Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing
Abstract
:1. Introduction
2. The Skin
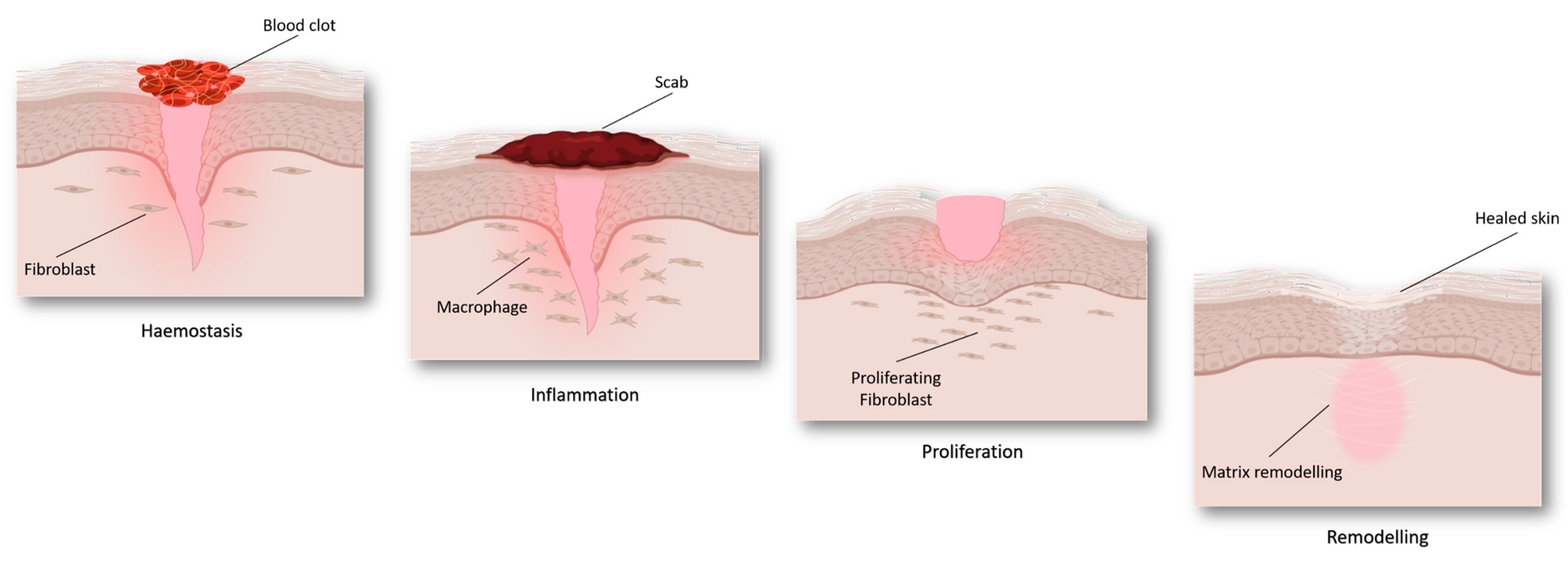
Wound Healing Phases
3. Wound Dressing
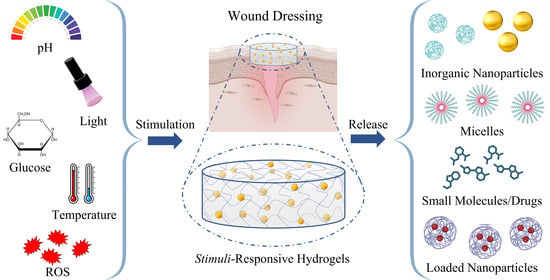
4. Stimuli-Responsive Systems
4.1. pH-Responsive
4.2. ROS-Responsive
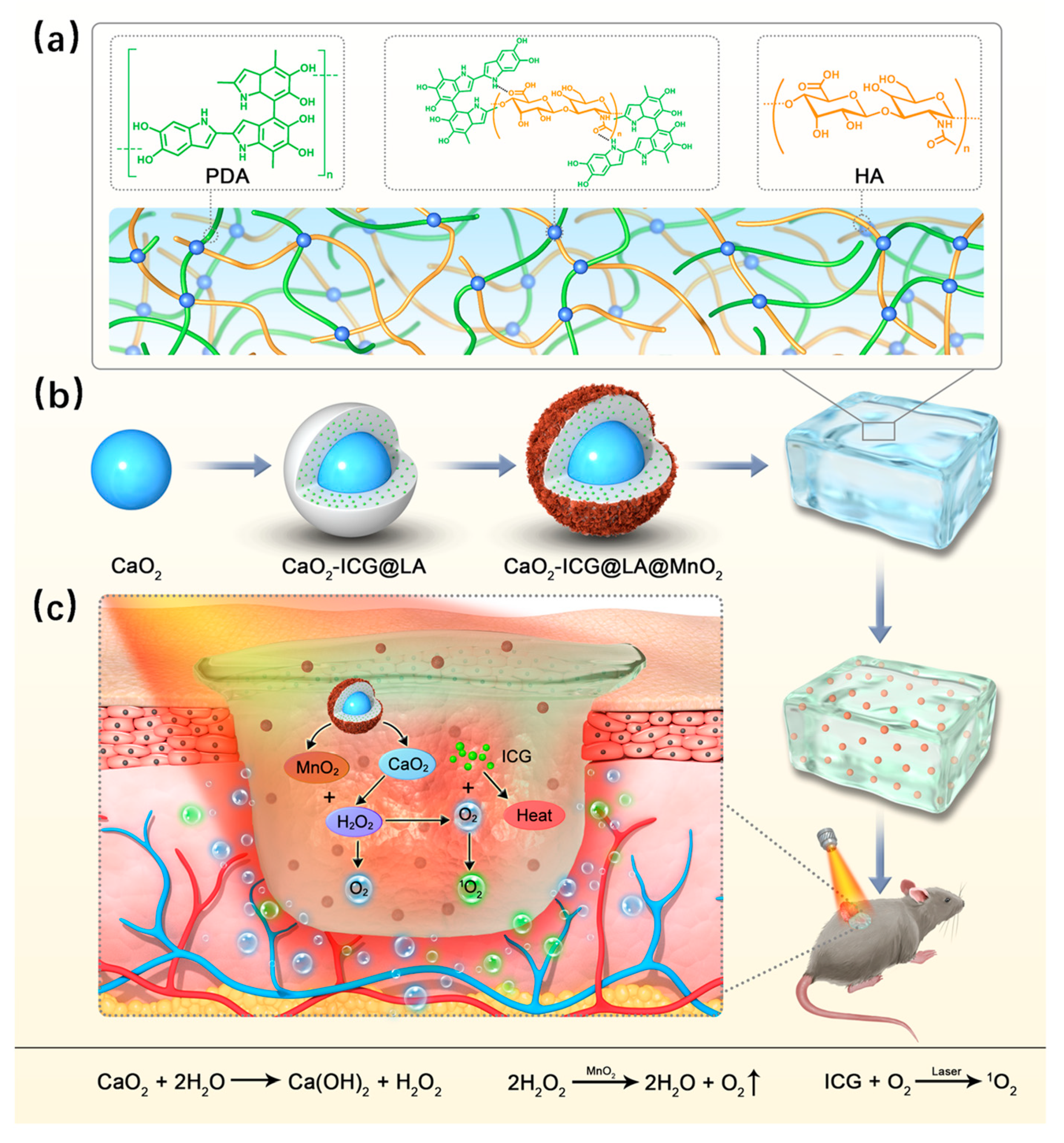
4.3. Light-Sensitive
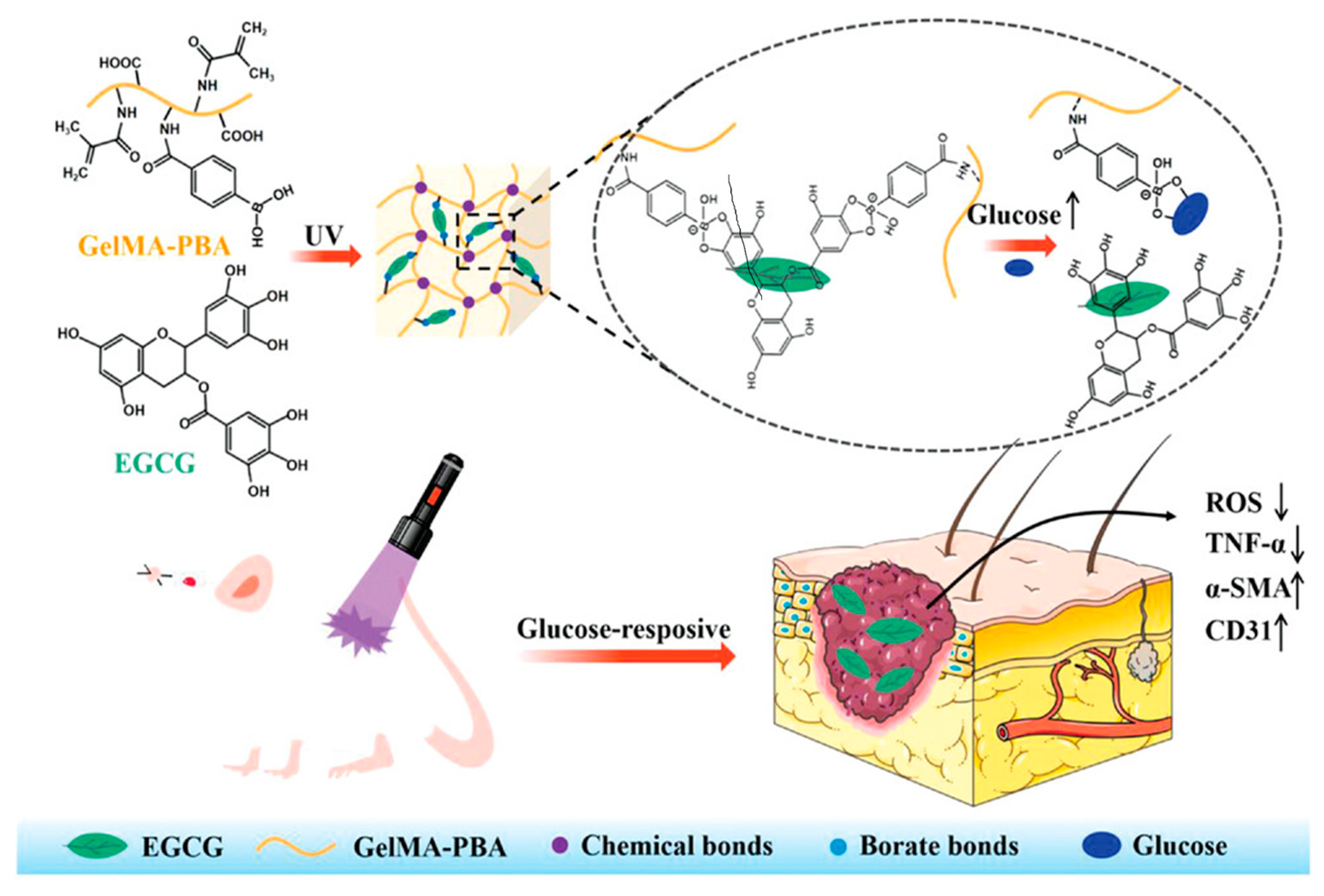
4.4. Glucose-Responsive
4.5. Thermosensitive
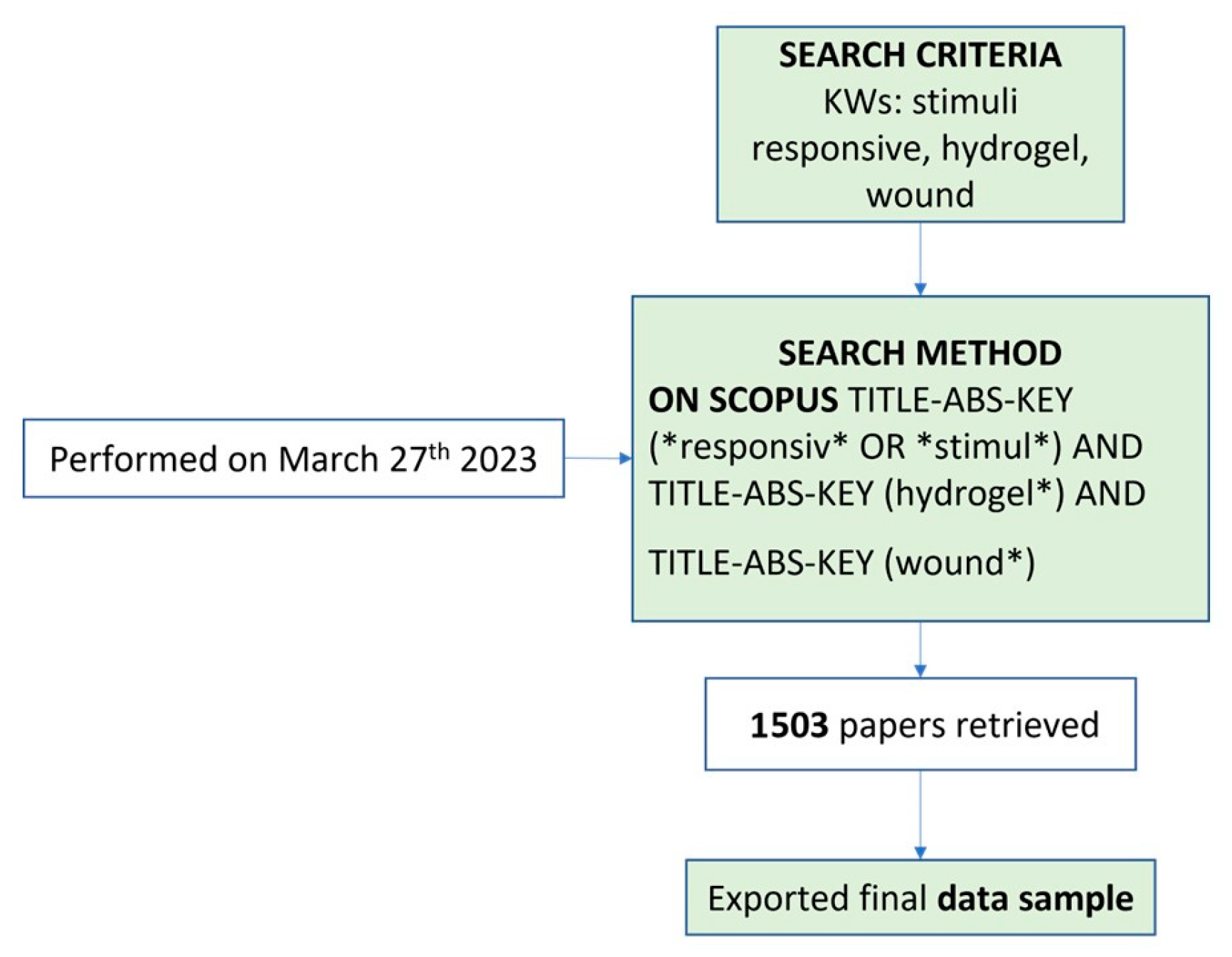
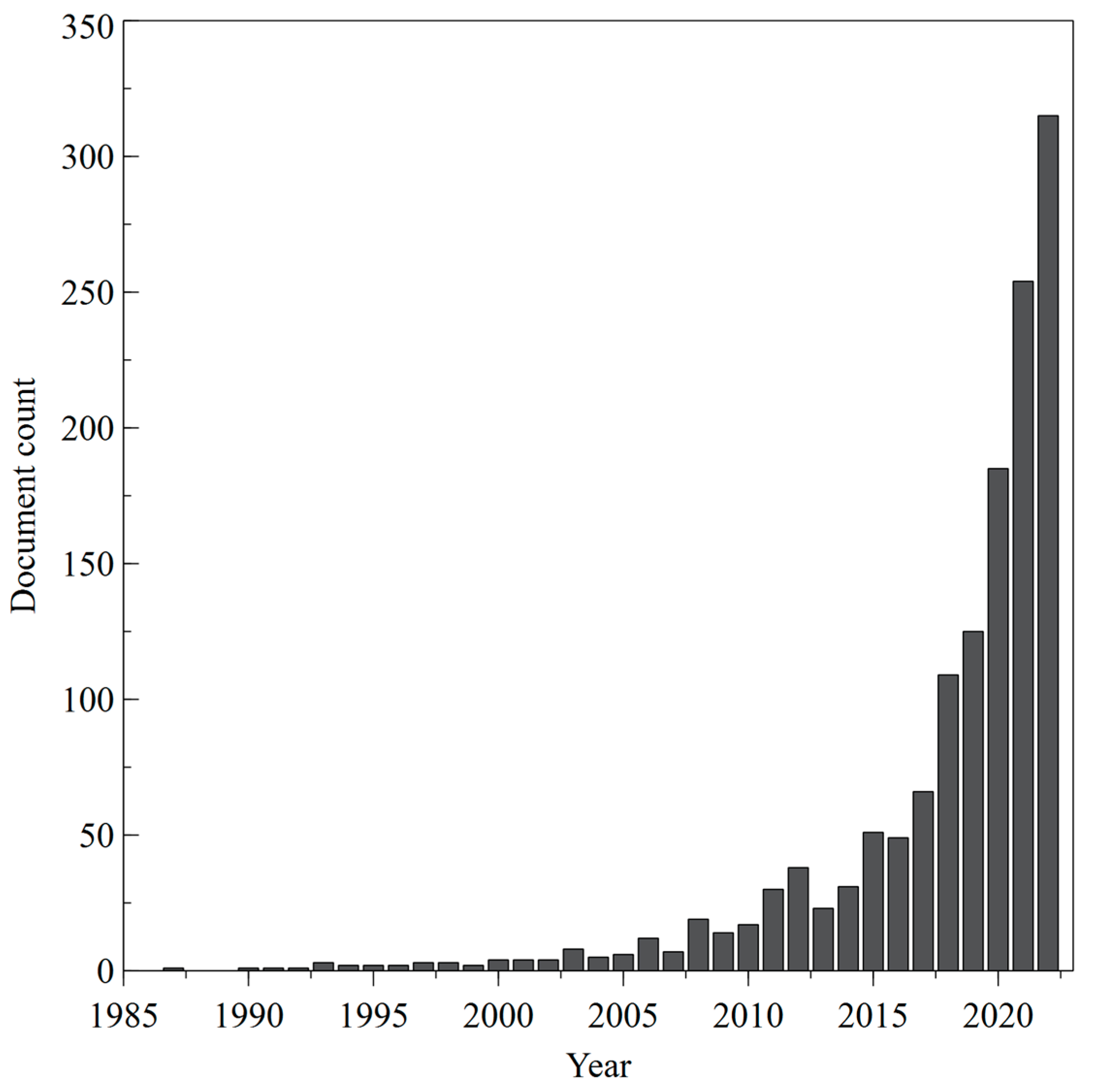
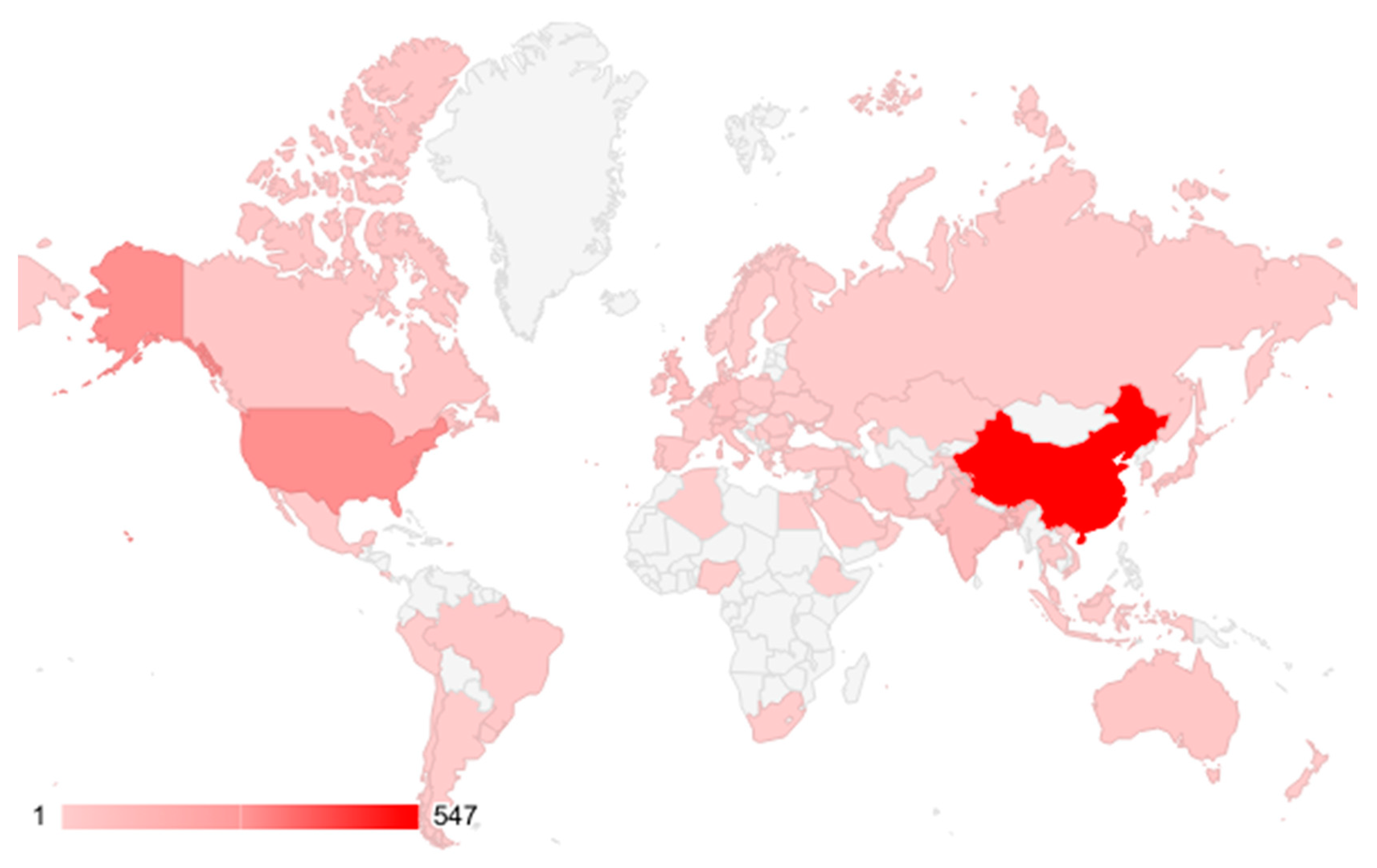
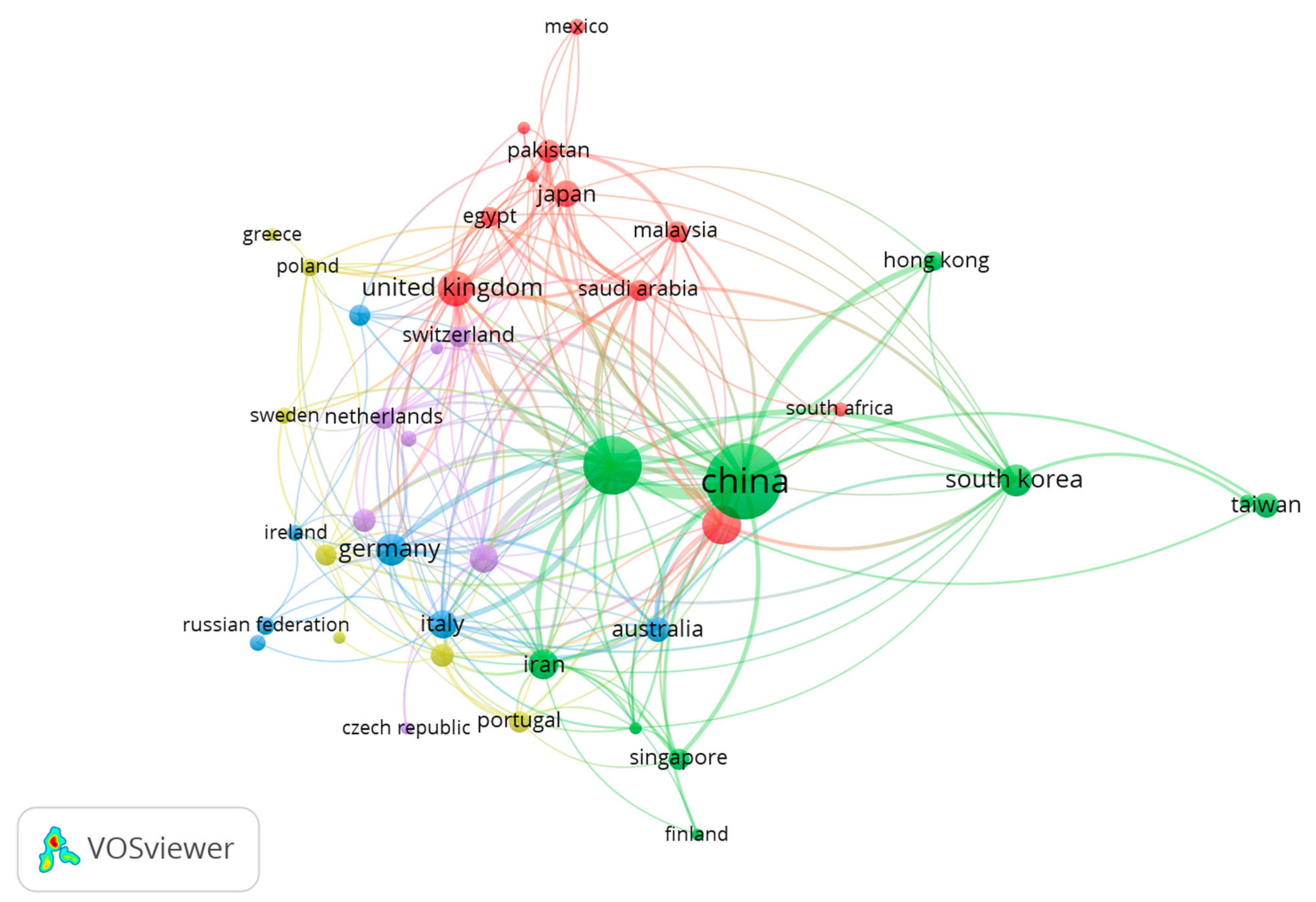
5. Bibliometric Data Collection and Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, Y.K.; Kim, S.W. Recent Advances in Polymeric Drug Delivery Systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Calori, I.R.; Braga, G.; de Jesus, P.D.C.C.; Bi, H.; Tedesco, A.C. Polymer Scaffolds as Drug Delivery Systems. Eur. Polym. J. 2020, 129, 109621. [Google Scholar] [CrossRef]
- Pires, P.C.; Mascarenhas-Melo, F.; Pedrosa, K.; Lopes, D.; Lopes, J.; Macário-Soares, A.; Peixoto, D.; Giram, P.S.; Veiga, F.; Paiva-Santos, A.C. Polymer-Based Biomaterials for Pharmaceutical and Biomedical Applications: A Focus on Topical Drug Administration. Eur. Polym. J. 2023, 187, 111868. [Google Scholar] [CrossRef]
- Riccio, B.V.F.; Silvestre, A.L.P.; Meneguin, A.B.; de Ribeiro, T.C.; Klosowski, A.B.; Ferrari, P.C.; Chorilli, M. Exploiting Polymeric Films as a Multipurpose Drug Delivery System: A Review. AAPS PharmSciTech 2022, 23, 269. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Das, S.S.; Sabir, F.; Sundaramahalingam, M.A.; Colmenares, J.C.; Prasannakumar, S.; Rajan, M.; Rahdar, A.; Kyzas, G.Z. Progress in Natural Polymer Engineered Biomaterials for Transdermal Drug Delivery Systems. Mater. Today Chem. 2021, 19, 100382. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, H.; Wu, D. Recent Advances on Polymeric Hydrogels as Wound Dressings. APL Bioeng. 2021, 5, 011504. [Google Scholar] [CrossRef]
- Kalai Selvan, N.; Shanmugarajan, T.S.; Uppuluri, V.N.V.A. Hydrogel Based Scaffolding Polymeric Biomaterials: Approaches towards Skin Tissue Regeneration. J. Drug Deliv. Sci. Technol. 2020, 55, 101456. [Google Scholar] [CrossRef]
- Cao, J.; Wang, P.; Liu, Y.; Zhu, C.; Fan, D. Double Crosslinked HLC-CCS Hydrogel Tissue Engineering Scaffold for Skin Wound Healing. Int. J. Biol. Macromol. 2020, 155, 625–635. [Google Scholar] [CrossRef]
- Su, T.; Zhang, M.; Zeng, Q.; Pan, W.; Huang, Y.; Qian, Y.; Dong, W.; Qi, X.; Shen, J. Mussel-Inspired Agarose Hydrogel Scaffolds for Skin Tissue Engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef]
- Lih, E.; Lee, J.S.; Park, K.M.; Park, K.D. Rapidly Curable Chitosan-PEG Hydrogels as Tissue Adhesives for Hemostasis and Wound Healing. Acta Biomater. 2012, 8, 3261–3269. [Google Scholar] [CrossRef]
- Wu, S.; Deng, L.; Hsia, H.; Xu, K.; He, Y.; Huang, Q.; Peng, Y.; Zhou, Z.; Peng, C. Evaluation of Gelatin-Hyaluronic Acid Composite Hydrogels for Accelerating Wound Healing. J. Biomater. Appl. 2017, 31, 1380–1390. [Google Scholar] [CrossRef]
- Harrison, I.P.; Spada, F. Hydrogels for Atopic Dermatitis and Wound Management: A Superior Drug Delivery Vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Varghese, S.A.; Rangappa, S.M.; Siengchin, S.; Parameswaranpillai, J. Chapter 2—Natural Polymers and the Hydrogels Prepared from Them. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–47. ISBN 9780128164211. [Google Scholar]
- Koehler, J.; Brandl, F.P.; Goepferich, A.M. Hydrogel Wound Dressings for Bioactive Treatment of Acute and Chronic Wounds. Eur. Polym. J. 2018, 100, 1–11. [Google Scholar] [CrossRef]
- Archer, C.B.; Eedy, D.J. The Skin and the Nervous System. In Rook’s Textbook of Dermatology; Wiley-Blackwell: Oxford, UK, 2010; pp. 1–25. ISBN 9781444317633. [Google Scholar]
- Bangert, C.; Brunner, P.M.; Stingl, G. Immune Functions of the Skin. Clin. Dermatol. 2011, 29, 360–376. [Google Scholar] [CrossRef]
- Brown, T.M.; Krishnamurthy, K. Histology, Dermis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Janis, J.E.; Harrison, B. Wound Healing. Plast. Reconstr. Surg. 2016, 138, 9S–17S. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S. Redox Signals in Wound Healing. Biochim. Biophys. Acta 2008, 1780, 1348–1361. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound Healing: Cellular Mechanisms and Pathological Outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.A.; Basehore, B.M.; Zito, P.M. Wound Healing Phases. Available online: https://europepmc.org/books/nbk470443 (accessed on 28 April 2023).
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and Impaired Wound Healing: Pathophysiology and Current Methods for Drug Delivery, Part 2: Role of Growth Factors in Normal and Pathological Wound Healing: Therapeutic Potential and Methods of Delivery. Adv. Ski. Wound Care 2012, 25, 349–370. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the Scab and the Rate of Epithelization of Superficial Wounds in the Skin of the Young Domestic Pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Junker, J.P.E.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate Polymer-Based Silver Nanocomposites: Recent Progress in the Antimicrobial Wound Dressings. Carbohydr. Polym. 2020, 231, 115696. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; El-Naggar, M.E. Chapter 2—Traditional and Modern Wound Dressings—Characteristics of Ideal Wound Dressings. In Antimicrobial Dressings; Khan, R., Gowri, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 21–42. ISBN 9780323950749. [Google Scholar]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic Insight into Diabetic Wounds: Pathogenesis, Molecular Targets and Treatment Strategies to Pace Wound Healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent Advances in Responsive Hydrogels for Diabetic Wound Healing. Mater. Today Bio 2023, 18, 100508. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable Antibacterial Conductive Nanocomposite Cryogels with Rapid Shape Recovery for Noncompressible Hemorrhage and Wound Healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Myers, T. The Phases of Wound Healing and Types of Wound Closure. In Fast Facts for Wound Care Nursing; Springer: New York, NY, USA, 2021; ISBN 9780826195029. [Google Scholar]
- Thakur, S.; Thakur, V.K.; Arotiba, O.A. History, Classification, Properties and Application of Hydrogels: An Overview. In Hydrogels: Recent Advances; Thakur, V.K., Thakur, M.K., Eds.; Springer: Singapore, 2018; pp. 29–50. ISBN 9789811060779. [Google Scholar]
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-Responsive Hydrogels: Theory, Modern Advances, and Applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Heydarpour, R.; Tehrani, Z.M. Multi-Stimuli-Responsive Hydrogels and Their Medical Applications. New J. Chem. 2021, 45, 15705–15717. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Xin, H.; Naficy, S. Drug Delivery Based on Stimuli-Responsive Injectable Hydrogels for Breast Cancer Therapy: A Review. Gels 2022, 8, 45. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Stimuli-Responsive Hydrogels for Intratumoral Drug Delivery. Drug Discov. Today 2021, 26, 2397–2405. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple Stimuli-Responsive MXene-Based Hydrogel as Intelligent Drug Delivery Carriers for Deep Chronic Wound Healing. Small 2022, 18, e2104368. [Google Scholar] [CrossRef]
- Chang, W.-H.; Lee, Y.-F.; Liu, Y.-W.; Willner, I.; Liao, W.-C. Stimuli-Responsive Hydrogel Microcapsules for the Amplified Detection of microRNAs. Nanoscale 2021, 13, 16799–16808. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Z.; Jia, X.; Chen, R.; Qin, Y.; Bian, Y.; Sheng, W.; Li, S.; Gao, Z. Stimulus-Responsive Hydrogels: A Potent Tool for Biosensing in Food Safety. Trends Food Sci. Technol. 2023, 131, 91–103. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhong, Y.; Jiang, X. Thermosensitive and pH-Responsive Tannin-Containing Hydroxypropyl Chitin Hydrogel with Long-Lasting Antibacterial Activity for Wound Healing. Carbohydr. Polym. 2020, 236, 116096. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Long, L.; Cao, J.; Zhang, S.; Wang, Y. Dual-Crosslinked Mussel-Inspired Smart Hydrogels with Enhanced Antibacterial and Angiogenic Properties for Chronic Infected Diabetic Wound Treatment via pH-Responsive Quick Cargo Release. Chem. Eng. J. 2021, 411, 128564. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, L.; Wang, T.; Sun, T.; Lu, S. pH-Responsive Cellulose-Based Dual Drug-Loaded Hydrogel for Wound Dressing. Eur. Polym. J. 2019, 121, 109290. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S.; Yang, X.; Pang, S.; Wang, X.; Yang, S. Preparation of a Recombinant Collagen-Peptide (RHC)-Conjugated Chitosan Thermosensitive Hydrogel for Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111555. [Google Scholar] [CrossRef]
- Jin, L.; Guo, X.; Gao, D.; Liu, Y.; Ni, J.; Zhang, Z.; Huang, Y.; Xu, G.; Yang, Z.; Zhang, X.; et al. An NIR Photothermal-Responsive Hybrid Hydrogel for Enhanced Wound Healing. Bioact. Mater. 2022, 16, 162–172. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, H.; Li, Z.; Zhangji, A.; Guo, B. Bioinspired Injectable Self-Healing Hydrogel Sealant with Fault-Tolerant and Repeated Thermo-Responsive Adhesion for Sutureless Post-Wound-Closure and Wound Healing. Nanomicro Lett. 2022, 14, 185. [Google Scholar]
- Qiao, B.; Pang, Q.; Yuan, P.; Luo, Y.; Ma, L. Smart Wound Dressing for Infection Monitoring and NIR-Triggered Antibacterial Treatment. Biomater. Sci. 2020, 8, 1649–1657. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Huang, J.; Wu, J. Novel Glucose-Responsive Antioxidant Hybrid Hydrogel for Enhanced Diabetic Wound Repair. ACS Appl. Mater. Interfaces 2022, 14, 7680–7689. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Li, Q.; Wu, J. A Novel Hydrogel with Glucose-Responsive Hyperglycemia Regulation and Antioxidant Activity for Enhanced Diabetic Wound Repair. Nano Res. 2022, 15, 5305–5315. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Liu, P.; Hu, Y.; Chen, Y.; Fang, Y.; Sun, G.; Huang, H.; Wu, J. Hyaluronic Acid-Based Glucose-Responsive Antioxidant Hydrogel Platform for Enhanced Diabetic Wound Repair. Acta Biomater. 2022, 147, 147–157. [Google Scholar] [CrossRef]
- Rui, J.-Z.; Peng, H.-H.; Guan, Y.-X.; Yao, S.-J. Preparation of ROS-Responsive Drug-Loaded Hydrogels Applied in Wound Dressings Using Supercritical Solvent Impregnation. J. Supercrit. Fluids 2022, 188, 105682. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Long, L.; Hu, C.; Kong, Q.; Wang, Y. A Spatiotemporal Release Platform Based on pH/ROS Stimuli-Responsive Hydrogel in Wound Repairing. J. Control. Release 2022, 341, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Elpa, D.P.; Chiu, H.-Y.; Wu, S.-P.; Urban, P.L. Skin Metabolomics. Trends Endocrinol. Metab. 2021, 32, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Rippke, F.; Schreiner, V.; Schwanitz, H.-J. The Acidic Milieu of the Horny Layer. Am. J. Clin. Dermatol. 2002, 3, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial Peptides: Key Components of the Innate Immune System. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The Effects of pH on Wound Healing, Biofilms, and Antimicrobial Efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Cui, T.; Yu, J.; Wang, C.-F.; Chen, S.; Li, Q.; Guo, K.; Qing, R.; Wang, G.; Ren, J. Micro-Gel Ensembles for Accelerated Healing of Chronic Wound via pH Regulation. Adv. Sci. 2022, 9, e2201254. [Google Scholar] [CrossRef]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of pH, Exudate Composition and Temperature in Wound Healing: A Systematic Review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef]
- Jiang, H.; Ochoa, M.; Waimin, J.F.; Rahimi, R.; Ziaie, B. A pH-Regulated Drug Delivery Dermal Patch for Targeting Infected Regions in Chronic Wounds. Lab Chip 2019, 19, 2265–2274. [Google Scholar] [CrossRef]
- Shi, L.; Ramsay, S.; Ermis, R.; Carson, D. pH in the Bacteria-Contaminated Wound and Its Impact on Clostridium Histolyticum Collagenase Activity. J. Wound Ostomy Cont. Nurs. 2011, 38, 514–521. [Google Scholar] [CrossRef]
- Bostancı, N.S.; Büyüksungur, S.; Hasirci, N.; Tezcaner, A. pH Responsive Release of Curcumin from Photocrosslinked Pectin/gelatin Hydrogel Wound Dressings. Biomater. Adv. 2022, 134, 112717. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Liu, H.; Ren, M.; Wang, Z.; Wang, X.; Liu, H.; Feng, Y.; Lin, Q.; Wang, C.; et al. pH-Responsive Hydrogel Loaded with Insulin as a Bioactive Dressing for Enhancing Diabetic Wound Healing. Mater. Des. 2021, 210, 110104. [Google Scholar] [CrossRef]
- Ahmadian, Z.; Correia, A.; Hasany, M.; Figueiredo, P.; Dobakhti, F.; Eskandari, M.R.; Hosseini, S.H.; Abiri, R.; Khorshid, S.; Hirvonen, J.; et al. A Hydrogen-Bonded Extracellular Matrix-Mimicking Bactericidal Hydrogel with Radical Scavenging and Hemostatic Function for pH-Responsive Wound Healing Acceleration. Adv. Healthc. Mater. 2021, 10, e2001122. [Google Scholar] [CrossRef]
- Serpico, L.; Dello Iacono, S.; De Stefano, L.; De Martino, S.; Battisti, M.; Dardano, P.; Pedatella, S.; De Nisco, M. pH-Sensitive Release of Antioxidant Se-Glycoconjugates through a Flexible Polymeric Patch. Eur. Polym. J. 2022, 178, 111486. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal Wound Healing Is Subject to Redox Control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive Oxygen Species (ROS) and Wound Healing: The Functional Role of ROS and Emerging ROS-Modulating Technologies for Augmentation of the Healing Process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Tamimi, M.A.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting Deregulated Oxidative Stress in Skin Inflammatory Diseases: An Update on Clinical Importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef]
- Xu, H.; Cao, W.; Zhang, X. Selenium-Containing Polymers: Promising Biomaterials for Controlled Release and Enzyme Mimics. Acc. Chem. Res. 2013, 46, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, Y.; Xu, H.; Wang, Z.; Zhang, X. Dual Redox Responsive Assemblies Formed from Diselenide Block Copolymers. J. Am. Chem. Soc. 2010, 132, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, S.; Escuin-Ordinas, H.; Dimatteo, R.; Xi, W.; Ribas, A.; Segura, T. Accelerated Wound Healing by Injectable Star Poly(ethylene Glycol)-B-Poly(propylene Sulfide) Scaffolds Loaded with Poorly Water-Soluble Drugs. J. Control. Release 2018, 282, 156–165. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, Z.; Yu, Q.; Ma, T. Preparation of Reactive Oxygen Species-Responsive Antibacterial Hydrogels for Efficient Anti-Infection Therapy. Mater. Lett. 2020, 263, 127254. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Huang, H.; Fan, S.; Yang, C.; Wang, L.; Li, W.; Niu, W.; Liao, J. ROS-Eliminating Carboxymethyl Chitosan Hydrogel to Enhance Burn Wound-Healing Efficacy. Front. Pharmacol. 2021, 12, 679580. [Google Scholar] [CrossRef]
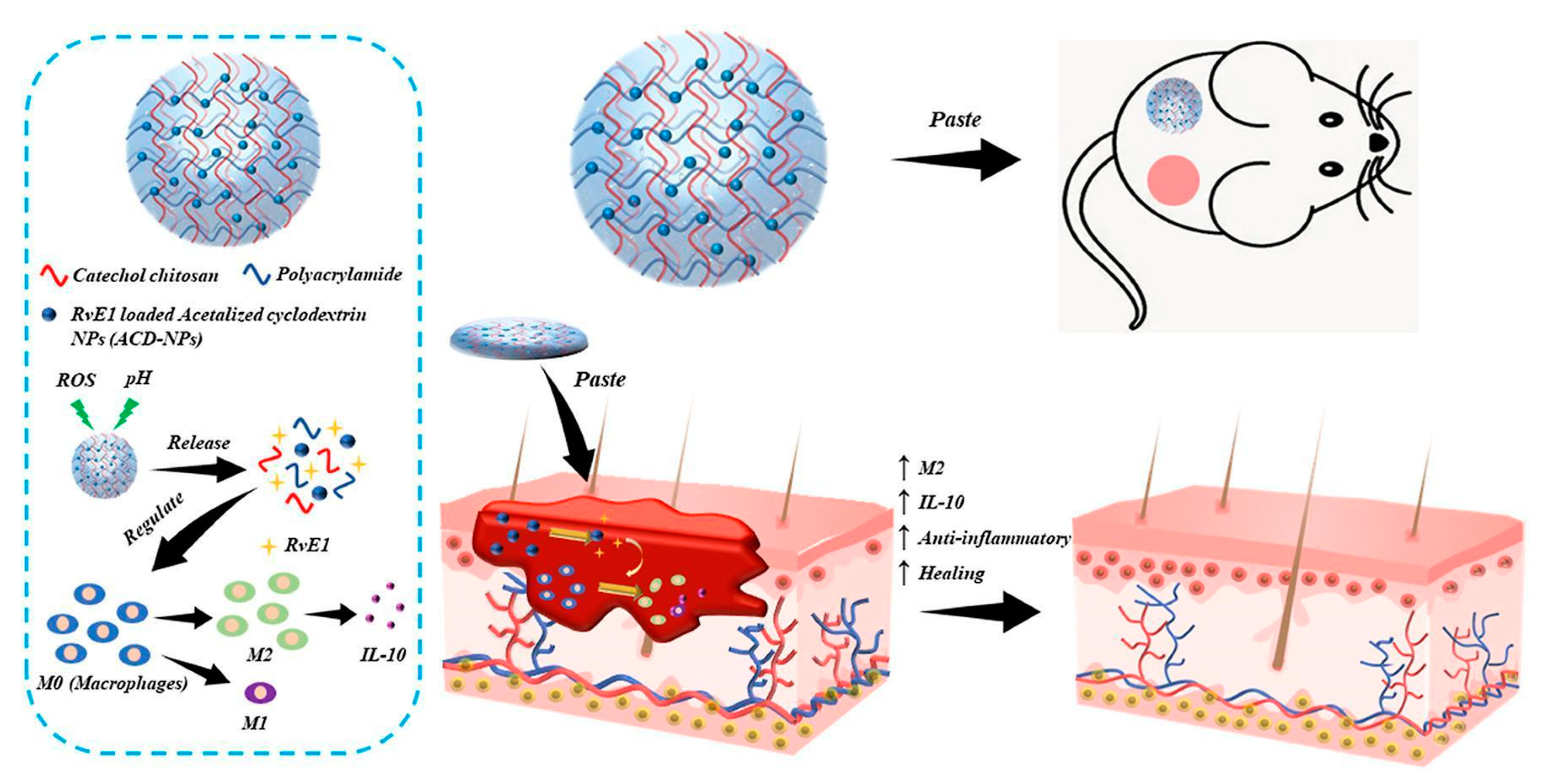
- Lu, B.; Han, X.; Zou, D.; Luo, X.; Liu, L.; Wang, J.; Maitz, M.F.; Yang, P.; Huang, N.; Zhao, A. Catechol-Chitosan/polyacrylamide Hydrogel Wound Dressing for Regulating Local Inflammation. Mater. Today Bio 2022, 16, 100392. [Google Scholar] [CrossRef]
- Lee, H.P.; Gaharwar, A.K. Light-Responsive Inorganic Biomaterials for Biomedical Applications. Adv. Sci. 2020, 7, 2000863. [Google Scholar] [CrossRef]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.-A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; He, Y.; Lu, Q.; Chen, R.; Zhao, C.; Dong, D.; Sun, Y.; He, H. Dual Light-Responsive Cellulose Nanofibril-Based in Situ Hydrogel for Drug-Resistant Bacteria Infected Wound Healing. Carbohydr. Polym. 2022, 297, 120042. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-Infrared Light-Responsive Multifunctional Nanocomposite Hydrogel for Efficient and Synergistic Antibacterial Wound Therapy and Healing Promotion. J. Mater. Chem. B Mater. Biol. Med. 2020, 8, 3908–3917. [Google Scholar] [CrossRef]
- Li, C.; He, X.; Li, Q.; Lv, M.; Shen, J.; Jin, L.; He, D. A Photothermal-Response Oxygen Release Platform Based on a Hydrogel for Accelerating Wound Healing. NPG Asia Mater. 2023, 15, 1–10. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhao, C.; Yang, L.; Qi, X.; Wang, X.; Zhou, Q.; Shi, W. Hyperglycemia-Reduced NAD+ Biosynthesis Impairs Corneal Epithelial Wound Healing in Diabetic Mice. Metabolism 2021, 114, 154402. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, Q.; Ni, R.; Tang, F.; Shan, L.; Cepinskas, I.; Cepinskas, G.; Wang, W.; Schiller, P.W.; Peng, T. Inhibition of Calpain Reduces Oxidative Stress and Attenuates Endothelial Dysfunction in Diabetes. Cardiovasc. Diabetol. 2014, 13, 88. [Google Scholar] [CrossRef]
- Sawaya, A.P.; Stone, R.C.; Brooks, S.R.; Pastar, I.; Jozic, I.; Hasneen, K.; O’Neill, K.; Mehdizadeh, S.; Head, C.R.; Strbo, N.; et al. Deregulated Immune Cell Recruitment Orchestrated by FOXM1 Impairs Human Diabetic Wound Healing. Nat. Commun. 2020, 11, 4678. [Google Scholar] [CrossRef]
- Wan, R.; Weissman, J.P.; Grundman, K.; Lang, L.; Grybowski, D.J.; Galiano, R.D. Diabetic Wound Healing: The Impact of Diabetes on Myofibroblast Activity and Its Potential Therapeutic Treatments. Wound Repair Regen. 2021, 29, 573–581. [Google Scholar] [CrossRef]
- Nowak, N.C.; Menichella, D.M.; Miller, R.; Paller, A.S. Cutaneous Innervation in Impaired Diabetic Wound Healing. Transl. Res. 2021, 236, 87–108. [Google Scholar] [CrossRef]
- Jodheea-Jutton, A.; Hindocha, S.; Bhaw-Luximon, A. Health Economics of Diabetic Foot Ulcer and Recent Trends to Accelerate Treatment. Foot 2022, 52, 101909. [Google Scholar] [CrossRef]
- Bardill, J.R.; Laughter, M.R.; Stager, M.; Liechty, K.W.; Krebs, M.D.; Zgheib, C. Topical Gel-Based Biomaterials for the Treatment of Diabetic Foot Ulcers. Acta Biomater. 2022, 138, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Qin, J.; Wu, P.; Gao, W.; Sun, G. Glucose-Responsive Antioxidant Hydrogel Accelerates Diabetic Wound Healing. Adv. Healthc. Mater. 2023, 2300074. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, W.; Xu, P.; Fu, X.; Yu, X.; Chen, L.; Leng, F.; Yu, C.; Yang, Z. Glucose-Responsive Multifunctional Metal–organic Drug-Loaded Hydrogel for Diabetic Wound Healing. Acta Biomater. 2022, 140, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, F.; Collins, M.N. Cell Based Therapeutics in Type 1 Diabetes Mellitus. Int. J. Pharm. 2017, 521, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Md Akil, H. Classification, Processing and Application of Hydrogels: A Review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Coughlan, D.C.; Quilty, F.P.; Corrigan, O.I. Effect of Drug Physicochemical Properties on Swelling/deswelling Kinetics and Pulsatile Drug Release from Thermoresponsive poly(N-Isopropylacrylamide) Hydrogels. J. Control. Release 2004, 98, 97–114. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical Properties of PNIPAM Based Hydrogels: A Review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Cao, M.; Wang, Y.; Hu, X.; Gong, H.; Li, R.; Cox, H.; Zhang, J.; Waigh, T.A.; Xu, H.; Lu, J.R. Reversible Thermoresponsive Peptide–PNIPAM Hydrogels for Controlled Drug Delivery. Biomacromolecules 2019, 20, 3601–3610. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Aliakbar Ahovan, Z.; Khosravimelal, S.; Eftekhari, B.S.; Mehrabi, S.; Hashemi, A.; Eftekhari, S.; Brouki Milan, P.; Mobaraki, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Thermo-Responsive Chitosan Hydrogel for Healing of Full-Thickness Wounds Infected with XDR Bacteria Isolated from Burn Patients: In Vitro and in Vivo Animal Model. Int. J. Biol. Macromol. 2020, 164, 4475–4486. [Google Scholar] [CrossRef]
- Yan, X.; Fang, W.-W.; Xue, J.; Sun, T.-C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.-H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol-Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus Aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Citation-Based Clustering of Publications Using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef]
- Verma, S.; Gustafsson, A. Investigating the Emerging COVID-19 Research Trends in the Field of Business and Management: A Bibliometric Analysis Approach. J. Bus. Res. 2020, 118, 253–261. [Google Scholar] [CrossRef]
- Bird, D.; Ravindra, N.M. Transdermal Drug Delivery and patches—An Overview. Med. Devices Sens. 2020, 3, e10069. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Park, W.H. Dual-Crosslinked, Self-Healing and Thermo-Responsive Methylcellulose/chitosan Oligomer Copolymer Hydrogels. Carbohydr. Polym. 2021, 258, 117705. [Google Scholar] [CrossRef]
- Morton, L.M.; Phillips, T.J. Wound Healing and Treating Wounds: Differential Diagnosis and Evaluation of Chronic Wounds. J. Am. Acad. Dermatol. 2016, 74, 589–605; quiz 605–606. [Google Scholar] [CrossRef]
- Ying, H.; Zhou, J.; Wang, M.; Su, D.; Ma, Q.; Lv, G.; Chen, J. In Situ Formed Collagen-Hyaluronic Acid Hydrogel as Biomimetic Dressing for Promoting Spontaneous Wound Healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 487–498. [Google Scholar] [CrossRef]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Della Pepa, C.; Dosio, F.; Serpe, L. Recent Developments in Antibacterial Therapy: Focus on Stimuli-Responsive Drug-Delivery Systems and Therapeutic Nanoparticles. Molecules 2019, 24, 1991. [Google Scholar] [CrossRef]
- Fan, D.-Y.; Tian, Y.; Liu, Z.-J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]



| Stimulus | Hydrogel-Based System | System Operation | System Performance | Ref. |
|---|---|---|---|---|
| Temperature/ NIR | poly(N-isopropyl acrylamide)- polydopamine NPs | phase transitions and volume changes in response to NIR | improved cell affinity, good tissue adhesiveness, and growth factor/protein immobilization ability | [50] |
| Temperature/ pH | hydroxypropyl chitin/tannic acid/ferric ion (HPCH/TA/Fe) | pH-dependent thermosensitivity due to the coordination between TA and Fe3+ | inhibited bacterial infection | [51] |
| pH | oxidized dextran-dopamine (OD-DA)+AgNPs+deferoxamine (DFO) | pH-responsive Schiff base structure | antimicrobial capacities to gram-positive and gram-negative bacteria and improved angiogenesis | [52] |
| pH | carboxylated agarose/tannic acid hydrogel cross + zinc ions | intermolecular ionic and H-bonding strongly affected by pH value | antibacterial and anti-inflammatory properties and low cytotoxicity | [53] |
| pH | dialdehyde carboxymethyl cellulose (DCMC)/Tobramycin (TB)/borneol/mono-6-(2-hydroxy-3-(trimethylammonio)propyl)-β-cyclodextrin (BN/EPTAC-β-CD) | Imine bonds break in response to the weakly acidic environment | anti-inflammatory function | [54] |
| Temperature | human collagen-peptide (RHC)/chitosan | thermoreversible sol–gel transition | promoted cell infiltration, vessel formation, and wound healing in second-degree burns | [55] |
| NIR/ Temperature | MXene nanofibers (MNFs)/dopamine-hyaluronic acid hydrogel (H)/vascular endothelial growth factor (V)/diallyl trisulfide as H2S donor (DA) | V release from the MXene nanofibrous skeleton induced by NIR light exposure and photothermal effect | inhibition of excessive neovascularization and extracellular matrix deposition at the wound site | [56] |
| Temperature | sodium alginate (SA)/gelatin (GT), protocatechualdehyde/ferric ions | temperature-dependent dynamic hydrogel crosslinked through Schiff base bond, catechol-Fe coordinate bond, and strong interactions between GT and SA | shape adaptability, antibacterial activity, and good biocompatibility facilitated post-wound-closure care | [57] |
| NIR | polyvinyl alcohol (PVA)/poly prodrug (GS-Linker-MPEG)/ up-conversion nanoparticles (UCNP)/ gentamicin sulfate (GS) | NIR light-triggered GS release via cleavage of physical UV-susceptible crosslinks between PVA and GS-Linker-MPEG; thanks to UCNP that converts NIR light to UV light | biocompatibility and antibacterial activity | [58] |
| Glucose | polyethylene glycol diacrylates (PEG-DA), phenylboronic acid (PBA) modified hyaluronic acid (HA), myricetin (MY) | glucose-triggered release of strongly antioxidant MY and immobilized in dynamic borate bond polyphenol group | efficient ROS-scavenging, ameliorated inflammatory response, accelerated angiogenesis, and increased tissue remodeling | [59] |
| Glucose | gallic acid (GA) grafted onto chitosan (CS) poly (ethylene glycol) diacrylate (PEG-DA) + phenylboronic acid (PBA), modified polyethyleneimine (PEI), insulin NPs | glucose-responsive insulin release through dynamic borate bond between the phenylboronic acid groups on the PEI-PBA and the polyphenol groups on the CS-GA | biocompatibility, antioxidant properties, protection of cells from oxidative damage, promoted angiogenesis, and accelerated wound closure | [60] |
| Glucose | hyaluronic acid methacrylate (HAMA) with phenylboronic acid (PBA)/catechin (HMPC) | glucose-responsive catechin release allowed by sensitive borate ester bond between HAMA-PBA and catechin | biocompatibility, antioxidant capability, elimination of intracellular reactive oxygen species, cell protection from oxidative stress damage, angiogenesis promotion, and reduced inflammatory responses | [61] |
| ROS | PBA grafted sodium alginate (Alg-PBA)/polyvinyl alcohol (PVA)/sodium hyaluronate PBA (HA-PBA) | ROS-responsive drug release due to network structure destruction under H2O2 and diffusion of Doxycycline hydrochloride | antibacterial activity and improvement of infected wounds’ treatment | [62] |
| pH/ROS | caffeic acid-grafted ε-polylysine (CE) + phenylboronic acid-grafted oxidized dextran (POD) | hydrogel network breakage due to hydrolysis of ROS-sensitive Schiff base and boronic ester bonds under acidic and oxidative conditions | inhibition of inflammatory response and promotion of wound healing in infected diabetic wounds | [63] |
| Funding Sponsor | Country | Documents |
|---|---|---|
| National Natural Science Foundation of China | China | 368 |
| Fundamental Research Funds for the Central Universities | China | 94 |
| National Key Research and Development Program of China | China | 82 |
| National Institutes of Health | United States | 73 |
| China Postdoctoral Science Foundation | China | 49 |
| National Science Foundation | United States | 42 |
| National Research Foundation of Korea | Korea | 30 |
| National Heart, Lung, and Blood Institute | United States | 25 |
| Natural Science Foundation of Shaanxi Province | China | 21 |
| National Institute of Arthritis and Musculoskeletal and Skin Diseases | United States | 20 |
| Subject Area | Documents | % |
|---|---|---|
| Materials Science | 765 | 24 |
| Engineering | 544 | 17 |
| Biochemistry | 421 | 13 |
| Chemistry | 351 | 11 |
| Chemical Engineering | 325 | 10 |
| Medicine | 257 | 8 |
| Pharmacology, Toxicology and Pharmaceutics | 205 | 6 |
| Physics | 142 | 4 |
| Environmental Science | 36 | 1 |
| Immunology and Microbiology | 31 | 1 |
| Keywords | ||||
|---|---|---|---|---|
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 |
| alginate biocompatibility chitosan gelatin hydrogel injectable polysaccharides self-healing sodium alginate stimuli-responsive thermoresponsive wound dressing | 3d printing biomaterials biomedical applications chronic wounds inflammation injectable hydrogels macrophages nanotechnology skin regeneration tissue regeneration vascularization | biodegradable collagen controlled release extracellular matrix growth factors hyaluronic acid infection platelet-rich plasma regenerative medicine scaffolds tissue engineering | antibacterial antimicrobial biopolymers curcumin drug delivery hemostasis nanoparticles pH-responsive self-assembly silk fibroin | angiogenesis conductive hydrogel diabetic wound electrical stimulation fibroblasts wound healing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serpico, L.; Dello Iacono, S.; Cammarano, A.; De Stefano, L. Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels 2023, 9, 451. https://doi.org/10.3390/gels9060451
Serpico L, Dello Iacono S, Cammarano A, De Stefano L. Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels. 2023; 9(6):451. https://doi.org/10.3390/gels9060451
Chicago/Turabian StyleSerpico, Luigia, Stefania Dello Iacono, Aniello Cammarano, and Luca De Stefano. 2023. "Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing" Gels 9, no. 6: 451. https://doi.org/10.3390/gels9060451
APA StyleSerpico, L., Dello Iacono, S., Cammarano, A., & De Stefano, L. (2023). Recent Advances in Stimuli-Responsive Hydrogel-Based Wound Dressing. Gels, 9(6), 451. https://doi.org/10.3390/gels9060451