Rheological Properties of Gel Foam Co-Stabilized with Nanoparticles, Xanthan Gum, and Multiple Surfactants
Abstract
1. Introduction
2. Results and Discussion
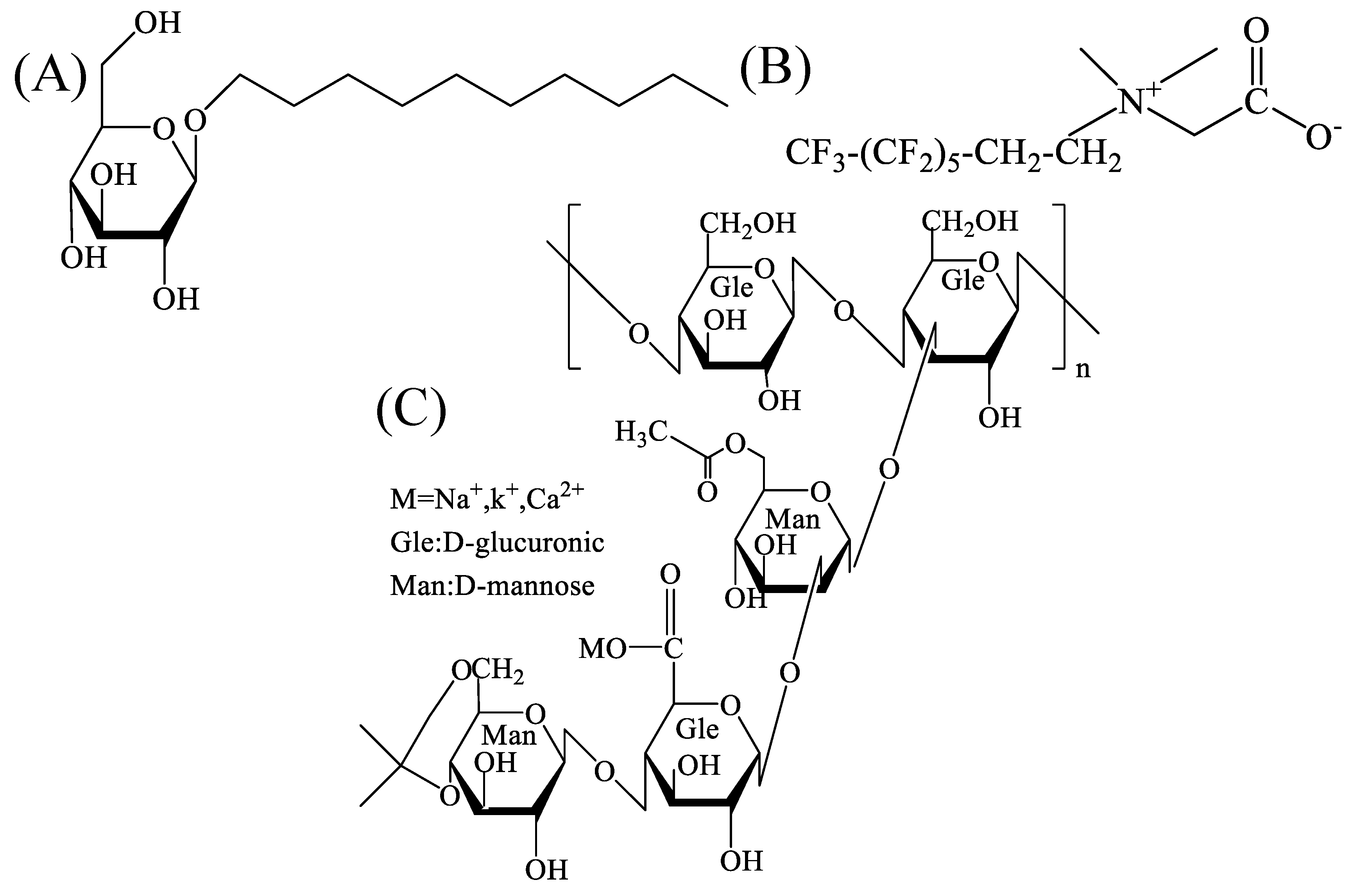
2.1. Characterization of the Mixed Dispersions
2.2. Foam Properties
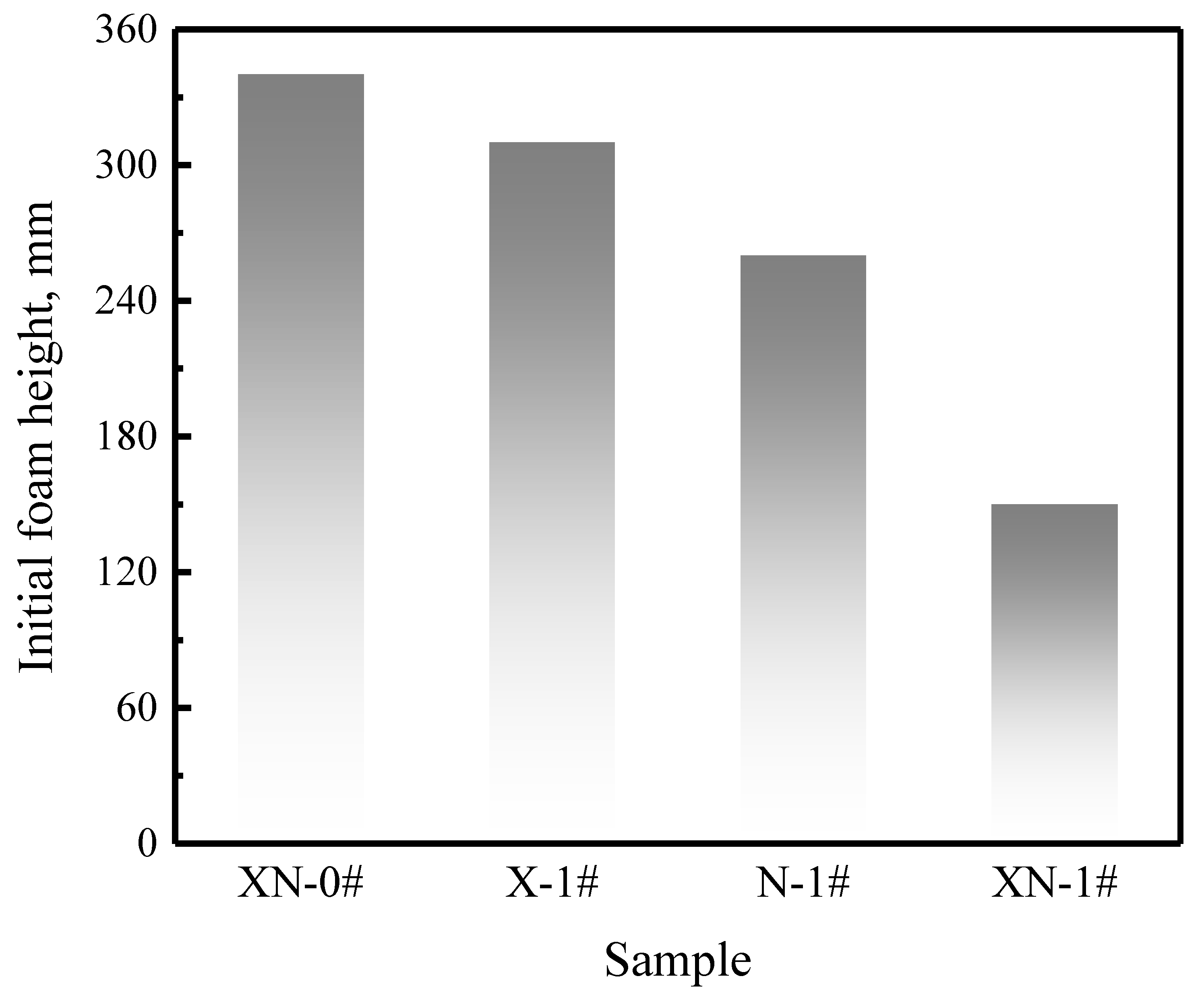
2.2.1. Foaming Ability
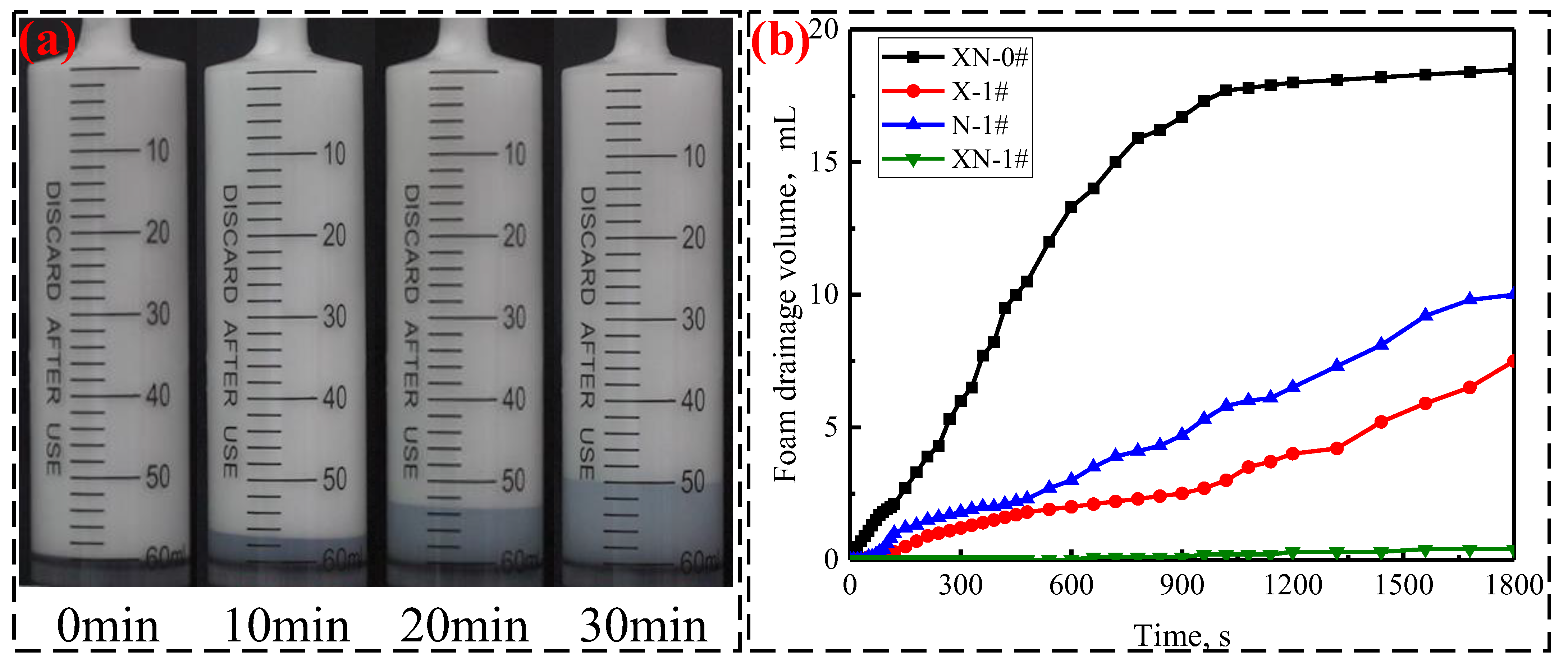
2.2.2. Foam Drainage
2.3. Foam Rheology
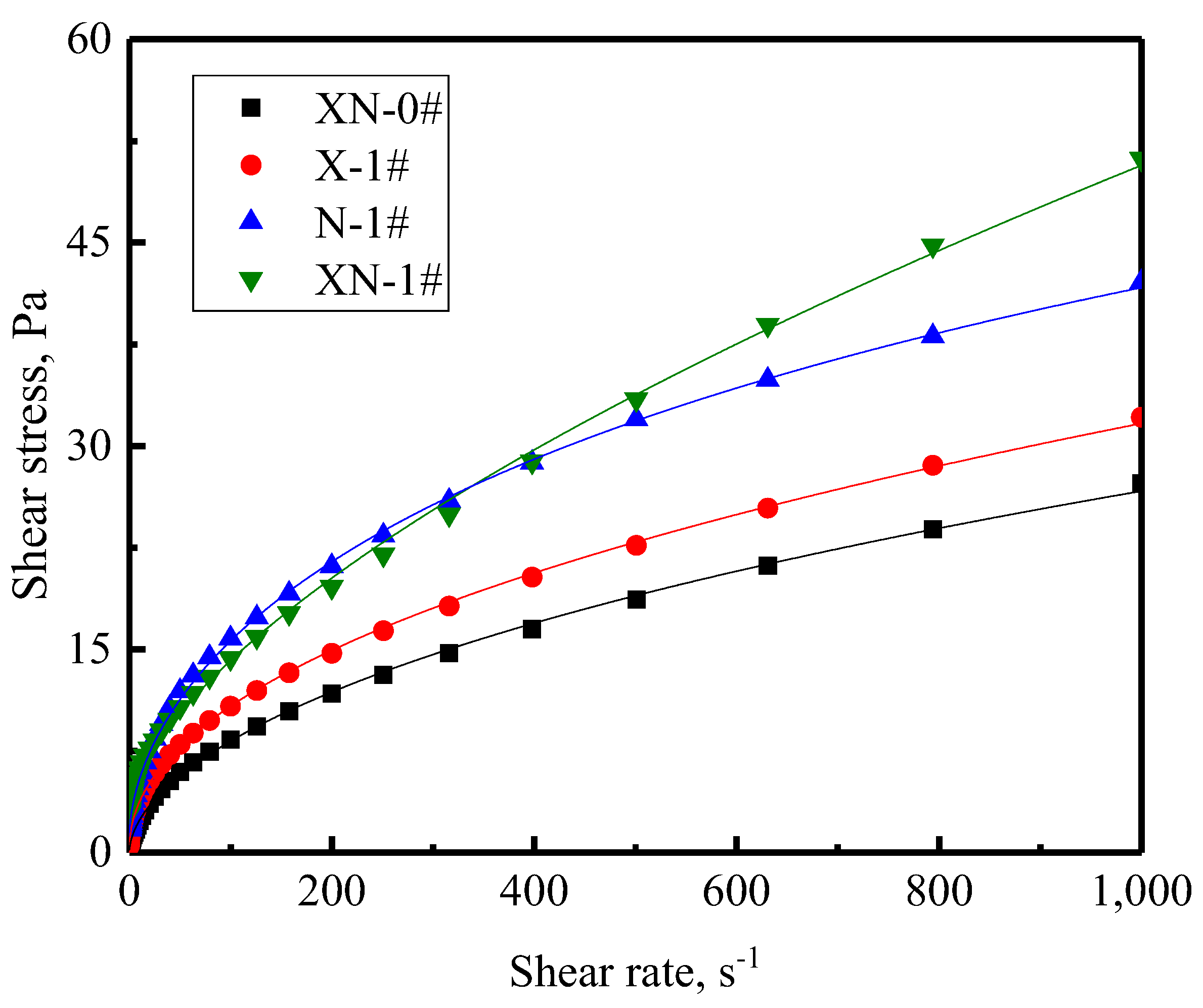
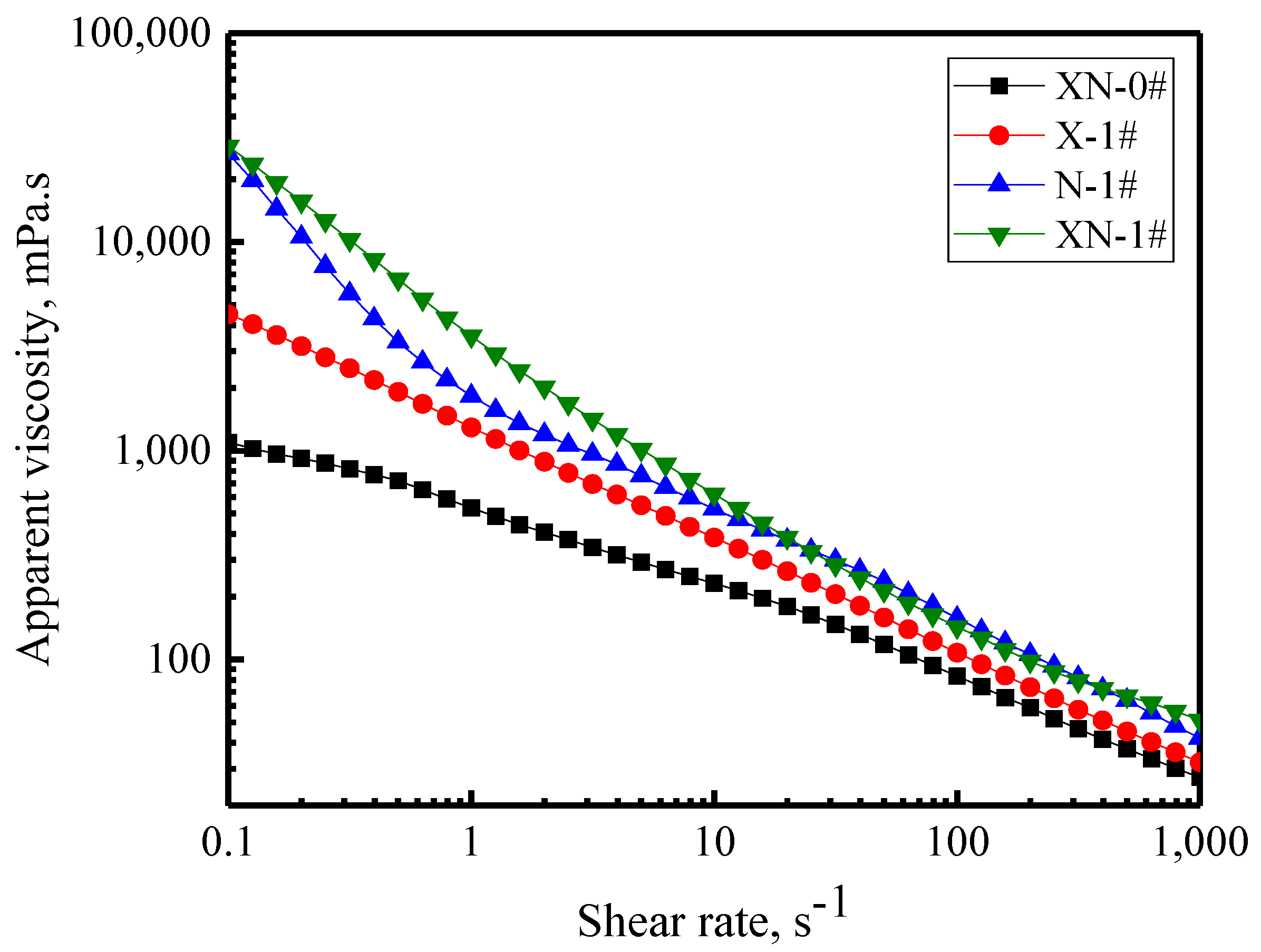
2.3.1. Flow Behavior
2.3.2. Viscoelasticity
3. Conclusions
4. Experimental
4.1. Materials
4.2. Apparatus and Methods
4.2.1. Generation Testing of Mixed Dispersions
4.2.2. Characterization of Foam Rheology and Foam Properties
Foam Rheology
Foaming Ability and Foam Drainage
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, A.J.; Littlejohn, K.A.; Hooley, P. Formation and stability of food foams and aerated emulsions: Hydrophobins as novel functional ingredients. Curr. Opin. Colloid Interface Sci. 2013, 18, 292–301. [Google Scholar] [CrossRef]
- Reddy, M.S.; Okuda, T.; Kurose, K.; Tsai, T.-Y.; Nakai, S.; Nishijima, W.; Okada, M. Surface ozonation of polyvinyl chloride for its separation from waste plastic mixture by froth floatation. J. Mater. Cycles Waste Manag. 2010, 12, 326–331. [Google Scholar] [CrossRef]
- Tran, D.N.; Whitby, C.P.; Fornasiero, D. Foamability of aqueous suspensions of fine graphite and quartz particles with a triblock copolymer. J. Colloid Interface Sci. 2010, 348, 460–468. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, H.; Wang, Q. Interfacial molecular array behaviors of mixed surfactant systems based on sodium laurylglutamate and the effect on the foam properties. J. Dispers. Sci. Technol. 2018, 39, 1427–1434. [Google Scholar] [CrossRef]
- Sheng, Y.; Yan, C.; Li, Y. Thermal stability of gel foams stabilized by xanthan gum, silica nanoparticles and surfactants. Gels 2021, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Qiu, K.; Li, H.; Miao, X.; Wang, J.; Li, Q.; Lu, S. Interfacial and rheological properties of long-lived foams stabilized by rice proteins complexed to transition metal ions in the presence of alkyl polyglycoside. J. Colloid Interface Sci. 2023, 630, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Ye, H.; Yang, L.; Luo, D.; Peng, B. Nanoparticles as foam stabilizer: Mechanism, control parameters and application in foam flooding for enhanced oil recovery. J. Pet. Sci. Eng. 2021, 202, 108561. [Google Scholar] [CrossRef]
- Palaniraj, A.; Jayaraman, V. Production, recovery and applications of xanthan gum by Xanthomonas campestris. J. Food Eng. 2011, 106, 1–12. [Google Scholar] [CrossRef]
- Ma, L.; Fan, X.; Wei, G.; Sheng, Y.; Liu, S.; Liu, X. Preparation and characterization of antioxidant gel foam for preventing coal spontaneous combustion. Fuel 2023, 338, 127270. [Google Scholar] [CrossRef]
- Polanish, E. Foam Applicators to Apply Cosmetics or Nail Polish. U.S. Patent No. 8,584,686, 19 November 2013. [Google Scholar]
- Verma, A.; Chauhan, G.; Baruah, P.P.; Ojha, K. Morphology, rheology, and kinetics of nanosilica stabilized gelled foam fluid for hydraulic fracturing application. Ind. Eng. Chem. Res. 2018, 57, 13449–13462. [Google Scholar] [CrossRef]
- Sheng, Y.; Lu, S.; Xu, M. Effect of Xanthan gum on the performance of aqueous film-forming foam. J. Dispers. Sci. Technol. 2016, 37, 1664–1670. [Google Scholar] [CrossRef]
- Yang, W.; Wang, T.; Fan, Z. Highly stable foam stabilized by alumina nanoparticles for EOR: Effects of sodium cumenesulfonate and electrolyte concentrations. Energy Fuels 2017, 31, 9016–9025. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Lee, S.; Lim, J. Surface modification of calcium carbonate nanoparticles by fluorosurfactant. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 213–223. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants—Similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Binks, B.P.; Kirkland, M.; Rodrigues, J.A. Origin of stabilisation of aqueous foams in nanoparticle–surfactant mixtures. Soft Matter 2008, 4, 2373–2382. [Google Scholar] [CrossRef]
- Sun, Q.; Li, Z.; Wang, J. Aqueous foam stabilized by partially hydrophobic nanoparticles in the presence of surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2015, 471, 54–64. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Lawson, J.B. Mechanisms of foam flow in porous media: Apparent viscosity in smooth capillaries. Soc. Pet. Eng. J. 1985, 25, 176–190. [Google Scholar] [CrossRef]
- Maxwell, R.; Costache, M.C.; Giarrosso, A.; Bosques, C.; Amin, S. Optimizing interactions between soluble silk fibroin and capryl glucoside for design of a natural and high-performance co-surfactant system. Int. J. Cosmet. Sci. 2021, 43, 68–77. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, H.; Song, X.; Wang, Z.; Wang, X.; Li, Y. Comparative study on foaming and foam stability of multiple mixed systems of fluorocarbon, hydrocarbon, and amino acid surfactants. J. Surfactants Deterg. 2023. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Drenckhan, W.; Salonen, A.; Rodrigues, J.A.; Íñiguez-Palomares, R.; Rio, E.; Langevin, D. On the long-term stability of foams stabilised by mixtures of nano-particles and oppositely charged short chain surfactants. Soft Matter 2012, 8, 11085–11097. [Google Scholar] [CrossRef]
- Garcıa-Ochoa, F.; Santos, V.E.; Casas, J.A. Xanthan gum: Production, recovery, and properties. Biotechnol. Adv. 2000, 18, 549–579. [Google Scholar] [CrossRef]
- Sheng, Y.; Zhang, H.; Guo, Y.; Ma, L.; Wang, Q.; Hu, D. Stability and rheological properties of foams co-stabilized by hydrophilic silica nanoparticles and amino acid/alkyl glycoside surfactants. J. Mol. Liq. 2023, 382, 122009. [Google Scholar] [CrossRef]
- Kennedy, J.R.; Kent, K.E.; Brown, J.R. Rheology of dispersions of xanthan gum, locust bean gum and mixed biopolymer gel with silicon dioxide nanoparticles. Mater. Sci. Eng. C 2015, 48, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cellesi, F.; Tirelli, N. Sol–gel synthesis at neutral pH in W/O microemulsion: A method for enzyme nanoencapsulation in silica gel nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 52–61. [Google Scholar] [CrossRef]
- Binks, B.P.; Campbell, S.; Mashinchi, S. Dispersion behavior and aqueous foams in mixtures of a vesicle-forming surfactant and edible nanoparticles. Langmuir 2015, 31, 2967–2978. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Sun, Q. CO2 foam properties and the stabilizing mechanism of sodium bis (2-ethylhexyl) sulfosuccinate and hydrophobic nanoparticle mixtures. Soft Matter 2016, 12, 946–956. [Google Scholar] [CrossRef]
- Koerner, C. Integral Foam Molding of Light Metals: Technology, Foam Physics and Foam Simulation; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- Rosen, M.J. Surfactants and Interfacial Phenomena; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Denkov, N.D.; Ivanov, I.B.; Kralchevsky, P.A.; Wasan, D.T. A possible mechanism of stabilization of emulsions by solid particles. J. Colloid Interface Sci. 1992, 150, 589–593. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Ilekti, P.; Persello, J. Dispersions of silica particles in surfactant phases. Langmuir 1996, 12, 2392–2401. [Google Scholar] [CrossRef]
- AlYousef, Z.; Almobarky, M.; Schechter, D. Enhancing the stability of foam by the use of nanoparticles. Energy Fuels 2017, 31, 10620–10627. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, N.; Miao, X. Formation of stable aqueous foams on the ethanol layer: Synergistic stabilization of fluorosurfactant and polymers. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124545. [Google Scholar] [CrossRef]
- Krishnan, J.M.; Deshpande, A.P.; Kumar, P.S. (Eds.) Rheology of Complex Fluids; Springer: New York, NY, USA, 2010; pp. 3–34. [Google Scholar]
- Stevenson, P. (Ed.) Foam Engineering: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sheng, Y.; Xue, M.; Zhang, S. Effect of xanthan gum and silica nanoparticles on improving foam properties of mixed solutions of short-chain fluorocarbon and hydrocarbon surfactants. Chem. Eng. Sci. 2021, 245, 116952. [Google Scholar] [CrossRef]
- Oh, M.H.; So, J.H.; Yang, S.M. Rheological evidence for the silica-mediated gelation of xanthan gum. J. Colloid Interface Sci. 1999, 216, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Marze, S.; Guillermic, R.M.; Saint-Jalmes, A. Oscillatory rheology of aqueous foams: Surfactant, liquid fraction, experimental protocol and aging effects. Soft Matter 2009, 5, 1937–1946. [Google Scholar] [CrossRef]
- Saint-Jalmes, A.; Durian, D.J. Vanishing elasticity for wet foams: Equivalence with emulsions and role of polydispersity. J. Rheol. 1999, 43, 1411–1422. [Google Scholar] [CrossRef]
- Simões, A.; Miranda, M.; Cardoso, C. Rheology by design: A regulatory tutorial for analytical method validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- Wang, J.; Chai, J.; Wang, G. Strong and thermally insulating polylactic acid/glass fiber composite foam fabricated by supercritical carbon dioxide foaming. Int. J. Biol. Macromol. 2019, 138, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Sheng, Y.; Li, C. Surface activity, foam properties and aggregation behavior of mixtures of short-chain fluorocarbon and hydrocarbon surfactants. J. Mol. Liq. 2018, 268, 249–255. [Google Scholar] [CrossRef]
- Sheng, Y.; Peng, Y.; Zhang, S.; Guo, Y.; Ma, L.; Zhang, H. Thermal stability of foams stabilized by fluorocarbon and hydrocarbon surfactants in presence of nanoparticles with different specific surface areas. J. Mol. Liq. 2022, 365, 120187. [Google Scholar] [CrossRef]
- Cherizol, R.; Sain, M.; Tjong, J. Review of non-Newtonian mathematical models for rheological characteristics of viscoelastic composites. Green Sustain. Chem. 2015, 5, 6. [Google Scholar] [CrossRef]
- Song, X.; Cui, X.; Su, X.; Munir, B.; Du, D. Laboratory study on the rheology properties of nanoparticle-stabilized supercritical CO2 foam. J. Pet. Sci. Eng. 2022, 218, 111065. [Google Scholar] [CrossRef]
- Escudier, M.P.; Gouldson, I.W.; Pereira, A.S.; Pinho, F.T.; Poole, R.J. On the reproducibility of the rheology of shear-thinning liquids. J. Non-Newton. Fluid Mech. 2001, 97, 99–124. [Google Scholar] [CrossRef]
- Cross, M.M. Rheology of non-newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]

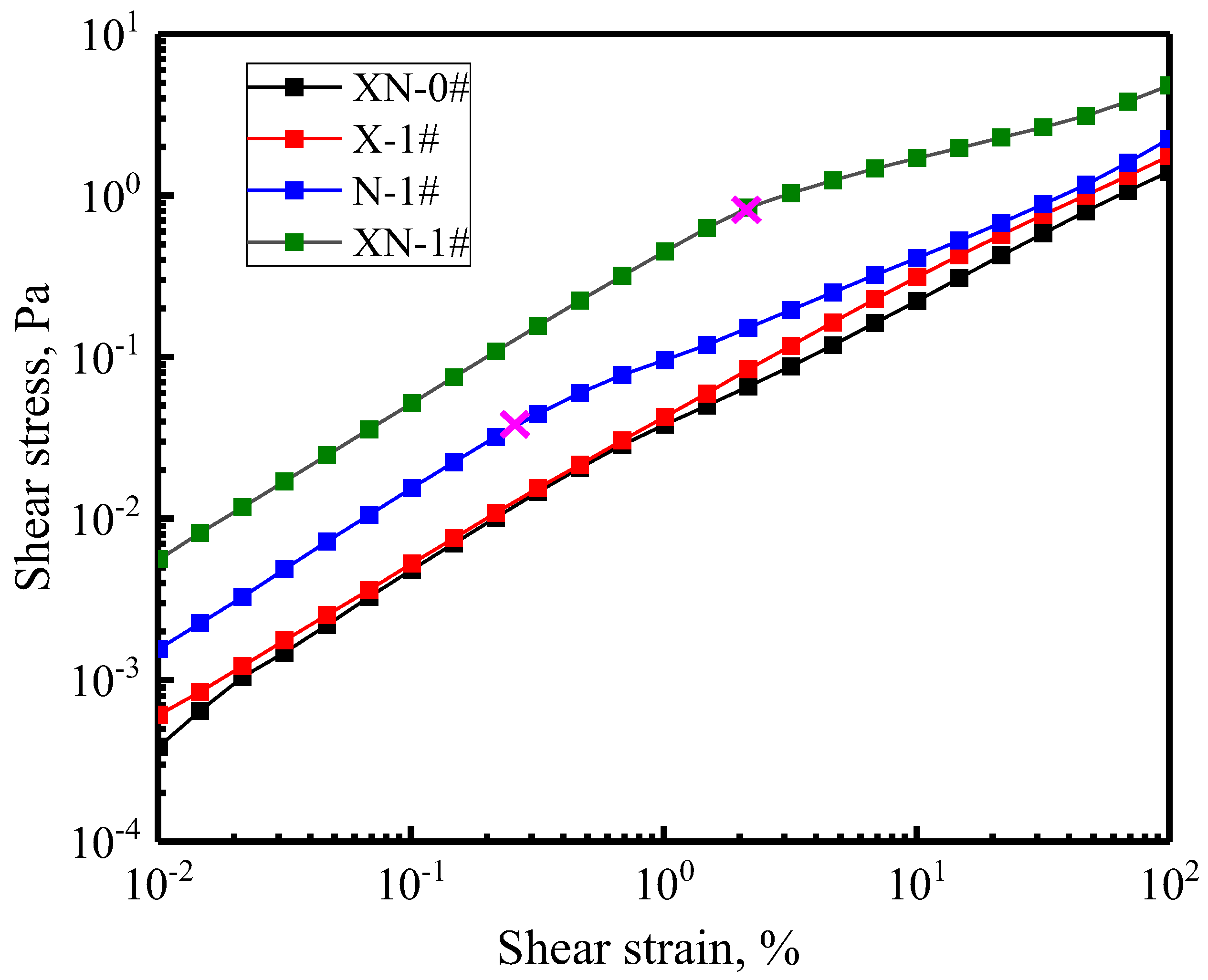

| Sample | Model Parameter | ||||
|---|---|---|---|---|---|
| τ0 (Pa) | τ∞ (Pa) | λ (s) | m | R2 | |
| XN-0# | 0 | 205.15 | 3.24 × 10−5 | 0.56 | 0.9993 |
| X-1# | 0.15 | 2582.29 | 9.69 × 10−8 | 0.48 | 0.9997 |
| N-1# | 1.17 | 119.57 | 3.19 × 10−4 | 0.57 | 0.9985 |
| XN-1# | 3.22 | 9214.19 | 2.67 × 10−7 | 0.64 | 0.9986 |
| Sample | FS-50(%) | APG0810(%) | XG(%) | NP(%) |
|---|---|---|---|---|
| XN-0# | 0.25 | 0.5 | 0 | 0 |
| X-1# | 0.25 | 0.5 | 0.05 | 0 |
| N-1# | 0.25 | 0.5 | 0 | 5 |
| XN-1# | 0.25 | 0.5 | 0.05 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, Y.; Zhang, H.; Ma, L.; Wang, Z.; Hu, D.; Zhang, S. Rheological Properties of Gel Foam Co-Stabilized with Nanoparticles, Xanthan Gum, and Multiple Surfactants. Gels 2023, 9, 534. https://doi.org/10.3390/gels9070534
Sheng Y, Zhang H, Ma L, Wang Z, Hu D, Zhang S. Rheological Properties of Gel Foam Co-Stabilized with Nanoparticles, Xanthan Gum, and Multiple Surfactants. Gels. 2023; 9(7):534. https://doi.org/10.3390/gels9070534
Chicago/Turabian StyleSheng, Youjie, Hanling Zhang, Li Ma, Zhenping Wang, Die Hu, and Shanwen Zhang. 2023. "Rheological Properties of Gel Foam Co-Stabilized with Nanoparticles, Xanthan Gum, and Multiple Surfactants" Gels 9, no. 7: 534. https://doi.org/10.3390/gels9070534
APA StyleSheng, Y., Zhang, H., Ma, L., Wang, Z., Hu, D., & Zhang, S. (2023). Rheological Properties of Gel Foam Co-Stabilized with Nanoparticles, Xanthan Gum, and Multiple Surfactants. Gels, 9(7), 534. https://doi.org/10.3390/gels9070534







