Hyaluronic Acid–Alginate Homogeneous Structures with Polylactide Coating Applied in Controlled Antibiotic Release
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of the Mechanical Strength of Carriers
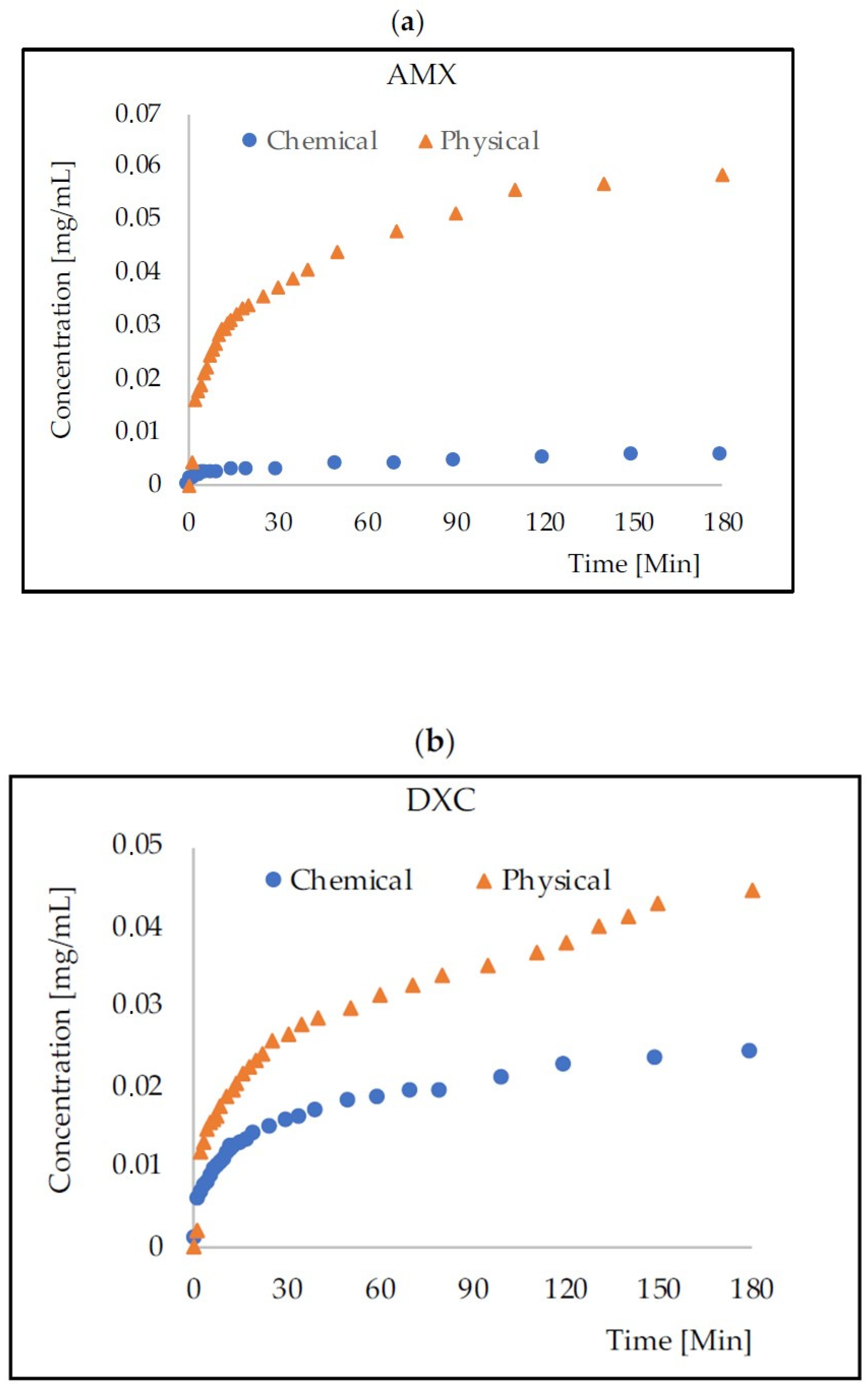
2.2. Mass Transport from HA–SAL Homogeneus Carriers
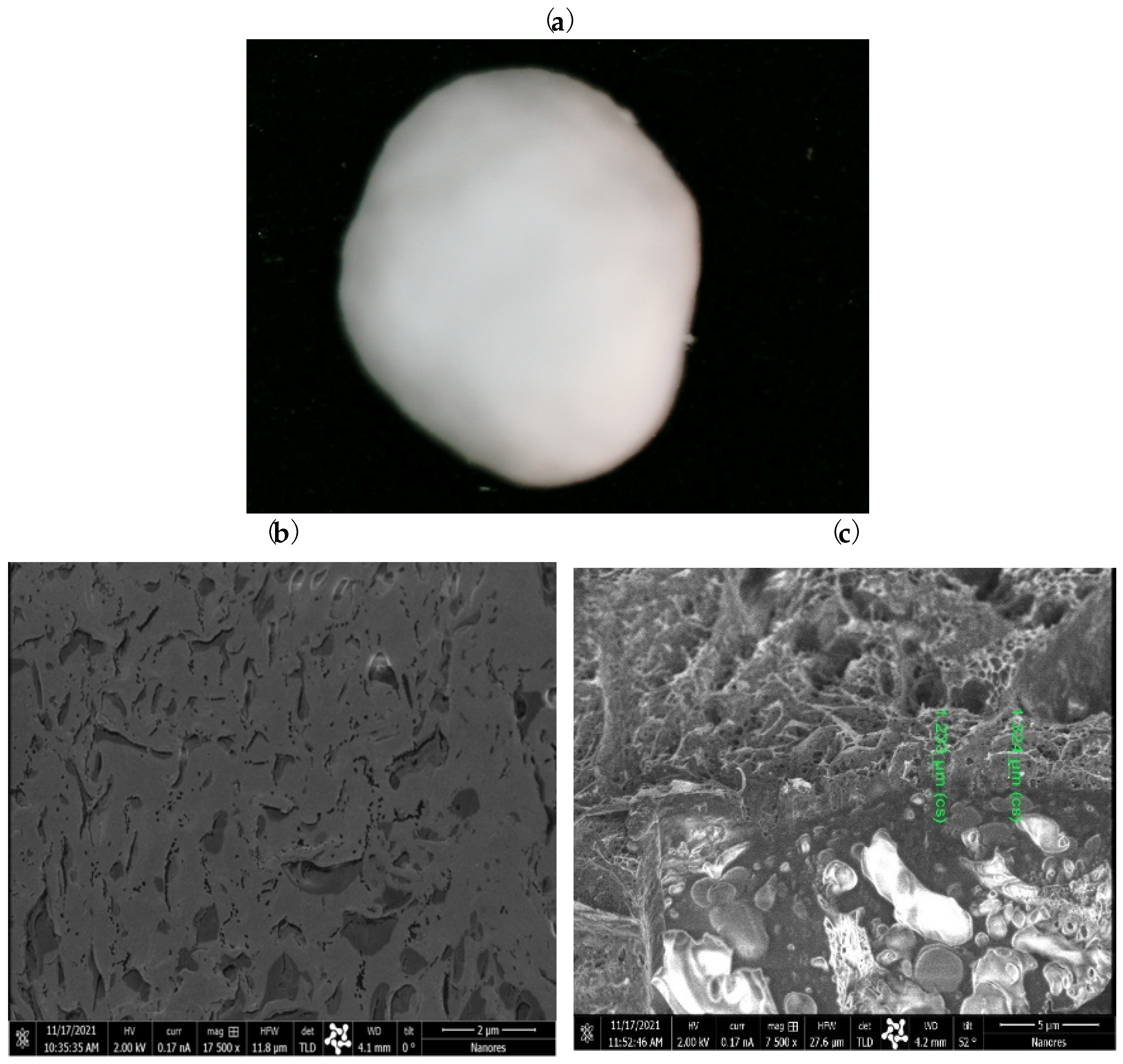
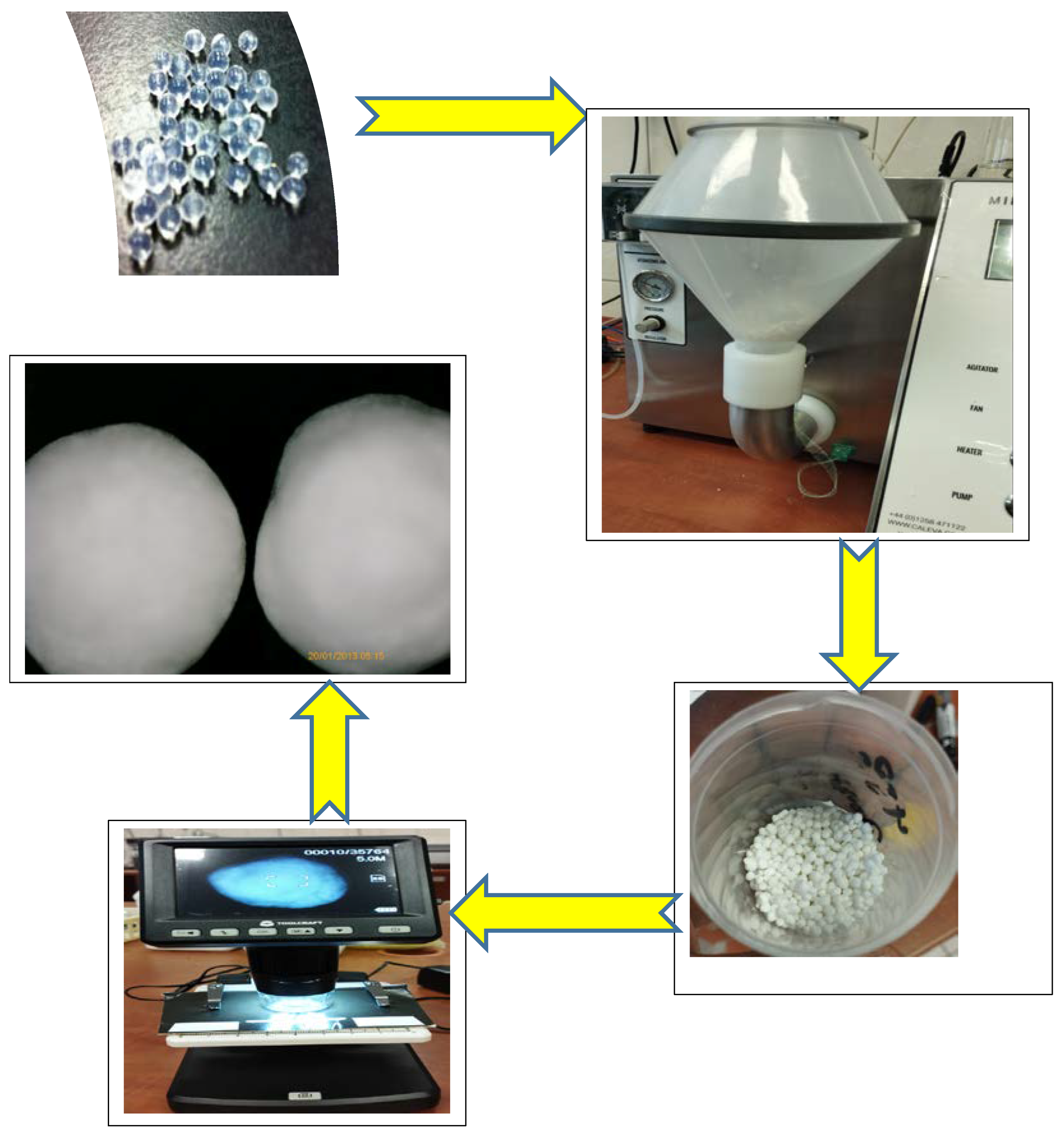
2.3. Coating HA–SAL Carriers with PLA
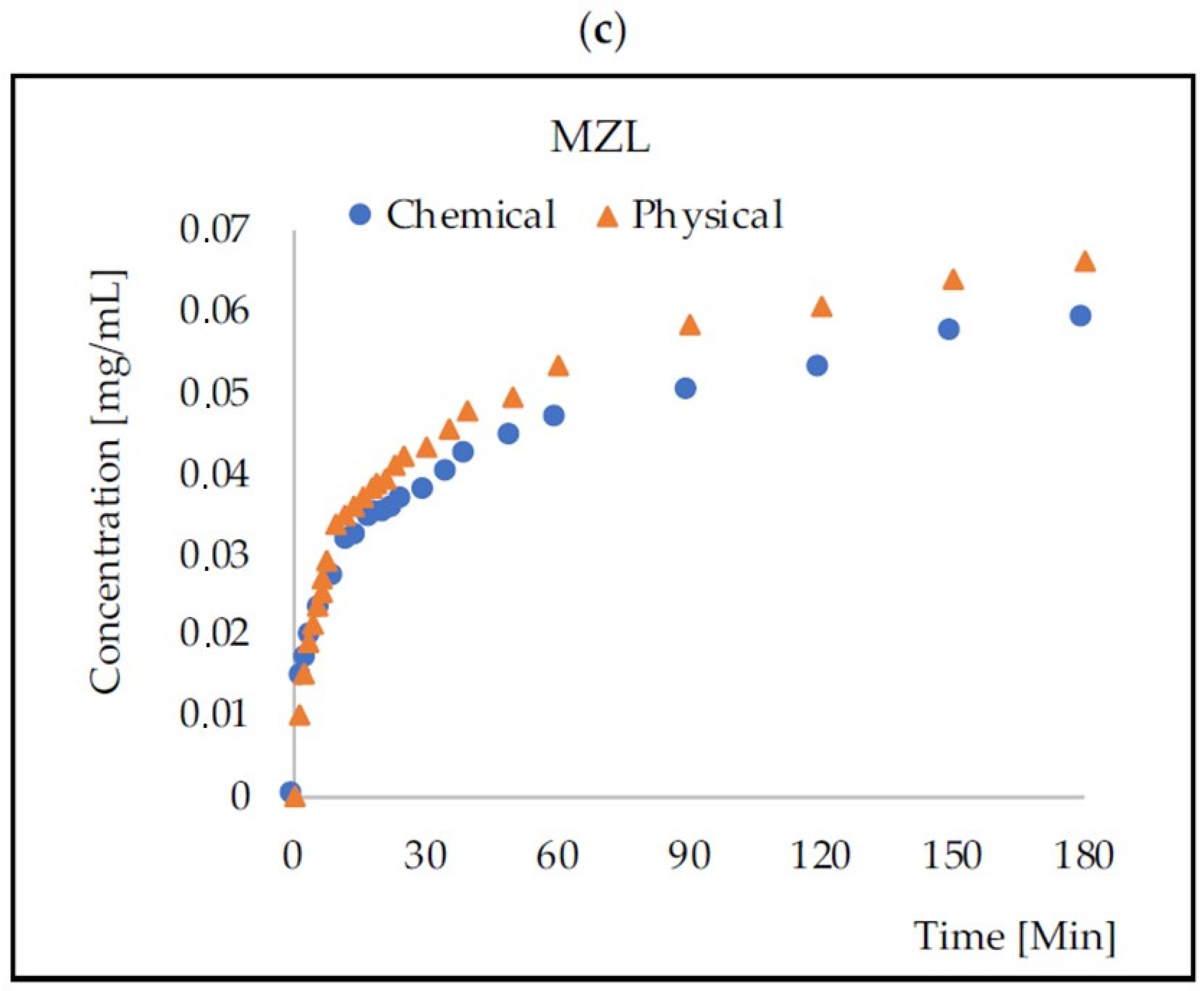
2.4. Mass Transport from PLA–HA–SAL Carriers
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Equipment
4.3. HA–SAL Composite Carrier Preparation by Physical Gelation
4.4. HA–SAL Composite Carrier Preparation by Chemical Gelation
4.5. Coating HA–SAL Carriers with PLA
4.6. Characteristics of the Obtained Carriers
4.7. Mass Transport Monitoring
- Y = mt/m0·100 [%]
- m0, mt—initial and released mass [mg]
- t—time [Min]
- k—coefficient
- n—coefficient [1/Min]
Author Contributions
Funding
Conflicts of Interest
References
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- O’sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from Marine Macroalgae for Human and Animal Health Applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Laurent, T.C. Chemistry and the Molecular Biology in the Intracellular Matrix; Academic Press: London, UK, 1970. [Google Scholar]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; Broek, L.A.M.V.D.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 624967. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Li, J.; Hao, Y.-B.; Qi, J.-X.; Dong, N.-G.; Wu, C.-L.; Wang, Q. Preparation and characterization of composite hydrogels based on crosslinked hyaluronic acid and sodium alginate. J. Appl. Polym. Sci. 2015, 132, 19. [Google Scholar] [CrossRef]
- Catanzano, O.; D’esposito, V.; Acierno, S.; Ambrosio, M.; De Caro, C.; Avagliano, C.; Russo, P.; Russo, R.; Miro, A.; Ungaro, F.; et al. Alginate–hyaluronan composite hydrogels accelerate wound healing process. Carbohydr. Polym. 2015, 131, 407–414. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhong, N.; Huang, Y.; He, K.; Ye, X. Injectable in situ dual-crosslinking hyaluronic acid and sodium alginate based hydrogels for drug release. J. Biomater. Sci. Polym. Ed. 2019, 30, 995–1007. [Google Scholar] [CrossRef]
- Luo, J.-W.; Liu, C.; Wu, J.-H.; Zhao, D.-H.; Lin, L.-X.; Fan, H.-M.; Sun, Y.-L. In situ forming gelatin/hyaluronic acid hydrogel for tissue sealing and hemostasis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 790–797. [Google Scholar] [CrossRef]
- Sakai, S.; Ohi, H.; Taya, M. Gelatin/Hyaluronic Acid Content in Hydrogels Obtained through Blue Light-Induced Gelation Affects Hydrogel Properties and Adipose Stem Cell Behaviors. Biomolecules 2019, 9, 342. [Google Scholar] [CrossRef]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for abdominal tissue regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Chen, J.-P.; Leu, Y.-L.; Hu, J.-W. Temperature-sensitive hydrogels composed of chitosan and hyaluronic acid as injectable carriers for drug delivery. Eur. J. Pharm. Biopharm. 2008, 68, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wang, Q.; Tariq, Z.; You, R.; Li, X.; Li, M.; Zhang, Q. Facile preparation of bioactive silk fibroin/hyaluronic acid hydrogels. Int. J. Biol. Macromol. 2018, 118, 775–782. [Google Scholar] [CrossRef]
- Poveda-Reyes, S.; Moulisova, V.; Sanmartín, A.; Salzer, E.; Barbero, A.; Martin, I.; Grad, S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. 2020, 120, 111701. [Google Scholar]
- Wang, M.-D.; Zhai, P.; Schreyer, D.J.; Zheng, R.-S.; Sun, X.-D.; Cui, F.-Z.; Chen, X.-B. Novel crosslinked alginate/hyaluronic acid hydrogels for nerve tissue engineering. Front. Mater. Sci. 2013, 7, 269–284. [Google Scholar] [CrossRef]
- Brzeziński, M.; Basko, M. Polylactide-Based Materials: Synthesis and Biomedical Applications. Molecules 2023, 28, 1386. [Google Scholar] [CrossRef]
- Guzzo, T.; Barile, F.; Marras, C.; Bellini, D.; Mandaliti, W.; Nepravishta, R.; Paci, M.; Topai, A. Stability evaluation and degradation studies of DAC(R) hyaluronic-polylactide based hHydrogel by DOSY NMR Spectroscopy. Biomolecules 2020, 10, 1478. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.W.; Jang, I.K.; Bae, J.W.; Suh, S.W.; Chung, I.K.; Song, Y.J.; Kim, J.H.; Chung, D.J. Cytotoxicity and in vivo biosafety of the polylactide based bone composite materials. Macromol. Res. 2017, 25, 648–655. [Google Scholar] [CrossRef]
- Grebenik, E.A.; Grinchenko, V.D.; Churbanov, S.N.; Minaev, N.V.; Shavkuta, B.S.; Melnikov, P.A.; Butnaru, D.V.; Rochev, Y.A.; Bagratashvili, V.N.; Timashev, P.S. Osteoinducing scaffolds with multi-layered biointerface. Biomed. Mater. 2018, 13, 054103. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. Drugs for Long Acting Injections and Implants; Springer: Berlin/Heidelberg, Germany, 2011; pp. 73–91. [Google Scholar] [CrossRef]
- Aqil, F.; Gupta, R.C. Controlled Delivery of Chemopreventive Agents by Polymeric Implants. In Cancer Chemoprevention: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–11. [Google Scholar] [CrossRef]
- He, X.; Yuan, Z.; Gaeke, S.; Kao, W.W.-Y.; Li, S.K.; Miller, D.; Williams, B.; Park, Y.C. Laser-Activated Drug Implant for Controlled Release to the Posterior Segment of the Eye. ACS Appl. Bio Mater. 2021, 4, 1461–1469. [Google Scholar] [CrossRef]
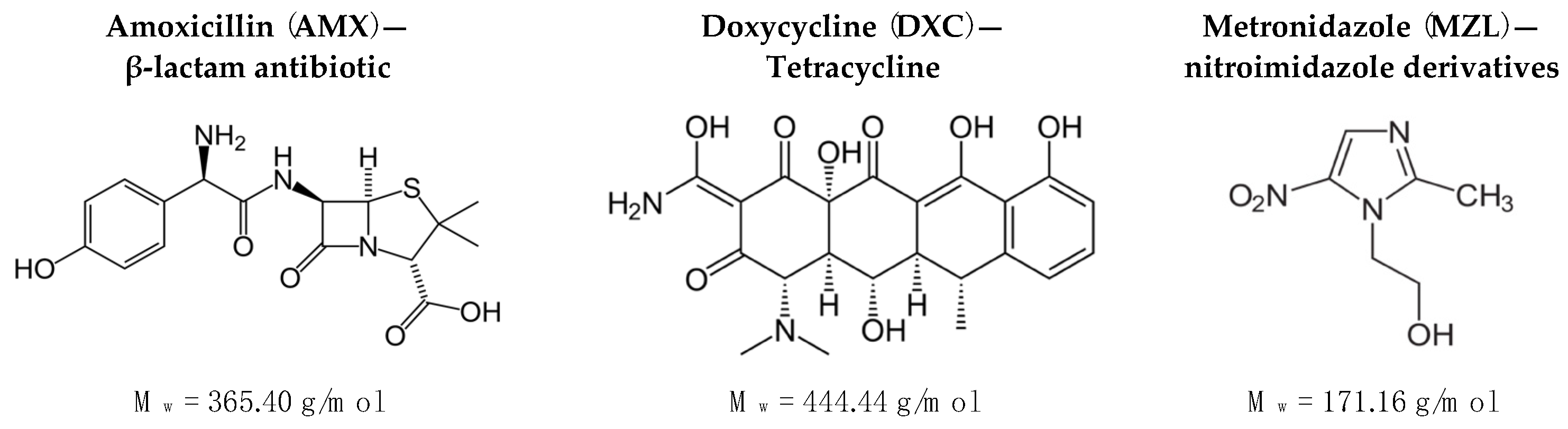
- Thornhill, M. Amoxicillin Is the Most Effective Oral Antibiotic Prophylaxis Regimen for Reducing Post-Invasive Dental Procedure Bacteremia. J. Evid. Based Dent. Pract. 2020, 20, 101464. [Google Scholar] [CrossRef]
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 105, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Chomarat, M.; Dubost, J.; Kalfon, F. Comparative study of amoxicillin-clavulanic acid versus amoxicillin+metronidazole in pyogenic infections of dental origin. Patholog. Biol. 1991, 39, 558–560. [Google Scholar]
- Mestnik, M.J.; Feres, M.; Figueiredo, L.C.; Duarte, P.M.; Lira, E.A.G.; Faveri, M. Short-term benefits of the adjunctive use of metronidazole plus amoxicillin in the microbial profile and in the clinical parameters of subjects with generalized aggressive periodontitis. J. Clin. Periodontol. 2010, 37, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Lan, S.-F.; Kehinde, T.; Zhang, X.; Khajotia, S.; Schmidtke, D.W.; Starly, B. Controlled release of metronidazole from composite poly-epsilon-caprolactone/alginate (PCL/alginate) rings for dental implants. Dent. Mater. 2013, 29, 656–665. [Google Scholar] [CrossRef]
- Akincibay, H.; Örsal, S.Ö.; Şengün, D.; Tözüm, T.F. Systemic administration of doxycycline versus metronidazole plus amoxicillin in the treatment of localized aggressive periodontitis: A clinical and microbiologic study. Quintessence Int. 2008, 39, 33–39. [Google Scholar]
- Kau, Y.-C.; Chen, D.W.-C.; Hsieh, Y.-T.; Lee, F.-Y.; Liu, S.-J. Compression molding of biodegradable drug-eluting implants for sustained release of metronidazole and doxycycline. J. Appl. Polym. Sci. 2012, 127, 554–560. [Google Scholar] [CrossRef]
- Ferreira, C.F.; Babu, J.; Hamlekhan, A.; Patel, S.; Shokuhfar, T. Efficiency of Nanotube Surface–Treated Dental Implants Loaded with Doxycycline on Growth Reduction of Porphyromonas gingivalis. Int. J. Oral Maxillofac. Implant. 2017, 32, 322–328. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Trusek, A.; Kijak, E.; Granicka, L. Graphene oxide as a potential drug carrier—Chemical carrier activation, drug attachment and its enzymatic controlled release. Mater. Sci. Eng. C 2020, 116, 111240. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.M.; Okabe, H.; Hidaka, Y.; Dafader, N.C.; Rahman, N.; Hara, K. Synthesis of pectin-N, N-dimethyl acrylamide hydrogel by gamma radiation and application in drug delivery (in vitro). J. Macromol. Sci. Part A 2018, 55, 369–376. [Google Scholar] [CrossRef]
- Bacaita, E.S.; Ciobanu, B.C.; Popa, M.; Agop, M.; Desbrieres, J. Phases in the temporal multiscale evolution of the drug release mechanism in IPN-type chitosan based hydrogels. Phys. Chem. Chem. Phys. 2014, 16, 25896–25905. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Christoper, G.V.P. Release Kinetics-Concepts and applications. Intern. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar]
- Keikha, M.; Entekhabi, E.; Shokrollahi, M.; Nazarpak, M.H.; Hassannejad, Z.; Akbari, S.; Vaccaro, A.R.; Rahimi-Movaghar, V. Fabrication and characterization of methylprednisolone-loaded polylactic acid/hyaluronic acid nanofibrous scaffold for soft tissue engineering. J. Ind. Text. 2022, 52, 1–24. [Google Scholar] [CrossRef]
- Das, M.; Sharabani-Yosef, O.; Eliaz, N.; Mandler, D. Hydrogel-integrated 3D-printed poly(lactic acid) scaffolds for bone tissue engineering. J. Mater. Res. 2021, 36, 3833–3842. [Google Scholar] [CrossRef]
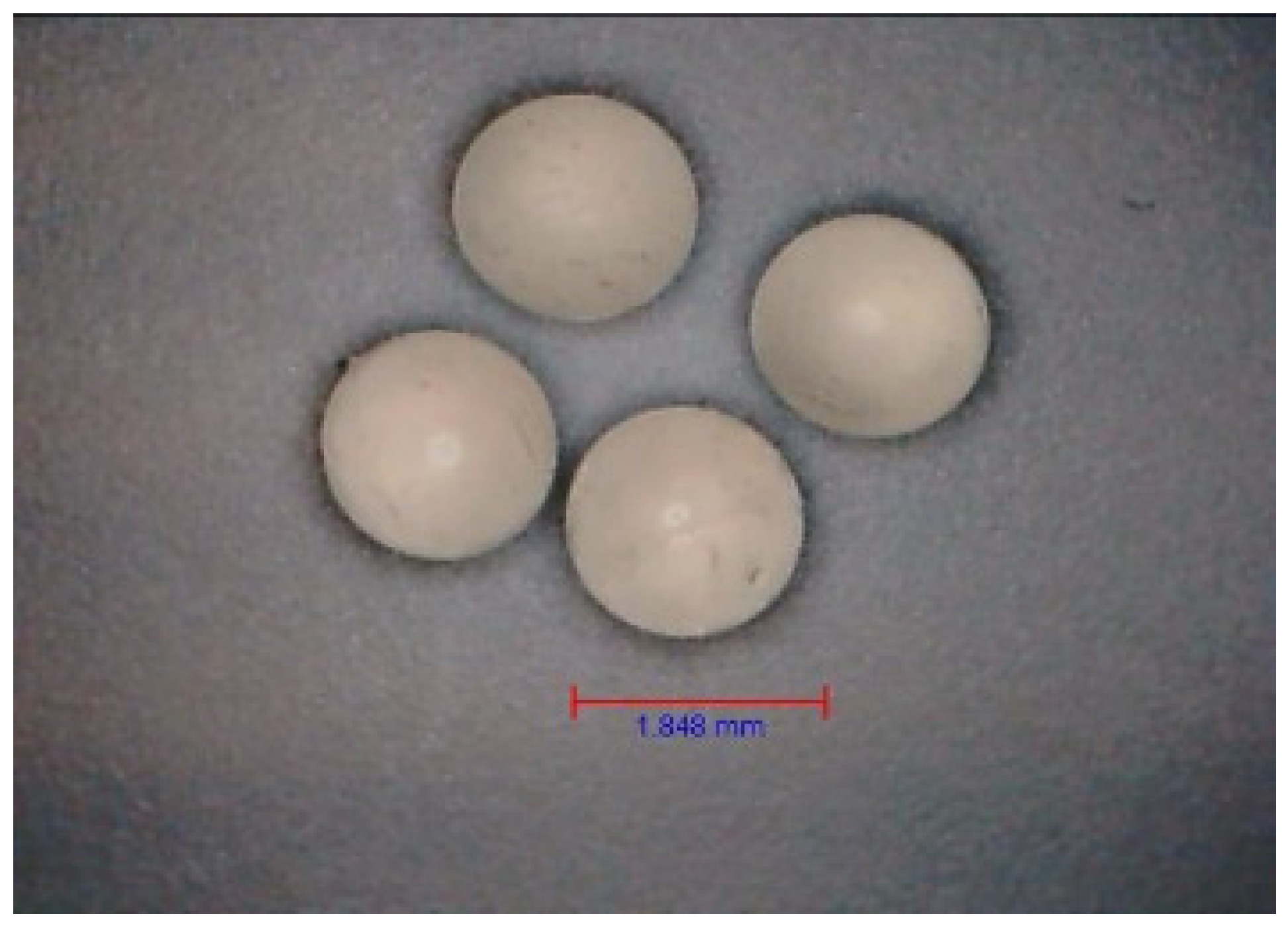



| Antibiotic | AMX | DXC | MZL | |||
|---|---|---|---|---|---|---|
| Physical | Chemical | Physical | Chemical | Physical | Chemical | |
| Pressure force [N] | 1.490 ± 0.125 | 0.425 ± 0.075 | 1.625 ± 0.175 | 0.600 ± 0.05 | 1.675 ± 0.15 | 0.625 ± 0.05 |
| PLA-coated core–shell carriers | ||||||
| Pressure force [N] | 4.625 ± 0.125 | 4.525 ± 0.125 | 4.765 ± 0.175 | |||
| Antibiotic | AMX | DXC | MZL | |||
|---|---|---|---|---|---|---|
| Physical | Chemical | Physical | Chemical | Physical | Chemical | |
| Time [h] | - | - | - | - | - | - |
| 1 | 21.3% | 1.8% | 14.8% | 9.1% | 29.9% | 17.8% |
| 2 | 38.7% | 3.7% | 27.7% | 16.5% | 50.1% | 41.1% |
| 3 | 55.7% | 5.3% | 44.2% | 22.9% | 62.6% | 55.9% |
| 5 | 82.2% | 7.8% | 72.2% | 30.2% | 81.0% | 78.2% |
| 10 | 90.7% | 9.7% | 88.0% | 37.8% | 96.7% | 91.1% |
| 24 | 100.0% | 14.4% | 100.0% | 55.3% | 100.0% | 100.0% |
| Antibiotic | AMX | DXC | MZL | |||
|---|---|---|---|---|---|---|
| Physical | Chemical | Physical | Chemical | Physical | Chemical | |
| n [1/Min] | 0.259 | 0.212 | 0.167 | 0.318 | 0.296 | 0.300 |
| k [-] | 1.491 | 0.146 | 1.303 | 1.995 | 1.562 | 1.348 |
| R2 | 0.956 | 0.925 | 0.976 | 0.983 | 0.972 | 0.978 |
| Antibiotic | AMX | DXC | MZL |
|---|---|---|---|
| n [1/Min] | 0.922 | 1.061 | 0.774 |
| k [-] | 0.003 | 0.001 | 0.013 |
| R2 | 0.976 | 0.995 | 0.994 |
| Antibiotic | AMX | DXC | MZL |
|---|---|---|---|
| Wavelength [nm] | 274 | 367 | 320 |
| Standard curve | A = 2.67 C [mg/mL] | A = 28.283 C [mg/mL] | A = 54.508 C [mg/mL] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trusek, A.; Grabowski, M.; Ajayi, O.; Kijak, E. Hyaluronic Acid–Alginate Homogeneous Structures with Polylactide Coating Applied in Controlled Antibiotic Release. Gels 2023, 9, 526. https://doi.org/10.3390/gels9070526
Trusek A, Grabowski M, Ajayi O, Kijak E. Hyaluronic Acid–Alginate Homogeneous Structures with Polylactide Coating Applied in Controlled Antibiotic Release. Gels. 2023; 9(7):526. https://doi.org/10.3390/gels9070526
Chicago/Turabian StyleTrusek, Anna, Maciej Grabowski, Omoyemi Ajayi, and Edward Kijak. 2023. "Hyaluronic Acid–Alginate Homogeneous Structures with Polylactide Coating Applied in Controlled Antibiotic Release" Gels 9, no. 7: 526. https://doi.org/10.3390/gels9070526
APA StyleTrusek, A., Grabowski, M., Ajayi, O., & Kijak, E. (2023). Hyaluronic Acid–Alginate Homogeneous Structures with Polylactide Coating Applied in Controlled Antibiotic Release. Gels, 9(7), 526. https://doi.org/10.3390/gels9070526








