Gels That Serve as Mucus Simulants: A Review
Abstract
:1. Introduction
2. Native Human Mucus
- (a)
- Collection of native human mucus
- (b)
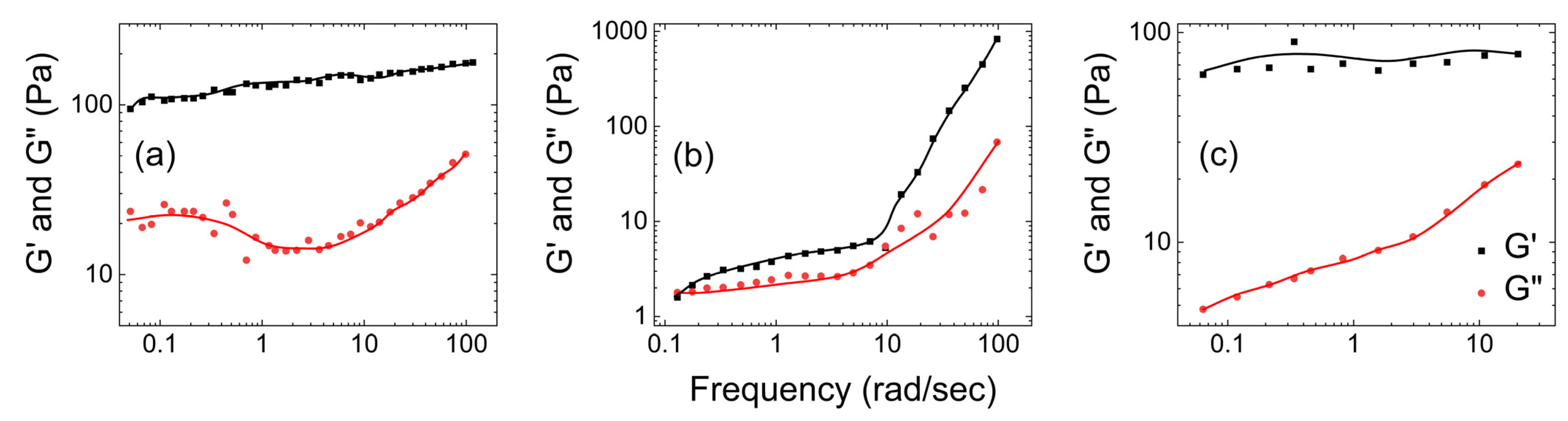
- Rheological properties of human mucus
- (c)
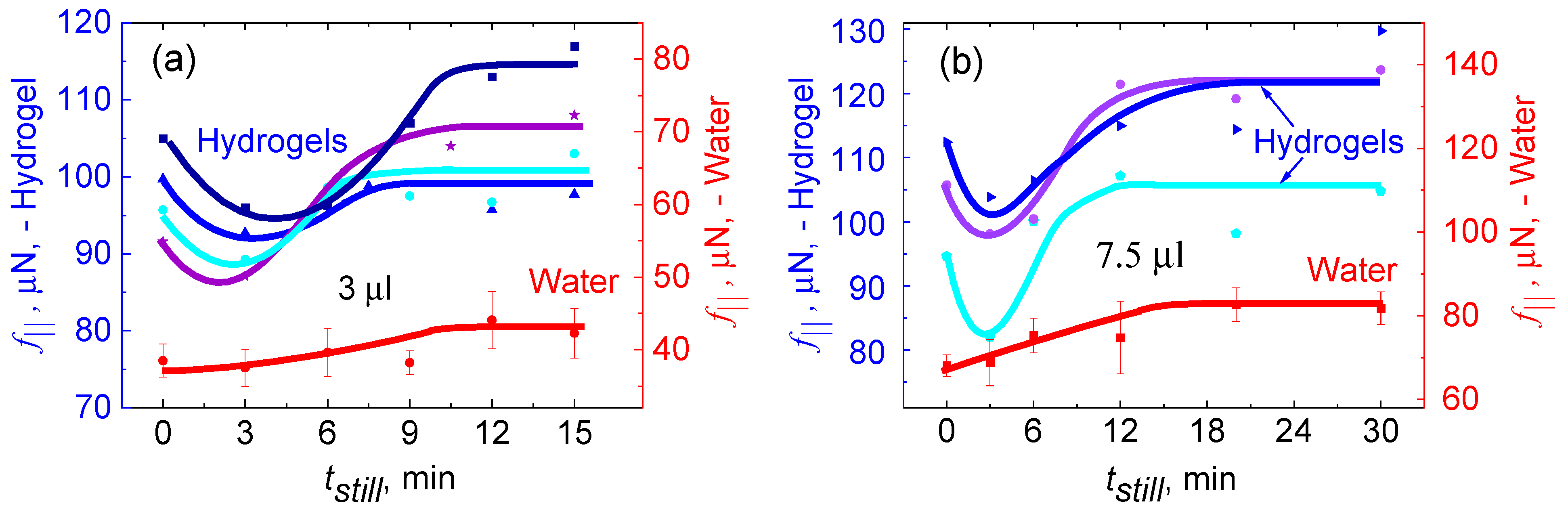
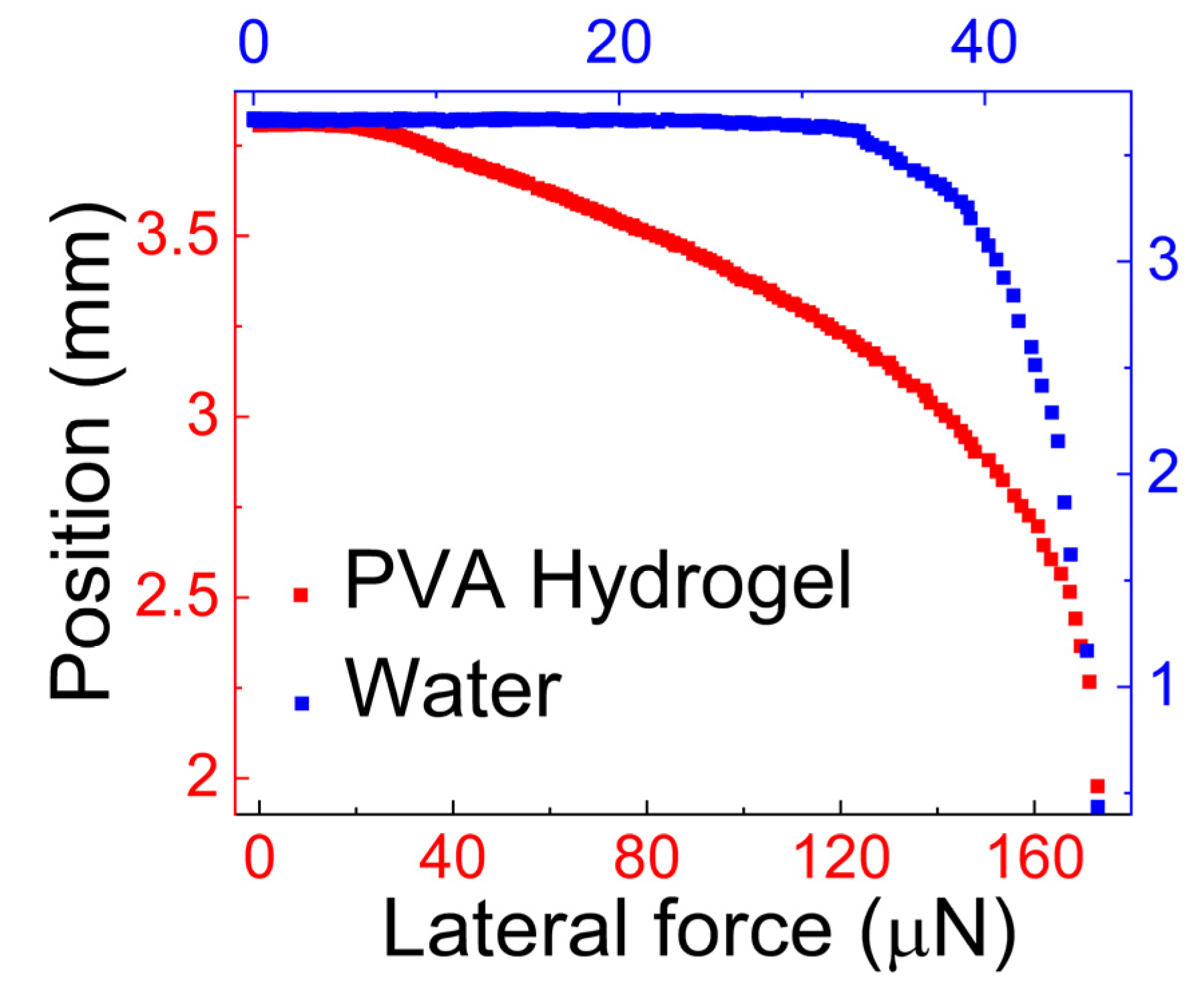
- Tribological properties of human mucus
3. Synthetic Mucus
3.1. Synthetic Mucus Prepared by Polyvinyl Alcohol
- (a)
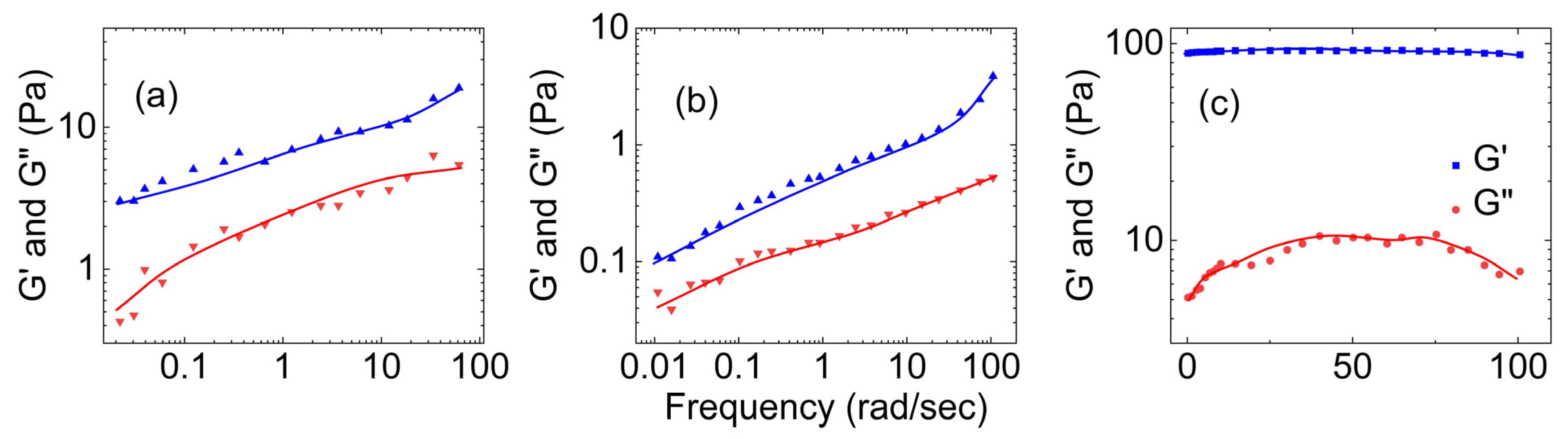
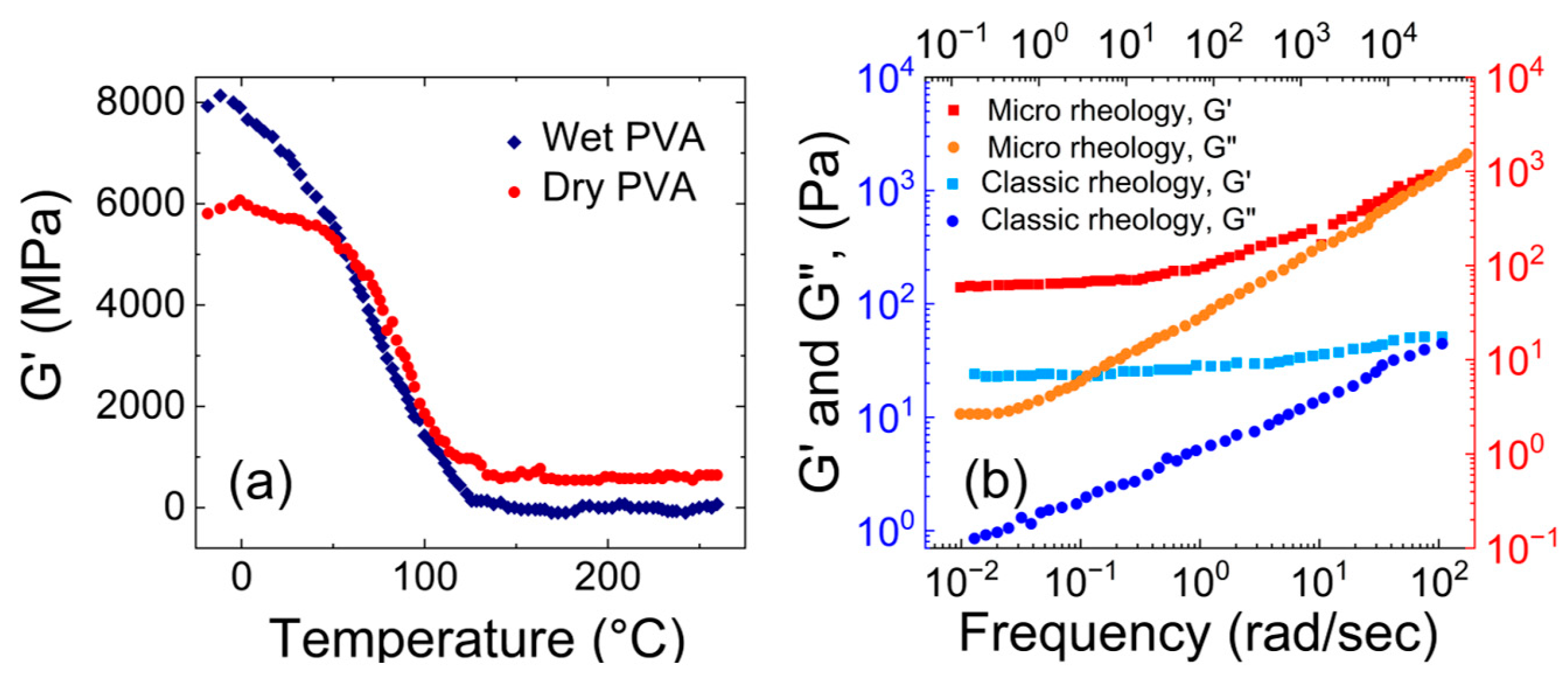
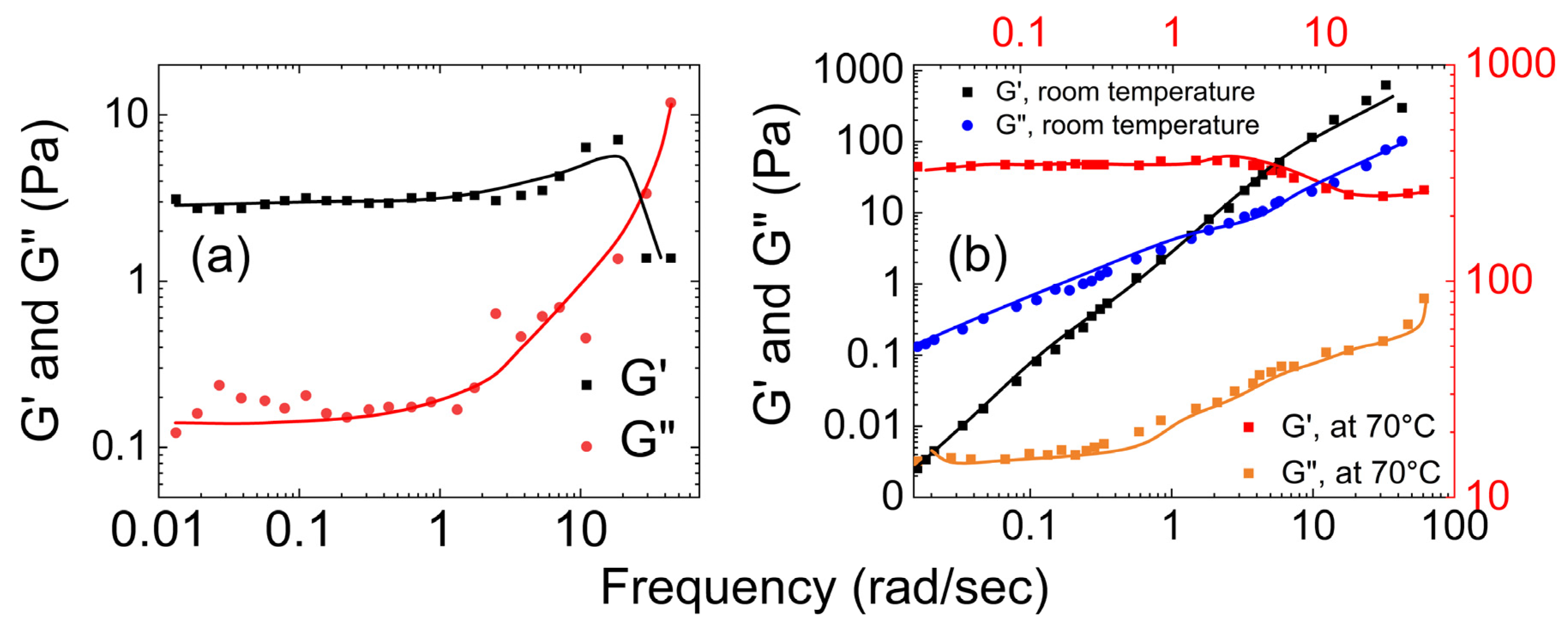
- Rheological properties of polyvinyl alcohol hydrogels
- (b)
- Tribological properties of polyvinyl alcohol hydrogels
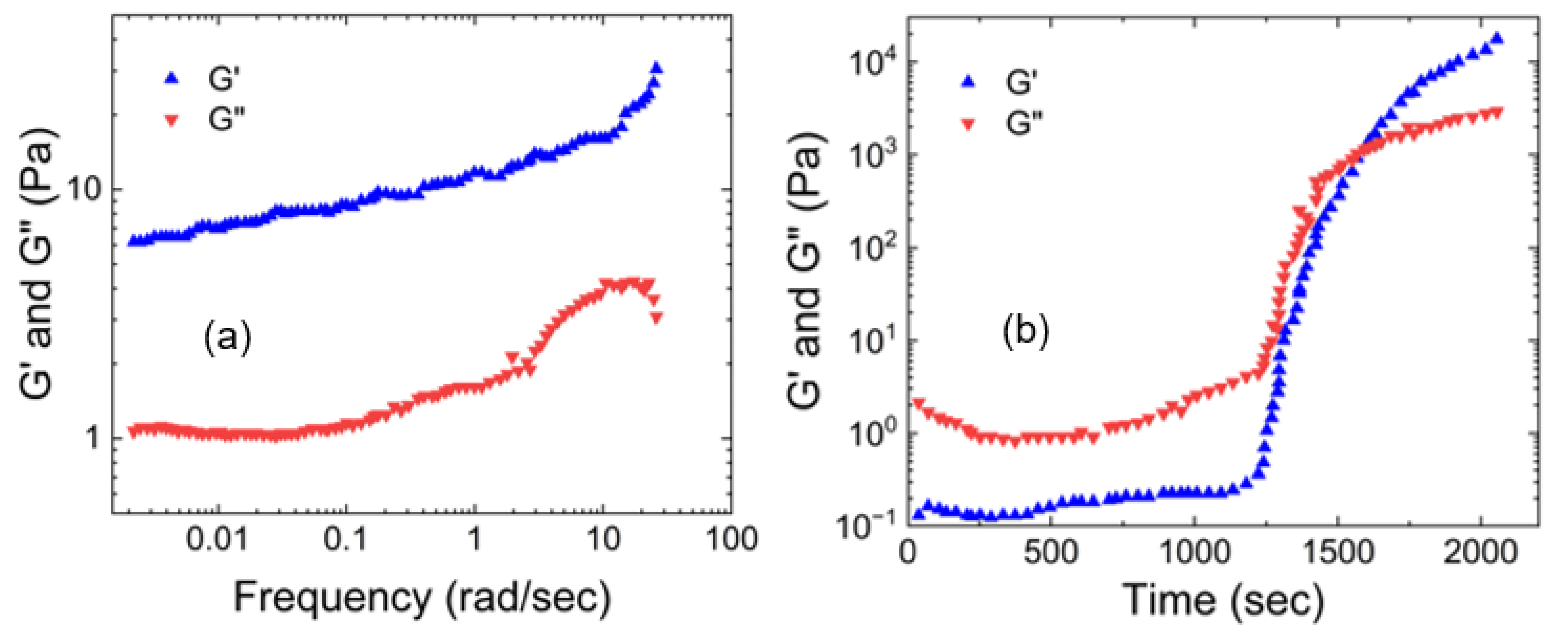
3.2. Synthetic Mucus Prepared by Polyvinyl Alcohol Crosslinked Using Borax
- (a)
- Rheological properties of polyvinyl alcohol hydrogels crosslinked using boron
- (b)
- Tribological properties of polyvinyl alcohol hydrogels crosslinked using boron

3.3. Synthetic Mucus Prepared by Using Guar Gum
- (i)
- Guar gum with scleroglucan
- (a)
- Rheological properties of guar gum and scleroglucan mucus simulant
- (b)
- Tribological properties of mucus simulated using guar gum and scleroglucan mucus simulant
- (ii)
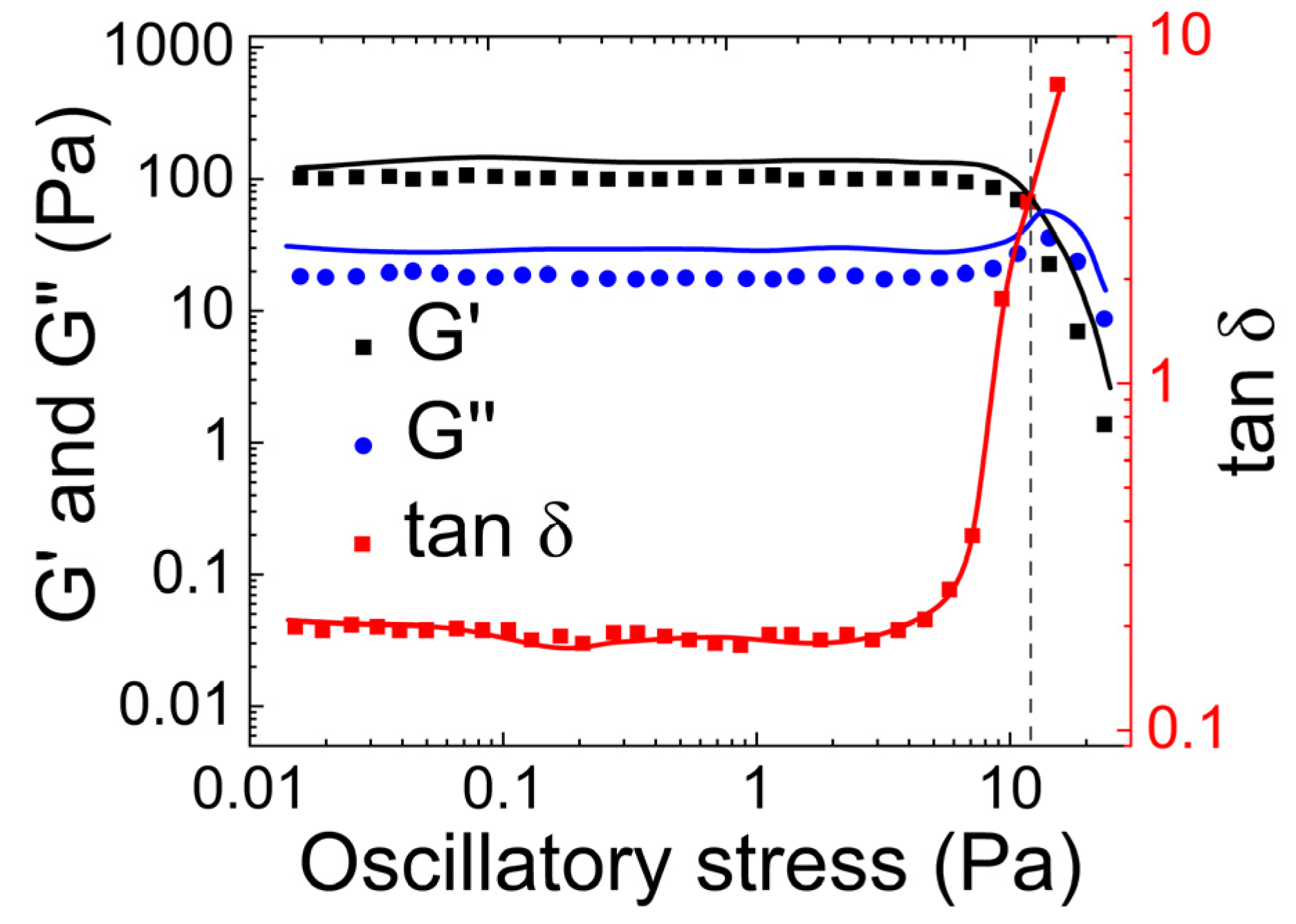
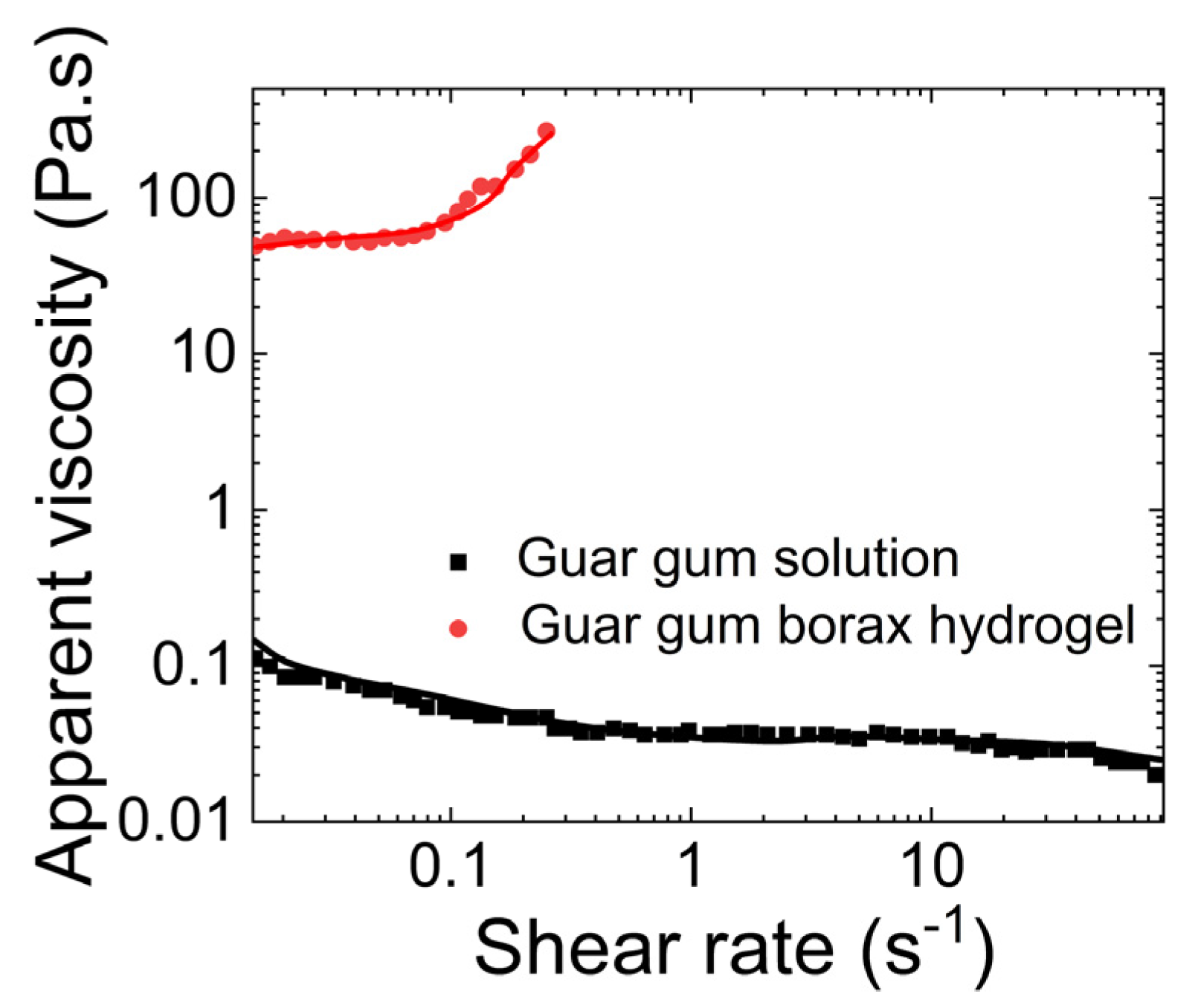
- Guar gum with borax
- (a)
- Rheological properties of guar gum with borax
- (b)
- Tribological properties of mucus simulated using guar gum and borax
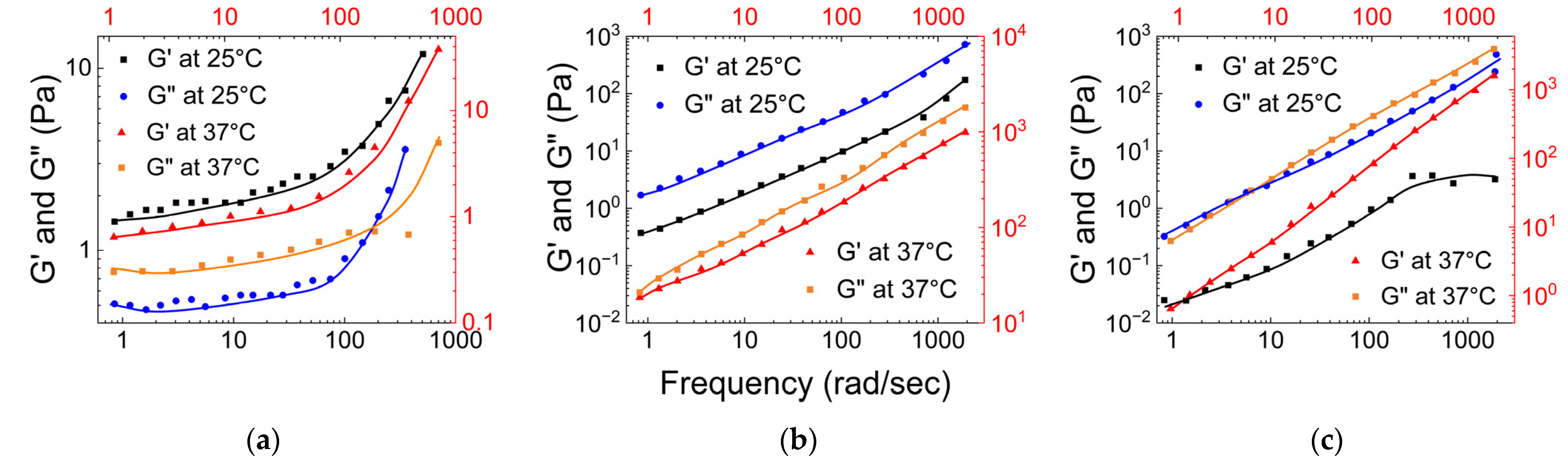
3.4. Synthetic Mucus Based on Polyglycerol
- (a)
- Rheological properties of linear polyglycerol based synthetic hydrogel
- (b)
- Tribological properties of linear glycerol-based polymer
3.5. Synthetic Mucus Prepared by Polyacrylic Acid Hydrogels/Carbopols
- (a)
- Rheological properties of polyacrylic acid hydrogels
- (b)
- Tribological properties of polyacrylic acid hydrogels
4. Discussion and Summary
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jeffery, P.; Gaillard, D.; Moret, S. Human Airway Secretory Cells during Development and in Mature Airway Epithelium. Eur. Respir. J. 1992, 5, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lieleg, O.; Ribbeck, K. Biological Hydrogels as Selective Diffusion Barriers. Trends Cell. Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girod, S.; Zahm, J.-M.; Plotkowski, C.; Beck, G.; Puchelle, E. Role of the Physicochemical Properties of Mucus in the Protection of the Respiratory Epithelium. Eur. Respir. J. 1992, 5, 477–487. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The Gastrointestinal Mucus System in Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensign, L.M.; Tang, B.C.; Wang, Y.Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-Penetrating Nanoparticles for Vaginal Drug Delivery Protect against Herpes Simplex Virus. Sci. Transl. Med. 2012, 4, 138ra79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Button, B.; Boucher, R.C. Role of Mechanical Stress in Regulating Airway Surface Hydration and Mucus Clearance Rates. Respir. Physiol. Neurobiol. 2008, 163, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Cone, R.A. Barrier Properties of Mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Ramos, F.L.; Krahnke, J.S.; Kim, V. Clinical Issues of Mucus Accumulation in COPD. Int. J. COPD 2014, 9, 139–150. [Google Scholar]
- Gutmark, E.; Anand, V.; Wheeler, A.; Zahn, A.; Cavari, Y.; Eluk, T.; Hay, M.; Katoshevski, D.; Gutmark-Little, I. Demonstration of Mucus Simulant Clearance in a Bench-Model Using Acoustic Field-Integrated Intrapulmonary Percussive Ventilation. J. Biomech. 2022, 144, 111305. [Google Scholar] [CrossRef]
- Smart, J.D. The Basics and Underlying Mechanisms of Mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Zheng, H. Introduction: Measuring Rheological Properties of Foods. In Rheology of Semisolid Foods; Food Engineering Series; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Friedrich, K.; Reinicke, P. Friction and Wear of Polymer Composites. Polym. Mech. 1986, 34, 503–514. [Google Scholar]
- Bej, R.; Haag, R. Mucus-Inspired Dynamic Hydrogels: Synthesis and Future Perspectives. J. Am. Chem. Soc. 2022, 144, 20137–20152. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.L.; Markovetz, M.; Wang, Y.; Ehre, C.; Sheikh, S.Z.; Allbritton, N.L.; Hill, D.B. Biochemical and Rheological Analysis of Human. Colonic Culture Mucus Reveals Similarity to Gut Mucus. Biophys. J. 2021, 120, 5384–5394. [Google Scholar] [CrossRef]
- Kirch, J.; Schneider, A.; Abou, B.; Hopf, A.; Schaefer, U.F.; Schneider, M.; Schall, C.; Wagner, C.; Lehr, C.M. Optical Tweezers Reveal. Relationship between Microstructure and Nanoparticle Penetration of Pulmonary Mucus. Proc. Natl. Acad. Sci. USA 2012, 109, 18355–18360. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Dickey, B.F. Airway Mucus Function and Dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.V.; Katz, A.; Maharjan, R.S.; Gadicherla, A.K.; Richter, M.H.; Heyda, J.; del Pino, P.; Laux, P.; Luch, A. Coronavirus-Mimicking Nanoparticles (CorNPs) in Artificial Saliva Droplets and Nanoaerosols: Influence of Shape and Environmental Factors on Particokinetics/Particle Aerodynamics. Sci. Total Environ. 2023, 860, 160503. [Google Scholar] [CrossRef]
- Lock, J.Y.; Carlson, T.L.; Carrier, R.L. Mucus Models to Evaluate the Diffusion of Drugs and Particles. Adv. Drug Deliv. Rev. 2018, 124, 24–49. [Google Scholar] [CrossRef]
- Bossi, R. Methods for Collecting and Measuring Mucus in Human. Methods Bronchial Mucol. 1988, 13–20. [Google Scholar]
- Jeanneret-Grosjean, A.; King, M.; Michoud, M.C.; Liote, H.; Amyot, R. Sampling Technique and Rheology of Human Tracheobronchial Mucus. Am. Rev. Respir. Dis. 1988, 137, 707–710. [Google Scholar] [CrossRef]
- Proctor, D.F.; Aharonson, E.F.; Reasor, M.J.; Bucklen, K.R. A Method for Collecting Normal Respiratory Mucus. Bull. Physio-Path. Respir. 1973, 9, 351–358. [Google Scholar]
- Zayas, J.G.; Man, G.C.W.; King, M. Tracheal Mucus Rheology in Patients Undergoing Diagnostic Bronchoscopy: Interrelations with Smoking and Cancer. Am. Rev. Respir. Dis. 1990, 141, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Macklem, P.T. Rheological Properties of Microliter Quantities of Normal Mucus. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 42, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.P.; Blasco, L.; Khan, M.A.; Litt, M. Human Cervical Mucus. I. Rheologic Characteristics. Fertil. Steril. 1977, 28, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.H.; Lim, S.T.; Choi, H.J.; John, M.S. Rheology of Poly(Ethylene Oxide)/Organoclay Nanocomposites. Macromolecules 2001, 34, 8084–8093. [Google Scholar] [CrossRef]
- Hill, D.B.; Button, B.; Rubinstein, M.; Boucher, R.C. Physiology and Pathophysiology of Human airwaymucus. Physiol. Rev. 2022, 102, 1757–1836. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Crosslinking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Lin, H.L.; Liu, W.H.; Shen, K.S.; Yu, T.L.; Cheng, C.H. Weak Gel Behaviour of Poly(Vinyl Alcohol)-Borax Aqueous Solutions. J. Polym. Res. 2003, 10, 171–179. [Google Scholar] [CrossRef]
- Winter, H.H. Can the Gel Point of a Cross-linking Polymer Be Detected by the G′—G″ Crossover? Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Martin, J.E.; Adolf, D.; Wilcoxon, J.P. Viscoelasticity near the Sol-Gel Transition. Phys. Rev. A 1989, 39, 1325–1332. [Google Scholar] [CrossRef]
- Izuka, A.; Winter, H.H.; Hashimoto, T. Molecular Weight Dependence of Viscoelasticity of Polycaprolactone Critical Gels. Macromolecules 1992, 25, 2422–2428. [Google Scholar] [CrossRef]
- Hamed, R.; Fiegel, J. Synthetic Tracheal Mucus with Native Rheological and Surface Tension Properties. J. Biomed. Mater. Res. A 2014, 102, 1788–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, D.B.; Vasquez, P.A.; Mellnik, J.; McKinley, S.A.; Vose, A.; Mu, F.; Henderson, A.G.; Donaldson, S.H.; Alexis, N.E.; Boucher, R.C.; et al. A Biophysical Basis for Mucus Solids Concentration as a Candidate Biomarker for Airways Disease. PLoS ONE 2014, 9, e87681. [Google Scholar] [CrossRef]
- Lucas, A.M.; Douglas, L.C. Principles underlying ciliary activity in the respiratory tract: II A comparison of nasal clearance in man, monkey and other mammals. Arch. Otolaryngol. Head. Neck Surg. 1934, 20, 285–296. [Google Scholar] [CrossRef]
- Albers, G.M.; Tomkiewicz, R.P.; May, M.K.; Ramirez, O.E.; Rubin, B.K. Ring Distraction Technique for Measuring Surface Tension of Sputum: Relationship to Sputum Clearability. J. Appl. Physiol. 1996, 81, 2690–2695. [Google Scholar] [CrossRef] [Green Version]
- Schrader, M.E. Young-Dupre Revisited. Langmuir 1995, 11, 3585–3589. [Google Scholar] [CrossRef]
- Lecomte Du Noüy, P. An Interfacial Tensiometer for Universal Use. J. Gen. Physiol. 1925, 7, 625–631. [Google Scholar] [CrossRef]
- Lozinsky, V.I.; Vainerman, E.S.; Domotenko, L.V.; Mamtsis, A.M.; Titova, E.F.; Belavtseva, E.M.; Rogozhin, S.V. Study of Cryostructurization of Polymer Systems VII. Structure Formation under Freezing of Poly(Vinyl Alcohol) Aqueous Solutions. Colloids Polym. Sci. 1986, 264, 19–24. [Google Scholar] [CrossRef]
- Watase, M.; Nishinari, K. Thermal and Rheological Properties of Poly(Vinyl Alcohol) Hydrogels Prepared by Repeated Cycles of Freezing and Thawing. Die Makromol. Chem. 1988, 189, 871–880. [Google Scholar] [CrossRef]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(Vinyl Alcohol) as Versatile Biomaterial for Potential. Biomedical Applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA Hydrogel Properties for Biomedical Application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Peppas, N.A.; Mongia, N.K. Ultrapure Poly(Viny1 Alcohol) Hydrogels with Mucoadhesive Drug Delivery Characteristics. Eur. J. Pharm. Biopharm. 1997, 43, 51–58. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Sterculia Crosslinked PVA and PVA-Poly(AAm) Hydrogel Wound Dressings for Slow Drug Delivery: Mechanical, Mucoadhesive, Biocompatible and Permeability Properties. J. Mech. Behav. Biomed. Mater. 2012, 9, 9–21. [Google Scholar] [CrossRef]
- Krise, K.M.; Hwang, A.A.; Sovic, D.M.; Milosavljevic, B.H. Macro- and Microscale Rheological Properties of Poly(Vinyl Alcohol) Aqueous Solutions. J. Phys. Chem. B 2011, 115, 2759–2764. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Maekawa, E. Viscosity Behavior of the System Polymethyl Acrylate and Diethyl Phthalate over the Complete Range of Composition. J. Phys. Chem. 1962, 66, 1053–1058. [Google Scholar] [CrossRef]
- Samadi, F.; Wolf, B.A.; Guo, Y.; Zhang, A.; Schlüter, A.D. Branched versus Linear Polyelectrolytes: Intrinsic Viscosities of Peripherically Charged Dendronized Poly(Methyl Methacrylate)s and of Their Uncharged Analogues. Macromolecules 2008, 41, 8173–8180. [Google Scholar] [CrossRef]
- Lămătic, I.E.; Bercea, M.; Morariu, S. Intrinsic Viscosity of Aqueous Polyvinyl Alcohol Solutions. Rev. Roum. Chim. 2009, 54, 981–986. [Google Scholar]
- Park, J.S.; Park, J.W.; Ruckenstein, E. On the Viscoelastic Properties of Poly(Vinyl Alcohol) and Chemically Crosslinked Poly(Vinyl Alcohol). J. Appl. Polym. Sci. 2001, 82, 1816–1823. [Google Scholar] [CrossRef]
- Narita, T.; Mayumi, K.; Ducouret, G.; Hébraud, P. Viscoelastic Properties of Poly(Vinyl Alcohol) Hydrogels Having Permanent and Transient Cross-Links Studied by Microrheology, Classical Rheometry, and Dynamic Light Scattering. Macromolecules 2013, 46, 4174–4183. [Google Scholar] [CrossRef]
- Tadmor, R.; Bahadur, P.; Leh, A.; N’Guessan, H.E.; Jaini, R.; Dang, L. Measurement of Lateral Adhesion Forces at the Interface between a Liquid Drop and a Substrate. Phys. Rev. Lett. 2009, 103, 266101. [Google Scholar] [CrossRef] [Green Version]
- Vinod, A.; Reddy Bhimavarapu, Y.V.; Hananovitz, M.; Stern, Y.; Gulec, S.; Jena, A.K.; Yadav, S.; Gutmark, E.J.; Patra, P.K.; Tadmor, R. Mucus-Inspired Tribology, a Sticky Yet Flowing Hydrogel. ACS Appl. Polym. Mater. 2022, 4, 8527–8535. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.R.S. Crosslinked Poly(Vinyl Alcohol) Hydrogels for Wound Dressing Applications: A Review of Remarkably Blended Polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Stable, Self-Healing Hydrogels from Nanofibrillated Cellulose, Poly(Vinyl Alcohol) and Borax via Reversible Crosslinking. Eur. Polym. J. 2014, 56, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Lin, F.; Jiang, X.; Cheng, J.; Lu, Q.; Song, J.; Chen, C.; Huang, B. One-Pot Assembly of Microfibrillated Cellulose Reinforced PVA-Borax Hydrogels with Self-Healing and PH-Responsive Properties. ACS Sustain. Chem. Eng. 2017, 5, 312. [Google Scholar] [CrossRef]
- Cui, L.; Chen, J.; Yan, C.; Xiong, D. Mechanical and Biotribological Properties of PVA/SB Triple-Network Hydrogel for Biomimetic Artificial Cartilage. J. Bionic Eng. 2022, 20, 1072–1082. [Google Scholar] [CrossRef]
- Hussain, M.; Bakalis, S.; Gouseti, O.; Zahoor, T.; Anjum, F.M.; Shahid, M. Dynamic and Shear Stress Rheological Properties of Guar Galactomannans and Its Hydrolyzed Derivatives. Int. J. Biol. Macromol. 2015, 72, 687–691. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Alhaique, F.; Farra, R.; Tesei, G.; Fiorentino, S.; Asaro, F.; Milcovich, G.; Grassi, M. Guar Gum/Borax Hydrogel: Rheological, Low Field NMR and Release Characterizations. Express Polym. Lett. 2013, 7, 733–746. [Google Scholar] [CrossRef]
- Coviello, T.; Alhaique, F.; Dorigo, A.; Matricardi, P.; Grassi, M. Two Galactomannans and Scleroglucan as Matrices for Drug Delivery: Preparation and Release Studies. Eur. J. Pharm. Biopharm. 2007, 66, 200–209. [Google Scholar] [CrossRef]
- Gliko-Kabir, I.; Yagen, B.; Penhasi, A.; Rubinstein, A. Low Swelling, Crosslinked Guar and Its Potential Use as Colon-Specific Drug Carrier. Pharm. Res. 1998, 15, 1019–1025. [Google Scholar] [CrossRef]
- Dangi, N.; Yadav, B.S.; Yadav, R.B. Pasting, Rheological, Thermal and Gel Textural Properties of Pearl Millet Starch as Modified by Guar Gum and Its Acid Hydrolysate. Int. J. Biol. Macromol. 2019, 139, 387–396. [Google Scholar] [CrossRef]
- Zahm, J.M.; King, M.; Duvivier, C.; Pierrot, D.; Girod, S.; Puchelle, E. Role of Simulated Repetitive Coughing in Mucus Clearance. Eur. Respir. J. 1991, 4, 311–315. [Google Scholar] [CrossRef]
- Quemada, D.; Droz, R. Blood Viscoelasticity and Thixotropy from Stress Formation and Relaxation Measurements: A Unified Model. Biorheology 1983, 20, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Lafforgue, O.; Bouguerra, N.; Poncet, S.; Seyssiecq, I.; Favier, J.; Elkoun, S. Thermo-Physical Properties of Synthetic Mucus for the Study of Airway Clearance. J. Biomed. Mater. Res. A 2017, 105, 3025–3033. [Google Scholar] [CrossRef] [Green Version]
- Mezger, T.G. The Rheology Handbook—For Users of Rotational and Oscillatory Rheometers; European Coatings: Nuremberg, Germany, 2006. [Google Scholar]
- Lai, S.K.; Wang, Y.Y.; Wirtz, D.; Hanes, J. Micro- and Macrorheology of Mucus. Adv. Drug Deliv. Rev. 2009, 61, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesavan, S.; Prud’homme, R.K. Rheology of Guar and HPG Cross-Linked by Borate. Macromolecules 1992, 25, 2026–2032. [Google Scholar] [CrossRef]
- Tayal, A.; Pai, V.B.; Khan, S.A. Rheology and Microstructural Changes during Enzymatic Degradation of a Guar-Borax Hydrogel. Macromolecules 1999, 32, 5567–5574. [Google Scholar] [CrossRef]
- Pan, X.; Wang, Q.; Ning, D.; Dai, L.; Liu, K.; Ni, Y.; Chen, L.; Huang, L. Ultraflexible Self-Healing Guar Gum-Glycerol Hydrogel with Injectable, Antifreeze, and Strain-Sensitive Properties. ACS Biomater. Sci. Eng. 2018, 4, 3397–3404. [Google Scholar] [CrossRef]
- Sun, C.; Boluk, Y. Rheological Behavior and Particle Suspension Capability of Guar Gum: Sodium Tetraborate Decahydrate Gels Containing Cellulose Nanofibrils. Cellulose 2016, 23, 3013–3022. [Google Scholar] [CrossRef]
- Pouyan, P.; Nie, C.; Bhatia, S.; Wedepohl, S.; Achazi, K.; Osterrieder, N.; Haag, R. Inhibition of Herpes Simplex Virus Type 1 Attachment and Infection by Sulfated Polyglycerols with Different Architectures. Biomacromolecules 2021, 22, 1545–1554. [Google Scholar] [CrossRef]
- Nie, C.; Pouyan, P.; Lauster, D.; Trimpert, J.; Kerkhoff, Y.; Szekeres, G.P.; Wallert, M.; Block, S.; Sahoo, A.K.; Dernedde, J.; et al. Polysulfates Block SARS-CoV-2 Uptake through Electrostatic Interactions. Angew. Chem. Int. Ed. 2021, 60, 15870–15878. [Google Scholar] [CrossRef]
- Sharma, A.; Thongrom, B.; Bhatia, S.; von Lospichl, B.; Addante, A.; Graeber, S.Y.; Lauster, D.; Mall, M.A.; Gradzielski, M.; Haag, R. Polyglycerol-Based Mucus-Inspired Hydrogels. Macromol. Rapid Commun. 2021, 42, 2100303. [Google Scholar] [CrossRef]
- Mahadevegowda, S.H.; Stuparu, M.C. Thermoresponsive Corannulene. Eur. J. Org. Chem. 2017, 2017, 570–576. [Google Scholar] [CrossRef]
- von Lospichl, B.; Hemmati-Sadeghi, S.; Dey, P.; Dehne, T.; Haag, R.; Sittinger, M.; Ringe, J.; Gradzielski, M. Injectable Hydrogels for Treatment of Osteoarthritis—A Rheological Study. Colloids Surf. B. Biointerfaces 2017, 159, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Ekinci, D.; Sisson, A.L.; Lendlein, A. Polyglycerol-Based Polymer Network Films for Potential Biomedical Applications. J. Mater. Chem. 2012, 22, 21100–21109. [Google Scholar] [CrossRef] [Green Version]
- Orafai, H.; Kallinteri, P.; Garnett, M.; Huggins, S.; Hutcheon, G.; Pourcain, C. Novel Poly(Glycerol-Adipate) Polymers Used for Nanoparticle Making: A Study of Surface Free Energy. Iran. J. Pharm. Res. 2008, 7, 11–19. [Google Scholar]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact Angle Measurement and Contact Angle Interpretation. Adv. Colloids Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Fowkes, F.M. Determination of Interfacial Tensions, Contact Angles, and Dispersion Forces in Surfaces by Assuming Additivity of Intermolecular Interactions in Surfaces. J. Phys. Chem. 1962, 66, 382. [Google Scholar] [CrossRef]
- Park, H.; Robinson, J.R. Mechanisms of Mucoadhesion of Poly(Acrylic Acid) Hydrogels. Pharm. Res. An. Off. J. Am. Assoc. Pharm. Sci. 1987, 4, 457–464. [Google Scholar]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Chauhan, N.P.S.; Jadoun, S.; Soltani, M.D.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Gupta, P.; Vermani, K.; Garg, S. Hydrogels: From Controlled Release to PH-Responsive Drug Delivery. Drug Discov. Today 2002, 7, 569–579. [Google Scholar] [CrossRef]
- Tamburic, S.; Craig, D.Q.M. An Investigation into the Rheological, Dielectric and Mucoadhesive Properties of Poly(Acrylic Acid) Gel Systems. J. Control. Release 1995, 37, 59–68. [Google Scholar] [CrossRef]
- Barry, B.W.; Meyer, M.C. The Rheological Properties of Carbopol Gels I. Continuous Shear and Creep Properties of Carbopol Gels. Int. J. Pharm. 1979, 2, 27–40. [Google Scholar] [CrossRef]
- Tamburic, S.; Craig, D.Q.M. A Comparison of Different in Vitro Methods for Measuring Mucoadhesive Performance. Eur. J. Pharm. Biopharm. 1997, 44, 159–167. [Google Scholar] [CrossRef]
- Hägerström, H.; Paulsson, M.; Edsman, K. Evaluation of Mucoadhesion for Two Polyelectrolyte Gels in Simulated Physiological Conditions Using a Rheological Method. Eur. J. Pharm. Sci. 2000, 9, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.G.; Smart, J.D.; Tsibouklis, J.; Dettmar, P.W.; Hampson, F.; Davis, J.A.; Kelly, G.; Wilber, W.R. An Investigation of Mucus/Polymer Rheological Synergism Using. Synthesised and Characterised Poly(Acrylic Acid)s. Int. J. Pharm. 2001, 217, 87–100. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, J.Y.; Lee, E.J.; Park, S.K. Rheological Properties and Microstructures of Carbopol Gel Network System. Colloids Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Bonacucina, G.; Martelli, S.; Palmieri, G.F. Rheological, Mucoadhesive and Release Properties of Carbopol Gels in Hydrophilic Cosolvents. Int. J. Pharm. 2004, 282, 115–130. [Google Scholar] [CrossRef]
- Baek, G.; Kim, C. Rheological Properties of Carbopol Containing Nanoparticles. J. Rheol. 2011, 55, 313–330. [Google Scholar] [CrossRef] [Green Version]
- Schenck, D.M.; Fiegel, J. Tensiometric and Phase Domain Behavior of Lung Surfactant on Mucus-like Viscoelastic Hydrogels. ACS Appl. Mater. Interfaces 2016, 8, 5917–5928. [Google Scholar] [CrossRef] [Green Version]
- De Vicente, J.; Stokes, J.R.; Spikes, H.A. Soft Lubrication of Model Hydrocolloids. Food Hydrocoll. 2006, 20, 483–491. [Google Scholar] [CrossRef]
- Koenderink, G.H.; Atakhorrami, M.; MacKintosh, F.C.; Schmidt, C.F. High-Frequency Stress Relaxation in Semiflexible Polymer Solutions and Networks. Phys. Rev. Lett. 2006, 96, 138307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco-Fuente, H.; Anguiano-Igea, S.; Otero-Espinar, F.J.; Blanco-Méndez, J. In-Vitro Bioadhesion of Carbopol Hydrogels. Int. J. Pharm. 1996, 142, 169–174. [Google Scholar] [CrossRef]
- Chau, A.L.; Getty, P.T.; Rhode, A.R.; Bates, C.M.; Hawker, C.J.; Pitenis, A.A. Superlubricity of PH-Responsive Hydrogels in Extreme Environments. Front. Chem. 2022, 10, 862. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent Advances of Injectable Hydrogels for Drug Delivery and Tissue Engineering Applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug. Delivery: Progress. and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.S.; Cunha, A.M.; Reis, R.L.; Vázquez, B.; San Román, J. New starch-based thermoplastic hydrogels for use as bone cements or drug-delivery carriers. J. Mater. Sci. Mater. Med. 1998, 9, 825–833. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.; Chen, G.; Zhou, Z.; Li, Q. A High Water-Content and High Elastic Dual-Responsive Polyurethane Hydrogel for Drug Delivery. J. Mater. Chem. B. 2015, 3, 8401–8409. [Google Scholar] [CrossRef]
- Islam, A.; Riaz, M.; Yasin, T. Structural and Viscoelastic Properties of Chitosan-Based Hydrogel and Its Drug Delivery Application. Int. J. Biol. Macromol. 2013, 59, 119–124. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 3, 1869–1879. [Google Scholar] [CrossRef]
- Vázquez-Portalatĺn, N.; Kilmer, C.E.; Panitch, A.; Liu, J.C. Characterization of Collagen Type i and II Blended Hydrogels for Articular Cartilage Tissue Engineering. Biomacromolecules 2016, 17, 3145–3152. [Google Scholar] [CrossRef] [Green Version]
- Madsen, F.; Eberth, K.; Smart, J.D. A Rheological Examination of the Mucoadhesive/Mucus Interaction: The Effect of Mucoadhesive Type and Concentration. J. Control. Release 1998, 50, 167–178. [Google Scholar] [CrossRef]
- Mortazavi, S.A.; Smart, J.D. An Investigation into the Role of Water Movement and Mucus Gel Dehydration in Mucoadhesion. J. Control. Release 1993, 25, 197–203. [Google Scholar] [CrossRef]
- Wright, L.; Joyce, P.; Barnes, T.J.; Prestidge, C.A. Mimicking the Gastrointestinal Mucus Barrier: Laboratory-Based Approaches to Facilitate an Enhanced Understanding of Mucus Permeation. ACS Biomater. Sci. Eng. 2021, 9, 2819–2837. [Google Scholar] [CrossRef] [PubMed]
- Murgia, X.; Yasar, H.; Carvalho-Wodarz, C.; Loretz, B.; Gordon, S.; Schwarzkopf, K.; Schaefer, U.; Lehr, C.M. Modelling the Bronchial Barrier in Pulmonary Drug Delivery: A Human Bronchial Epithelial Cell Line Supplemented with Human Tracheal Mucus. Eur. J. Pharm. Biopharm. 2017, 118, 79–88. [Google Scholar] [CrossRef]
- Liu, X.; Inda, M.E.; Lai, Y.; Lu, T.K.; Zhao, X. Engineered Living Hydrogels. Adv. Mater. 2022, 34, 2201326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-Based Biopolymer Hydrogels for Heavy Metal Detection and Adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Kofinas, P.; Kioussis, D.R. Reactive Phosphorus Removal from Aquaculture and Poultry Productions Systems Using. Polymeric Hydrogels. Environ. Sci. Technol. 2003, 37, 423–427. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene Oxide-Chitosan Composite Hydrogels as Broad-Spectrum Adsorbents for Water Purification. J. Mater. Chem. A Mater. 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, J.F.; Rojas, D.; Escarpa, A. Electrochemical Sensing Directions for Next-Generation Healthcare: Trends, Challenges, and Frontiers. Anal. Chem. 2021, 93, 167–183. [Google Scholar] [CrossRef]
- Guenther, M.; Gerlach, G.; Wallmersperger, T.; Avula, M.N.; Cho, S.H.; Xie, X.; Devener, B.V.; Solzbacher, F.; Tathireddy, P.; Magda, J.J.; et al. Smart Hydrogel-Based Biochemical Microsensor Array for Medical Diagnostics. Wearable/Wireless Body Sensor Networks for Healthcare Applications. 2012, Volume 85. Available online: https://www.scientific.net/AST.85.47 (accessed on 30 May 2023).
- Cheng, W.; Wu, X.; Zhang, Y.; Wu, D.; Meng, L.; Chen, Y.; Tang, X. Recent Applications of Hydrogels in Food Safety Sensing: Role of Hydrogels. Trends Food Sci. Technol. 2022, 129, 224–257. [Google Scholar] [CrossRef]
- Du, X.; Zhai, J.; Li, X.; Zhang, Y.; Li, N.; Xie, X. Hydrogel-Based Optical Ion Sensors: Principles and Challenges for Point-of-Care Testing and Environmental Monitoring. ACS Sens. 2021, 6, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.; Xu, H.; Peng, S.; Li, L.; Chen, S.; Lu, T.; Guo, X. Integrated Soft Ionotronic Skin with Stretchable and Transparent Hydrogel-Elastomer Ionic Sensors for Hand-Motion Monitoring. Soft Robot. 2019, 6, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.L.; Yu, T.L.; Cheng, C.H. Reentrant behavior of poly(vinyl alcohol)-Borax semidilute aqueous solutions. Colloid Polym Sci. 2000, 278, 187–194. [Google Scholar] [CrossRef]
- Le, V.N.A.; Lin, J.W. Tribological properties of aluminum nanoparticles as additives in an aqueous glycerol solution. Appl. Sci. 2017, 7, 80. [Google Scholar] [CrossRef] [Green Version]





| Mucus Samples Collected from People with: | Calculated Work of Adhesion to Separate Mucus from Airway Duct | |
|---|---|---|
| du Nouy Ring | Using Equation (3) | |
| Cystic fibrosis | 140 ± 30 mN/m | 160 ± 20 mN/m |
| Chronic bronchitis | 130 ± 20 mN/m | 150 ± 20 mN/m |
| MIH Studied | Polymer Used as Backbone | Crosslinker to Polymer Ratio |
|---|---|---|
| MIH-1 | LPG5(SH)2 | MIH-1 shown in the plot (Figure 12a) is an average of the ratios 1:3, 1:7, 1:10, 1:14. |
| MIH-2 | PEG3(SH)2 | 1:3 |
| MIH-3 | PEG6(SH)2 | 1:3 |
| Adipate Concentration (%) | Surface Energy (mJ/m2) |
|---|---|
| 0 | 62.4 |
| 20 | 60.7 |
| 40 | 31.4 |
| 100 | 57.3 |
| Carbopol Concentration (wt.%) | Surface Tension (mN/m) |
|---|---|
| 0.025 | 72.3 ± 0.10 |
| 0.04 | 71.7 ± 0.20 |
| 0.05 | 71.8 ± 0.40 |
| Hydrogel Used | Research Conducted by | Comments by the Research Group on the Hydrogel’s Rheological Properties |
|---|---|---|
| Native mucus | Wolf et al. [24] | G′ > G″ |
| Hill et al. [26] | G′ > G″ | |
| Polyvinyl alcohol | Park et al. [48] | G′ decreased as the temperature increased. |
| Narita et al. [49] | At lower frequencies, G′ < G″, and at higher frequencies, G′ > G″. | |
| Lu et al. [54] | At lower frequencies, G″ > G′, and at higher frequencies, G′ > G″ | |
| Polyvinyl alcohol with boron | Lin et al. [54] | At lower frequency G′ < G″, and at higher frequency the G′ > G″. |
| Vinod et al. [51] | G′ > G″ | |
| Guar gum borax | Pan et al. [68] | G′ > G″ |
| Sun et al. [69] | G′ > G″ | |
| Sharma et al. [72] | For MIH-1, G′ > G″ For MIH-2 and MIH-3, G″ > G′ | |
| Polyglycerol | Lospichl et al. [74] | G′ > G″ |
| Ekinci et al. [75] | G′ < G″ | |
| Kim et al. [88] | G′ > G″ | |
| Polyacrylic acid | Bonacucina et al. [89] | G′ > G″ |
| Baek et al. [90] | G′ > G″ | |
| Schenck et al. [91] | G′ > G″ | |
| Vicente et al. [92] | G′ > G″ |
| Rheological Moduli of Native Airway Mucus | Group | Hydrogels with Rheological Moduli Falling in the Range of Native Airway Mucus | |
|---|---|---|---|
| Storage Modulus (G′, Pa) | Loss Modulus (G″, Pa) | ||
| ~1 | ~0.1 | Hill et al. [26] |
|
| ~10 | ~1.0 | Wolf et al. [24] |
|
| ~100 | ~10 | Hamed et al. [32] |
|
| Hydrogel Used | Research Conducted by | Comments by the Research Group on the Hydrogel’s Tribological Properties |
|---|---|---|
| Native mucus | Albers et al. [35] | Measured the work of adhesion needed to move native mucus from the airway duct:
|
| King et al. [61] | As the work of adhesion increased, the transportability of the mucus decreased. | |
| Polyvinyl alcohol | Vinod et al. [51] | The lateral force, f||, for sliding = 111 μN. |
| Polyvinyl alcohol with boron | Cui et al. [55] | The coefficient of friction (COF):
|
| Vinod et al. [51] | The lateral force, f||, needed to slide the hydrogel drop was around 166 μN. | |
| Guar gum with scleroglucan | Lafforgue et al. [63] | The surface tension of the hydrogel = 72 to 90 mN/m. |
| Guar gum borax | Pan et al. [68] | The work of adhesion required to detach the hydrogel from the ‘human skin-mimicking surface’ was 2.5 KPa. |
| Polyglycerol | Orafai et al. [76] | Surface energy of the hydrogel = 31.4 mJ/m to 62.4 mJ/m. |
| Polyacrylic acid | Schenck et al. [91] | Surface tensions were recorded for different weight percentage of polymer in the hydrogel:
|
| Chau et al. [95] | The coefficient of friction (COF) was recorded based on the pH of the hydrogel:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinod, A.; Tadmor, R.; Katoshevski, D.; Gutmark, E.J. Gels That Serve as Mucus Simulants: A Review. Gels 2023, 9, 555. https://doi.org/10.3390/gels9070555
Vinod A, Tadmor R, Katoshevski D, Gutmark EJ. Gels That Serve as Mucus Simulants: A Review. Gels. 2023; 9(7):555. https://doi.org/10.3390/gels9070555
Chicago/Turabian StyleVinod, Appu, Rafael Tadmor, David Katoshevski, and Ephraim J. Gutmark. 2023. "Gels That Serve as Mucus Simulants: A Review" Gels 9, no. 7: 555. https://doi.org/10.3390/gels9070555





