Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy
Abstract
:1. Introduction
2. Drug Delivery
3. Tissue Engineering
4. Bone Repair
5. Wound Healing
6. Photothermal
7. Other Biomedical Applications
7.1. Angiogenesis
7.2. Antibacterial
7.3. Immiunotherapy
7.4. Cartilage Repair
7.5. Other Applications
7.6. Perspective
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bar, A.; Cohen, S. Inducing Endogenous Cardiac Regeneration: Can Biomaterials Connect the Dots? Front. Bioeng. Biotechnol. 2020, 8, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, M.C.; Cristiano, M.C.; Celia, C.; D’Avanzo, N.; Mancuso, A.; Paolino, D.; Wolfram, J.; Fresta, M. Injectable Drug Delivery Systems for Osteoarthritis and Rheumatoid Arthritis. ACS Nano 2022, 16, 19665–19690. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lai, J.Y.; Zhou, R.F.; Ouyang, Y.F.; Fu, H. The Analgesic Effect of Dexmedetomidine Loaded with Nano-Hydrogel as a Novel Nano-Drug Delivery System for Thoracic Paravertebral Block After Thoracic Surgery. J. Biomed. Nanotechnol. 2022, 18, 1604–1612. [Google Scholar] [CrossRef]
- Du Toit, L.C.; Choonara, Y.E.; Pillay, V. An Injectable Nano-Enabled Thermogel to Attain Controlled Delivery of p11 Peptide for the Potential Treatment of Ocular Angiogenic Disorders of the Posterior Segment. Pharmaceutics 2021, 13, 176. [Google Scholar] [CrossRef]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int. J. Nanomed. 2019, 14, 4333–4351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsi, N.M.; Nabil Shamma, R.; Osama Eladawy, N.; Abdelkhalek, A.A. Bioactive injectable triple acting thermosensitive hydrogel enriched with nano-hydroxyapatite for bone regeneration: In-vitro characterization, Saos-2 cell line cell viability and osteogenic markers evaluation. Drug Dev. Ind. Pharm. 2019, 45, 787–804. [Google Scholar] [CrossRef] [PubMed]
- Gilarska, A.; Lewandowska-Lancucka, J.; Horak, W.; Nowakowska, M. Collagen/chitosan/hyaluronic acid—Based injectable hydrogels for tissue engineering applications—Design, physicochemical and biological characterization. Colloids Surf. B Biointerfaces 2018, 170, 152–162. [Google Scholar] [CrossRef]
- Mishra, D.; Bhunia, B.; Banerjee, I.; Datta, P.; Dhara, S.; Maiti, T.K. Enzymatically crosslinked carboxymethyl-chitosan/gelatin/nano-hydroxyapatite injectable gels for in situ bone tissue engineering application. Mater. Sci. Eng. C 2011, 31, 1295–1304. [Google Scholar] [CrossRef]
- He, B.; Zhang, M.Z.; Yin, L.F.; Quan, Z.X.; Ou, Y.S.; Huang, W. bFGF-incorporated composite biomaterial for bone regeneration. Mater. Des. 2022, 215, 110469. [Google Scholar] [CrossRef]
- Amirthalingam, S.; Lee, S.S.; Pandian, M.; Ramu, J.; Iyer, S.; Hwang, N.S.; Jayakumar, R. Combinatorial effect of nano whitlockite/nano bioglass with FGF-18 in an injectable hydrogel for craniofacial bone regeneration. Biomater. Sci. 2021, 9, 2439–2453. [Google Scholar] [CrossRef]
- Lu, W.; Zeng, M.; Liu, W.B.; Ma, T.L.; Fan, X.L.; Li, H.; Wang, Y.A.; Wang, H.Y.; Hu, Y.H.; Xie, J. Human urine-derived stem cell exosomes delivered via injectable GelMA templated hydrogel accelerate bone regeneration. Mater. Today Bio 2023, 19, 100569. [Google Scholar] [CrossRef]
- Tan, R.W.; Feng, Q.L.; She, Z.D.; Wang, M.B.; Jin, H.; Li, J.Y.; Yu, X. In vitro and in vivo degradation of an injectable bone repair composite. Polym. Degrad. Stab. 2010, 95, 1736–1742. [Google Scholar] [CrossRef]
- Alipour, M.; Ashrafihelan, J.; Salehi, R.; Aghazadeh, Z.; Rezabakhsh, A.; Hassanzadeh, A.; Firouzamandi, M.; Heidarzadeh, M.; Rahbarghazi, R.; Aghazadeh, M.; et al. In vivo evaluation of biocompatibility and immune modulation potential of poly(caprolactone)-poly(ethylene glycol)-poly(caprolactone)-gelatin hydrogels enriched with nano-hydroxyapatite in the model of mouse. J. Biomater. Appl. 2021, 35, 1253–1263. [Google Scholar] [CrossRef]
- Deng, L.Z.; Liu, Y.; Yang, L.Q.; Yi, J.Z.; Deng, F.L.; Zhang, L.M. Injectable and bioactive methylcellulose hydrogel carrying bone mesenchymal stem cells as a filler for critical-size defects with enhanced bone regeneration. Colloids Surf. B Biointerfaces 2020, 194, 111159. [Google Scholar] [CrossRef]
- Fu, S.Z.; Ni, P.Y.; Wang, B.Y.; Chu, B.Y.; Zheng, L.; Luo, F.; Luo, J.C.; Qian, Z.Y. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials 2012, 33, 4801–4809. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.S.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.J.; Jiang, K.X.; Yang, X.T.; Watanabe, N.; Mayo, K.H.; et al. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater. Sci. Eng. C 2020, 116, 111158. [Google Scholar] [CrossRef]
- Cai, M.; Li, X.J.; Xu, M.; Zhou, S.Q.; Fan, L.; Huang, J.Y.; Xiao, C.R.; Lee, Y.C.; Yang, B.; Wang, L.; et al. Injectable Tumor Microenvironment-Modulated Hydrogels with Enhanced Chemosensitivity and Osteogenesis for Tumor-Associated Bone Defects Closed-Loop Management. Chem. Eng. J. 2022, 450, 138086. [Google Scholar] [CrossRef]
- Luo, S.Y.; Wu, J.; Jia, Z.R.; Tang, P.F.; Sheng, J.; Xie, C.M.; Liu, C.; Gan, D.L.; Hu, D.; Zheng, W.; et al. An Injectable, Bifunctional Hydrogel with Photothermal Effects for Tumor Therapy and Bone Regeneration. Macromol. Biosci. 2019, 19, 1900047. [Google Scholar] [CrossRef]
- Abdul-Monem, M.M.; Kamoun, E.A.; Ahmed, D.M.; El-Fakharany, E.M.; Al-Abbassy, F.H.; Aly, H.M. Light-cured hyaluronic acid composite hydrogels using riboflavin as a photoinitiator for bone regeneration applications. J. Taibah Univ. Med. Soc. 2021, 16, 529–539. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Park, S.S.; Kang, H.J.; Sultana, T.; Gwon, J.G.; Lee, B.T. Liver tissue-derived ECM loaded nanocellulose-alginate-TCP composite beads for accelerated bone regeneration. Biomed. Mater. 2022, 17, 055016. [Google Scholar] [CrossRef]
- Kumar, A.; Sivashanmugam, A.; Deepthi, S.; Bumgardner, J.D.; Nair, S.V.; Jayakumar, R. Nano-fibrin stabilized CaSO4 crystals incorporated injectable chitin composite hydrogel for enhanced angiogenesis & osteogenesis. Carbohydr. Polym. 2016, 140, 144–153. [Google Scholar] [CrossRef]
- Ding, Z.Z.; Han, H.Y.; Fan, Z.H.; Lu, H.J.; Sang, Y.H.; Yao, Y.L.; Cheng, Q.Q.; Lu, Q.; Kaplan, D.L. Nanoscale Silk-Hydroxyapatite Hydrogels for Injectable Bone Biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 16914–16922. [Google Scholar] [CrossRef]
- Zhou, X.H.; Sun, J.W.; Wo, K.Q.; Wei, H.J.; Lei, H.Q.; Zhang, J.Y.; Lu, X.F.; Mei, F.; Tang, Q.M.; Wang, Y.F.; et al. nHA-loaded gelatin/alginate hydrogel with combined physical and bioactive features for maxillofacial bone repair. Carbohydr. Polym. 2022, 298, 120127. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, P.; Reyes, R.; Segredo-Morales, E.; Perez-Herrero, E.; Delgado, A.; Evora, C. PLGA-BMP-2 and PLA-17 β-Estradiol Microspheres Reinforcing a Composite Hydrogel for Bone Regeneration in Osteoporosis. Pharmaceutics 2019, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.Q.; Chen, S.Y.; Chen, D.J. Preparation and characterization of chitosan based injectable hydrogels enhanced by chitin nano-whiskers. J. Mech. Behav. Biomed. Mater. 2017, 65, 466–477. [Google Scholar] [CrossRef]
- Kuang, L.J.; Ma, X.Y.; Ma, Y.F.; Yao, Y.; Tariq, M.; Yuan, Y.; Liu, C.S. Self-Assembled Injectable Nanocomposite Hydrogels Coordinated by in Situ Generated CaP Nanoparticles for Bone Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17234–17246. [Google Scholar] [CrossRef]
- Tan, R.W.; Feng, Q.L.; Jin, H.; Li, J.Y.; Yu, X.; She, Z.D.; Wang, M.B.; Liu, H.Y. Structure and Biocompatibility of an Injectable Bone Regeneration Composite. J. Biomater. Sci. Polym. Ed. 2011, 22, 1861–1879. [Google Scholar] [CrossRef]
- Shin, D.Y.; Cheon, K.H.; Song, E.H.; Seong, Y.J.; Park, J.U.; Kim, H.E.; Jeong, S.H. Fluorine-ion-releasing injectable alginate nanocomposite hydrogel for enhanced bioactivity and antibacterial property. Int. J. Biol. Macromol. 2019, 123, 866–877. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Moretti, G.; Petralito, S.; Di Giacomo, S.; Vitalone, A.; Casadei, M.A. Gellan gum methacrylate and laponite as an innovative nanocomposite hydrogel for biomedical applications. Eur. Polym. J. 2016, 77, 114–123. [Google Scholar] [CrossRef]
- Melo, B.L.; Lima-Sousa, R.; Alves, C.G.; Moreira, A.F.; Correia, I.J.; de Melo-Diogo, D. Chitosan-based injectable in situ forming hydrogels containing dopamine-reduced graphene oxide and resveratrol for breast cancer chemo-photothermal therapy. Biochem. Eng. J. 2022, 185, 108529. [Google Scholar] [CrossRef]
- Lee, W.T.; Yoon, J.; Kim, S.S.; Kim, H.; Nguyen, N.T.; Le, X.T.; Lee, E.S.; Oh, K.T.; Choi, H.G.; Youn, Y.S. Combined Antitumor Therapy Using in Situ Injectable Hydrogels Formulated with Albumin Nanoparticles Containing Indocyanine Green, Chlorin e6, and Perfluorocarbon in Hypoxic Tumors. Pharmaceutics 2022, 14, 148. [Google Scholar] [CrossRef]
- Zhou, L.P.; Pi, W.; Hao, M.D.; Li, Y.S.; An, H.; Li, Q.C.; Zhang, P.X.; Wen, Y.Q. An injectable and biodegradable nano-photothermal DNA hydrogel enhances penetration and efficacy of tumor therapy. Biomater. Sci. 2021, 9, 4904–4921. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.J.; Qian, Z.Y.; Yuan, W.Z.; Li, Z.H. Injectable and self-healing nanocomposite hydrogel loading needle-like nano-hydroxyapatite and graphene oxide for synergistic tumour proliferation inhibition and photothermal therapy. J. Mater. Chem. B 2021, 9, 9734–9743. [Google Scholar] [CrossRef]
- Wang, H.H.; Wang, B.Y.; Wang, S.S.; Chen, J.Q.; Zhi, W.W.; Guan, Y.B.; Cai, B.R.; Zhu, Y.H.; Jia, Y.Y.; Huang, S.N.; et al. Injectable in situ intelligent thermo-responsive hydrogel with glycyrrhetinic acid-conjugated nano graphene oxide for chemo-photothermal therapy of malignant hepatocellular tumor. J. Biomater. Appl. 2022, 37, 151–165. [Google Scholar] [CrossRef]
- Rong, L.D.; Liu, Y.; Fan, Y.; Xiao, J.; Su, Y.H.; Lu, L.G.; Peng, S.J.; Yuan, W.Z.; Zhan, M.X. Injectable nano-composite hydrogels based on hyaluronic acid-chitosan derivatives for simultaneous photothermal-chemo therapy of cancer with anti-inflammatory capacity. Carbohydr. Polym. 2023, 310, 120721. [Google Scholar] [CrossRef]
- He, W.; Li, P.; Zhu, Y.; Liu, M.M.; Huang, X.N.; Qi, H. An injectable silk fibroin nanofiber hydrogel hybrid system for tumor upconversion luminescence imaging and photothermal therapy. New J. Chem. 2019, 43, 2213–2219. [Google Scholar] [CrossRef]
- Xu, X.Y.; Huang, Z.Y.; Huang, Z.Q.; Zhang, X.F.; He, S.Y.; Sun, X.Q.; Shen, Y.F.; Yan, M.N.; Zhao, C.S. Injectable, NIR/pH-Responsive Nanocomposite Hydrogel as Long-Acting Implant for Chemophotothermal Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 20361–20375. [Google Scholar] [CrossRef]
- Liao, J.F.; Han, R.X.; Wu, Y.Z.; Qian, Z.Y. Review of a new bone tumor therapy strategy based on bifunctional biomaterials. Bone Res. 2021, 9, 18. [Google Scholar] [CrossRef]
- Veisi, H.; Varshosaz, J.; Rostami, M.; Mirian, M. Thermosensitive TMPO-oxidized lignocellulose/cationic agarose hydrogel loaded with deferasirox nanoparticles for photothermal therapy in melanoma. Int. J. Biol. Macromol. 2023, 238, 124126. [Google Scholar] [CrossRef]
- Gangrade, A.; Mandal, B.B. Injectable Carbon Nanotube Impregnated Silk Based Multifunctional Hydrogel for Localized Targeted and On-Demand Anticancer Drug Delivery. ACS Biomater. Sci. Eng. 2019, 5, 2365–2381. [Google Scholar] [CrossRef]
- Gil, M.S.; Thambi, T.; Phan, V.H.G.; Kim, S.H.; Lee, D.S. Injectable hydrogel-incorporated cancer cell-specific cisplatin releasing nanogels for targeted drug delivery. J. Mater. Chem. B 2017, 5, 7140–7152. [Google Scholar] [CrossRef]
- Pei, Y.H.; Huang, L.F.; Wang, T.; Yao, Q.H.; Sun, Y.R.; Zhang, Y.; Yang, X.M.; Zhai, J.L.; Qin, L.H.; Xue, J.J.; et al. Bone marrow mesenchymal stem cells loaded into hydrogel/nanofiber composite scaffolds ameliorate ischemic brain injury. Mater. Today Adv. 2023, 17, 100349. [Google Scholar] [CrossRef]
- Min, K.; Tae, G. Cellular infiltration in an injectable sulfated cellulose nanocrystal hydrogel and efficient angiogenesis by VEGF loading. Biomater Res. 2023, 27, 28. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.S.; Yang, X.S.; Deng, H.; Hao, Y.T.; Mao, L.Z.; Zhang, R.J.; Liao, W.Z.; Yuan, M.M. Injectable Hydrogel-Based Nanocomposites for Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 8, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.Y.; Li, J.J.; Hu, Y.C.; Gao, F.; Leung, G.P.H.; Geng, F.N.; Fu, C.M.; Zhang, J.M. Injectable thermo-responsive nano-hydrogel loading triptolide for the anti-breast cancer enhancement via localized treatment based on "two strikes" effects. Acta Pharm. Sin. B 2020, 10, 2227–2245. [Google Scholar] [CrossRef]
- Song, H.J.; Su, Q.; Shi, W.F.; Huang, P.S.; Zhang, C.N.; Zhang, C.; Liu, Q.; Wang, W.W. Antigen epitope-TLR7/8a conjugate as self-assembled carrier-free nanovaccine for personalized immunotherapy. Acta Biomater. 2022, 141, 398–407. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Thambi, T.; Yin, Y.; Kim, S.H.; Nguyen, T.L.; Phan, V.H.G.; Kim, J.; Jeong, J.H.; Lee, D.S. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials 2020, 230, 119599. [Google Scholar] [CrossRef]
- Cellesi, F.; Tirelli, N. Injectable Nanotechnology; Woodhead Publ Ltd.: Cambridge, UK, 2011; pp. 298–322. [Google Scholar]
- Bai, Y.T.; Wang, T.R.; Zhang, S.L.; Chen, X.S.; He, C.L. Recent advances in organic and polymeric carriers for local tumor chemo-immunotherapy. Sci. China Technol. Sci. 2022, 65, 1011–1028. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, T.G.; Zhuang, Y.P.; He, T.D.; Wu, X.L.; Su, L.; Kang, J.; Chang, J.; Wang, H.J. Sodium Alginate Hydrogel-Mediated Cancer Immunotherapy for Postoperative In Situ Recurrence and Metastasis. ACS Biomater. Sci. Eng. 2021, 7, 5717–5726. [Google Scholar] [CrossRef]
- Fan, W.; Yuan, L.; Li, J.; Wang, Z.; Chen, J.; Guo, C.; Mo, X.; Yan, Z. Injectable double-crosslinked hydrogels with kartogenin-conjugated polyurethane nano-particles and transforming growth factor β3 for in-situ cartilage regeneration. Mater. Sci. Eng. C 2020, 110, 110705. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Subramanian, A.; Sethuraman, S. Injectable glycosaminoglycan-protein nano-complex in semi-interpenetrating networks: A biphasic hydrogel for hyaline cartilage regeneration. Carbohydr. Polym. 2017, 175, 63–74. [Google Scholar] [CrossRef]
- Koushki, N.; Tavassoli, H.; Katbab, A.A.; Katbab, P.; Bonakdar, S. A New Injectable Biphasic Hydrogel Based on Partially Hydrolyzed Polyacrylamide and Nano Hydroxyapatite, Crosslinked with Chromium Acetate, as Scaffold for Cartilage Regeneration. In Proceedings of the 30th International Conference of the Polymer-Processing-Society (PPS), Cleveland, OH, USA, 6–12 June 2014. [Google Scholar]
- Chyzy, A.; Tomczykowa, M.; Plonska-Brzezinska, M.E. Hydrogels as Potential Nano-, Micro- and Macro-Scale Systems for Controlled Drug Delivery. Materials 2020, 13, 188. [Google Scholar] [CrossRef] [Green Version]
- Holyoak, D.T.; Wheeler, T.A.; van der Meulen, M.C.H.; Singh, A. Injectable mechanical pillows for attenuation of load-induced post-traumatic osteoarthritis. Regen. Biomater. 2019, 6, 211–219. [Google Scholar] [CrossRef]
- Li, X.Q.; Shi, Y.L.; Xu, S.X. Local delivery of tumor-targeting nano-micelles harboring GSH-responsive drug release to improve antitumor efficiency. Polym. Adv. Technol. 2022, 33, 2835–2844. [Google Scholar] [CrossRef]
- Gosecka, M.; Gosecki, M. Antimicrobial Polymer-Based Hydrogels for the Intravaginal Therapies—Engineering Considerations. Pharmaceutics 2021, 13, 1393. [Google Scholar] [CrossRef]
- GuhaSarkar, S.; More, P.; Banerjee, R. Urothelium-adherent, ion-triggered liposome-in-gel system as a platform for intravesical drug delivery. J. Control. Release 2017, 245, 147–156. [Google Scholar] [CrossRef]
- GuhaSarkar, S.; Pathak, K.; Sudhalkar, N.; More, P.; Goda, J.S.; Gota, V.; Banerjee, R. Synergistic locoregional chemoradiotherapy using a composite liposome-in-gel system as an injectable drug depot. Int. J. Nanomed. 2016, 11, 6435–6448. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.J.; Guo, X.L.; Ruan, C.P.; Hu, H.L.; Jiang, B.P.; Liang, H.; Shen, X.C. An injectable thermosensitive photothermal-network hydrogel for near-infrared-triggered drug delivery and synergistic photothermal-chemotherapy. Acta Biomater. 2019, 96, 281–294. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Sedghi Aminabad, N.; Sabzi, A.; Zarebkohan, A.; Razavi, M.; Vosough, M.; Bodaghi, M.; Maleki, H. Smart and Biomimetic 3D and 4D Printed Composite Hydrogels: Opportunities for Different Biomedical Applications. Biomedicines 2021, 9, 1537. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.; Kim, Y.M.; Choi, I.; Han, H.S.; Kim, T.; Choi, K.Y.; Roh, Y.H. Crystallinity-tuned ultrasoft polymeric DNA networks for controlled release of anticancer drugs. J. Control. Release 2023, 355, 7–17. [Google Scholar] [CrossRef]
- Parameswaran-Thankam, A.; Parnell, C.M.; Watanabe, F.; RanguMagar, A.B.; Chhetri, B.P.; Szwedo, P.K.; Biris, A.S.; Ghosh, A. Guar-Based Injectable Thermoresponsive Hydrogel as a Scaffold for Bone Cell Growth and Controlled Drug Delivery. ACS Omega 2018, 3, 15158–15167. [Google Scholar] [CrossRef]
- Ren, B.W.; Chen, X.Y.; Du, S.K.; Ma, Y.; Chen, H.A.; Yuan, G.L.; Li, J.L.; Xiong, D.S.; Tan, H.P.; Ling, Z.H.; et al. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int. J. Biol. Macromol. 2018, 118, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Salehi, S.; Naghib, S.M.; Garshasbi, H.R.; Ghorbanzadeh, S.; Zhang, W. Smart stimuli-responsive injectable gels and hydrogels for drug delivery and tissue engineering applications: A review. Front. Bioeng. Biotechnol. 2023, 11, 1104126. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, I.; Khalil, I.; Ali, I.; Yacoub, M. Updates on smart polymeric carrier systems for protein delivery. Drug Dev. Ind. Pharm. 2017, 43, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.T.; Chen, C.H.; Chen, J.P. Intratumoral Delivery of Doxorubicin on Folate-Conjugated Graphene Oxide by In-Situ Forming Thermo-Sensitive Hydrogel for Breast Cancer Therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Niu, H.S.; Wang, Z.X.; Wang, Y.; Li, X.C.; Hao, J.L. Comparative analysis of different drug delivery methods of injectable hydrogel nanomaterials of insulin biomaterials via multiple daily injections and continuous subcutaneous insulin infusion in the treatment of type 1 diabetes mellitus in children. Mater. Express 2021, 11, 1154–1160. [Google Scholar] [CrossRef]
- Tong, S.; Li, Q.Y.; Liu, Q.Y.; Song, B.; Wu, J.Z. Recent advances of the nanocomposite hydrogel as a local drug delivery for diabetic ulcers. Front. Bioeng. Biotechnol. 2022, 10, 14. [Google Scholar] [CrossRef]
- Wanakule, P.; Roy, K. Disease-Responsive Drug Delivery: The Next Generation of Smart Delivery Devices. Curr. Drug Metab. 2012, 13, 42–49. [Google Scholar] [CrossRef]
- Wu, M.; Chen, J.S.; Huang, W.J.; Yan, B.; Peng, Q.Y.; Liu, J.F.; Chen, L.Y.; Zeng, H.B. Injectable and Self-Healing Nanocomposite Hydrogels with Ultrasensitive pH-Responsiveness and Tunable Mechanical Properties: Implications for Controlled Drug Delivery. Biomacromolecules 2020, 21, 2409–2420. [Google Scholar] [CrossRef]
- Xiong, J.J.; Yan, J.J.; Li, C.; Wang, X.Y.; Wang, L.Z.; Pan, D.H.; Xu, Y.P.; Wang, F.; Li, X.X.; Wu, Q.; et al. Injectable liquid metal nanoflake hydrogel as a local therapeutic for enhanced postsurgical suppression of tumor recurrence. Chem. Eng. J. 2021, 416, 129092. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.M.; Ma, Q.Y.; Di, X.; Wu, G.L. A bio-inspired fluorescent nano-injectable hydrogel as a synergistic drug delivery system. New J. Chem. 2021, 45, 3079–3087. [Google Scholar] [CrossRef]
- Yang, S.H.; Ren, J.Y.; Wang, H. Injectable Micromotor@Hydrogel System for Antibacterial Therapy. Chem. Eur. J. 2022, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Mohandas, A.; Jayakumar, R. Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel for angiogenic drug delivery. Colloids Surf. B Biointerfaces 2019, 177, 41–49. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, Y.; Xu, C.; Lin, J.T.; Jin, J.K.; Xu, A.K.; Lou, J.N.; Qian, C.; Yu, W.; Wu, Y.L.; et al. Novel optimized drug delivery systems for enhancing spinal cord injury repair in rats. Drug Deliv. 2021, 28, 2548–2561. [Google Scholar] [CrossRef]
- Zhou, X.H.; He, X.L.; Shi, K.; Yuan, L.P.; Yang, Y.; Liu, Q.Y.; Ming, Y.; Yi, C.; Qian, Z.Y. Injectable Thermosensitive Hydrogel Containing Erlotinib-Loaded Hollow Mesoporous Silica Nanoparticles as a Localized Drug Delivery System for NSCLC Therapy. Adv. Sci. 2020, 7, 2001442. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhang, P.; Zhang, F.; Pu, M.Y.; Zhong, W.T.; Zhang, Y.; Shen, Y.C.; Zuo, B.Q. Injectable PEG-induced silk nanofiber hydrogel for vancomycin delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103596. [Google Scholar] [CrossRef]
- Rodriguez-Velazquez, E.; Alatorre-Meda, M.; Mano, J.F. Polysaccharide-Based Nanobiomaterials as Controlled Release Systems for Tissue Engineering Applications. Curr. Pharm. Des. 2015, 21, 4837–4850. [Google Scholar] [CrossRef]
- Bheri, S.; Davis, M.E. Nanoparticle-Hydrogel System for Post-myocardial Infarction Delivery of MicroRNA. ACS Nano 2019, 13, 9702–9706. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Qiao, C.Y.; Ning, J.; Ding, X.X.; Wang, H.Y.; Zhou, Y.M. A Polysaccharide-based Hydrogel and PLGA Microspheres for Sustained P24 Peptide Delivery: An In vitro and In vivo Study Based on Osteogenic Capability. Chem. Res. Chin. Univ. 2019, 35, 908–915. [Google Scholar] [CrossRef]
- Chamradova, I.; Vojtova, L.; Castkova, K.; Divis, P.; Peterek, M.; Jancar, J. The effect of hydroxyapatite particle size on viscoelastic properties and calcium release from a thermosensitive triblock copolymer. Colloid Polym. Sci. 2017, 295, 107–115. [Google Scholar] [CrossRef]
- Forsback, A.P.; Noppari, P.; Viljanen, J.; Mikkola, J.; Jokinen, M.; Leino, L.; Bjerregaard, S.; Borglin, C.; Halliday, J. Sustained In-Vivo Release of Triptorelin Acetate from a Biodegradable Silica Depot: Comparison to Pamorelin® LA. Nanomaterials 2021, 11, 1578. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, H.; Hosseinkhani, M.; Khademhosseini, A.; Kobayashi, H. Bone regeneration through controlled release of bone morphogenetic protein-2 from 3-D tissue engineered nano-scaffold. J. Control. Release 2007, 117, 380–386. [Google Scholar] [CrossRef]
- Hu, Z.C.; Tang, Q.; Yan, D.Y.; Zheng, G.; Gu, M.B.; Luo, Z.C.; Mao, C.; Qian, Z.Y.; Ni, W.F.; Shen, L.Y. A multi-functionalized calcitriol sustainable delivery system for promoting osteoporotic bone regeneration both in vitro and in vivo. Appl. Mater. Today 2021, 22, 100906. [Google Scholar] [CrossRef]
- Huang, P.S.; Song, H.J.; Zhang, Y.M.; Liu, J.J.; Cheng, Z.; Liang, X.J.; Wang, W.W.; Kong, D.L.; Liu, J.F. FRET-enabled monitoring of the thermosensitive nanoscale assembly of polymeric micelles into macroscale hydrogel and sequential cognate micelles release. Biomaterials 2017, 145, 81–91. [Google Scholar] [CrossRef]
- Huang, P.S.; Zhang, Y.M.; Wang, W.W.; Zhou, J.H.; Sun, Y.; Liu, J.J.; Kong, D.L.; Liu, J.F.; Dong, A.J. Co-delivery of doxorubicin and I-131 by thermosensitive micellar-hydrogel for enhanced in situ synergetic chemoradiotherapy. J. Control. Release 2015, 220, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Park, M.R.; Song, S.C. An injectable cell penetrable nano-polyplex hydrogel for localized siRNA delivery. Biomaterials 2013, 34, 4493–4500. [Google Scholar] [CrossRef] [PubMed]
- Klubthawee, N.; Bovone, G.; Marco-Dufort, B.; Guzzi, E.A.; Aunpad, R.; Tibbitt, M.W. Biopolymer Nano-Network for Antimicrobial Peptide Protection and Local Delivery. Adv. Healthc. Mater. 2022, 11, e2101426. [Google Scholar] [CrossRef]
- Kumar, P.; Choonara, Y.E.; Modi, G.; Naidoo, D.; Pillay, V. Multifunctional Therapeutic Delivery Strategies for Effective Neuro-Regeneration Following Traumatic Spinal Cord Injury. Curr. Pharm. Des. 2015, 21, 1517–1528. [Google Scholar] [CrossRef]
- Ligorio, C.; Zhou, M.; Wychowaniec, J.K.; Zhu, X.Y.; Bartlam, C.; Miller, A.F.; Vijayaraghavan, A.; Hoyland, J.A.; Saiani, A. Graphene oxide containing self-assembling peptide hybrid hydrogels as a potential 3D injectable cell delivery platform for intervertebral disc repair applications. Acta Biomater. 2019, 92, 92–103. [Google Scholar] [CrossRef]
- Lim, H.J.; Do Ghim, H.; Choi, J.H.; Chung, H.Y.; Lim, J.O. Controlled Release of BMP-2 from Alginate Nanohydrogels Enhanced Osteogenic Differentiation of Human Bone Marrow Stromal Cells. Macromol. Res. 2010, 18, 787–792. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.Y.; Li, L.; Xia, Y.M.; Hu, X.Y.; Fang, Y. The incorporated hydrogel of chitosan-oligoconjugated linoleic acid vesicles and the protective sustained release for curcumin in the gel. Int. J. Biol. Macromol. 2023, 227, 17–26. [Google Scholar] [CrossRef]
- Perez-Herrero, E.; Garcia-Garcia, P.; Gomez-Morales, J.; Llabres, M.; Delgado, A.; Evora, C. New injectable two-step forming hydrogel for delivery of bioactive substances in tissue regeneration. Regen. Biomater. 2019, 6, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.B.; Koh, J.T.; Song, S.C. Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 2017, 122, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.B.; Park, M.R.; Song, S.C. Sustained Release of Exendin 4 Using Injectable and lonic-Nano-Complex Forming Polymer Hydrogel System for Long-Term Treatment of Type 2 Diabetes Mellitus. ACS Appl. Mater. Interfaces 2019, 11, 15201–15211. [Google Scholar] [CrossRef]
- Sun, X.J.; Li, Z.Y.; Cui, Z.D.; Wu, S.L.; Zhu, S.L.; Liang, Y.Q.; Yang, X.J. Preparation and physicochemical properties of an injectable alginate-based hydrogel by the regulated release of divalent ions via the hydrolysis of d-glucono-δ-lactone. J. Biomater. Appl. 2020, 34, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.W.; She, Z.D.; Wang, M.B.; Yu, X.; Jin, H.; Feng, Q.L. Repair of rat calvarial bone defects by controlled release of rhBMP-2 from an injectable bone regeneration composite. J. Tissue Eng. Regen. Med. 2012, 6, 614–621. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges. Antioxidants 2020, 9, 791. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Weir, M.D.; Simon, C.G. Injectable and strong nano-apatite scaffolds for cell/growth factor delivery and bone regeneration. Dent. Mater. 2008, 24, 1212–1222. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Q.; Kim, Y.M.; Song, S.C. Injectable and Quadruple-Functional Hydrogel as an Alternative to Intravenous Delivery for Enhanced Tumor Targeting. ACS Appl. Mater. Interfaces 2019, 11, 34634–34644. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Song, S.C. Multiple hyperthermia-mediated release of TRAIL/SPION nanocomplex from thermosensitive polymeric hydrogels for combination cancer therapy. Biomaterials 2017, 132, 16–27. [Google Scholar] [CrossRef]
- Boffito, M.; Pontremoli, C.; Fiorilli, S.; Laurano, R.; Ciardelli, G.; Vitale-Brovarone, C. Injectable Thermosensitive Formulation Based on Polyurethane Hydrogel/Mesoporous Glasses for Sustained Co-Delivery of Functional Ions and Drugs. Pharmaceutics 2019, 11, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.D.; Ma, Y.D.; Guo, S.; He, B.F.; Jiang, T.Y. Topical delivery of chemotherapeutic drugs using nano-hybrid hydrogels to inhibit post-surgical tumour recurrence. Biomater. Sci. 2021, 9, 4356–4363. [Google Scholar] [CrossRef] [PubMed]
- Soh, W.W.M.; Teoh, R.Y.P.; Zhu, J.L.; Xun, Y.R.; Wee, C.Y.; Ding, J.; San Thian, E.; Li, J. Facile Construction of a Two-in-One Injectable Micelleplex-Loaded Thermogel System for the Prolonged Delivery of Plasmid DNA. Biomacromolecules 2022, 23, 3477–3492. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Y.B.; Hu, T.L.; Ma, P.X.; Guo, B.L. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019, 96, 175–187. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Agarwal, T.; Rathnam, V.S.S.; Pal, K.; Banerjee, I. Cobalt doped nano-hydroxyapatite incorporated gum tragacanth-alginate beads as angiogenic-osteogenic cell encapsulation system for mesenchymal stem cell based bone tissue engineering. Int. J. Biol. Macromol. 2021, 179, 101–115. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Ashrafihelan, J.; Salehi, R.; Rahbarghazi, R.; Firouzamandi, M.; Ahmadi, M.; Khaksar, M.; Alipour, M.; Aghazadeh, M. Development and biocompatibility of the injectable collagen/nano-hydroxyapatite scaffolds as in situ forming hydrogel for the hard tissue engineering application. Artif. Cells Nanomed. Biotechnol. 2021, 49, 136–146. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Manigandan, A.; Chinnaswamy, P.; Subramanian, A.; Sethuraman, S. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials 2018, 162, 82–98. [Google Scholar] [CrossRef]
- Saludas, L.; Pascual-Gil, S.; Prosper, F.; Garbayo, E.; Blanco-Prieto, M. Hydrogel based approaches for cardiac tissue engineering. Int. J. Pharm. 2017, 523, 454–475. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Zaszczynska, A.; Zabielski, K.; Sajkiewicz, P. Hydrogel, Electrospun and Composite Materials for Bone/Cartilage and Neural Tissue Engineering. Materials 2021, 14, 6899. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F.; Firouzi, Z.; Mousavi, S.D. The impact of zirconium oxide nanoparticles content on alginate dialdehyde-gelatin scaffolds in cartilage tissue engineering. J. Mol. Liq. 2021, 335, 116531. [Google Scholar] [CrossRef]
- Jaikumar, D.; Sajesh, K.M.; Soumya, S.; Nimal, T.R.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Injectable alginate-O-carboxymethyl chitosan/nano fibrin composite hydrogels for adipose tissue engineering. Int. J. Biol. Macromol. 2015, 74, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Mohandas, A.; Hwang, N.S.; Jayakumar, R. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2019, 122, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Z.; Gun, G.; Gong, C.Y.; Zeng, S.; Liang, H.; Luo, F.; Zhang, X.N.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. Injectable Biodegradable Thermosensitive Hydrogel Composite for Orthopedic Tissue Engineering. 1. Preparation and Characterization of Nanohydroxyapatite/Poly(ethylene glycol)-Poly(ε-caprolactone)-Poly(ethylene glycol) Hydrogel Nanocomposites. J. Phys. Chem. B 2009, 113, 16518–16525. [Google Scholar] [CrossRef]
- Kaur, K.; Paiva, S.S.; Caffrey, D.; Cavanagh, B.L.; Murphy, C.M. Injectable chitosan/collagen hydrogels nano-engineered with functionalized single wall carbon nanotubes for minimally invasive applications in bone. Mater. Sci. Eng. C 2021, 128, 112340. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.X.; Zhang, X.L.; Wu, A.M.; Xu, H.Z. An injectable nano-hydroxyapatite (n-HA)/glycol chitosan (G-CS)/hyaluronic acid (HyA) composite hydrogel for bone tissue engineering. RSC Adv. 2016, 6, 33529–33536. [Google Scholar] [CrossRef]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Visage, C.L.; Tassin, J.F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef]
- Qiao, M.X.; Xu, Z.Y.; Pei, X.B.; Liu, Y.H.; Wang, J.; Chen, J.Y.; Zhu, Z.; Wan, Q.B. Nano SIM@ZIF-8 modified injectable High-intensity biohydrogel with bidirectional regulation of osteogenesis and Anti-adipogenesis for bone repair. Chem. Eng. J. 2022, 434, 134583. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, Y.; Mulatibieke, R.; Zhong, Q.; Pan, X.; Chen, Y.; Lian, Q.; Luo, X.; Shi, Z.; Zhu, Q. Nano-Silicate-Reinforced and SDF-1α-Loaded Gelatin-Methacryloyl Hydrogel for Bone Tissue Engineering. Int. J. Nanomed. 2020, 15, 9337–9353. [Google Scholar] [CrossRef]
- Cernencu, A.I.; Dinu, A.I.; Stancu, I.C.; Lungu, A.; Iovu, H. Nanoengineered biomimetic hydrogels: A major advancement to fabricate 3D-printed constructs for regenerative medicine. Biotechnol. Bioeng. 2022, 119, 762–783. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, H.H.; Kowitsch, A.; Mader, K.; Hause, G.; Ulrich, J.; Groth, T. Novel mineralized heparin-gelatin nanoparticles for potential application in tissue engineering of bone. J. Mater. Sci. Mater. Med. 2014, 25, 669–680. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Yin, P.J.; Huang, J.F.; Yang, L.N.; Liu, Z.; Fu, D.L.; Hu, Z.Q.; Huang, W.H.; Miao, Y. Scalable and high-throughput production of an injectable platelet-rich plasma (PRP)/cell-laden microcarrier/hydrogel composite system for hair follicle tissue engineering. J. Nanobiotechnol. 2022, 20, 22. [Google Scholar] [CrossRef]
- Cao, Z.; Bai, X.; Wang, C.B.; Ren, L.L.; Ma, D.Y. A simple polysaccharide based injectable hydrogel compositing nano-hydroxyapatite for bone tissue engineering. Mater. Lett. 2021, 293, 129755. [Google Scholar] [CrossRef]
- Pal, A.; Das Karmakar, P.; Vel, R.; Bodhak, S. Synthesis and Characterizations of Bioactive Glass Nanoparticle-Incorporated Triblock Copolymeric Injectable Hydrogel for Bone Tissue Engineering. ACS Appl. Bio Mater. 2023, 6, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Latifi, N.; Asgari, M.; Vali, H.; Mongeau, L. A tissue-mimetic nano-fibrillar hybrid injectable hydrogel for potential soft tissue engineering applications. Sci. Rep. 2018, 8, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.K.; Alexander, A.; Sivaram, M.; Agrawal, M.; Singhvi, G.; Sharma, S.; Dayaramani, R. Uncovering the Diversification of Tissue Engineering on the Emergent Areas of Stem Cells, Nanotechnology and Biomaterials. Curr. Stem Cell Res. Ther. 2020, 15, 187–201. [Google Scholar] [CrossRef]
- Wei, H.Q.; Zhang, B.; Lei, M.; Lu, Z.; Liu, J.P.; Guo, B.L.; Yu, Y. Visible-Light-Mediated Nano-biomineralization of Customizable Tough Hydrogels for Biomimetic Tissue Engineering. ACS Nano 2022, 16, 4734–4745. [Google Scholar] [CrossRef]
- Jabbari, E. Challenges for Natural Hydrogels in Tissue Engineering. Gels 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Nageeb, M.; Nouh, S.R.; Bergman, K.; Nagy, N.B.; Khamis, D.; Kisiel, M.; Engstrand, T.; Hilborn, J.; Marei, M.K. Bone Engineering by Biomimetic Injectable Hydrogel. Mol. Cryst. Liq. Cryst. 2012, 555, 177–188. [Google Scholar] [CrossRef]
- Sultan, N.; Jayash, S.N. Evaluation of osteogenic potential of demineralized dentin matrix hydrogel for bone formation. BMC Oral Health 2023, 23, 247. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Bai, X.; Yang, J.J.; Li, J.S.; Xing, J.Q.; Yuan, H.; Xie, J.; Li, J.Y. Preparation and characterisation of a gellan gum-based hydrogel enabling osteogenesis and inhibiting Enterococcus faecalis. Int. J. Biol. Macromol. 2020, 165, 2964–2973. [Google Scholar] [CrossRef]
- Zeng, D.; Shen, S.H.; Fan, D.D. Molecular design, synthesis strategies and recent advances of hydrogels for wound dressing applications. Chin. J. Chem. Eng. 2021, 30, 308–320. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Bian, S.Q.; Li, Z.Q.; Zhang, Z.Y.; Liu, Y.; Zhai, X.Y.; Pan, H.B.; Zhao, X.L. Catechol modified quaternized chitosan enhanced wet adhesive and antibacterial properties of injectable thermo-sensitive hydrogel for wound healing. Carbohydr. Polym. 2020, 249, 116826. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Sohail, M.; Karperien, M.; Johnbosco, C.; Mahmood, A.; Kousar, M. Chitosan and carboxymethyl cellulose-based 3D multifunctional bioactive hydrogels loaded with nano-curcumin for synergistic diabetic wound repair. Int. J. Biol. Macromol. 2023, 227, 1203–1220. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Ma, B.; Xue, J.M.; Wu, J.F.; Chang, J.; Wu, C.T. Defective Black Nano-Titania Thermogels for Cutaneous Tumor-Induced Therapy and Healing. Nano Lett. 2019, 19, 2138–2147. [Google Scholar] [CrossRef]
- Gou, L.; Xiang, M.L.; Ni, X.L. Development of wound therapy in nursing care of infants by using injectable gelatin-cellulose composite hydrogel incorporated with silver nanoparticles. Mater. Lett. 2020, 277, 128340. [Google Scholar] [CrossRef]
- Yin, X.C.; Fan, X.Y.; Zhou, Z.P.; Li, Q. Encapsulation of berberine decorated ZnO nano-colloids into injectable hydrogel using for diabetic wound healing. Front. Chem. 2022, 10, 14. [Google Scholar] [CrossRef]
- Qi, X.L.; Huang, Y.J.; You, S.Y.; Xiang, Y.J.; Cai, E.Y.; Mao, R.T.; Pan, W.H.; Tong, X.Q.; Dong, W.; Ye, F.F.; et al. Engineering Robust Ag-Decorated Polydopamine Nano-Photothermal Platforms to Combat Bacterial Infection and Prompt Wound Healing. Adv. Sci. 2022, 9, 2106015. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, S.; Zhang, B.J.; Li, M.; Diao, K.; Zhang, Z.L.; Li, J.; Xu, Y.; Wang, X.H.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Tan, Y.F.; Chen, X.Y.; Ran, Y.Q.; Tong, Q.L.; Tang, L.W.; Su, W.; Wang, X.L.; Li, X.D. Injectable oxidized alginate/carboxylmethyl chitosan hydrogels functionalized with nanoparticles for wound repair. Carbohydr. Polym. 2022, 293, 119733. [Google Scholar] [CrossRef]
- Mei, J.W.; Zhou, J.; Kong, L.T.; Dai, Y.; Zhang, X.Z.; Song, W.Q.; Zhu, C. An injectable photo-cross-linking silk hydrogel system augments diabetic wound healing in orthopaedic surgery through spatiotemporal immunomodulation. J. Nanobiotechnol. 2022, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.Q.; Hao, L.Z.; Qiu, X.; Wu, J.; Chang, H.; Kuang, G.M.; Zhang, S.; Hu, X.H.; Dai, Y.K.; Zhou, Z.Y.; et al. An Injectable Rapid-Adhesion and Anti-Swelling Adhesive Hydrogel for Hemostasis and Wound Sealing. Adv. Funct. Mater. 2022, 32, 2207741. [Google Scholar] [CrossRef]
- Hu, Q.S.; Nie, Y.; Xiang, J.; Xie, J.W.; Si, H.B.; Li, D.H.; Zhang, S.Y.; Li, M.; Huang, S.S. Injectable sodium alginate hydrogel loaded with plant polyphenol-functionalized silver nanoparticles for bacteria-infected wound healing. Int. J. Biol. Macromol. 2023, 234, 123691. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Su, Y.; Wang, C.X.; Lei, B.; Song, X.J.; Wang, W.J.; Wu, P.; Liu, X.Y.; Dong, X.C.; Zhong, L.P. Injectable Tissue-Adhesive Hydrogel for Photothermal/Chemodynamic Synergistic Antibacterial and Wound Healing Promotion. ACS Appl. Mater. Interfaces 2023, 15, 2714–2724. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, G.P.; Bu, H.T.; Zhang, H.Y.; Li, W.X.; Song, K.; Jiang, G.B. An injectable, adhesive, and self-healable composite hydrogel wound dressing with excellent antibacterial activity. Chem. Eng. J. 2022, 450, 138201. [Google Scholar] [CrossRef]
- Wang, L.; Hussain, Z.; Zheng, P.H.; Zhang, Y.J.; Cao, Y.; Gao, T.; Zhang, Z.Z.; Zhang, Y.H.; Pei, R.J. A mace-like heterostructural enriched injectable hydrogel composite for on-demand promotion of diabetic wound healing. J. Mater. Chem. B 2023, 11, 2166–2183. [Google Scholar] [CrossRef]
- Ha, S.S.; Kim, J.H.; Savitri, C.; Choi, D.; Park, K. Nano-Sized Extracellular Matrix Particles Lead to Therapeutic Improvement for Cutaneous Wound and Hindlimb Ischemia. Int. J. Mol. Sci. 2021, 22, 13265. [Google Scholar] [CrossRef]
- Perez-Rafael, S.; Ivanova, K.; Stefanov, I.; Puiggali, J.; del Valle, L.J.; Todorova, K.; Dimitrov, P.; Hinojosa-Caballero, D.; Tzanov, T. Nanoparticle-driven self-assembling injectable hydrogels provide a multi-factorial approach for chronic wound treatment. Acta Biomater. 2021, 134, 131–143. [Google Scholar] [CrossRef]
- Palem, R.R.; Madhusudana Rao, K.; Kang, T.J. Self-healable and dual-functional guar gum-grafted-polyacrylamidoglycolic acid-based hydrogels with nano-silver for wound dressings. Carbohydr. Polym. 2019, 223, 115074. [Google Scholar] [CrossRef]
- Cheng, W.H.; Chen, Y.H.; Teng, L.J.; Lu, B.H.; Ren, L.; Wang, Y.J. Antimicrobial colloidal hydrogels assembled by graphene oxide and thermo-sensitive nanogels for cell encapsulation. J. Colloid Interface Sci. 2018, 513, 314–323. [Google Scholar] [CrossRef]
- Niu, Y.L.; Guo, T.T.; Yuan, X.Y.; Zhao, Y.H.; Ren, L.X. An injectable supramolecular hydrogel hybridized with silver nanoparticles for antibacterial application. Soft Matter 2018, 14, 1227–1234. [Google Scholar] [CrossRef]
- Ocampo, J.I.G.; Bassous, N.; Orozco, C.P.O.; Webster, T.J. Evaluation of cytotoxicity and antimicrobial activity of an injectable bone substitute of carrageenan and nano hydroxyapatite. J. Biomed. Mater. Res. Part A 2018, 106, 2984–2993. [Google Scholar] [CrossRef]
- Wang, M.; Sa, Y.; Li, P.; Guo, Y.R.; Du, Y.M.; Deng, H.B.; Jiang, T.; Wang, Y.N. A versatile and injectable poly(methyl methacrylate) cement functionalized with quaternized chitosan-glycerophosphate/nanosized hydroxyapatite hydrogels. Mater. Sci. Eng. C 2018, 90, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhao, N.U.; Huang, Y.X.; He, R.; Xu, S.C.; Yuan, W.Z. Coordination of injectable self-healing hydrogel with Mn-Zn ferrite@mesoporous silica nanospheres for tumor MR imaging and efficient synergistic magnetothermal-chemo-chemodynamic therapy. Chem. Eng. J. 2020, 401, 126100. [Google Scholar] [CrossRef]
- Jooken, S.; Deschaume, O.; Bartic, C. Nanocomposite Hydrogels as Functional Extracellular Matrices. Gels 2023, 9, 153. [Google Scholar] [CrossRef]
- Xu, J.P.; Chen, T.Y.; Tai, C.H.; Hsu, S.H. Bioactive self-healing hydrogel based on tannic acid modified gold nano-crosslinker as an injectable brain implant for treating Parkinson’s disease. Biomater. Res. 2023, 27, 8. [Google Scholar] [CrossRef] [PubMed]
- Osi, B.; Khoder, M.; Al-Kinani, A.A.; Alany, R.G. Pharmaceutical, biomedical and ophthalmic applications of biodegradable polymers (BDPs): Literature and patent review. Pharm. Dev. Technol. 2022, 27, 341–356. [Google Scholar] [CrossRef]
- Guan, X.F.; Avci-Adali, M.; Alarcin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.X.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Tan, B.Y.; Chen, J.R.; Bao, R.; Zhang, X.R.; Liang, S.; Shang, Y.Y.; Liang, W.; Cui, Y.L.; Fan, G.W.; et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 2018, 160, 69–81. [Google Scholar] [CrossRef]
- Jeong, S.H.; Fan, Y.F.; Baek, J.U.; Song, J.; Choi, T.H.; Kim, S.W.; Kim, H.E. Long-lasting and bioactive hyaluronic acid-hydroxyapatite composite hydrogels for injectable dermal fillers: Physical properties and in vivo durability. J. Biomater. Appl. 2016, 31, 464–474. [Google Scholar] [CrossRef]
- Wang, A.Y.; Podlasek, C.A. Role of Nanotechnology in Erectile Dysfunction Treatment. J. Sex. Med. 2017, 14, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.H.; Wu, H.; Ren, X.Y.; Wang, J.W.; Liu, R.Q.; Hu, B.H.; Gu, N. Dual-network hydrogel based on ionic nano-reservoir for gastric perforation sealing. Sci. China Mater. 2022, 65, 827–835. [Google Scholar] [CrossRef]





| Hydrogel Composition | Outcomes | Ref. |
|---|---|---|
| Polyurethane hydrogel and copper-substituted bioactive mesoporous glasses (Cu-MBGs) | Injectable hybrid formulations based on polyurethane hydrogel and Cu-MBGs enable simultaneous localized co-delivery of functional ions and drugs with sustained release profiles and tunable residence time at the pathological site. | [103] |
| Dexmedetomidine-loaded nano-hydrogel | Injectable nano-drug delivery system combined with Dexmedetomidine for thoracic paravertebral block significantly relieved pain, improved sleep quality, and reduced the need for remedial analgesia and side effects after thoracic surgery. | [3] |
| Nano-thermogel system of polyethylene glycol-polycaprolactone-polyethylene glycol (PEG-PCL-PEG) triblock with poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded p11 peptide | Controlled release of p11 peptide achieved with nano-thermogel system, showing potential for effective treatment of ocular disorders characterized by angiogenesis. | [4] |
| Hybrid silk hydrogel with carbon nanotubes | Hybrid silk hydrogel with carbon nanotubes enables localized, targeted, and on-demand delivery of anticancer drugs, reducing systemic side effects. | [40] |
| pH- and temperature-responsive hydrogels poly(ethylene glycol)-poly(beta-aminoester urethane) | Chondroitin sulfate nanogels incorporated into pH- and temperature-responsive hydrogels deliver cisplatin selectively to cancer cells, improving targeted therapy. | [41] |
| Urothelium-adherent, ion-triggered liposome-in-gel system | Liposome-in-gel system enhances drug penetration and adhesion in the bladder, showing prolonged drug retention and potential use in intravesical applications. | [58] |
| Composite liposome-in-gel system (gellan hydrogel) | Liposome-in-gel system delivers radiosensitizer paclitaxel to tumor site, enhancing the effect of concurrent radiotherapy and improving tumor volume reduction and animal survival. | [59] |
| Four-arm maleimide-functionalized polyethylene glycol (PEG-4MAL) hydrogel system | PEG-4MAL hydrogel acted as a mechanical pillow to protect the knee joint, inhibit cartilage degradation, and prevent osteophyte formation in an in vivo load-induced osteoarthritis mouse model. | [55] |
| Injectable hydrogel (amphiphilic polymers) system with tumor-targeting nano-micelles | The injectable hydrogel system sustainedly released tumor-targeting nano-micelles, which exhibited GSH-responsive drug release behavior, leading to enhanced antitumor efficiency and improved bioavailability of the drug. | [56] |
| Injectable thermosensitive photothermal-network hydrogel | The thermosensitive photothermal-network hydrogel demonstrated high photothermal conversion efficiency, reversible gel–sol transition, and on-demand drug release, enabling effective near-infrared-triggered photothermal-chemotherapy for breast cancer treatment. | [60] |
| Ultrasoft polymeric DNA networks of variable crystallinities | Ultrasoft self-supporting polymerized DNA networks with variable crystallinities showed tunable mechanical properties, pH-responsive drug release, and crystallinity-dependent antitumor efficacy, providing a favorable microenvironment for demand-localized drug delivery. | [62] |
| Sugar-based injectable thermoresponsive hydrogel | Injectable thermoresponsive hydroxypropyl guar-graft-poly(N-vinylcaprolactam) (HPG-g-PNVCL) hydrogel and its composite with nano-hydroxyapatite (n-HA) showed biocompatibility, thermoreversibility, slow drug release, and supported osteoblastic cell growth, making them potential scaffolds for bone tissue engineering. | [63] |
| Injectable polysaccharide hydrogel with hydroxyapatite and calcium carbonate | Injectable and degradable polysaccharide-based hydrogels integrated with hydroxyapatite and calcium carbonate show controlled gelation, enhanced mechanical properties, sustained drug release, antibacterial properties, and self-healing capabilities, making them promising for bone regeneration. | [64] |
| Injectable hydrogel nanomaterials (PNIPAAM with CS, APS and cross-linked with PEGDMA) | Continuous subcutaneous insulin infusion (CSII) showed better blood glucose control and lower incidence of hypoglycemia compared with multiple daily injections (MDI) in children with type 1 diabetes mellitus (T1DM). | [68] |
| Injectable liquid metal nanoflake hydrogel | The LM-doxorubicin nanoflake hydrogel with pH-triggered drug release shows enhanced therapeutic efficacy in preventing postoperative tumor relapse. | [72] |
| Bio-inspired fluorescent nano-injectable hydrogel prepared by copolymerization of N-isopropylacrylamide (NIPAM) and acrylic functionalized nucleobase (adenine) | The injectable hydrogel with a phase-separated structure enables sequential release of different drugs and exhibits fluorescence characteristics, making it suitable for dual drug delivery and imaging. | [73] |
| Injectable micromotor@hydrogel system | The micromotor@hydrogel drug delivery system protects micromotors and enables sustained release of erythromycin, exhibiting excellent antibacterial effect for the treatment of bacterial infections. | [74] |
| Nano polydopamine crosslinked thiol-functionalized hyaluronic acid hydrogel | The hydrogel, crosslinked using polydopamine nanoparticles, shows good injectability, mechanical stability, sustained drug release, and enhanced endothelial cell behavior, making it suitable for angiogenic drug delivery and tissue engineering. | [75] |
| poly(lactic-co-glycolic acid) (PLGA) MS loaded with melatonin(Mel) + Laponite hydrogels | The injectable micro-gel compound and nano-PM compound based on sustained-release microspheres provide stable and prolonged drug release, repair neural function, and reduce biomaterial loss for the treatment of spinal cord injury. | [76] |
| Injectable thermosensitive hydrogel (poly(d,l-lactide)-poly(ethylene glycol)-poly(d,l-lactide))containing erlotinib-loaded hollow mesoporous silica nanoparticles | The injectable ERT@HMSNs/gel composite provides sustained release of erlotinib, improves efficacy against NSCLC, and demonstrates an impressive balance between antitumor efficacy and systemic safety. | [77] |
| Injectable PEG-induced silk nanofiber hydrogel | The injectable silk fibroin nanofiber hydrogel, prepared using a dissolving technique and PEG, exhibits fast gelation, amorphous structure, and superior antibacterial properties, making it suitable for vancomycin delivery in tissue engineering. | [78] |
| Injectable hydrogel and nanoparticle system | The injectable hydrogel-nanoparticle system provides a promising approach for delivering microRNAs to cardiac tissue, improving cardiac function after myocardial infarction. | [80] |
| Sustained delivery system incorporating P24-loaded PLGA microspheres and nano-hydroxyapatite in composite hydrogel | The composite hydrogel with sustained P24 peptide release enhances bone tissue regeneration and shows potential for improving bone defect treatment in tissue engineering. | [81] |
| Injectable hydrogel (PLGA-PEG-PLGA) modified with hydroxyapatite particles | The hydrogel modified with nano- and core-shell hydroxyapatite particles enables controlled release of calcium cations, offering potential applications in bone regeneration. | [82] |
| Injectable thermo-sensitive hydrogel (hyaluronic acid-chitosan-g-poly(N-isopropylacrylamide) | The injectable hydrogel, combined with folic acid-conjugated graphene oxide (GOFA) nano-carrier, provides controlled and targeted intratumoral delivery of doxorubicin for breast cancer therapy. | [67] |
| Silica-triptorelin acetate depot | The silica-triptorelin acetate depot demonstrates sustained release of triptorelin, comparable to Pamorelin(R), and maintains equivalent pharmacodynamic effects with lower Cmax values, offering potential for prolonged therapeutic effects. | [83] |
| Injectable 3-D nano-scaffold hydrogel | Mixing peptide-amphiphile (PA) with BMP-2 formed a transparent hydrogel that induced significant ectopic bone formation, offering potential for tissue regeneration. | [84] |
| Multi-functional calcitriol delivery system for osteoporotic bone regeneration based on poly(D, L-lactide)-poly(ethylene glycol)-poly(D, L-lactide) hydrogel | PDLLA-PEG-PDLLA hydrogel integrated with HA-D and PCL-PEG-NH2 micelles enabled sustained release of calcitriol, promoting osteogenesis and bone regeneration both in vitro and in vivo. | [85] |
| FRET-enabled monitoring of thermosensitive micellar hydrogel assembly (poly(epsilon-caprolactone-co-1,4,8-trioxa[4.6]spiro-9-undecanone)-b-poly(ethylene glycol)-b-poly(epsilon-caprolactone-co-1,4,8-trioxa[4.6]spiro-9-undecanone) triblock copolymer. | PECT triblock copolymer facilitated hydrogel formation and sustained release of micelles, allowing precise imaging of the fate of macro biodegradable materials and potential for co-delivery of therapeutic agents. | [86] |
| Thermosensitive micellar hydrogel (PECT triblock copolymer) | Injectable MHg depot composed of PECT micelles immobilized DOX and I-131-HA, enabling localized delivery, sustained release, and enhanced antitumor effect with reduced side effects. | [87] |
| Cell penetrable nano-polyplex hydrogel | Protamine-conjugated poly(organo-phosphazene) hydrogel forms after injection, releasing nano-polyplexes for effective siRNA delivery and long-term gene silencing on target site. | [88] |
| Biopolymer nano-network (Chitosan and dextran sulfate) | Colloidal nano-network made of chitosan and dextran sulfate encapsulates PA-13 antimicrobial peptide, protecting it from degradation, and delivers it locally, eliminating bacteria without impacting bioactivity. | [89] |
| Graphene oxide-containing self-assembling peptide hybrid hydrogels | GO-reinforced peptide hydrogels promote high cell viability and metabolic activity, showing potential as injectable scaffolds for in vivo delivery of nucleus pulposus cells. | [91] |
| Alginate nanohydrogels | BMP-2@ANH system promotes proliferation and differentiation of human bone marrow stromal cells into osteoblasts, offering a potential method for facilitating stem cell differentiation in vivo. | [92] |
| Nano-hybrid oligopeptide hydrogel | Topical delivery of docetaxel using DTX-CTs/Gel inhibited post-surgical tumor recurrence and enhanced cell death, showing promise for cancer therapy. | [104] |
| Chitosan-incorporated fatty acid vesicles hydrogel | Curcumin-loaded OCLAVs-CS hydrogel effectively reduced burst release, exhibited enhanced antioxidant activity, and can serve as an injectable or 3D printable drug delivery system. | [93] |
| Injectable two-step forming hydrogel (chitosan, collagen, hydroxypropyl-gamma-cyclodextrin and polyethylene glycol) | Hydrogel composed of chitosan, collagen, hydroxypropyl-gamma-cyclodextrin, and polyethylene glycol exhibited controlled release properties, adaptability for minimally invasive implantation, and support for cell proliferation. | [94] |
| Injectable poly(phosphazene) hydrogels with different anionic sidechains | Tunable hydrogel systems with optimized physical properties and BMP-2 release rates were identified, enabling effective bone regeneration in a critical-sized calvarial defect model. | [95] |
| Injectable hydrogel depot system using Exendin 4 (Ex-4) interactive and complex-forming polymeric ionic nanoparticles | The hydrogel system demonstrated prolonged release of Exendin 4 (Ex-4), offering potential as a long-term effective and reproducible treatment for type 2 diabetes mellitus. | [96] |
| Two-in-one injectable micelleplex-loaded thermogel system composed with polymerization of poly(ethylene glycol), poly(propylene glycol), and poly(3-hydroxybutyrate) | The novel nanoparticle-hydrogel system enabled prolonged release of pDNA micelleplexes, indicating its potential for sustained gene delivery applications. | [105] |
| Injectable alginate-based hydrogel cross-linked via the regulated release of divalent ions from the hydrolysis of D-glucono-delta-lactone | The hydrogel exhibited improved mechanical properties through the slow release of divalent ions from D-glucono-delta-lactone, making it suitable for bone tissue engineering applications. | [97] |
| Injectable bone regeneration composite (IBRC) with nano-hydroxyapatite/collagen particles in an alginate hydrogel carrier | The controlled release of rhBMP-2 from IBRC promoted bone formation, highlighting its potential as a bone defect repair material for clinical applications. | [98] |
| Moldable/injectable calcium phosphate cement (CPC) composite scaffolds | Strong, macroporous CPC scaffolds were developed, suitable for bone regeneration, cell delivery, and growth factor release, with potential applications in dental, craniofacial, and orthopedic reconstructions. | [100] |
| Injectable and quadruple-functional hydrogel (folate/polyethylenimine-conjugated poly(organophosphazene) polymer) encapsulated with siRNA and Au-Fe3O4 nanoparticles | The hydrogel-based delivery method improved tumor targeting efficiency compared with intravenous delivery, enabling sustained release, passive targeting, active targeting, and magnetic targeting for enhanced therapeutic effects. | [101] |
| Injectable thermosensitive polymeric hydrogel of poly(organophosphazene) combined with superparamagnetic iron oxide nanoparticles | The designed injectable hydrogel allowed controlled release of TRAIL/SPION nanocomplex under hyperthermia, resulting in enhanced cytotoxicity against TRAIL-resistant cancer cells and significant tumor reduction in vivo. | [102] |
| Hydrogel Composition | Outcomes | Ref. |
|---|---|---|
| Core-shell scaffold based on aligned conductive nanofiber yarns (NFYs) within a methacrylated gelatin (GelMA) hydrogel | Aligned nanofiber yarns within a hydrogel scaffold induce neurite alignment and extension, promoting the alignment and elongation of nerve cells, offering potential for nerve tissue engineering applications. | [106] |
| In situ forming thermosensitive chitosan-glycerol phosphate hydrogel loaded with risedronate and nano-hydroxyapatite | The prepared hydrogel formulation with risedronate and nano-hydroxyapatite showed sustained drug release, enhanced Saos-2 cell proliferation, alkaline phosphatase activity, and calcium deposition, making it a promising option for bone tissue engineering. | [6] |
| Protein-based hydrogels derived from natural tissues | Investigating the nano-/micro-structure and composition of protein-based hydrogels derived from natural tissues is crucial for their widespread use in tissue engineering and regenerative medicine. | [129] |
| Calcium alginate-gum tragacanth hydrogels incorporated with cobalt-doped nano-hydroxyapatite | The hydrogels exhibited enhanced swelling, degradation, diffusion, long-term viability of encapsulated cells, osteogenic differentiation, and angiogenic properties, making them suitable for bone tissue engineering applications. | [107] |
| Chemically crosslinked collagen/chitosan/hyaluronic acid hydrogels | Optimization of the hydrogel composition showed that using high concentrations of crosslinking agent and adjusting the hyaluronic acid content resulted in hydrogels with compact structure, good mechanical properties, prolonged degradation profile, and suitable biocompatibility for bone regeneration applications. | [7] |
| Injectable PCL-PEG-PCL-Col/nHA hydrogels | PCL-PEG-PCL-Col/nHA hydrogels showed successful integration of collagen and nano-hydroxyapatite, delayed biodegradation rate, no prominent pro-inflammatory response, and increased expression of CD31 and IL-10, indicating biocompatibility for hard tissue regeneration. | [108] |
| Enzymatically crosslinked CMC/gelatin/nHAp injectable gels | The enzymatically crosslinked injectable gels exhibited rigidity, adjustable crosslinking degree and strength, increased pore sizes with higher gelatin concentration, and support for osteoblast cell proliferation and differentiation, making them suitable for in situ bone tissue engineering applications. | [8] |
| Injectable semi-interpenetrating network hydrogel with chondroitin sulfate nanoparticles (ChS-NP)s and nanohydroxyapatite (nHA) | The gradient hydrogel construct demonstrated mineralized subchondral and chondral zones, higher osteoblast proliferation in the subchondral zone, porous structure with gradient interface, layer-specific retention of cells, and in vivo osteochondral regeneration with hyaline cartilage formation and subchondral bone integration. | [109] |
| Alginate dialdehyde-gelatin scaffolds with zirconium oxide nanoparticles | Incorporation of ZrO2 nanoparticles into alginate-gelatin hydrogels enhances mechanical and chemical properties. Nanocomposite hydrogels exhibit improved swelling behavior, controlled biodegradation, cell viability, and attachment, making them suitable for cartilage tissue regeneration. | [112] |
| Alginate-O-carboxymethyl chitosan/nano fibrin composite hydrogels | Alginate/O-CMC hydrogel blend demonstrated superior properties for tissue engineering applications, supporting the survival, adhesion, proliferation, and differentiation of adipose-derived stem cells. | [113] |
| Injectable carrageenan nanocomposite hydrogel | Carrageenan nanocomposite hydrogel incorporated with whitlockite nanoparticles and an angiogenic drug promoted osteogenesis and angiogenesis in vitro, showing potential for bone tissue engineering. | [114] |
| Injectable thermosensitive hydrogel made of poly(ethylene glycol)-poly(epsilon-caprolactone)-poly(ethylene glycol) (PECE) and nanohydroxyapatite (n-HA) | Thermosensitive hydrogel nanocomposites exhibited good thermosensitivity, injectability, and 3D network structure, making them promising for injectable orthopedic tissue engineering. | [115] |
| Chitosan/collagen hydrogels nano-engineered with functionalized single-wall carbon nanotubes | Integration of COOH-SWCNTs into chitosan and collagen hydrogels increased mechanical strength, bioactivity, and potential for bone tissue engineering and regenerative medicine. | [116] |
| Nano-hydroxyapatite/glycol chitosan/hyaluronic acid composite hydrogel | Composite hydrogel exhibited porous structure, enzymatic degradation, and cytocompatibility, making it suitable for bone tissue engineering applications. | [117] |
| Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose hydrogel | Incorporation of laponites into silated hydroxypropylmethyl cellulose hydrogel resulted in an interpenetrating network that improved mechanical properties without compromising cytocompatibility, oxygen diffusion, or chondrogenic cell functionality. | [118] |
| Nano SIM@ZIF-8-modified injectable high-intensity biohydrogel composed of composed of poly (ethylene glycol) diacrylate (PEGDA) and sodium alginate (SA) + nano simvastatin-laden zeolitic imidazolate framework-8 | nSZPS hydrogel stimulates osteogenic differentiation, inhibits adipogenic differentiation, exhibits excellent injectability, mechanical strength, and promotes bone regeneration in hyperlipidemic microenvironments. | [119] |
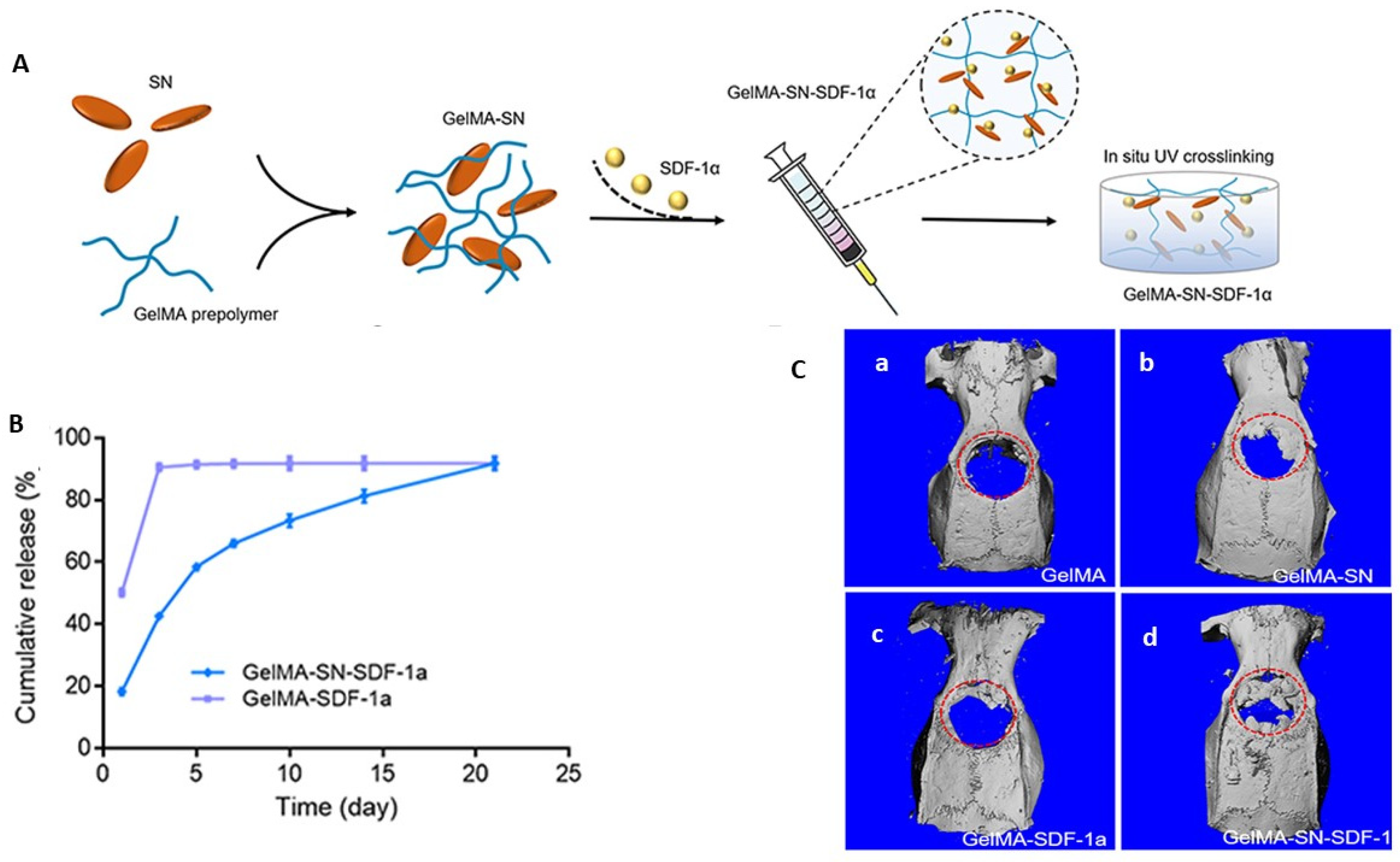
| Nano-silicate-reinforced and SDF-1alpha-loaded gelatin-methacryloyl hydrogel | GelMA-SN-SDF-1alpha hydrogel demonstrates injectability, controlled release of SDF-1alpha, MSC migration and homing, and excellent bone regeneration ability in critical-sized calvaria defects. | [120] |
| Succinylated gelatin cross-linked with aldehyde heparin formed nanoparticles, which were mineralized with hydroxyapatite (mineralized heparin-gelatin nanoparticles) | These nanoparticles may enhance the mechanical properties of injectable hydrogels for bone regeneration. | [122] |
| Injectable platelet-rich plasma (PRP)/cell-laden microcarrier/hydrogel composite system | Gelatin methacryloyl (GelMA) and chitosan hydrogels were used to prepare scalable interpenetrating network GelMA/chitosan-microcarriers (IGMs) loaded with PRP and dermal papilla cells (DPCs). The composite system promoted DPC viability, hair inducibility, and hair follicle regeneration. | [123] |
| Polysaccharide-based injectable hydrogel compositing nano-hydroxyapatite | N-carboxyethyl chitosan (NCEC) and oxidized dextran (ODex) were cross-linked via Schiff base linkage to form an injectable hydrogel. The hydrogel, composited with nano-hydroxyapatite (nHAP), exhibited interconnected porous structure and showed excellent bone repair effect in vivo. | [124] |
| Bioactive glass nanoparticle-incorporated triblock copolymeric injectable hydrogel | Injectable hydrogel with bioactive glass nanoparticles showed good gelling and injectability properties, excellent swelling properties, enhanced bone cell proliferation, ALP activity, and apatite mineralization for accelerated in vitro osteogenesis. | [125] |
| Nano-fibrillar hybrid injectable hydrogel with heterotypic collagen fibrils | Injectable hydrogel with semi-interpenetrating networks of heterotypic collagen fibrils in a glycol-chitosan matrix showed nano-fibrillar porous structure, mechanical stability, prolonged half-life, and support for cell implantation. | [126] |
| Visible-light-mediated nano-biomineralization of customizable tough hydrogels | Rapid preparation of biomineralized tough hydrogels with improved mechanical and biological properties under visible light irradiation, suitable for customizable skin repair and bone regeneration. | [128] |
| Hydrogel Composition | Outcomes | Ref. |
|---|---|---|
| RADA16 peptide hydrogel filled with porous calcium sulfate/nano-hydroxyapatite (CaSO4/HA) composite biomaterial | Controlled and sustainable release of bFGF for more than 32 days from RADA16/CaSO4/HA composite biomaterial, leading to enhanced osteogenic differentiation in vitro and improved bone formation in vivo. | [9] |
| Injectable chitin-PLGA hydrogel containing bioglass nanoparticles (nBG) or whitlockite nanoparticles (nWH) with FGF-18 | CGnWHF (nWH + FGF-18 containing CG) showed the highest osteogenic potential and near-complete bone regeneration in critical-sized defect region compared to other groups, indicating its potential for craniofacial bone defects. | [10] |
| GelMA-HAMA/nHAP composite hydrogel with human-urine-derived stem cell exosomes | Composite hydrogel with controlled release of USCEXOs promotes osteogenesis and angiogenesis, enhancing bone regeneration in vivo. | [11] |
| Injectable bone regeneration composite (IBRC) with nano-hydroxyapatite/collagen (nHAC) particles in alginate hydrogel carrier | IBRC exhibited controllable degradability and biocompatibility, making it a promising material for bone repair and tissue engineering. | [12] |
| poly (caprolactone)-poly(ethylene glycol)-poly(caprolactone) + gelatin and nano-hydroxyapatite | Hydrogels showed successful integration of Gel and nHA, lacked inflammation, and exhibited biocompatibility without toxic effects in in vivo conditions. | [13] |
| nano-hydroxyapatite hybrid methylcellulose hydrogel carrying bone mesenchymal stem cells | Addition of nHA to MC hydrogel enhances cell survival, osteogenic differentiation, and remediation efficiency in vivo. | [14] |
| Thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite | Composite hydrogel exhibits thermosensitivity, biocompatibility, and better performance in guided bone regeneration compared to self-healing processes. | [15] |
| Injectable polysaccharide hydrogel-loaded nano-hydroxyapatite | Hydrogel/hydroxyapatite composite scaffold enhances new bone area and alveolar ridge promotion, while promoting soft tissue healing. | [16] |
| TME-modulated hydrogel (MBGN/Gel/OCS) | Hydrogel interferes with tumor microenvironment, overcomes cancer resistance, and promotes sustained drug release and osteogenesis. | [17] |
| Injectable hydrogel containing cisplatin (DDP) and polydopamine-decorated nano-hydroxyapatite (DDP/PDA/nHA) | Exhibits dual functions of tumor therapy and bone regeneration, effectively ablating tumor cells and inducing bone regeneration. | [18] |
| Light-cured hyaluronic acid composite hydrogels (nano-HA/chitosan) | Enhance mechanical properties and osteogenic potential, promising for bone regeneration applications. | [19] |
| Nanocellulose reinforced alginate hydrogel(AC) that carried beta-tricalcium phosphate (beta-TCP) nano-powder and liver-derived extracellular matrix (ECM) from porcine | ETAC Show enhanced cytocompatibility, accelerated bone regeneration, and improved healing quality compared to TAC and AC beads. | [20] |
| Chitin-CaSO4-nFibrin gel | Demonstrates improved rheology, angiogenic potential, and osteo-regeneration compared to chitin control. | [21] |
| Silk-hydroxyapatite composite | Exhibits injectability, thixotropy, and osteodifferentiation potential, supporting improved osteogenesis and bone defect healing. | [22] |
| nHA@Gel/ADA hydrogel with gelatin, alginate dialdehyde, Ca2+, borax, and nano-sized hydroxyapatite | Promotes efficient repair of critical-size skull bone defects and supports macrophage-BMSC crosstalk. | [23] |
| Composite hydrogel system incorporating PLGA-BMP-2 and PLA-17 beta-estradiol microspheres in a hydrogel core | Shows controlled release, refilling of bone defects, and regeneration in osteoporotic rats. | [24] |
| Injectable hydrogel with chitosan/beta-glycerophosphate disodium salt (CS/GP) and chitin nano-whiskers (CNWs) | Exhibits improved mechanical properties, gelation speed, and biocompatibility, suitable for tissue engineering scaffold applications. | [25] |
| PDH/mICPN hydrogel composed of DMAEMA, HEMA, CaP nanoparticles (ICPNs), and poly-L-glutamic acid (PGA) | Self-assembles in situ, demonstrating enhanced mechanical strength, cell adhesion, and osteodifferentiation for bone regeneration. | [26] |
| Injectable bone regeneration composite (IBRC) with calcium alginate hydrogel matrix carrying nano-hydroxyapatite/collagen | Demonstrates structural homogeneity, good biocompatibility, and the ability to promote bone healing. | [27] |
| Biomimetic/osteoinductive injectable hyaluronan-based hydrogel loaded with nano-hydroxyapatite crystals (Hya/HA) | Shows potential for enhancing bone architecture, with an osteoinductive effect and improved bone density and architecture in the rabbit distal femur. | [130] |
| Demineralized dentin matrix hydrogel (DDMH) | Exhibits a porous structure and supports viability and differentiation of BMMSCs, with potential for promoting bone formation. A 50% concentration of DDMH shows promising results. | [131] |
| Gellan gum (GG)-based injectable hydrogel loaded with chlorhexidine (CHX) and nanohydroxyapatite (nHA) | Demonstrates superior biocompatibility, mechanical strength, osteogenic properties, and antibacterial effect against E. faecalis. Shows potential for treating infectious bone defects. | [132] |
| Hydrogel Composition | Outcomes | Ref. |
|---|---|---|
| PLEL-nBG-QCS-C hydrogel: poly(d,llactide)-poly(ethylene glycol)-poly(d,l-lactide) PLEL, nano-scaled bioactive glass (nBG), and catechol modified quaternized chitosan (QCS-C) | Exhibits thermo-sensitivity, antibacterial properties, tissue adhesion, and accelerates wound healing | [134] |
| Chitosan-CMC-g-PF127 injectable hydrogels loaded with nano-curcumin | Show controlled release, biocompatibility, and promote diabetic wound repair | [135] |
| BT-CTS thermogel: Injectable thermosensitive hydrogel with black titania nanoparticles (B-TiO2-x) in chitosan matrix | Provides effective tumor therapy, wound closure, and tissue regeneration for skin tumors | [136] |
| Injectable silver-gelatin-cellulose ternary hydrogel dressing with aminated silver nanoparticles | Exhibits antibacterial properties and enhances cutaneous wound healing in infant nursing care | [137] |
| ZnO-Ber/H: Berberine-modified ZnO nano-colloids hydrogel | Promotes diabetic wound healing by enhancing wound healing rate, regulating antioxidant stress factors, downregulating inflammatory factors, and promoting the expression of vascular and epithelial tissue-related factors | [138] |
| CG/PDA@Ag hydrogel: Cationic guar gum hydrogel encapsulating Polydopamine NPs with Ag (PDA@Ag) | Combines high photothermal conversion efficiency and inherent antibacterial ability, demonstrating superior antibacterial efficacy for photothermal antibacterial therapy | [139] |
| Injectable alginate nanocomposite hydrogel containing nano-sized calcium fluoride particles | Enhances bioactivity, antibacterial property, cell proliferation, migration, and extracellular matrix deposition for accelerated wound healing | [28] |
| GG-MA/Laponite hydrogel: Gellan gum methacrylate (GG-MA) combined with laponite (R) XLG | Shows improved mechanical properties and potential as wound dressing materials for infected wounds | [29] |
| Nano-curcumin/CCS-OA hydrogel: In situ injectable hydrogel composed of curcumin, N,O-carboxymethyl chitosan, and oxidized alginate | Accelerates wound healing by promoting re-epithelialization and collagen deposition in rat dorsal wounds | [140] |
| KA hydrogel: Injectable oxidized alginate/carboxymethyl chitosan hydrogel functionalized with keratin nanoparticles (Ker NPs) and nanosized-EGCG covered with Ag nanoparticles (AE NPs) | Accelerates wound healing, particularly in the early stage, and improves the thickness of renascent epidermis | [141] |
| M@M-Ag-Sil-MA hydrogel: Photocurable methacryloxylated silk fibroin hydrogel (Sil-MA) co-encapsulated with metformin-loaded mesoporous silica microspheres (MET@MSNs) and silver nanoparticles (Ag NPs) | Resolves immune contradiction in diabetic wounds, promotes fibroblast migration and endothelial cell angiogenesis, and accelerates diabetic wound healing in a diabetic mouse model | [142] |
| RAAS hydrogel: Injectable hydrogel adhesive with rapid adhesion to wet tissues and anti-swelling properties. | Achieves rapid adhesion to wet tissues, exhibits excellent anti-swelling properties, and demonstrates fast hemostasis and stable adhesion strength in diverse hemorrhage models | [143] |
| GA@AgNPs-SA hydrogel: Injectable sodium alginate hydrogel loaded with gallic acid-functionalized silver nanoparticles (GA@AgNPs) | Exhibits long-term antimicrobial effect, reduces inflammatory response, and accelerates the repair of bacteria-infected wounds through sustained release of silver ions and promotion of angiogenesis | [144] |
| Injectable hydrogel with Ag-doped Mo2C-derived polyoxometalate (AgPOM) nanoparticles, urea, gelatin, and tea polyphenols (TPs) | Exhibits antibacterial activity, accelerates wound healing, and shows potential as a therapeutic agent for drug-resistant bacteria-infected wounds | [145] |
| CMCS-brZnO hydrogel: Injectable hydrogel synthesized by incorporating fusiform-like zinc oxide nanorods (brZnO) into carboxymethyl chitosan (CMCS) | Demonstrates injectability, self-healing, tissue adhesion, antibacterial activity, and promotion of wound healing through sustained release of antibacterial Zn(2+) ions | [146] |
| Silk fibroin-hyaluronic acid based injectable hydrogel incorporated with mace-like Au-CuS heterostructural nanoparticles (gAu-CuS HSs) | Enhances hemostasis, exhibits antibacterial activity, regulates cytokine expression, promotes angiogenesis, and accelerates wound healing, making it a promising strategy for diabetic wound healing | [147] |
| PH/sFDM hydrogel containing nano-sized suspended formulation and Pluronic F127/hyaluronic acid (HA) | Promotes neovessel formation, collagen deposition, blood reperfusion, and reduces necrosis and fibrosis in cutaneous wound and hindlimb ischemia models | [148] |
| Self-assembling hydrogels based on thiolated hyaluronic acid (HA-SH) and bioactive silver-lignin nanoparticles (Ag@Lig NPs) | Inhibits proteolytic enzymes, oxidative enzymes, and bacteria, while promoting tissue remodeling and skin integrity restoration in chronic wounds | [149] |
| Guar gum-grafted-polyacrylamidoglycolic acid (GG-g-PAGA) polymer-based silver nanocomposite (AgNC) hydrogels | Exhibits self-healing ability, injectability, stretchability, flowability, high swelling, porosity, mechanical behavior, and biodegradability, suitable for wound-healing applications | [150] |
| Hydrogel Materials and Composition | Outcomes | Ref. |
|---|---|---|
| Chitosan-based injectable in-situ-forming hydrogels containing dopamine-reduced graphene oxide (DOPA-rGO) and resveratrol (RES) | Exhibits injectability, in situ gelation, suitable physicochemical properties, and good cytocompatibility, and significantly enhances the efficacy of chemo-photothermal therapy in breast cancer cells. | [30] |
| In situ injectable PEG hydrogel system formulated with albumin nanoparticles | Exhibits hyperthermia, singlet oxygen ((1)O(2)) generation, and enhanced killing of tumor cells, showing potential for ablation of poorly responsive hypoxic tumors. | [31] |
| Injectable and biodegradable nano-photothermal DNA hydrogel | Exhibits improved penetration, sensitivity to photothermal therapy (PTT) and photodynamic treatment (PDT), easy cellular uptake, enhanced anti-tumor activity, and reduced drug resistance, providing a safe and efficient supplement for cancer therapy. | [32] |
| Injectable and self-healing nanocomposite hydrogel loaded with needle-like nano-hydroxyapatite (HAP) and graphene oxide (GO) | Effectively inhibits tumor cell proliferation, realizes the synergistic effect of photothermal therapy, and shows potential as an effective treatment approach for tumors. | [33] |
| Injectable in situ intelligent thermo-responsive hydrogel with glycyrrhetinic acid (GA)-conjugated nano graphene oxide (NGO) | Exhibits sustained and temperature-dependent drug release, enhanced anti-tumor activity when combined with laser irradiation, and shows potential for clinical treatment of malignant tumors. | [34] |
| Injectable nano-composite hydrogel based on hyaluronic acid-chitosan derivatives | Demonstrates tumor inhibition through a comprehensive approach of photothermal therapy, chemotherapy, and anti-inflammatory effects. | [35] |
| Silk fibroin nanofiber (SF) hydrogel system complexed with upconversion nanoparticles and nano-graphene oxide (SF/UCNP@NGO) | Shows potential for tumor imaging and therapy, with excellent biocompatibility, efficient cancer cell ablation, and outstanding antitumor efficacy. | [36] |
| Injectable, near-infrared (NIR)/pH-responsive nanocomposite hydrogel | Demonstrates potential as a long-term drug delivery platform for chemophotothermal synergistic cancer therapy, reducing adverse effects and enabling prolonged drug retention in the tumor region. | [37] |
| Thermosensitive TMPO-oxidized lignocellulose/cationic agarose hydrogel | Shows potential for photothermal therapy in melanoma, with short gelation time, high mechanical strength, efficient drug release, and reduced cytotoxicity with laser light irradiation. | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Chowdhury, S.D. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels 2023, 9, 533. https://doi.org/10.3390/gels9070533
Omidian H, Chowdhury SD. Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels. 2023; 9(7):533. https://doi.org/10.3390/gels9070533
Chicago/Turabian StyleOmidian, Hossein, and Sumana Dey Chowdhury. 2023. "Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy" Gels 9, no. 7: 533. https://doi.org/10.3390/gels9070533
APA StyleOmidian, H., & Chowdhury, S. D. (2023). Advancements and Applications of Injectable Hydrogel Composites in Biomedical Research and Therapy. Gels, 9(7), 533. https://doi.org/10.3390/gels9070533