electrochemical performance of a flexible solid-state supercapacitor using a gel polymer electrolyte. Mater. Today Commun. 2021, 26, 102102. [Google Scholar] [CrossRef]
- Genovese, M.; Wu, H.; Virya, A.; Li, J.; Shen, P.; Lian, K. Ultrathin all-solid-state supercapacitor devices based on chitosan activated carbon electrodes and polymer electrolytes. Electrochim. Acta 2018, 273, 392–401. [Google Scholar] [CrossRef]
- Ulihin, A.S.; Mateyshina, Y.G.; Uvarov, N.F. All-solid-state asymmetric supercapacitors with solid composite electrolytes. Solid State Ion. 2013, 251, 62–65. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Chen, C.; Feng, X.; Zhang, Z.; Liu, P. A high-performance solid electrolyte assisted with hybrid biomaterials for lithium metal batteries. J. Colloid Interface Sci. 2022, 608, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.M.; Venâncio, R.; Peterlevitz, A.C.; Zanin, H. Recent advances on quasi-solid-state electrolytes for supercapacitors. J. Energy Chem. 2022, 67, 697–717. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. Composite gel-polymer electrolyte for high-loading polysulfide cathodes. J. Mater. Chem. A 2022, 10, 13719. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184. [Google Scholar] [CrossRef]
- Ye, T.; Zou, Y.; Xu, W.; Zhan, T.; Sun, J.; Xia, Y.; Zhang, X.; Yang, D. Poorly-crystallized poly(vinyl alcohol)/carrageenan matrix: Highly ionic conductive and flame-retardant gel polymer electrolytes for safe and flexible solid-state supercapacitors. J. Power Sources 2020, 475, 228688. [Google Scholar] [CrossRef]
- Tian, B.; Wang, X.-Y.; Zhang, L.-N.; Shi, F.-N.; Zhang, Y.; Li, S.-X. Preparation of PVDF anionic exchange membrane by chemical grafting of GMA onto PVDF macromolecule. Solid State Ion. 2016, 293, 56–63. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Guo, Y.; Hou, Y.; Li, P.; Niu, Q.J. Engineering a superwettable polyolefin membrane for highly efficient oil/water separation with excellent self-cleaning and photo-catalysis degradation property. J. Membr. Sci. 2020, 611, 118409. [Google Scholar] [CrossRef]
- Di Mauro, A.; Farrugia, C.; Abela, S.; Refalo, P.; Grech, M.; Falqui, L.; Privitera, V.; Impellizzeri, G. Synthesis of ZnO/PMMA nanocomposite by low-temperature atomic layer deposition for possible photocatalysis applications. Mater. Sci. Semicond. Process. 2020, 118, 105214. [Google Scholar] [CrossRef]
- Mhatre, A.; Bhagwat, A.; Bangde, P.; Jain, R.; Dandekar, P. Chitosan/gelatin/PVA membranes for mammalian cell culture. Carbohydr. Polym. Technol. Appl. 2021, 2, 100163. [Google Scholar] [CrossRef]
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, D.; Gao, Y.; Chen, T.; Huang, Q.; Wang, D. Stable Li metal anode by a polyvinyl alcohol protection layer via modifying solid-electrolyte interphase layer. Nano Energy 2019, 64, 103893. [Google Scholar] [CrossRef]
- Alipoori, S.; Torkzadeh, M.M.; Mazinani, S.; Aboutalebi, S.H.; Sharif, F. Performance-tuning of PVA-based gel electrolytes by acid/PVA ratio and PVA molecular weight. SN Appl. Sci. 2021, 3, 310. [Google Scholar] [CrossRef]
- Putri, R.M.; Sundari, C.D.D.; Floweri, O.; Mayangsari, T.R.; Ivansyah, A.L.; Santosa, S.P.; Arcana, I.M.; Iskandar, F. PEO/PVA/LiOH Solid Polymer Electrolyte Prepared via Ultrasound-assisted Solution Cast Method. J. Non. Cryst. Solids 2021, 556, 120549. [Google Scholar] [CrossRef]
- Pei, X.; Wang, J.; Cong, Y.; Fu, J. Recent progress in polymer hydrogel bioadhesives. J. Polym. Sci. 2021, 59, 1312–1337. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, Z.-J. Plasma techniques for the fabrication of polymer electrolyte membranes for fuel cells. J. Membr. Sci. 2014, 456, 85–106. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Wahid, K.A.A. Polymer electrolyte membrane modification in direct ethanol fuel cells: An update. J. Appl. Polym. Sci. 2023, 140, e53383. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Yalcinkaya, B.; Pazourek, A.; Mullerova, J.; Stuchlik, M.; Maryska, J. Surface Modification of Electrospun PVDF/PAN Nanofibrous Layers by Low Vacuum Plasma Treatment. Int. J. Polym. Sci. 2016, 2016, 4671658. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Arabi, H.; Salem, A.; Asiaban, S. Effect of low-pressure O2 and Ar plasma treatments on the wettability and morphology of biaxial-oriented polypropylene (BOPP) film. Prog. Org. Coat. 2007, 60, 105–111. [Google Scholar] [CrossRef]
- Kim, J.; Lee, G.; Lee, K.; Yu, H.; Lee, J.W.; Yoon, C.-M.; Kim, S.G.; Kim, S.K.; Jang, J. Fluorine plasma treatment on carbon-based perovskite solar cells for rapid moisture protection layer formation and performance enhancement. Chem. Commun. 2020, 56, 535–538. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Shao, H.; Dong, Y. Effects of low-vacuum helium cold plasma treatment on seed germination, plant growth and yield of oilseed rape. Plasma Sci. Technol. 2018, 20, 095502. [Google Scholar] [CrossRef]
- Merche, D.; Vandencasteele, N.; Reniers, F. Atmospheric plasmas for thin film deposition: A critical review. Thin Solid Film. 2012, 520, 4219–4236. [Google Scholar] [CrossRef]
- Boaretti, C.; Rossignolo, G.; Roso, M.; Modesti, M.; Kandola, B.; Vendrame, A.; Lorenzetti, A. Investigation and Optimization of Vacuum Plasma Treatment of PA66 Fabric for Reduced Fire Retardant Consumption. ACS Omega 2022, 7, 21775–21787. [Google Scholar] [CrossRef] [PubMed]
- Junkar, I.; Vesel, A.; Cvelbar, U.; Mozetič, M.; Strnad, S. Influence of oxygen and nitrogen plasma treatment on polyethylene terephthalate (PET) polymers. Vacuum 2009, 84, 83–85. [Google Scholar] [CrossRef]
- Lee, C.; Han, Y.-J.; Seo, Y.D.; Nakabayashi, K.; Miyawaki, J.; Santamaría, R.; Menéndez, R.; Yoon, S.-H.; Jang, J. C4F8 plasma treatment as an effective route for improving rate performance of natural/synthetic graphite anodes in lithium ion batteries. Carbon 2016, 103, 28–35. [Google Scholar] [CrossRef]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical applications of cold atmospheric plasma: State of the science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef]
- Kim, K.-S.; Bang, J.-O.; Choa, Y.-H.; Jung, S.-B. The characteristics of Cu nanopaste sintered by atmospheric-pressure plasma. Microelectron. Eng. 2013, 107, 121–124. [Google Scholar] [CrossRef]
- Zille, A.; Oliveira, F.R.; Souto, P.A.P. Plasma treatment in textile industry. Plasma Process. Polym. 2015, 12, 98–131. [Google Scholar] [CrossRef]
- Domonkos, M.; Tichá, P. Wettability of SiOx nanofibrous mats and their modification using cold atmospheric plasma treatment. Acta Phys. Polon. A 2021, 140, 181–185. [Google Scholar] [CrossRef]
- Jucius, D.; Grigaliunas, V.; Kopustinskas, V.; Lazauskas, A.; Guobiene, A. Wettability and optical properties of O2 and CF4 plasma treated biaxially oriented semicrystalline poly(ethylene terephthalate) films. Appl. Surf. Sci. 2012, 263, 722–729. [Google Scholar] [CrossRef]
- Kim, K.S.; Lee, K.H.; Cho, K.; Park, C.E. Surface modification of polysulfone ultrafiltration membrane by oxygen plasma treatment. J. Membr. Sci. 2002, 199, 135–145. [Google Scholar] [CrossRef]
- Ozgen, O.; Aksoy, E.A.; Hasirci, V.; Hasirci, N. Surface characterization and radical decay studies of oxygen plasma-treated PMMA films. Surf. Interface Anal. 2013, 45, 844–853. [Google Scholar] [CrossRef]
- Fu, W.; Xu, R.; Zhang, X.; Tian, Z.; Huang, H.; Xie, J.; Lei, C. Enhanced wettability and electrochemical performance of separators for lithium-ion batteries by coating core-shell structured silica-poly(cyclotriphosphazene-co-4,4″-sulfonyldiphenol) particles. J. Power Sources 2019, 436, 226839. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, Q.; Ma, Z.; Liu, Q.; Wu, Z.; Wang, S. Oxygen plasma modified separator for lithium sulfur battery. RSC Adv. 2015, 5, 79473–79478. [Google Scholar] [CrossRef]
- Jin, S.Y.; Manuel, J.; Zhao, X.; Park, W.H.; Ahn, J.-H. Surface-modified polyethylene separator via oxygen plasma treatment for lithium ion battery. J. Ind. Eng. Chem. 2017, 45, 15–21. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, D.Y. Surface-modified membrane as a separator for lithium-ion polymer battery. Energies 2010, 3, 866–885. [Google Scholar] [CrossRef]
- Yoshida, A.; Tanahashi, I.; Nishino, A. Aluminum Collector Electrodes Formed by the Plasma Spraying Method for Electric Double-Layer Capacitors. IEEE Trans. Comp. Hybrids Manuf. Technol. 1988, 11, 318–323. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Hsu, C.-C.; Chen, J.-Z. Comparison between atmospheric-pressure-plasma-jet-processed and furnace-calcined rGO-MnOx nanocomposite electrodes for gel-electrolyte supercapacitors. J. Alloy. Compd. 2022, 911, 165006. [Google Scholar] [CrossRef]
- Liu, C.; Hung, C.-W.; Cheng, I.-C.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Dielectric barrier discharge plasma jet (Dbdjet) processed reduced graphene oxide/polypyrrole/chitosan nanocomposite supercapacitors. Polymers 2021, 13, 3585. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Koo, H.-J.; Lee, A.; Braun, P.V. Selective wetting-induced micro-electrode patterning for flexible micro-supercapacitors. Adv. Mater. 2014, 26, 5108–5112. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Michael, B.D.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, C.; Jiao, H.; Zhang, M. Polyvinyl Alcohol/SiO2 Hybrid Dielectric for Transparent Flexible/Stretchable All-Carbon-Nanotube Thin-Film-Transistor Integration. Adv. Electron. Mater. 2020, 6, 1901133. [Google Scholar]
- Ibrahim, M.M.; EI-Zawawy, W.K.; Nassar, M.A. Synthesis and characterization of polyvinyl alcohol/nanospherical cellulose particle films. Carbohydr. Polym. 2010, 79, 694–699. [Google Scholar] [CrossRef]
- Labbé, N.; Harper, D.; Rials, T.; Elder, T. Chemical structure of wood charcoal by infrared spectroscopy and multivariate analysis. J. Agric. Food Chem. 2006, 54, 3492–3497. [Google Scholar] [CrossRef]
- Korbag, I.; Mohamed Saleh, S. Studies on the formation of intermolecular interactions and structural characterization of polyvinyl alcohol/lignin film. Int. J. Environ. Stud. 2016, 73, 226–235. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS-PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, N.M.; Aziz, S.B.; Kadir, M.F.Z. Development of Flexible Plasticized Ion Conducting Polymer Blend Electrolytes Based on Polyvinyl Alcohol (PVA): Chitosan (CS) with High Ion Transport Parameters Close to Gel Based Electrolytes. Gels 2022, 8, 153. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stoica, A.; Stroescu, M.; Dobre, L.-M.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly(vinyl alcohol)-bacterial cellulose films. Chem. Zvesti 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Lim, M.; Zulkifli, A.Z.S. Investigation of biomass surface modification using non-thermal plasma treatment. Plasma Sci. Technol. 2018, 20, 115502. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C.; Gengembre, L.; Payen, E. Treatment of polymer films with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7066–7075. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; An, G.; He, X. Highly porous SnO2 fibers by electrospinning and oxygen plasma etching and its ethanol-sensing properties. Sens. Actuators B Chem. 2010, 144, 43–48. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal stability and reducibility of oxygen-containing functional groups on multiwalled carbon nanotube surfaces: A quantitative high-resolution xps and TPD/TPR study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Dawaymeh, F.; Abbas, Y.; Khaleel, M.; Alazzam, A.; Alamoodi, N. Tuning the Surface Wettability of Cyclic Olefin Copolymer by Plasma Treatment and Graphene Oxide Deposition and Reduction. Polymers 2021, 13, 2305. [Google Scholar] [CrossRef] [PubMed]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric constants of water, methanol, ethanol, butanol and acetone: Measurement and computational study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Kim, G.; Kang, J.; Choe, G.; Yim, S. Enhanced energy density of supercapacitors using hybrid electrodes based on Fe2O3 and MnO2 nanoparticles. Int. J. Electrochem. Sci. 2017, 12, 10015–10022. [Google Scholar] [CrossRef]
- Cho, S.; Patil, B.; Yu, S.; Ahn, S.; Hwang, J.; Park, C.; Do, K.; Ahn, H. Flexible, Swiss roll, fiber-shaped, asymmetric supercapacitor using MnO2 and Fe2O3 on carbon fibers. Electrochim. Acta 2018, 269, 499–508. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Ratnaji, T.; Konikkara, N.; Vijaya, J.J. Value added porous carbon from leather wastes as potential supercapacitor electrode using neutral electrolyte. J. Clean. Prod. 2018, 197, 930–936. [Google Scholar] [CrossRef]
- Lin, W.-C.; Razali, N.A.M. Temporary Wettability Tuning of PCL/PDMS Micro Pattern Using the Plasma Treatments. Materials 2019, 12, 644. [Google Scholar] [CrossRef]
- Xie, H.; Wu, Z.; Wang, Z.; Qin, N.; Li, Y.; Cao, Y.; Lu, Z. Solid electrolyte interface stabilization via surface oxygen species functionalization in hard carbon for superior performance sodium-ion batteries. J. Mater. Chem. A 2020, 8, 3606–3612. [Google Scholar] [CrossRef]
- Jekal, S.; Kim, M.-S.; Kim, D.-H.; Noh, J.; Kim, H.-Y.; Kim, J.; Yi, H.; Oh, W.-C.; Yoon, C.-M. Fabrication of Flexible All-Solid-State Asymmetric Supercapacitor Device via Full Recycling of Heated Tobacco Waste Assisted by PLA Gelation Template Method. Gels 2023, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Li, Q.; Smith, A.D.; Kuzmenko, V.; Köhler, E.; Lundgren, P.; Enoksson, P. Thermal influence on the electrochemical behavior of a supercapacitor containing an ionic liquid electrolyte. Electrochim. Acta 2018, 263, 249–260. [Google Scholar] [CrossRef]
- Kumbhar, V.S.; Jagadale, A.D.; Shinde, N.M.; Lokhande, C.D. Chemical synthesis of spinel cobalt ferrite (CoFe2O4) nano-flakes for supercapacitor application. Appl. Surf. Sci. 2012, 259, 39–43. [Google Scholar] [CrossRef]
- Emran, K.M.; Omar, I.M.A.; Arab, S.T.; Ouerfelli, N. On the pseudo-hyperbolic behavior of charge transfer resistance–temperature dependence in corrosion behavior of Nickel based glass alloy. Sci. Rep. 2022, 12, 6432. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, C.; Fukumoto, Y.; Matsuoka, M.; Kohno, T.; Shinmou, K. Electrochemical characterization of hydrogen storage alloys modified with metal oxides. J. Alloy. Compd. 1993, 192, 152–154. [Google Scholar] [CrossRef]
- Kharade, P.M.; Kulkarni, S.B.; Salunkhe, D.J. Nanoflakes like hydrophilic Mn2O3 thin film as a supercapacitor electrode. Chin. J. Phys. 2017, 55, 1684–1689. [Google Scholar] [CrossRef]
- Ueshima, M.; Toda, E.; Nakajima, Y.; Sugiyama, K. Effect of microwave non thermal plasma irradiation on the adsorptive properties of active carbon preliminarily impregnated with poly(vinyl alcohol). Jpn. J. Appl. Phys. 2010, 49, 08JA03. [Google Scholar] [CrossRef]
- Xue, Q.; Gan, H.; Huang, Y.; Zhu, M.; Pei, Z.; Li, H.; Deng, S.; Liu, F.; Zhi, C. Boron Element Nanowires Electrode for Supercapacitors. Adv. Energy Mater. 2018, 8, 1703117. [Google Scholar] [CrossRef]
- Pandit, B.; Dhakate, S.R.; Singh, B.P.; Sankapal, B.R. Free-standing flexible MWCNTs bucky paper: Extremely stable and energy efficient supercapacitive electrode. Electrochim. Acta 2017, 249, 395–403. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Lou, F.; Melandsø Buan, M.E.; Sheridan, E.; Chen, D. Enhancing capacitance of supercapacitor with both organic electrolyte and ionic liquid electrolyte on a biomass-derived carbon. RSC Adv. 2017, 7, 23859–23865. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, H.; Meng, C.; Wang, Z.; Akhtar, K.; Yuan, A. Cyanometallic framework-derived hierarchical Co3O4-NiO/graphene foam as high-performance binder-free electrodes for supercapacitors. Chem. Eng. J. 2019, 369, 57–63. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Jover, E.; Bertran, E. Nitrogen plasma functionalization of carbon nanotubes for supercapacitor applications. J. Mater. Sci. 2013, 48, 7620–7628. [Google Scholar] [CrossRef]
- Dulyaseree, P.; Yordsri, V.; Wongwiriyapan, W. Effects of microwave and oxygen plasma treatments on capacitive characteristics of supercapacitor based on multiwalled carbon nanotubes. Jpn. J. Appl. Phys. 2016, 55, 02BD05. [Google Scholar] [CrossRef]
- Kong, M.; Wang, Z.; Wang, W.; Ma, M.; Liu, D.; Hao, S.; Kong, R.; Du, G.; Asiri, A.M.; Yao, Y.; et al. NiCoP Nanoarray: A Superior Pseudocapacitor Electrode with High Areal Capacitance. Chem. Eur. J. 2017, 23, 4435–4441. [Google Scholar] [CrossRef] [PubMed]



| Materials | Target of the Treatment | Plasma Type | Specific Capacitance | Ref | |
|---|---|---|---|---|---|
| Without Plasma | With Plasma | ||||
| PVA-120W5/Na2SO4 | gel electrolyte | Oxygen atmospheric plasma | 3.89 mF cm−2 (at 100 mV s−1) | 7.89 mF cm−2 | This work |
| rGO-MnOx | active material or electrode | Nitrogen plasma | 9.92 mF cm−2 (at 2 mV s−1) | 52.23 mF cm−2 | [48] |
| MWCNTs | active material or electrode | Nitrogen plasma | 22 F g−1 (at 10 mV s−1) | 55 F g−1 | [82] |
| MWNTs | active material or electrode | Oxygen plasma | 61.5 F g−1 (at 100 mV s−1) | 238.2 F g−1 | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-H.; Jekal, S.; Kim, C.-G.; Chu, Y.-R.; Noh, J.; Kim, M.S.; Lee, N.; Song, W.-J.; Yoon, C.-M. Facile Enhancement of Electrochemical Performance of Solid-State Supercapacitor via Atmospheric Plasma Treatment on PVA-Based Gel-Polymer Electrolyte. Gels 2023, 9, 351. https://doi.org/10.3390/gels9040351
Kim D-H, Jekal S, Kim C-G, Chu Y-R, Noh J, Kim MS, Lee N, Song W-J, Yoon C-M. Facile Enhancement of Electrochemical Performance of Solid-State Supercapacitor via Atmospheric Plasma Treatment on PVA-Based Gel-Polymer Electrolyte. Gels. 2023; 9(4):351. https://doi.org/10.3390/gels9040351
Chicago/Turabian StyleKim, Dong-Hyun, Suk Jekal, Chan-Gyo Kim, Yeon-Ryong Chu, Jungchul Noh, Min Sang Kim, Neunghi Lee, Woo-Jin Song, and Chang-Min Yoon. 2023. "Facile Enhancement of Electrochemical Performance of Solid-State Supercapacitor via Atmospheric Plasma Treatment on PVA-Based Gel-Polymer Electrolyte" Gels 9, no. 4: 351. https://doi.org/10.3390/gels9040351
APA StyleKim, D.-H., Jekal, S., Kim, C.-G., Chu, Y.-R., Noh, J., Kim, M. S., Lee, N., Song, W.-J., & Yoon, C.-M. (2023). Facile Enhancement of Electrochemical Performance of Solid-State Supercapacitor via Atmospheric Plasma Treatment on PVA-Based Gel-Polymer Electrolyte. Gels, 9(4), 351. https://doi.org/10.3390/gels9040351