Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering
Abstract
1. Introduction
2. Results and Discussion
2.1. Egg White and Gelatin Hydrocolloids: Appearance and Rheology
2.2. Two-Dimensional Hydrocolloid Films of Egg White and Gelatin: Topography
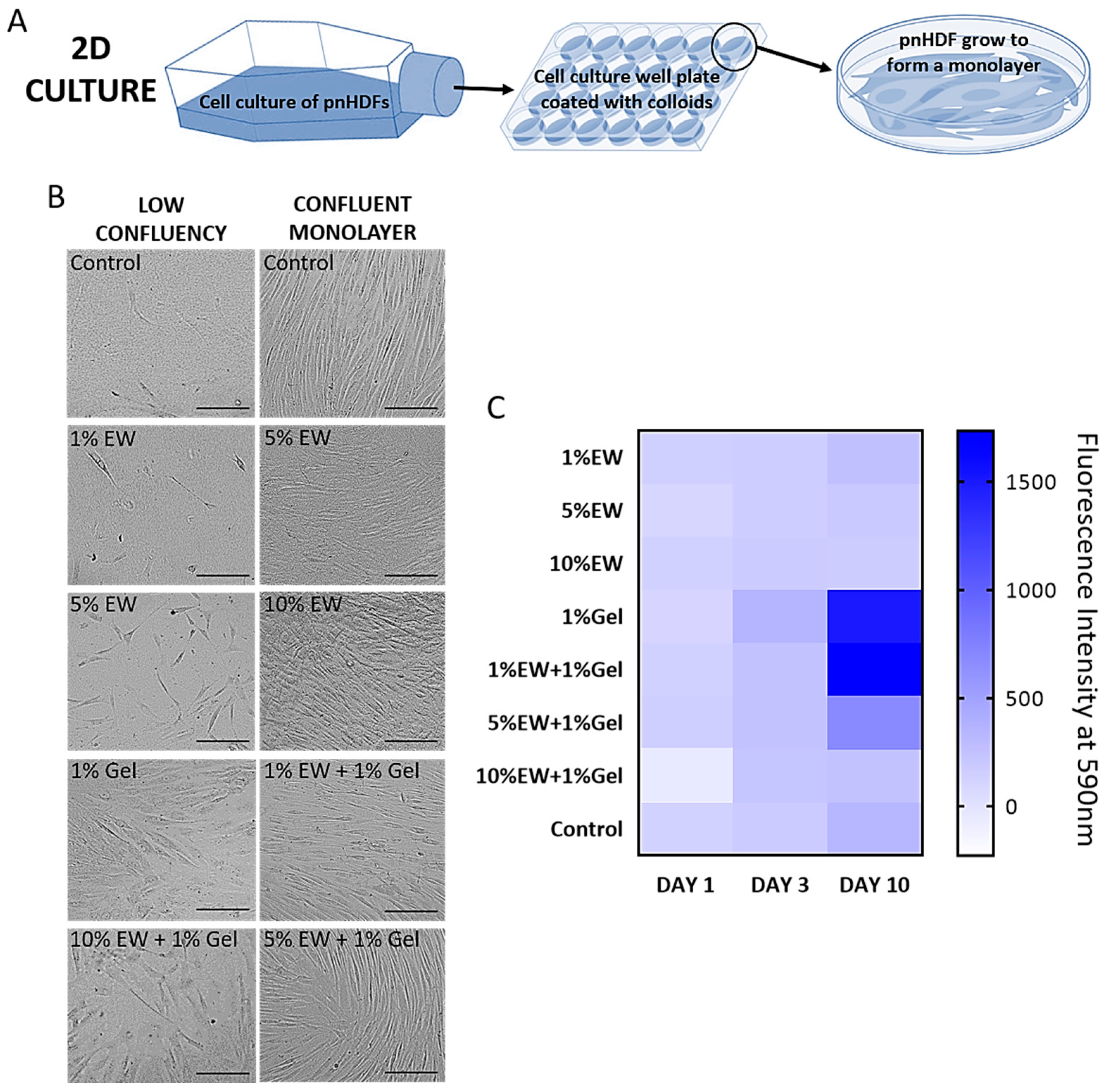
2.3. Two-Dimensional Hydrocolloid Films of Egg White and Gelatin: Cell Morphology, Viability, and Proliferation
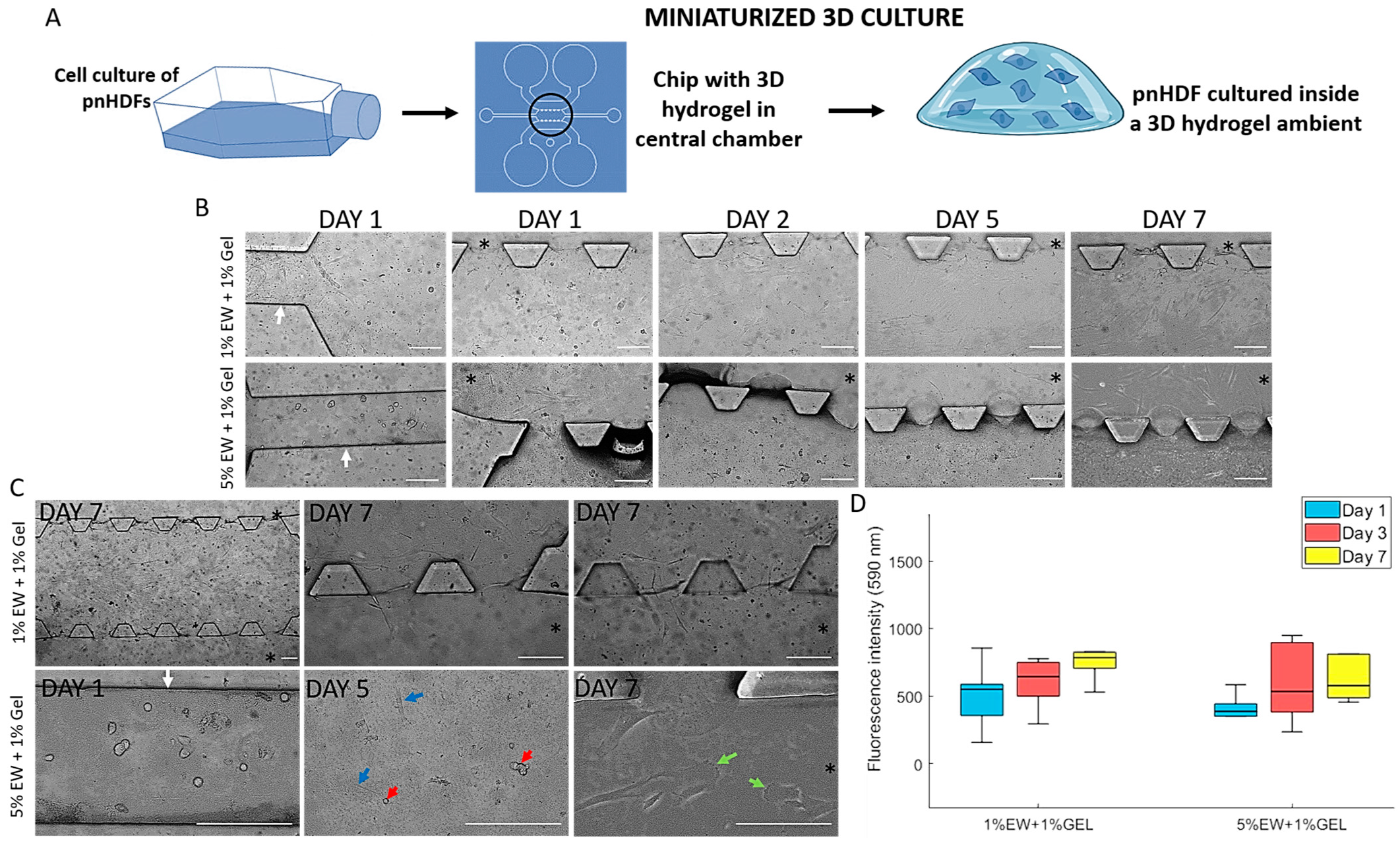
2.4. Miniaturized 3D Environments of Egg White and Gelatin
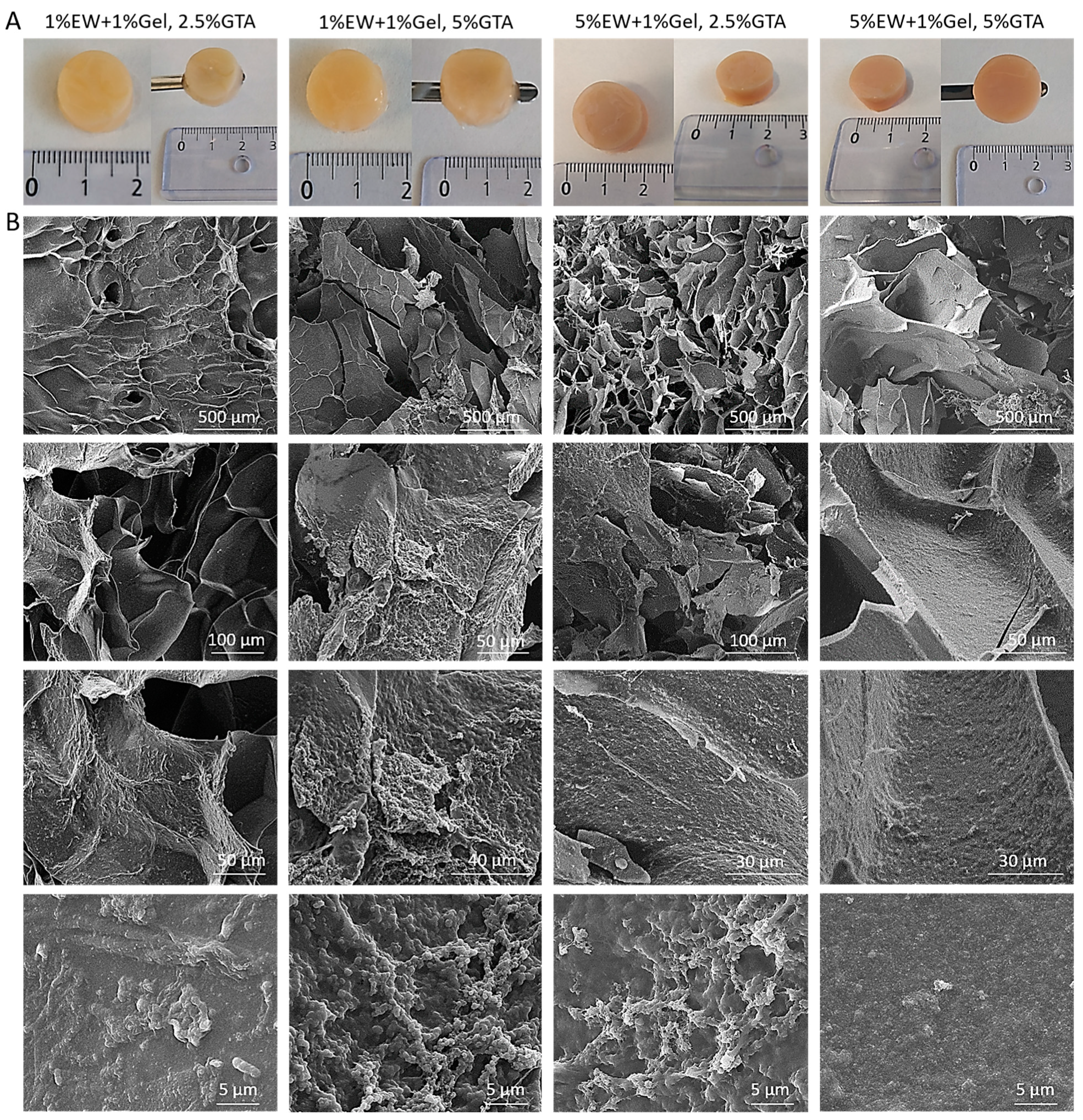
2.5. Three-Dimensional Hydrogel Scaffolds of Egg White and Gelatin: Morphology and Architecture
2.6. Three-Dimensional Hydrogel Scaffolds of Egg White and Gelatin: Rheology and Swelling
2.7. Three-Dimensional Hydrogel Scaffolds of Egg White and Gelatin: Cell Proliferation and Penetration

3. Conclusions
4. Materials and Methods
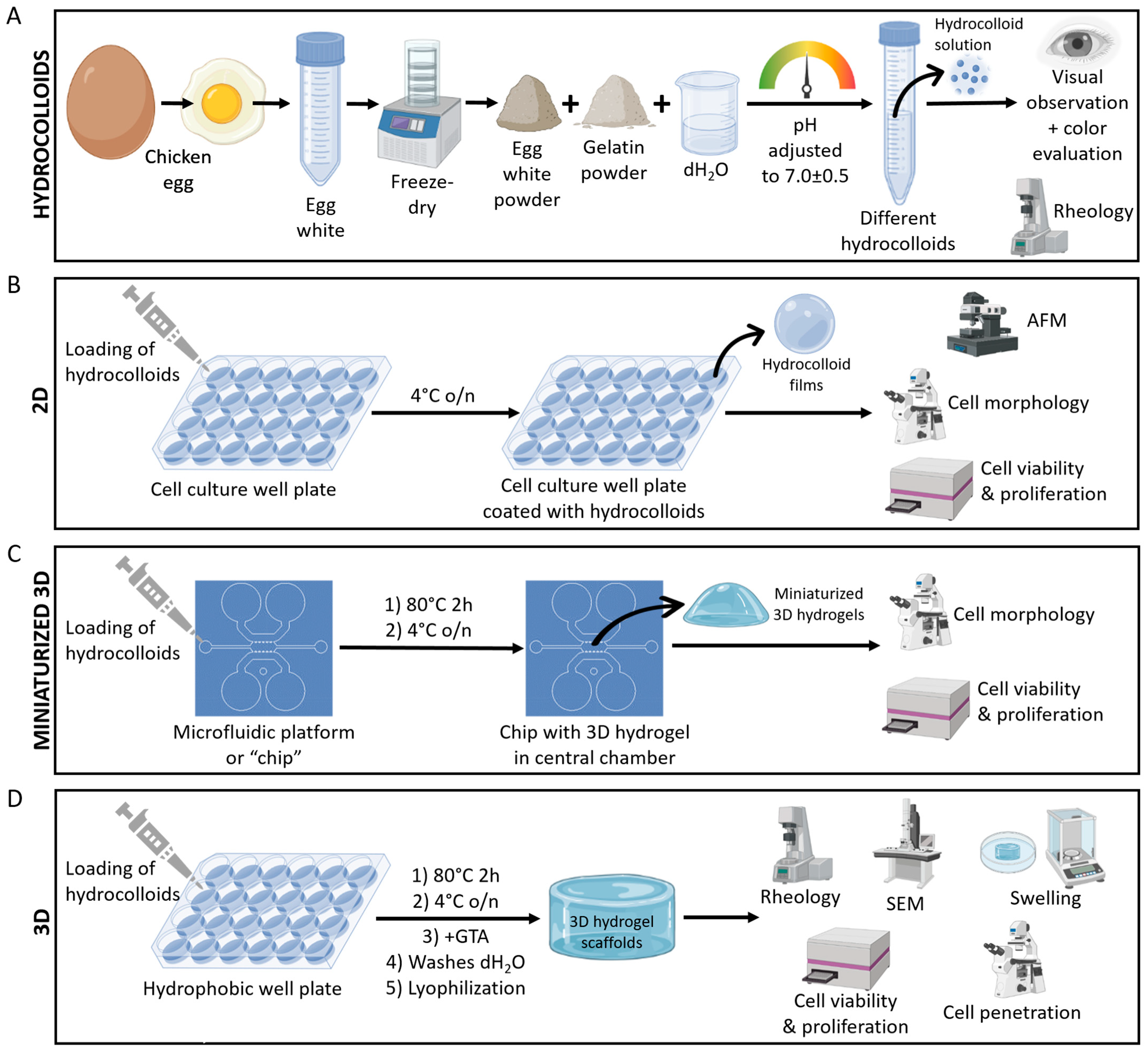
4.1. Experimental Design Summary
4.2. Egg White and Gelatin Hydrocolloids
4.3. Rheology of Hydrocolloids
4.4. Coating of Cell Culture Well Plates
4.5. Microfluidic Platform
4.6. Three-Dimensional Hydrogel Scaffolds
4.7. Atomic Force Microscopy (AFM)
4.8. Scanning Electron Microscopy (SEM)
4.9. Swelling Ratio of 3D Hydrogel Scaffolds
4.10. Rheology of 3D Hydrogel Scaffolds
4.11. Cell Culture
4.12. Cell Seeding
4.13. Cell Viability and Proliferation by AlamarBlue® Assay
4.14. Phase Contrast Light Microscopy
4.15. Cell Penetration into 3D Hydrogel Scaffolds
4.16. Data and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920. [Google Scholar] [CrossRef]
- García-Gareta, E. Collagen, from Tissue Culture to Biomaterials, Tissue Engineering, and Beyond, 1st ed.; Cambridge Scholars Publishing: Newcastle upon Tyne, UK, 2019. [Google Scholar]
- Rijal, G.; Li, W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 2017, 3, e1700764. [Google Scholar] [CrossRef]
- Karami, D.; Richbourg, N.; Sikavitsas, V. Dynamic in vitro models for tumor tissue engineering. Cancer Lett. 2019, 449, 178–185. [Google Scholar] [CrossRef]
- Sitarski, A.M.; Fairfield, H.; Falank, C.; Reagan, M.R. 3D Tissue Engineered in Vitro Models of Cancer in Bone. ACS Biomater. Sci. Eng. 2018, 4, 324–336. [Google Scholar] [CrossRef]
- Giobbe, G.G.; Crowley, C.; Luni, C.; Campinoti, S.; Khedr, M.; Kretzschmar, K.; De Santis, M.M.; Zambaiti, E.; Michielin, F.; Meran, L.; et al. Extracellular matrix hydrogel derived from decellularized tissues enables endodermal organoid culture. Nat. Commun. 2019, 10, 5658. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kikuchi, A.; Aoyagi, T.; Okano, T. Cell sheet tissue engineering: Cell sheet preparation, harvesting/manipulation, and transplantation. J. Biomed. Mater. Res. Part A 2019, 107, 955–967. [Google Scholar] [CrossRef]
- Mollica, P.A.; Booth-Creech, E.N.; Reid, J.A.; Zamponi, M.; Sullivan, S.M.; Palmer, X.-L.; Sachs, P.C.; Bruno, R.D. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. 2019, 95, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Nasello, G.; Alamán-Díez, P.; Schiavi, J.; Ángeles Pérez, M.; Mcnamara, L.; García-Aznar, J.M. Primary Human Osteoblasts Cultured in a 3D Microenvironment Create a Unique Representative Model of Their Differentiation Into Osteocytes. Front. Bioeng. Biotechnol. 2020, 1, 336. [Google Scholar] [CrossRef]
- Frost, O.G.; Owji, N.; Thorogate, R.; Kyriakidis, C.; Sawadkar, P.; Mordan, N.; Knowles, J.C.; Lali, F.; Garcia-Gareta, E. Cell morphology as a design parameter in the bioengineering of cell-biomaterial surface interactions. Biomater. Sci. 2021, 9, 8032–8050. [Google Scholar] [CrossRef] [PubMed]
- Jalili-Firoozinezhad, S.; Filippi, M.; Mohabatpour, F.; Letourneur, D.; Scherberich, A. Chicken egg white: Hatching of a new old biomaterial. Mater. Today 2020, 40, 193–214. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, Y.Q. An insight on egg white: From most common functional food to biomaterial application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 1045–1058. [Google Scholar] [CrossRef]
- Kaipparettu, B.A.; Kuiatse, I.; Chan, B.T.Y.; Kaipparettu, M.B.; Lee, A.V.; Oesterreich, S. Novel egg white-based 3-D cell culture system. Biotechniques 2008, 45, 165–171. [Google Scholar] [CrossRef]
- Nolasco, E.; Guha, S.; Majumder, K. Bioactive Egg Proteins. In Food Chemistry, Function and Analysis; Royal Society of Chemistry: London, UK, 2019; Chapter 13; pp. 223–258. Available online: https://pubs.rsc.org/en/content/chapter/bk9781788012133-00223/978-1-78801-213-3 (accessed on 16 June 2022).
- Sunwoo, H.H.; Gujral, N. Chemical Composition of Eggs and Egg Products. In Handbook of Food Chemistry; Springer: Berlin/Heidelberg, Germany, 2015; pp. 331–363. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-642-36605-5_28 (accessed on 13 June 2022).
- Chang, C.; Lahti, T.; Tanaka, T.; Nickerson, M.T. Egg proteins: Fractionation, bioactive peptides and allergenicity. J. Sci. Food Agric. 2018, 98, 5547–5558. [Google Scholar] [CrossRef]
- Croguennec, T.; Nau, F.; Brulé, G. Influence of pH and Salts on Egg White Gelation. J. Food Sci. 2002, 67, 608–614. [Google Scholar] [CrossRef]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.; Abduallah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2019, 42, 240–250. [Google Scholar] [CrossRef]
- Dai, Y.; Zhao, J.; Gao, J.; Deng, Q.; Wan, C.; Li, B.; Zhou, B. Heat- and cold-induced gels of desalted duck egg white/gelatin mixed system: Study on rheological and gel properties. Food Hydrocoll. 2021, 121, 107003. [Google Scholar] [CrossRef]
- Fletes-Vargas, G.; Espinosa-Andrews, H.; Cervantes-Uc, J.M.; Limón-Rocha, I.; Luna-Bárcenas, G.; Vázquez-Lepe, M.; Morales-Hernández, N.; Jiménez-Ávalos, J.A.; Mejía-Torres, D.G.; Ramos-Martínez, P.; et al. Porous Chitosan Hydrogels Produced by Physical Crosslinking: Physicochemical, Structural, and Cytotoxic Properties. Polymers 2023, 15, 2203. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Chakraborty, E. Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, D.; Xiang, T.; Li, X.; Sun, Q.; Li, Q. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. [Google Scholar]
- Babaei, J.; Mohammadian, M.; Madadlou, A. Gelatin as texture modifier and porogen in egg white hydrogel. Food Chem. 2019, 270, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Huertas, S.; Terpiłowski, K.; Tomczyńska-Mleko, M.; Nishinari, K.; Mleko, S. Surface and rheological properties of egg white albumin/gelatin dispersions gelled on cold plasma-activated glass. Food Hydrocoll. 2019, 96, 224–230. [Google Scholar] [CrossRef]
- Nour, S.; Imani, R.; Sharifi, A.M. Angiogenic Effect of a Nanoniosomal Deferoxamine-Loaded Poly(vinyl alcohol)-Egg White Film as a Promising Wound Dressing. ACS Biomater. Sci. Eng. 2022, 8, 3485–3497. [Google Scholar] [CrossRef] [PubMed]
- Renkler, N.Z.; Ergene, E.; Gokyer, S.; Tuzlakoglu Ozturk, M.; Yilgor Huri, P.; Tuzlakoglu, K. Facile modification of polycaprolactone nanofibers with egg white protein. J. Mater. Sci. Mater. Med. 2021, 32, 34. [Google Scholar] [CrossRef] [PubMed]
- Patty, D.J.; Nugraheni, A.D.; Ana, I.D.; Yusuf, Y. Dual functional carbonate-hydroxyapatite nanocomposite from Pinctada maxima and egg-white for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2022, 33, 1043–1062. [Google Scholar] [CrossRef]
- Carpena, N.T.; Abueva, C.D.G.; Padalhin, A.R.; Lee, B.-T. Evaluation of egg white ovomucin-based porous scaffold as an implantable biomaterial for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2107–2117. [Google Scholar] [CrossRef]
- Sarangapani, P.S.; Hudson, S.D.; Migler, K.B.; Pathak, J.A. The Limitations of an Exclusively Colloidal View of Protein Solution Hydrodynamics and Rheology. Biophys. J. 2013, 105, 2418. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Goudoulas, T.B.; Germann, N. Phase transition kinetics and rheology of gelatin-alginate mixtures. Food Hydrocoll. 2017, 66, 49–60. [Google Scholar] [CrossRef]
- Mad-Ali, S.; Benjakul, S.; Prodpran, T.; Maqsood, S. Characteristics and gelling properties of gelatin from goat skin as affected by drying methods. J. Food Sci. Technol. 2017, 54, 1646–1654. [Google Scholar] [CrossRef]
- Lueyot, A.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wonganu, B.; Wongsa-Ngasri, P.; Charoenlappanit, S.; Roytrakul, S.; Thumthanaruk, B. Influence of collagen and some proteins on gel properties of jellyfish gelatin. PLoS ONE 2021, 16, e0253254. [Google Scholar] [CrossRef]
- Tomas, M.; Amaveda, H.; Angurel, L.A.; Mora, M. Effect of silica sol on the dispersion-gelation process of concentrated silica suspensions for fibre-reinforced ceramic composites. J. Eur. Ceram. Soc. 2013, 33, 727–736. [Google Scholar] [CrossRef]
- Bianco, S.; Panja, S.; Adams, D.J. Using Rheology to Understand Transient and Dynamic Gels. Gels 2022, 8, 132. [Google Scholar] [CrossRef]
- Ghanbari, A.; Mousavi, Z.; Heuzey, M.C.; Patience, G.S.; Carreau, P.J. Experimental methods in chemical engineering: Rheometry. Can. J. Chem. Eng. 2020, 98, 1456–1470. [Google Scholar] [CrossRef]
- Oza, A.U.; Venerus, D.C. The dynamics of parallel-plate and cone–plate flows. Phys. Fluids. 2021, 33, 023102. [Google Scholar] [CrossRef]
- Ramya, K.A.; Deshpande, A.P. Selection of geometry for nonlinear rheology using large amplitude oscillatory shear: Poly (vinyl alcohol) based complex network systems. J. Vinyl Addit. Technol. 2023. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Thermoresponsive surfaces for cell culture and enzyme-free cell detachment. Prog. Polym. Sci. 2010, 35, 1311–1324. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, Z.; Li, C.; Zhou, N.; Liu, F.; Lan, J. The osteoinduction of RGD and Mg ion functionalized bioactive zirconia coating. J. Mater. Sci. Mater. Med. 2019, 30, 95. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Berdecka, D.; Rodrigues, M.T.; Reis, R.L.; Gomes, M.E. Tendon explant cultures to study the communication between adipose stem cells and native tendon niche. J. Cell. Biochem. 2018, 119, 3653–3662. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gareta, E.; Hua, J.; Orera, A.; Kohli, N.; Knowles, J.C.; Blunn, G.W. Biomimetic surface functionalization of clinically relevant metals used as orthopaedic and dental implants. Biomed. Mater. 2018, 13, 015008. [Google Scholar] [CrossRef]
- García-Gareta, E.; Hua, J.; Knowles, J.C.; Blunn, G.W. Comparison of mesenchymal stem cell proliferation and differentiation between biomimetic and electrochemical coatings on different topographic surfaces. J. Mater. Sci. Mater. Med. 2013, 24, 199–210. [Google Scholar] [CrossRef]
- Zahn, I.; Stöbener, D.D.; Weinhart, M.; Gögele, C.; Breier, A.; Hahn, J.; Schröpfer, M.; Meyer, M.; Schulze-Tanzil, G. Cruciate ligament cell sheets can be rapidly produced on thermoresponsive poly(Glycidyl ether) coating and successfully used for colonization of embroidered scaffolds. Cells 2021, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.J.; Phillips, A.S.R.; Shah, A.D.S.H.; Athey, A.D.; Lakey, A.J.H.; Przyborski, A.S.A. Enhanced cell attachment using a novel cell culture surface presenting functional domains from extracellular matrix proteins. Cytotechnology 2008, 56, 71–79. [Google Scholar] [CrossRef]
- Viheriälä, T.; Sorvari, J.; Ihalainen, T.O.; Mörö, A.; Grönroos, P.; Schlie-Wolter, S.; Chichkov, B.; Skottman, H.; Nymark, S.; Ilmarinen, T. Culture surface protein coatings affect the barrier properties and calcium signalling of hESC-RPE. Sci. Rep. 2021, 11, 933. [Google Scholar] [CrossRef]
- Van der Boon, T.A.B.; Yang, L.; Li, L.; Córdova Galván, D.E.; Zhou, Q.; de Boer, J.; Van Rijn, P. Well Plate Integrated Topography Gradient Screening Technology for Studying Cell-Surface Topography Interactions. Adv. Biosyst. 2020, 4, e1900218. [Google Scholar] [CrossRef] [PubMed]
- Orlowska, A.; Perera, P.T.; Al Kobaisi, M.A.; Dias, A.; Nguyen, H.K.D.; Ghanaati, S.; Baulin, V.; Crawford, R.J.; Ivanova, E.P. The effect of coatings and nerve growth factor on attachment and differentiation of Pheochromocytoma Cells. Materials 2017, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Kendall, R.T.; Feghali-Bostwick, C.A. Fibroblasts in fibrosis: Novel roles and mediators. Front. Pharmacol. 2014, 5, 123. [Google Scholar] [CrossRef]
- García-Gareta, E.; Levin, A.; Hook, L. Engineering the migration and attachment behaviour of primary dermal fibroblasts. Biotechnol. Bioeng. 2019, 116, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Ravindran, N.; Dye, J.F. Apoptotic primary normal human dermal fibroblasts for in vitro models of fibrosis. Anal. Biochem. 2014, 470, 22–24. [Google Scholar] [CrossRef]
- Ravindran, N.; García-Gareta, E. Kerr’s coining of “Apoptosis” and its relevance in skin wound healing and fibrosis. Exp. Dermatol. 2015, 24, 99–100. [Google Scholar] [CrossRef]
- Madl, C.M. Accelerating aging with dynamic biomaterials: Recapitulating aged tissue phenotypes in engineered platforms. iScience 2023, 26, 106825. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Plou, J.; Juste-Lanas, Y.; Olivares, V.; del Amo, C.; Borau, C.; García-Aznar, J.M. From individual to collective 3D cancer dissemination: Roles of collagen concentration and TGF-β. Sci. Rep. 2018, 8, 12723. [Google Scholar] [CrossRef]
- Alamán-Díez, P.; García-Gareta, E.; Arruebo, M.; Pérez, A. A bone-on-a-chip collagen hydrogel-based model using pre-differentiated adipose-derived stem cells for personalized bone tissue engineering. J. Biomed. Mater. Res. Part A 2022, 111, 88–105. [Google Scholar] [CrossRef]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016; Available online: https://www.intechopen.com/books/emerging-concepts-in-analysis-and-applications-of-hydrogels/an-introduction-to-hydrogels-and-some-recent-applications (accessed on 1 June 2023).
- Weiner, S.; Traub, W.; Wagner, H.D. Lamellar Bone: Structure–Function Relations. J. Struct. Biol. 1999, 126, 241–255. [Google Scholar] [CrossRef]
- Yu, X.; Turcotte, R.; Seta, F.; Zhang, Y. Micromechanics of elastic lamellae: Unravelling the role of structural inhomogeneity in multi-scale arterial mechanics. J. R. Soc. Interface 2018, 15, 20180492. [Google Scholar] [CrossRef]
- Gil, E.S.; Mandal, B.B.; Park, S.-H.; Marchant, J.K.; Omenetto, F.G.; Kaplan, D.L. Helicoidal multi-lamellar features of RGD-functionalized silk biomaterials for corneal tissue engineering. Biomaterials 2010, 31, 8953–8963. [Google Scholar] [CrossRef]
- Salmasi, S. Role of nanotopography in the development of tissue engineered 3D organs and tissues using mesenchymal stem cells. World J. Stem Cells 2015, 7, 266. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Patel, N.; Kohli, N.; Ravindran, N.; Hook, L.; Mason, C.; García-Gareta, E. Viscoelastic, physical, and bio-degradable properties of dermal scaffolds and related cell behaviour. Biomed. Mater. 2016, 11, 055001. [Google Scholar] [CrossRef]
- Helary, C.; Bataille, I.; Abed, A.; Illoul, C.; Anglo, A.; Louedec, L.; Letourneur, D.; Meddahi-Pellé, A.; Giraud-Guille, M.M. Concentrated collagen hydrogels as dermal substitutes. Biomaterials 2010, 31, 481–490. [Google Scholar] [CrossRef]
- Valero, C.; Amaveda, H.; Mora, M.; García-Aznar, J.M. Combined experimental and computational characterization of crosslinked collagen-based hydrogels. PLoS ONE 2018, 13, e0195820. [Google Scholar] [CrossRef] [PubMed]
- Sponton, O.E.; Perez, A.A.; Carrara, C.R.; Santiago, L.G. Impact of environment conditions on physicochemical characteristics of ovalbumin heat-induced nanoparticles and on their ability to bind PUFAs. Food Hydrocoll. 2015, 48, 165–173. [Google Scholar] [CrossRef]
- Dargaville, B.L.; Hutmacher, D.W. Water as the often neglected medium at the interface between materials and biology. Nat. Commun. 2022, 13, 4222. [Google Scholar] [CrossRef]
- Sawadkar, P.; Mohanakrishnan, J.; Rajasekar, P.; Rahmani, B.; Kohli, N.; Bozec, L.; Garcia-Gareta, E. A Synergistic Relationship between Polycaprolactone and Natural Polymers Enhances the Physical Properties and Biological Activity of Scaffolds. ACS Appl. Mater. Interfaces 2020, 12, 13587–13597. [Google Scholar] [CrossRef]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.R.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the cross-linking density on the swelling and rheological behavior of ester-bridged β-cyclodextrin nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dargaville, B.L.; Hutmacher, D.W. Elucidating the molecular mechanisms for the interaction of water with polyethylene glycol-based hydrogels: Influence of ionic strength and gel network structure. Polymers 2021, 13, 845. [Google Scholar] [CrossRef]
- El-Mohri, H.; Wu, Y.; Mohanty, S.; Ghosh, G. Impact of matrix stiffness on fibroblast function. Mater. Sci. Eng. C 2017, 74, 146–151. [Google Scholar] [CrossRef]
- Movilla, N.; Borau, C.; Valero, C.; García-Aznar, J.M. Degradation of extracellular matrix regulates osteoblast migration: A microfluidic-based study. Bone 2018, 107, 10–17. [Google Scholar] [CrossRef]
- Movilla, N.; Valero, C.; Borau, C.; García-Aznar, J.M. Matrix degradation regulates osteoblast protrusion dynamics and individual migration. Integr. Biol. 2019, 11, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Farahat, W.A.; Wood, L.B.; Zervantonakis, I.K.; Schor, A.; Ong, S. Ensemble Analysis of Angiogenic Growth in Three-Dimensional Microfluidic Cell Cultures. PLoS ONE 2012, 7, 37333. [Google Scholar] [CrossRef]
- Marcuello, C.; Frempong, G.A.; Balsera, M.; Medina, M.; Lostao, A. Atomic force microscopy to elicit conformational transitions of ferredoxin-dependent flavin thioredoxin reductases. Antioxidants 2021, 10, 1437. [Google Scholar] [CrossRef]
- Canet-Ferrer, J.; Coronado, E.; Forment-Aliaga, A.; Pinilla-Cienfuegos, E. Correction of the tip convolution effects in the imaging of nanostructures studied through scanning force microscopy. Nanotechnology 2014, 25, 395703. [Google Scholar] [CrossRef] [PubMed]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 13705. [Google Scholar] [CrossRef]
- Sawadkar, P.; Mandakhbayar, N.; Patel, K.D.; Buitrago, J.O.; Kim, T.H.; Rajasekar, P.; Lali, F.; Kyriakidis, C.; Rahmani, B.; Mohanakrishnan, J.; et al. Three dimensional porous scaffolds derived from collagen, elastin and fibrin proteins orchestrate adipose tissue regeneration. J. Tissue Eng. 2021, 12, 20417314211019238. [Google Scholar] [CrossRef] [PubMed]
- Pele, K.G. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering: A Feasibility Study. Bachelor’s Thesis, University of Zaragoza, Zaragoza, Aragon, Spain, 2022. [Google Scholar]

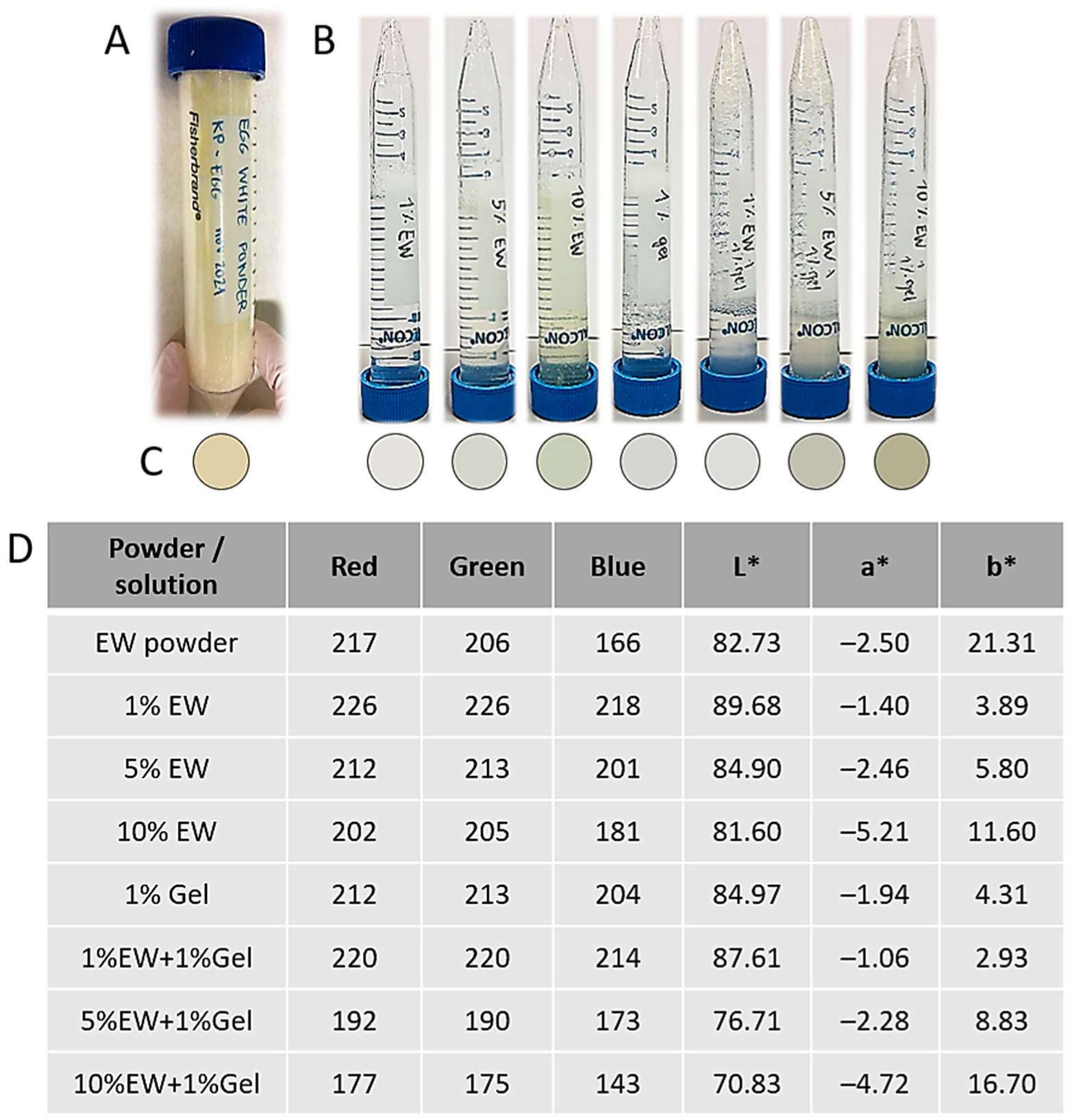



| Hydrocolloid (% in w/v) | Observations |
|---|---|
| 1% Gel | Liquid, yellowish in color, and translucid |
| 1% EW | Liquid, yellowish in color, and translucid |
| 5% EW | Viscosity in between 1% and 10% egg white colloids, yellowish in color, and translucid |
| 10% EW | Thick, viscous, yellowish in color, and translucid |
| 1% EW + 1% Gel | No observable differences compared with 1% egg white colloid |
| 5% EW + 1% Gel | No observable differences compared with 5% egg white colloid |
| 10% EW + 1% Gel | No observable differences compared with 10% egg white colloid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pele, K.G.; Amaveda, H.; Mora, M.; Marcuello, C.; Lostao, A.; Alamán-Díez, P.; Pérez-Huertas, S.; Ángeles Pérez, M.; García-Aznar, J.M.; García-Gareta, E. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels 2023, 9, 505. https://doi.org/10.3390/gels9060505
Pele KG, Amaveda H, Mora M, Marcuello C, Lostao A, Alamán-Díez P, Pérez-Huertas S, Ángeles Pérez M, García-Aznar JM, García-Gareta E. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels. 2023; 9(6):505. https://doi.org/10.3390/gels9060505
Chicago/Turabian StylePele, Karinna Georgiana, Hippolyte Amaveda, Mario Mora, Carlos Marcuello, Anabel Lostao, Pilar Alamán-Díez, Salvador Pérez-Huertas, María Ángeles Pérez, José Manuel García-Aznar, and Elena García-Gareta. 2023. "Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering" Gels 9, no. 6: 505. https://doi.org/10.3390/gels9060505
APA StylePele, K. G., Amaveda, H., Mora, M., Marcuello, C., Lostao, A., Alamán-Díez, P., Pérez-Huertas, S., Ángeles Pérez, M., García-Aznar, J. M., & García-Gareta, E. (2023). Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels, 9(6), 505. https://doi.org/10.3390/gels9060505











