The Characteristics of Whey Protein and Blueberry Juice Mixed Fermentation Gels Formed by Lactic Acid Bacteria
Abstract
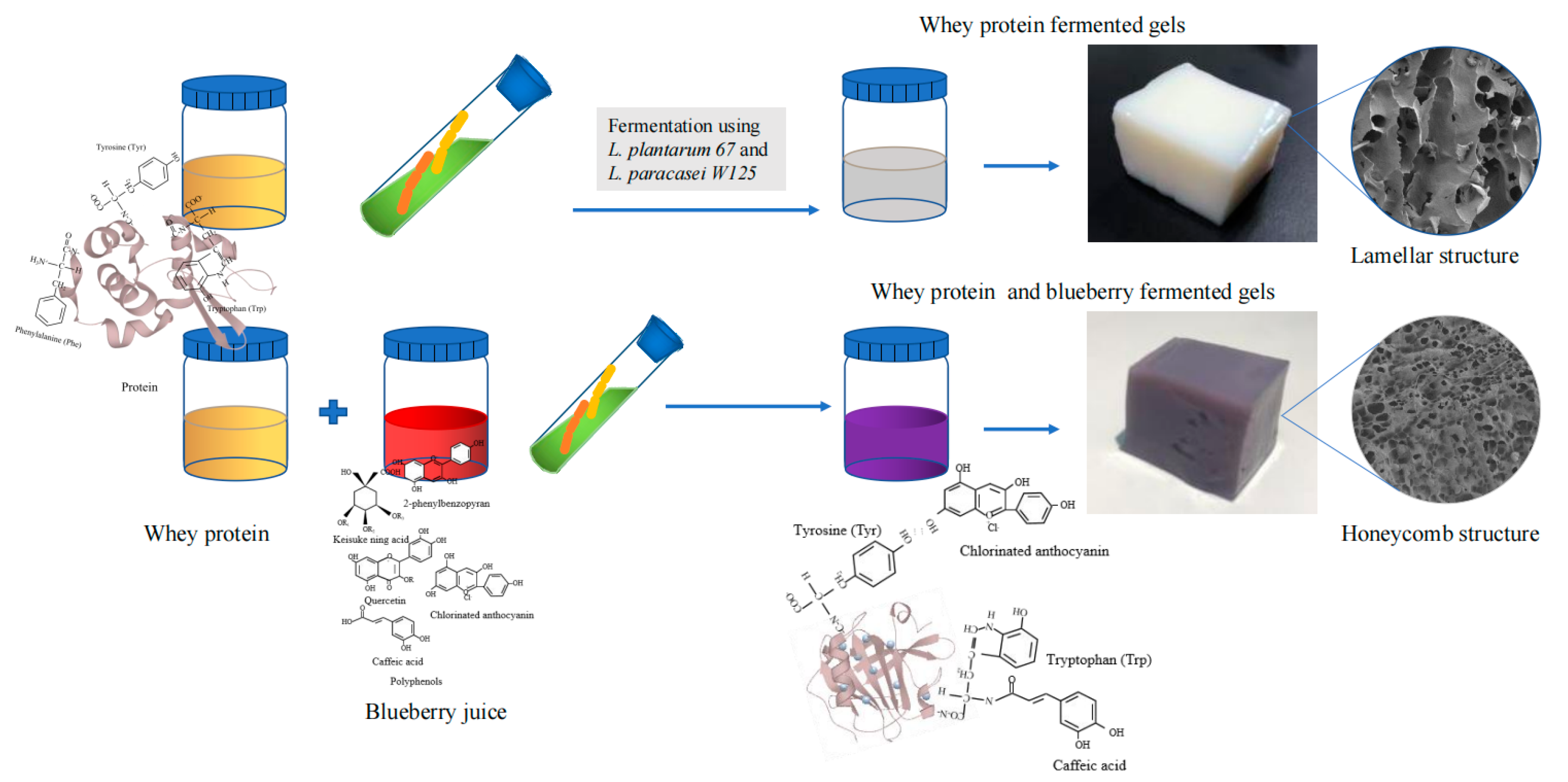
:1. Introduction
2. Results and Discussion
2.1. Macrostructure Observation
2.2. Colour Analysis
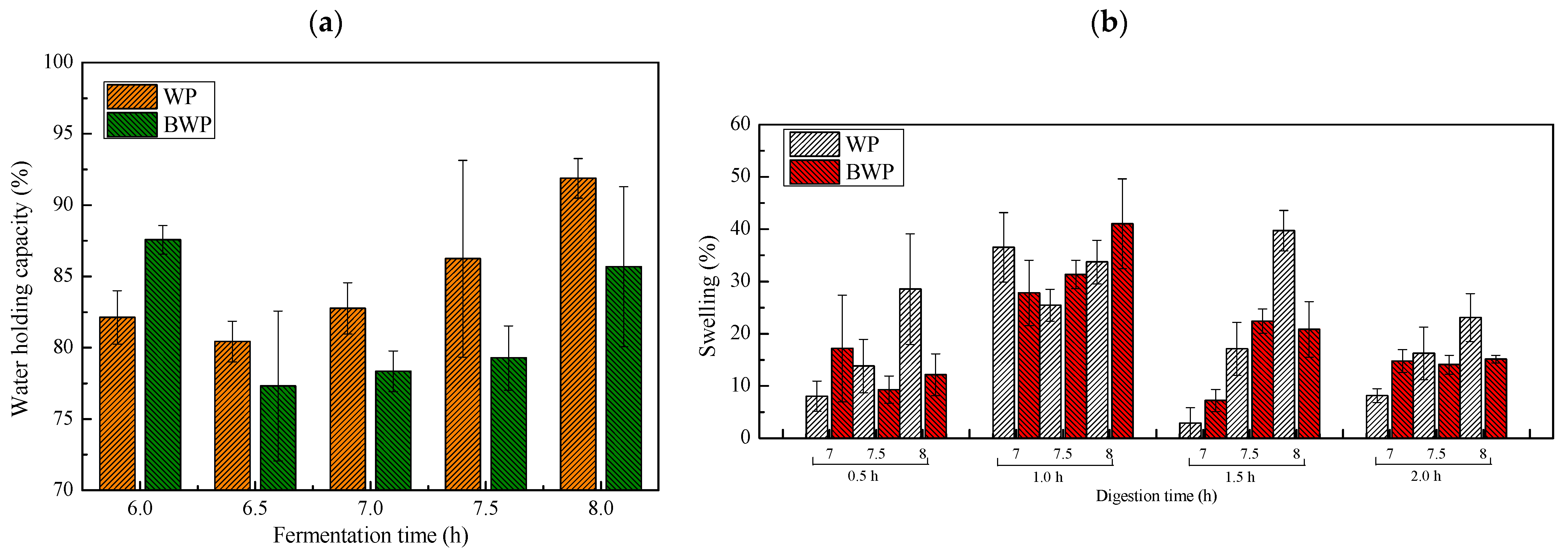
2.3. Water Holding Capacity and Swelling of the Fermentation Gels
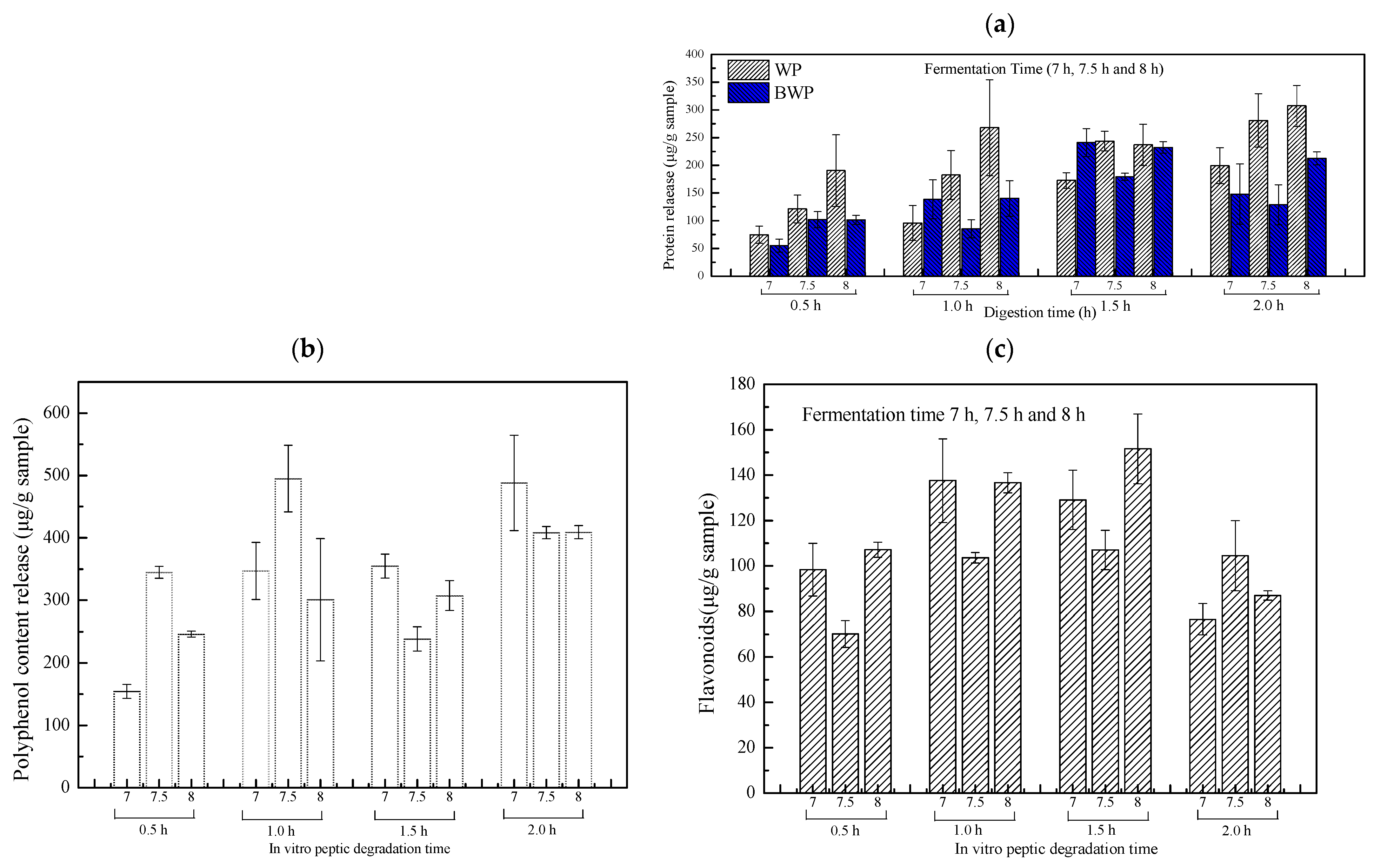
2.4. The Polyphenolic, Flavonoid, and Protein Release of Whey Protein or Combined Blueberry Juice Fermentation Gels
2.5. Rheological Measurements
2.6. Texture Analysis of Whey Protein Blueberry Gel Formation at Different Fermentation Times
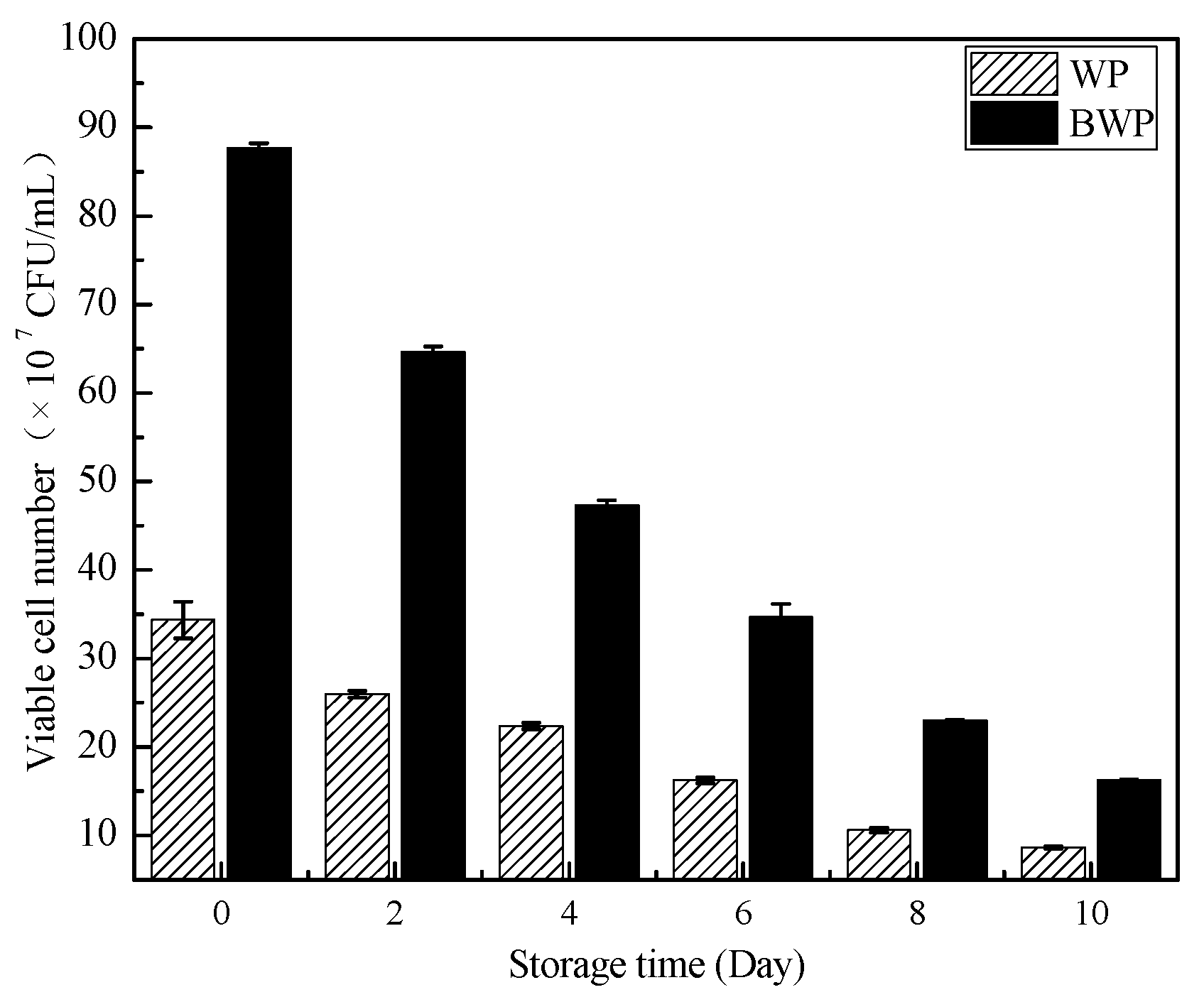
2.7. Monitoring of Live Bacteria during Storage of Whey Protein in Blueberry Gels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Experimental Preparation
4.3. Surface Structure Observation
4.4. Colour Measurement of Each Sample at Different Fermentation Times
4.5. Texture of Whey Protein or Blueberry Gel Formation at Different Fermentation Times
4.6. Water Holding Capacity of Whey Protein or Blueberry Gels
4.7. Swelling and Protein Release following In Vitro Digestion
4.8. Release of Total Phenolic Content (TPC) following In Vitro Digestion
4.9. Release of Anthocyanin Concentration (TAC) following In Vitro Digestion
4.10. Rheological Measurements
4.11. Monitoring of Live Bacteria during Storage of Whey Protein in Blueberry Gels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Ma, T.; Zhang, W.; Su, E.; Cao, F.; Huang, M.; Wang, Y. Heat-induced gel formation by whey protein isolate-Lycium barbarum polysaccharides at varying pHs. Food Hydrocoll. 2021, 115, 106607. [Google Scholar] [CrossRef]
- Lambrecht, M.A.; Rombouts, I.; Delcour, J.A. Denaturation and covalent network formation of wheat gluten, globular proteins and mixtures thereof in aqueous ethanol and water. Food Hydrocoll. 2016, 57, 122–131. [Google Scholar] [CrossRef]
- Mezzenga, R.; Fischer, P. The self-assembly, aggregation and phase transitions of food protein systems in one, two and three dimensions. Rep. Prog. Phys. 2013, 76, 046601. [Google Scholar] [CrossRef] [PubMed]
- Klost, M.; Giménez-Ribes, G.; Drusch, S. Enzymatic hydrolysis of pea protein: Interactions and protein fractions involved in fermentation induced gels and their influence on rheological properties. Food Hydrocoll. 2020, 105, 105793. [Google Scholar] [CrossRef]
- Doherty, S.B.; Gee, V.L.; Ross, R.P.; Stanton, C.; Fitzgerald, G.F.; Brodkorb, A. Efficacy of whey protein gel networks as potential viability-enhancing scaffolds for cell immobilization of Lactobacillus rhamnosus GG. J. Microbiol. Methods 2010, 80, 231–241. [Google Scholar] [CrossRef]
- Wang, W.-Q.; Sheng, H.-B.; Zhou, J.-Y.; Yuan, P.-P.; Zhang, X.-F.; Lu, M.-L.; Gu, R.-X. The effect of a variable initial pH on the structure and rheological properties of whey protein and monosaccharide gelation via the Maillard reaction. Int. Dairy J. 2021, 113, 104896. [Google Scholar] [CrossRef]
- Uribe-Alvarez, R.; Murphy, C.P.; Coleman-Vaughan, C.; O’Shea, N. Evaluation of ionic calcium and protein concentration on heat- and cold-induced gelation of whey protein isolate gels as a potential food formulation for 3D food printing. Food Hydrocoll. 2023, 142, 108777. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Nishinari, K.; Mleko, S.; Terpiłowski, K.; Pérez-Huertas, S. Cold gelation of whey protein isolate with sugars in an ultrasound environment. Food Hydrocoll. 2023, 139, 108510. [Google Scholar] [CrossRef]
- Wagner, J.; Andreadis, M.; Nikolaidis, A.; Biliaderis, C.G.; Moschakis, T. Effect of ethanol on the microstructure and rheological properties of whey proteins: Acid-induced cold gelation. LWT 2021, 139, 110518. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Euston, S.R.; Li, B.; Li, E.; Fu, C.; Chen, G. Structural and gelling properties of whey proteins influenced by various acids: Experimental and computational approaches. Food Hydrocoll. 2023, 144, 109003. [Google Scholar] [CrossRef]
- Ribeiro, J.C.B.; Granato, D.; Masson, M.L.; Andriot, I.; Mosca, A.C.; Salles, C.; Guichard, E. Effect of lactobionic acid on the acidification, rheological properties and aroma release of dairy gels. Food Chem. 2016, 207, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Liu, Y.; Jiang, J.; Xiong, Y.L.; Zhao, X. Rheological, structural, and water-immobilizing properties of mung bean protein-based fermentation-induced gels: Effect of pH-shifting and oil imbedment. Food Hydrocoll. 2022, 129, 107607. [Google Scholar] [CrossRef]
- Kleemann, C.; Schuster, R.; Rosenecker, E.; Selmer, I.; Smirnova, I.; Kulozik, U. In-vitro-digestion and swelling kinetics of whey protein, egg white protein and sodium caseinate aerogels. Food Hydrocoll. 2020, 101, 105534. [Google Scholar] [CrossRef]
- Wen-Qiong, W.; Jie-Long, Z.; Qian, Y.; Ji-Yang, Z.; Mao-Lin, L.; Rui-Xia, G.; Yujun, H. Structural and compositional changes of whey protein and blueberry juice fermented using Lactobacillus plantarum or Lactobacillus casei during fermentation. RSC Adv. 2021, 11, 26291–26302. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Beta, T.; Feng, J.; Huang, W. Blueberry anthocyanins: An updated review on approaches to enhancing their bioavailability. Trends Food Sci. Technol. 2021, 118, 808–821. [Google Scholar] [CrossRef]
- Hazrati, Z.; Madadlou, A. Gelation by bioactives: Characteristics of the cold-set whey protein gels made using gallic acid. Int. Dairy J. 2021, 117, 104952. [Google Scholar] [CrossRef]
- Deng, R.; Mars, M.; Van Der Sman, R.G.M.; Smeets, P.A.M.; Janssen, A.E.M. The importance of swelling for in vitro gastric digestion of whey protein gels. Food Chem. 2020, 330, 127182. [Google Scholar] [CrossRef]
- Hoskin, R.T.; Xiong, J.; Esposito, D.A.; Lila, M.A. Blueberry polyphenol-protein food ingredients: The impact of spray drying on the in vitro antioxidant activity, anti-inflammatory markers, glucose metabolism and fibroblast migration. Food Chem. 2019, 280, 187–194. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Mleko, S.; Terpiłowski, K.; Pérez-Huertas, S.; Nishinari, K. Aerated whey protein gels as a controlled release system of creatine investigated in an artificial stomach. Innov. Food Sci. Emerg. Technol. 2022, 79, 103060. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Meng, X.; Zhou, L.; Liu, W.; Liu, C.; Prakash, S.; Zhong, J. The gel mechanism and carrier quality of fibrous and granular whey protein self-assembly. Food Hydrocoll. 2023, 136, 108302. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, J.; Zhang, L. Influence of rose anthocyanin extracts on physicochemical properties and in vitro digestibility of whey protein isolate sol/gel: Based on different pHs and protein concentrations. Food Chem. 2023, 405, 134937. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Z.; Zheng, J.; Sun, W.; Xiao, Z.; Shao, J.-H. Effects of direct current magnetic field co-treated with stirring on gel properties of chicken batter: Hydration and textural properties. J. Food Eng. 2023, 339, 111279. [Google Scholar] [CrossRef]
- Rong, L.; Shen, M.; Wen, H.; Xiao, W.; Li, J.; Xie, J. Effects of xanthan, guar and Mesona chinensis Benth gums on the pasting, rheological, texture properties and microstructure of pea starch gels. Food Hydrocoll. 2022, 125, 107391. [Google Scholar] [CrossRef]
- Dong, X.; Zhuo, H.; Wang, K.; Wu, P.; Chen, X.D. Real-time spatial quantification of gastric acid diffusion in whey protein gels with different NaCl concentrations by wide-field fluorescence microscopy. Food Res. Int. 2023, 169, 112828. [Google Scholar] [CrossRef]
- Lan, W.; Wang, S.; Chen, M.; Sameen, D.E.; Lee, K.; Liu, Y. Developing poly(vinyl alcohol)/chitosan films incorporate with d-limonene: Study of structural, antibacterial, and fruit preservation properties. Int. J. Biol. Macromol. 2020, 145, 722–732. [Google Scholar] [CrossRef]
- Yamul, D.K.; Galmarini, M.V.; Lupano, C.E.; Zamora, M.C. Whey protein concentrate gels with different sucrose content: Instrumental texture measurements and sensory perception. Int. Dairy J. 2013, 28, 24–31. [Google Scholar] [CrossRef]
- Shen, X.; Sun, X.; Xie, Q.; Liu, H.; Zhao, Y.; Pan, Y.; Hwang, C.-A.; Wu, V.C.H. Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis. Food Control 2014, 35, 159–165. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Li, B.; Tan, H.; Li, D.; Li, L.; Liu, X.; Han, J.; Meng, X. Comparative transcriptome analysis of genes involved in anthocyanin synthesis in blueberry. Plant Physiol. Biochem. 2018, 127, 561–572. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhong, M.; Qi, B.; Li, Y. Soy and whey protein isolate mixture/calcium chloride thermally induced emulsion gels: Rheological properties and digestive characteristics. Food Chem. 2022, 380, 132212. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, W.; Liu, X.; Shen, W.; Gu, R.; Tang, C. The Antioxidant Activity and Protection of Probiotic Bacteria in the In Vitro Gastrointestinal Digestion of a Blueberry Juice and Whey Protein Fermentation System. Fermentation 2023, 9, 335. [Google Scholar] [CrossRef]
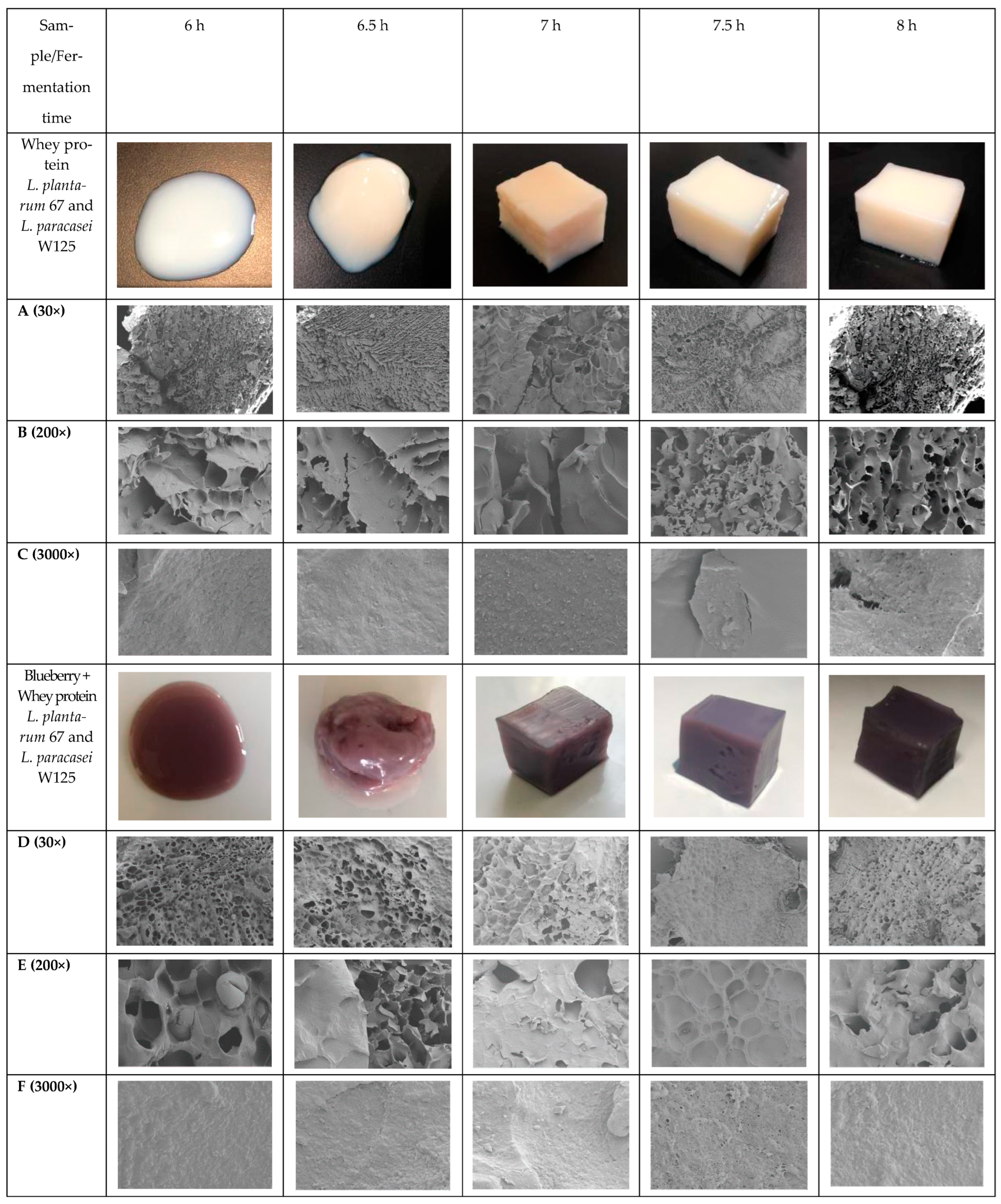

| Sample (pH 6.5) | Fermentation Time (h) | ΔL | Δa | Δb | ΔE |
|---|---|---|---|---|---|
| Whey protein L. plantarum 67 and L. paracasei W125 | 6 h | 4.167 ± 0.988 b | 0.180 ± 0.206 b | 2.647 ± 0.988 b | 4.968 ± 1.361 c |
| 6.5 h | 7.213 ± 0.859 a | 0.450 ± 0.408 a | 5.693 ± 0.859 a | 9.220 ± 1.211 a | |
| 7 h | 5.387 ± 0.596 b | 0.033 ± 0.269 c | 3.867 ± 0.596 b | 6.644 ± 0.834 b | |
| 7.5 h | 5.023 ± 1.337 b | 0.157 ± 0.239 b | 3.503 ± 1.337 b | 6.158 ± 1.854 b | |
| 8 h | 5.260 ± 0.601 b | 0.173 ± 0.272 b | 3.740 ± 0.601 b | 6.472 ± 0.835 b | |
| Blueberry + Whey protein L. plantarum 67 and L. paracasei W125 | 6 h | 8.980 ± 0.264 b | 3.510 ± 0.553 a | 0.210 ± 0.445 a | 9.683 ± 0.445 b |
| 6.5 h | 11.02 ± 0.220 a | 3.327 ± 0.533 a | 0.063 ± 0.258 b | 11.57 ± 0.332 a | |
| 7 h | 11.41 ± 0.728 a | 4.113 ± 0.408 a | −0.807 ± 0.373 c | 12.19 ± 0.657 a | |
| 7.5 h | 12.23 ± 0.429 a | 4.223 ± 0.187 a | 0.100 ± 0.274 b | 12.95 ± 0.469 a | |
| 8 h | 11.95 ± 0.797 a | 3.607 ± 0.176 a | −0.617 ± 0.784 b | 12.56 ± 0.721 a |
| Sample (pH 6.5) | Fermentation Time (h) | Rupture Strength (N) | Hardness (N) | Maximum Adhesion (N·M−2) | Adhesiveness (%) | Cohesiveness (%) | Elasticity | Glueyness | Chewiness |
|---|---|---|---|---|---|---|---|---|---|
| Whey protein L. plantarum 67 and L. paracasei W125 | 6.0 h | 0.619 ± 0.084 b | 0.674 ± 0.049 c | −0.086 ± 0.012 b | 1.023 ± 0.101 b | 0.490 ± 0.000 a | 16.890 ± 0.072 b | 0.322 ± 0.026 b | 5.440 ± 0.435 b |
| 6.5 h | 0.707 ± 0.052 b | 1.094 ± 0.503 b | −0.112 ± 0.014 b | 1.218 ± 0.205 b | 0.493 ± 0.012 a | 15.933 ± 0.409 b | 0.462 ± 0.091 b | 7.353 ± 1.384 b | |
| 7.0 h | 1.289 ± 0.535 a | 1.850 ± 0.111 a | −0.094 ± 0.019 b | 1.248 ± 0.290 b | 0.485 ± 0.007 a | 17.312 ± 0.160 b | 0.682 ± 0.064 a | 11.80 ± 1.088 a | |
| 7.5 h | 0.929 ± 0.429 b | 1.216 ± 0.274 b | −0.119 ± 0.027 b | 1.434 ± 0.647 b | 0.455 ± 0.037 a | 16.033 ± 4.397 b | 0.620 ± 0.064 a | 10.14 ± 3.370 a | |
| 8.0 h | 0.703 ± 0.156 b | 1.185 ± 0.077 b | −0.150 ± 0.006 a | 1.980 ± 0.073 a | 0.418 ± 0.062 a | 19.380 ± 0.125 a | 0.646 ± 0.072 a | 12.51 ± 1.365 a | |
| Blueberry + Whey protein L. plantarum 67 and L. paracasei W125 | 6.0 h | 0.975 ± 0.037 b | 1.096 ± 0.171 b | −0.140 ± 0.030 b | 1.638 ± 0.388 c | 0.480 ± 0.034 a | 17.277 ± 0.146 b | 0.504 ± 0.086 b | 8.713 ± 1.533 b |
| 6.5 h | 0.707 ± 0.052 b | 1.094 ± 0.503 b | −0.112 ± 0.014 b | 1.218 ± 0.205 d | 0.493 ± 0.012 a | 15.933 ± 0.409 b | 0.462 ± 0.091 b | 7.353 ± 1.384 b | |
| 7.0 h | 0.778 ± 0.426 b | 1.102 ± 0.110 b | −0.166 ± 0.025 b | 1.784 ± 0.241 c | 0.495 ± 0.037 a | 17.563 ± 0.297 b | 0.397 ± 0.106 b | 6.960 ± 1.785 b | |
| 7.5 h | 1.243 ± 0.303 a | 1.692 ± 0.133 a | −0.241 ± 0.045 a | 3.267 ± 0.427 a | 0.543 ± 0.065 a | 19.235 ± 0.202 a | 0.785 ± 0.124 a | 15.11 ± 2.374 a | |
| 8.0 h | 1.329 ± 0.232 a | 1.542 ± 0.115 a | −0.197 ± 0.024 a | 2.804 ± 0.249 b | 0.493 ± 0.031 a | 19.710 ± 0.295 a | 0.686 ± 0.102 a | 13.53 ± 2.180 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, Y.; Liu, X.; Yu, Q. The Characteristics of Whey Protein and Blueberry Juice Mixed Fermentation Gels Formed by Lactic Acid Bacteria. Gels 2023, 9, 565. https://doi.org/10.3390/gels9070565
Wang W, Wang Y, Liu X, Yu Q. The Characteristics of Whey Protein and Blueberry Juice Mixed Fermentation Gels Formed by Lactic Acid Bacteria. Gels. 2023; 9(7):565. https://doi.org/10.3390/gels9070565
Chicago/Turabian StyleWang, Wenqiong, Yuxian Wang, Xian Liu, and Qian Yu. 2023. "The Characteristics of Whey Protein and Blueberry Juice Mixed Fermentation Gels Formed by Lactic Acid Bacteria" Gels 9, no. 7: 565. https://doi.org/10.3390/gels9070565
APA StyleWang, W., Wang, Y., Liu, X., & Yu, Q. (2023). The Characteristics of Whey Protein and Blueberry Juice Mixed Fermentation Gels Formed by Lactic Acid Bacteria. Gels, 9(7), 565. https://doi.org/10.3390/gels9070565





