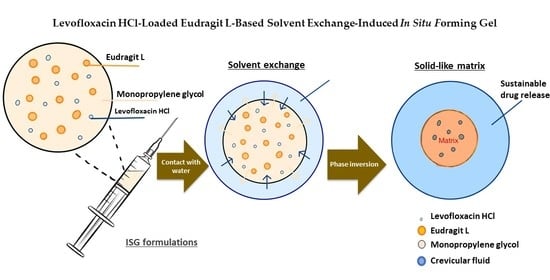
Levofloxacin HCl-Loaded Eudragit L-Based Solvent Exchange-Induced In Situ Forming Gel Using Monopropylene Glycol as a Solvent for Periodontitis Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Drug-Free Eudragit-Based ISG
2.1.1. Physical Appearance, Viscosity and Rheology, Injectability, Contact Angle, and Mechanical Properties
2.1.2. Gel Formation of Eudragit-Based ISGs
2.2. Levofloxacin HCl-Loaded Eudragit-Based ISG
2.2.1. Physical Appearance, Viscosity and Rheology, Injectability, Contact Angle, and Mechanical Properties
2.2.2. Gel Formation of Levofloxacin HCl-Loaded Eudragit-Based ISG
2.2.3. Microscopic Interface Interaction
2.2.4. Drug Content and Release of Levofloxacin HCl-Loaded Eudragit-Based ISGs
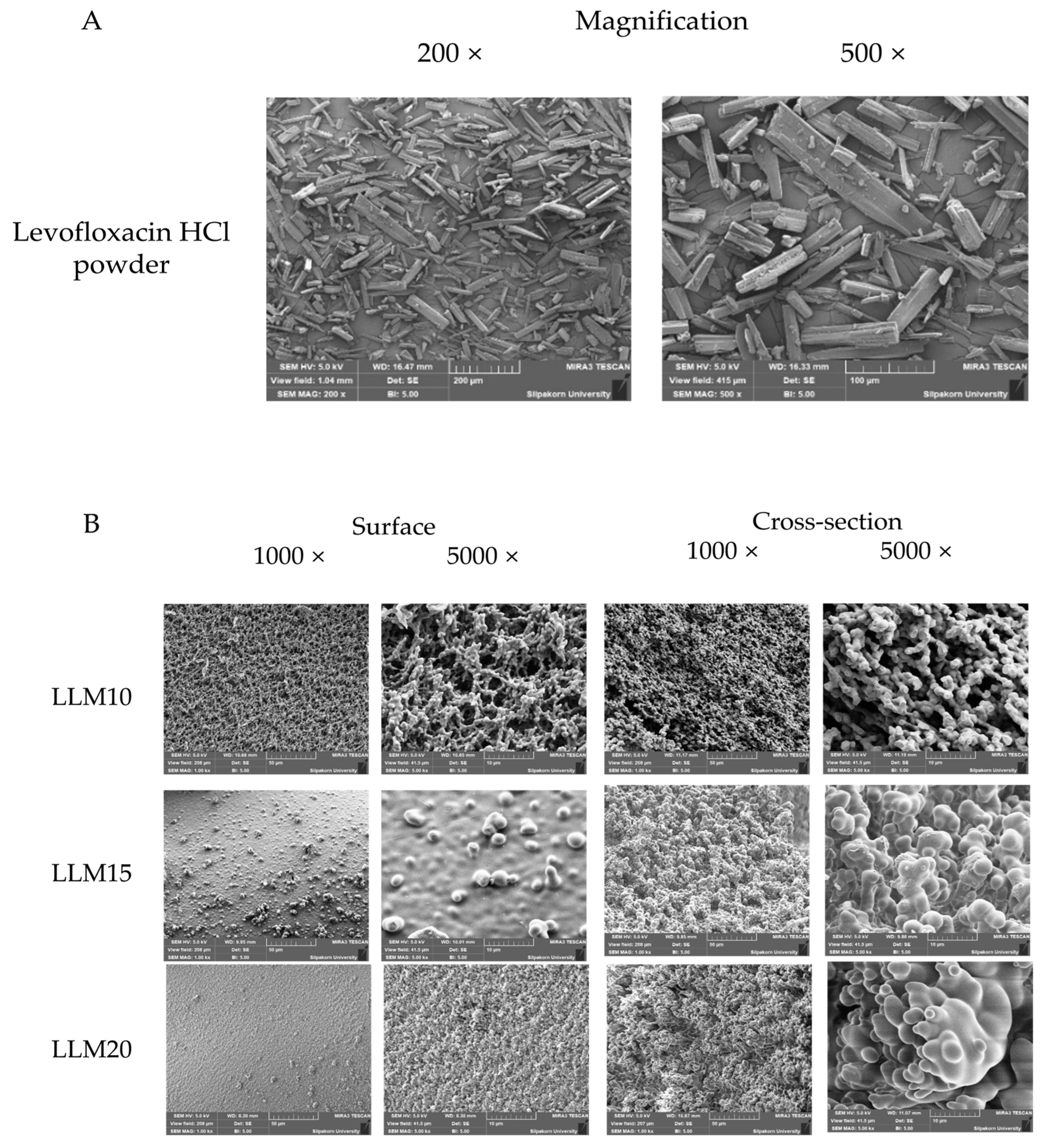
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. In Vitro Degradation
2.2.7. X-ray Computed Microtomography (μCT)
2.2.8. Antimicrobial Activities
2.2.9. FOURIER Transform Infrared Spectroscopy (FTIR)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of In Situ Forming Gel
4.3. Viscosity and Rheology Characterization
4.4. Contact Angle
4.5. Injectability
4.6. Mechanical Properties
4.7. Gel Formation Study
4.8. Interfacial Phenomena of Formulation-Aqueous Phase
4.9. Drug Content and In Vitro Drug Release Studies
4.10. Scanning Electron Microscopy (SEM)
4.11. In Vitro Degradation Test
- = initial weight of the sample
- = weight of remained sample at a specific time
4.12. X-ray Imaging and X-ray Tomographic Microscopy
4.13. Antimicrobial Activities
4.14. Fourier Transform Infrared (FTIR) Spectroscopy
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiorillo, L. Oral health: The first step to well-being. Medicina 2019, 55, 676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global prevalence of periodontal disease and lack of its surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Dental and Craniofacial Research. Periodontal (Gum) Disease Cause, Symptoms and Treatments; NIH: Bethesda, MD, USA, 2013. [Google Scholar]
- Könönen, E.; Gursoy, M.; Gursoy, U.K. Periodontitis: A Multifaceted Disease of Tooth-Supporting Tissues. J. Clin. Med. 2019, 8, 1135. [Google Scholar] [CrossRef] [Green Version]
- Papapanou, P.N.; Susin, C. Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontology 2017, 75, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Kikuchi, T.; Hayashi, J.I.; Mitani, A. Next-generation examination, diagnosis, and personalized medicine in periodontal disease. J. Pers. Med. 2022, 12, 1743. [Google Scholar] [CrossRef]
- Genco, R.J. Antibiotics in the treatment of human periodontal diseases. J. Periodontol. 1981, 52, 545–558. [Google Scholar] [CrossRef]
- Vyas, S.P.; Sihorkar, V.; Mishra, V. Controlled and targeted drug delivery strategies towards intraperiodontal pocket diseases. J. Clin. Pharm. Ther. 2000, 25, 21–42. [Google Scholar] [CrossRef]
- Pitcher, G.R.; Newman, H.N.; Strahan, J.D. Access to subgingival plaque by disclosing agents using mouthrinsing and direct irrigation. J. Clin. Periodontol. 1980, 7, 300–308. [Google Scholar] [CrossRef]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human chronic periodontitis microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Rao, M.; Jadhav, K.; Shaikh, S.; Puzhankara, L.; Jalalpure, S. Local drug delivery systems in the management of periodontitis: A scientific review. J. Control. Release 2019, 307, 393–409. [Google Scholar] [CrossRef]
- Nair, S.C.; Anoop, K.R. Intraperiodontal pocket: An ideal route for local antimicrobial drug delivery. J. Adv. Pharm. Technol. Res. 2012, 3, 9–15. [Google Scholar]
- Batool, F.; Agossa, K.; Lizambard, M.; Petit, C.; Bugueno, I.M.; Delcourt-Debruyne, E.; Benkirane-Jessel, N.; Tenenbaum, H.; Siepmann, J.; Siepmann, F.; et al. In-situ forming implants loaded with chlorhexidine and ibuprofen for periodontal treatment: Proof of concept study in vivo. Int. J. Pharm. 2019, 569, 118564. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and comparative evaluation of vancomycin HCl-loaded rosin-based in situ forming gel and microparticles. Gels. 2022, 8, 231. [Google Scholar] [CrossRef]
- Costa, J.V.; Portugal, J.; Neves, C.B.; Bettencourt, A.F. Should local drug delivery systems be used in dentistry? Drug Deliv. Transl. Res. 2022, 12, 1395–1407. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J.; Chuenbarn, T. In situ forming gel comprising bleached shellac loaded with antimicrobial drugs for periodontitis treatment. Mater. Des. 2016, 89, 294–303. [Google Scholar] [CrossRef]
- Lertsuphotvanit, N.; Santimaleeworagun, W.; Narakornwit, W.; Chuenbarn, T.; Mahadlek, J.; Chantadee, T.; Phaechamud, T. Borneol-based antisolvent-induced in situ forming matrix for crevicular pocket delivery of vancomycin hydrochloride. Int. J. Pharm. 2022, 617, 121603. [Google Scholar] [CrossRef]
- Ei Mon Khaing, T.I. Wichai Santimaleeworagun, Yaowalak Phorom, Tiraniti Chuenbarn, Thawatchai Phaechamud. Natural-resin in-situ-forming gels: Physicochemical characteristics and bioactivities. Pharm. Sci. Asia 2021, 48, 461–470. [Google Scholar] [CrossRef]
- Chantadee, T.; Santimaleeworagun, W.; Phorom, Y.; Chuenbarn, T.; Phaechamud, T. Vancomycin HCl-loaded lauric acid in situ-forming gel with phase inversion for periodontitis treatment. J. Drug Deliv. Sci. Technol. 2020, 57, 101615. [Google Scholar] [CrossRef]
- Phaechamud, T.; Jantadee, T.; Mahadlek, J.; Charoensuksai, P.; Pichayakorn, W. Characterization of antimicrobial agent loaded eudragit RS solvent exchange-induced in situ forming gels for periodontitis treatment. AAPS PharmSciTech 2017, 18, 494–508. [Google Scholar] [CrossRef]
- Phaechamud, T.; Thurein, S.M.; Chantadee, T. Role of clove oil in solvent exchange-induced doxycycline hyclate-loaded Eudragit RS in situ forming gel. Asian J. Pharm. Sci. 2018, 13, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Senarat, S.; Wai Lwin, W.; Mahadlek, J.; Phaechamud, T. Doxycycline hyclate-loaded in situ forming gels composed from bleached shellac, Ethocel, and Eudragit RS for periodontal pocket delivery. Saudi Pharm. J. 2021, 29, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Mahadlek, J.; Tuntarawongsa, S. Peppermint oil/doxycycline hyclate-loaded Eudragit RS in situ forming gel for periodontitis treatment. J. Pharm. Investig. 2018, 48, 451–464. [Google Scholar] [CrossRef]
- Lwin, W.; Puyathorn, N.; Senarat, S.; Mahadlek, J.; Phaechamud, T. Emerging role of polyethylene glycol on doxycycline hyclate-incorporated Eudragit RS in situ forming gel for periodontitis treatment. J. Pharm. Investig. 2019, 50, 81–94. [Google Scholar] [CrossRef]
- Senarat, S.; Phaechamud, T.; Mahadlek, J.; Tuntarawongsa, S. Fluid properties of various Eudragit® solutions in different solvent systems for periodontal pocket injection. Mater. Today Proc. 2022, 65, 2399–2406. [Google Scholar] [CrossRef]
- Tuntarawongsa, S.; Mahadlek, J.; Senarat, S.; Phaechamud, T. Eudragit® RL in 2-pyrrolidone as antisolvent-based in-situ forming matrix. Mater. Today Proc. 2022, 52, 2534–2538. [Google Scholar] [CrossRef]
- Mahadlek, J.; Tuntarawongsa, S.; Senarat, S.; Phaechamud, T. In situ solvent removal-based Eudragit®L/dimethyl sulfoxide forming gel for periodontal pocket drug delivery. Mater. Today Proc. 2021, 52, 2394–2399. [Google Scholar] [CrossRef]
- Patra, C.N.; Priya, R.; Swain, S.; Kumar Jena, G.; Panigrahi, K.C.; Ghose, D. Pharmaceutical significance of Eudragit: A review. Future J. Pharm. Sci. 2017, 3, 33–45. [Google Scholar] [CrossRef]
- Malipeddi, V.R.; Awasthi, R.; Ghisleni, D.D.; de Souza Braga, M.; Kikuchi, I.S.; de Jesus Andreoli Pinto, T.; Dua, K. Preparation and characterization of metoprolol tartrate containing matrix type transdermal drug delivery system. Drug Deliv. Transl. Res. 2017, 7, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, L.E.; Unnithan, A.R.; Amarjargal, A.; Tiwari, A.P.; Hong, S.T.; Park, C.H.; Kim, C.S. Electrospun polyurethane/Eudragit® L100-55 composite mats for the pH dependent release of paclitaxel on duodenal stent cover application. Int. J. Pharm. 2015, 478, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sareen, R.; Nath, K.; Jain, N.; Dhar, K.L. Curcumin loaded microsponges for colon targeting in inflammatory bowel disease: Fabrication, optimization, and in vitro and pharmacodynamic evaluation. BioMed Res. Int. 2014, 2014, 340701. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local delivery and controlled release drugs systems: A new approach for the clinical treatment of periodontitis therapy. Pharmaceutics. 2023, 15, 1312. [Google Scholar] [CrossRef]
- Chen, G.L.; Cai, H.Y.; Chen, J.P.; Li, R.; Zhong, S.Y.; Jia, X.J.; Liu, X.F.; Song, B.B. Chitosan/alginate nanoparticles for the enhanced oral antithrombotic activity of clam heparinoid from the clam coelomactra antiquata. Mar. Drugs 2022, 20, 136. [Google Scholar] [CrossRef]
- FDA. Generally Recognized as Safe; 21 Cfr 184.1666; FDA: Silver Spring, MD, USA, 1982.
- Mahmoud, D.B.; Shukr, M.H.; ElMeshad, A.N. Gastroretentive cosolvent-based in situ gel as a promising approach for simultaneous extended delivery and enhanced bioavailability of mitiglinide calcium. J. Pharm. Sci. 2019, 108, 897–906. [Google Scholar] [CrossRef]
- Souza de Araujo, G.R.; de Oliveira Porfírio, L.; Santos Silva, L.A.; Gomes Santana, D.; Ferreira Barbosa, P.; Pereira dos Santos, C.; Narain, N.; Vitorino Sarmento, V.H.; de Souza Nunes, R.; Ting, E.; et al. In situ microemulsion-gel obtained from bioadhesive hydroxypropyl methylcellulose films for transdermal administration of zidovudine. Colloids Surf. B 2020, 188, 110739. [Google Scholar] [CrossRef]
- Bansal, M.; Mittal, N.; Yadav, S.K.; Khan, G.; Mishra, B.; Nath, G. Clinical evaluation of thermoresponsive and mucoadhesive Chitosan in situ gel containing Levofloxacin and Metronidazole in the treatment of periodontal pockets—A split-mouth, clinical study. J. Pierre Fauchard Acad. 2016, 30, 6–14. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Zhang, C.; Liu, D.; Meng, S.; Zhang, W.; Meng, S. Effect of Polymer Permeability and Solvent Removal Rate on In Situ Forming Implants: Drug Burst Release and Microstructure. Pharmaceutics 2019, 11, 520. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, A.; Zhao, X.; Liu, X.; Wang, D.; Sun, F.; Li, Y. Design of a long-term antipsychotic in situ forming implant and its release control method and mechanism. Int. J. Pharm. 2012, 427, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A.; Ibrahim, H.M.; Samy, A.M.; Kaseem, A.; Nutan, M.T.H.; Hussain, M.D. Biodegradable Injectable In Situ Implants and Microparticles for Sustained Release of Montelukast: In Vitro Release, Pharmacokinetics, and Stability. Am. Assoc. Pharm. Sci. J. 2014, 15, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmoazzen, H.Y.; Poovadan, A.; Law, G.K.; Elliott, J.A.W.; McGann, L.E.; Jomha, N.M. Dimethyl sulfoxide toxicity kinetics in intact articular cartilage. Cell Tissue Bank. 2007, 8, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, R.; Mizuno, M.; Katano, H.; Otabe, K.; Ozeki, N.; Tsuji, K.; Koga, H.; Sekiya, I. Cryopreservation in 95% serum with 5% DMSO maintains colony formation and chondrogenic abilities in human synovial mesenchymal stem cells. BMC Musculoskelet. Disord. 2019, 20, 316. [Google Scholar] [CrossRef] [Green Version]
- Phaechamud, T.; Senarat, S.; Puyathorn, N.; Praphanwittaya, P. Solvent exchange and drug release characteristics of doxycycline hyclate-loaded bleached shellac in situ-forming gel and -microparticle. Int. J. Biol. Macromol. 2019, 135, 1261–1272. [Google Scholar] [CrossRef]
- dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C. Eudragit®: A versatile family of polymers for hot melt extrusion and 3D printing processes in pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef]
- Zhang, Q.; Fassihi, M.A.; Fassihi, R. Delivery considerations of highly viscous polymeric fluids mimicking concentrated biopharmaceuticals: Assessment of injectability via measurement of total work done “WT”. AAPS PharmSciTech 2018, 19, 1520–1528. [Google Scholar] [CrossRef] [Green Version]
- Seo, Y.P.; Seo, Y. Effect of molecular structure change on the melt rheological properties of a polyamide (Nylon 6). ACS Omega 2018, 3, 16549–16555. [Google Scholar] [CrossRef]
- Rein, S.M.T.; Lwin, W.W.; Tuntarawongsa, S.; Phaechamud, T. Meloxicam-loaded solvent exchange-induced in situ forming beta-cyclodextrin gel and microparticle for periodontal pocket delivery. Mater. Sci. Eng. C 2020, 117, 111275. [Google Scholar] [CrossRef]
- Thurein, S.M.; Lertsuphotvanit, N.; Phaechamud, T. Physicochemical properties of β-cyclodextrin solutions and precipitates prepared from injectable vehicles. Asian J. Pharm. Sci. 2018, 13, 438–449. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Chantadee, T.; Phaechamud, T. Doxycycline hyclate-loaded Eudragit® RS PO in situ-forming microparticles for periodontitis treatment. J. Drug Deliv. Sci. Technol. 2022, 71, 103294. [Google Scholar] [CrossRef]
- Khaing, E.M.; Mahadlek, J.; Okonogi, S.; Phaechamud, T. Lime peel oil-incorporated rosin-based antimicrobial in situ forming gel. Gels 2022, 8, 169. [Google Scholar] [CrossRef] [PubMed]
- Phaechamud, T.; Setthajindalert, O. Antimicrobial in-situ forming gels based on bleached shellac and different solvents. J. Drug Deliv. Sci. Technol. 2018, 46, 285–293. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Metz, H.; Delcourt, E.; Siepmann, J.; Mäder, K.; Siepmann, F. Mechanistic analysis of PLGA/HPMC-based in-situ forming implants for periodontitis treatment. Eur. J. Pharm. Biopharm. 2015, 94, 273–283. [Google Scholar] [CrossRef]
- Hoang, C.; Nguyen, A.K.; Nguyen, T.Q.; Fang, W.; Han, B.; Hoang, B.X.; Tran, H.D. Application of dimethyl sulfoxide as a therapeutic agent and drug vehicle for eye diseases. J. Ocul. Pharmacol. Ther. 2021, 37, 441–451. [Google Scholar] [CrossRef]
- Zheng, Z.-J.; Ye, H.; Guo, Z.-P. Recent progress in designing stable composite lithium anodes with improved wettability. Adv. Sci. 2020, 7, 2002212. [Google Scholar] [CrossRef]
- Mei, L.; Huang, X.; Xie, Y.; Chen, J.; Huang, Y.; Wang, B.; Wang, H.; Pan, X.; Wu, C. An injectable in situ gel with cubic and hexagonal nanostructures for local treatment of chronic periodontitis. Drug Deliv. 2017, 24, 1148–1158. [Google Scholar] [CrossRef] [Green Version]
- Senarat, S.; Charoenteeraboon, J.; Praphanwittaya, P.; Phaechamud, T. Phase behavior of doxycycline hyclate-incorporated bleached shellac in-situ forming gel/microparticle after solvent movement. Key Eng. Mater. 2020, 859, 21–26. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Delcourt, E.; Seixas Certo, T.; Siepmann, J.; Siepmann, F. In situ forming implants for periodontitis treatment with improved adhesive properties. Eur. J. Pharm. Biopharm. 2014, 88, 342–350. [Google Scholar] [CrossRef]
- Golmaghani-Ebrahimi, E.; Bagheri, A.; Fazli, M. The influence of temperature on surface concentration and interaction energy between components in binary liquid systems. J. Chem. Thermodyn. 2020, 146, 106105. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mahadlek, J. Solvent exchange-induced in situ forming gel comprising ethyl cellulose-antimicrobial drugs. Int. J. Pharm. 2015, 494, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, O.M.; Cook, M.T. In situ gelling drug delivery systems for topical drug delivery. Eur. J. Pharm. Biopharm. 2023, 184, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Puyathorn, N.; Sirirak, J.; Chantadee, T.; Phaechamud, T. Phase separation and intermolecular binding energy of ibuprofen in some organic solvents. Mater. Today Proc. 2022, 65, 2303–2308. [Google Scholar] [CrossRef]
- Ershad, A.L.; Rajabi-Siahboomi, A.; Missaghi, S.; Kirby, D.; Mohammed, A.R. Multi-analytical framework to assess the in vitro swallowability of solid oral dosage forms targeting patient acceptability and adherence. Pharmaceutics 2021, 13, 411. [Google Scholar] [CrossRef] [PubMed]
- Khaing, E.M.; Intaraphairot, T.; Mahadlek, J.; Okonogi, S.; Pichayakorn, W.; Phaechamud, T. Imatinib mesylate-loaded rosin cinnamon oil-based in situ forming gel against colorectal cancer cells. Gels 2022, 8, 526. [Google Scholar] [CrossRef] [PubMed]
- Baddam, D.O.; Ragi, S.D.; Tsang, S.H.; Ngo, W.K. Ophthalmic fluorescein angiography. Methods Mol. Biol. 2023, 2560, 153–160. [Google Scholar] [CrossRef]
- Gusmão, M.R.; Alves, T.C.; Lemes, A.P.; Bettiol, G.M.; Pedroso, A.d.F.; Barioni Junior, W.; Oliveira, P.P.A.; Grego, C.R. Sodium fluorescein as an internal tracer on the location of bovine urine patches in pastures. Grass Forage Sci. 2016, 71, 305–314. [Google Scholar] [CrossRef]
- Alemán-Nava, G.S.; Cuellar-Bermudez, S.P.; Cuaresma, M.; Bosma, R.; Muylaert, K.; Ritmann, B.E.; Parra, R. How to use Nile Red, a selective fluorescent stain for microalgal neutral lipids. J. Microbiol. Methods 2016, 128, 74–79. [Google Scholar] [CrossRef]
- Martinez, V.; Henary, M. Nile red and nile blue: Applications and syntheses of structural analogues. Chemistry. 2016, 22, 13764–13782. [Google Scholar] [CrossRef]
- Ahmed, T. Review: Approaches to develop PLGA based in situ gelling system with low initial burst. Pak. J. Pharm. Sci. 2015, 28, 657–665. [Google Scholar]
- Bansal, M.; Mittal, N.; Yadav, S.K.; Khan, G.; Gupta, P.; Mishra, B.; Nath, G. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: Preparation, in-vitro characterization and antimicrobial study. J. Oral Biol. Craniofacial Res. 2018, 8, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ashish, G.; Rahul, Y.; Mukesh, R.; Prakash, M. Formulation and evaluation of in situ gel containing ciprofloxacin hydrochloride in the treatment of periodontitis. Asian J. Pharm. Clin. Res. 2017, 10, 154. [Google Scholar] [CrossRef] [Green Version]
- Javali, M.A.; Vandana, K.L. A comparative evaluation of atrigel delivery system (10% doxycycline hyclate) Atridox with scaling and root planing and combination therapy in treatment of periodontitis: A clinical study. J. Indian Soc. Periodontol. 2012, 16, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Speck, S.; Wenke, C.; Feßler, A.T.; Kacza, J.; Geber, F.; Scholtzek, A.D.; Hanke, D.; Eichhorn, I.; Schwarz, S.; Rosolowski, M.; et al. Borderline resistance to oxacillin in Staphylococcus aureus after treatment with sub-lethal sodium hypochlorite concentrations. Heliyon 2020, 6, e04070. [Google Scholar] [CrossRef]
- Rattanaumpawan, P.; Nachamkin, I.; Bilker, W.B.; Roy, J.A.; Metlay, J.P.; Zaoutis, T.E.; Lautenbach, E. High fluoroquinolone MIC is associated with fluoroquinolone treatment failure in urinary tract infections caused by fluoroquinolone susceptible Escherichia coli. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Conrads, G.; Klomp, T.; Deng, D.; Wenzler, J.S.; Braun, A.; Abdelbary, M.M.H. The antimicrobial susceptibility of porphyromonas gingivalis: Genetic repertoire, global phenotype, and review of the literature. Antibiotics 2021, 10, 1438. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Azami, M.; Mozafari, M. Controllable synthesis and characterization of porous polyvinyl alcohol/hydroxyapatite nanocomposite scaffolds via an in situ colloidal technique. Colloids Surf. B 2011, 84, 310–316. [Google Scholar] [CrossRef]
- Machado, P.S.T.; Habert, A.C.; Borges, C.P. Membrane formation mechanism based on precipitation kinetics and membrane morphology: Flat and hollow fiber polysulfone membranes. J. Membr. Sci. 1999, 155, 171–183. [Google Scholar] [CrossRef]
- Dias, R.; Medeiros, V.; Silva, B.; Araujo, E.; Lira, H. Study of the influence of viscosity on the morphology of polyethersulfone hollow fiber membranes/additives. Mat. Res. 2019, 22, e20180913. [Google Scholar] [CrossRef] [Green Version]
- Pawar, I.A.; Joshi, P.J.; Kadam, A.D.; Pande, N.B.; Kamble, P.H.; Hinge, S.P.; Banerjee, B.S.; Mohod, A.V.; Gogate, P.R. Ultrasound-based treatment approaches for intrinsic viscosity reduction of polyvinyl pyrrolidone (PVP). Ultrason. Sonochem. 2014, 21, 1108–1116. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Koli, A.R.; Desai, D.T.; Parikh, R.K.; Shah, D.O. Tailored doxycycline hyclate loaded in situ gel for the treatment of periodontitis: Optimization, in vitro characterization, and antimicrobial studies. AAPS PharmSciTech 2021, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Tahtat, D.; Mahlous, M.; Benamer, S.; Nacer Khodja, A.; Larbi Youcef, S. Effect of molecular weight on radiation chemical degradation yield of chain scission of γ-irradiated chitosan in solid state and in aqueous solution. Radiat. Phys. Chem. 2012, 81, 659–665. [Google Scholar] [CrossRef]
- Zhang, P.; Lee, Y.I.; Zhang, J. A review of high-resolution X-ray computed tomography applied to petroleum geology and a case study. Micron 2019, 124, 102702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, D.; Zhou, R.; Zhang, Y. 3D X-ray micro-computed tomography imaging for the microarchitecture evaluation of porous metallic implants and scaffolds. Micron 2021, 142, 102994. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, J.; Chen, L.; Yu, L.; Xu, J.; Ding, J. Biodegradable and thermoreversible PCLA-PEG-PCLA hydrogel as a barrier for prevention of post-operative adhesion. Biomaterials 2011, 32, 4725–4736. [Google Scholar] [CrossRef]
- Yacoubi, D.A.; Bouziane, D.; Leila, M.; Bensoltane, A. Microbiological Study of Periodontitis in the West of Algeria. World J. Med. Sci. 2010, 5, 7–12. [Google Scholar]
- Rajendiran, M.; Trivedi, H.M.; Chen, D.; Gajendrareddy, P.; Chen, L. Recent development of active ingredients in mouthwashes and toothpastes for periodontal diseases. Molecules 2021, 26, 2001. [Google Scholar] [CrossRef]
- Nalawade, T.M.; Bhat, K.; Sogi, S.H. Bactericidal activity of propylene glycol, glycerine, polyethylene glycol 400, and polyethylene glycol 1000 against selected microorganisms. J. Int. Soc. Prev. Community Dent. 2015, 5, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Phaechamud, T.; Setthajindalert, O. Cholesterol in situ forming gel loaded with doxycycline hyclate for intra-periodontal pocket delivery. Eur. J. Pharm. Sci. 2017, 99, 258–265. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef]
- Wang, F.; Saidel, G.M.; Gao, J. A mechanistic model of controlled drug release from polymer millirods: Effects of excipients and complex binding. J. Control. Release 2007, 119, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) 5—Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Delhi, India, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Thomas, N.L.; Windle, A.H. A theory of case II diffusion. Polymer 1982, 23, 529–542. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Peterchristoper, G.V. Release Kinetics—Concepts and applications. IJPRT 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Aziz, I.H.; Abdullah, M.M.A.B.; Mohd Salleh, M.A.A.; Yoriya, S.; Chaiprapa, J.; Rojviriya, C.; Li, L.Y. Microstructure and porosity evolution of alkali activated slag at various heating temperatures. J. Mater. Res. Technol. 2020, 9, 15894–15907. [Google Scholar] [CrossRef]













| Formula | Zero Order | First Order | Higuchi’s | Korsmeyer–PEPPAS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r2 | msc | r2 | msc | r2 | msc | r2 | msc | k ± SD | n ± SD | Release Mechanism | |
| LLM10 | 0.8048 | 1.3742 | 0.8683 | 1.9759 | 0.9058 | 2.5144 | 0.9770 | 3.5961 | 3.344 ± 1.837 | 0.444 ± 0.050 | Fickian diffusion |
| LLM15 | 0.7118 | 0.9164 | 0.8872 | 2.0854 | 0.8945 | 2.0844 | 0.9503 | 2.6867 | 2.603 ± 0.666 | 0.367 ± 0.028 | Fickian diffusion |
| LLM20 | 0.7941 | 1.2387 | 0.9090 | 2.3402 | 0.9188 | 2.3419 | 0.9320 | 2.3732 | 1.856 ± 0.819 | 0.444 ± 0.055 | Fickian diffusion |
| Formula | Weight Loss (%) | ||||
|---|---|---|---|---|---|
| Day 1 | Day 3 | Day 5 | Day 7 | Day 14 | |
| LLM10 | 85.39 ± 0.32 a | 91.96 ± 0.98 b | 94.53 ± 1.46 c | 94.96 ± 1.21 d | 100.00 ± 0.00 |
| LLM15 | 80.22 ± 2.90 a | 86.95 ± 1.97 b | 87.91 ± 0.54 | 92.57 ± 1.92 | 100.00 ± 0.00 |
| LLM20 | 72.81 ± 1.85 a | 82.02 ± 1.46 b | 85.95 ± 1.98 c | 90.21 ± 1.80 d | 100.00 ± 0.00 |
| Formula | Clear Zone Diameter (mm.) Mean ± S.D. | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus 6538 | S. aureus 4430 | S. aureus 6532 | S. aureus 25923 | E. coli 8739 | C. albicans 10231 | P. gingivalis ATCC 33277 | A. actinomycetemcomitans ATCC 29522 | |
| MP | 12.7 ± 0.5 | 13.0 ± 0.8 | 12.3 ± 0.5 | 10.3 ± 0.5 | 14.7 ± 0.5 | 18.7 ± 1.2 | 17.0 ± 2.2 | 26.3 ± 0.5 |
| LM10 | 11.7 ± 0.5 | 10.7 ± 0.5 | 11.7 ± 0.5 | 9.8 ± 0.2 | 12.0 ± 0.8 | 16.7 ± 0.5 | 12.0 ± 0.8 | 23.7 ± 0.5 |
| LM15 | 10.5 ± 0.4 | 10.7 ± 0.5 | 10.7 ± 0.5 | - | 12.0 ± 1.4 | 16.0 ± 0.8 | 15.0 ± 1.4 | 23.3 ± 0.5 |
| LM20 | 10.3 ± 1.2 | 9.7 ± 0.5 | 11.3 ± 1.2 | - | 12.7 ± 1.2 | 15.0 ± 0.8 | 13.3 ± 1.2 | 22.3 ± 0.5 |
| LVM | 26.3 ± 0.9 a | 25.3 ± 0.9 b | 26.0 ± 0.8 c | 25.3 ± 0.5 d | 25.3 ± 0.9 e | 20.0 ± 1.6 f | 26.0 ± 0.8 g | >40 |
| LLM10 | 26.7 ± 0.5 | 23.3 ± 0.5 | 23.3 ± 1.2 | 23.3 ± 0.5 | 23.3 ± 0.9 | 15.3 ± 2.1 | 25.3 ± 1.2 | >40 |
| LLM15 | 25.3 ± 0.5 | 22.7 ± 0.5 b | 21.7 ± 0.5 c | 22.7 ± 1.2 d | 22.3 ± 0.5 e | 16.0 ± 0.8 f | 23.3 ± 1.2 | >40 |
| LLM20 | 24.3 ± 0.5 a | 21.3 ± 0.9 b | 20.3 ± 0.5 c | 20.7 ± 0.5 d | 22.0 ± 0.8 e | 14.7 ± 0.9 f | 21.3 ± 0.5 g | >40 |
| A | |||
| Formula | Amount (% w/w) | ||
| Eudragit L | MP | ||
| LM5 | 5 | 95 | |
| LM10 | 10 | 90 | |
| LM15 | 15 | 85 | |
| LM20 | 20 | 80 | |
| LM25 | 25 | 75 | |
| B | |||
| Formula | Amount (% w/w) | ||
| Eudragit S | MP | ||
| SM5 | 5 | 95 | |
| SM10 | 10 | 90 | |
| SM15 | 15 | 85 | |
| SM20 | 20 | 80 | |
| SM25 | 25 | 75 | |
| C | |||
| Formula | Amount (% w/w) | ||
| Levofloxacin HCl | Eudragit L | MP | |
| LLM10 | 0.5 | 10 | 89.5 |
| LLM15 | 0.5 | 15 | 84.5 |
| LLM20 | 0.5 | 20 | 79.5 |
| LVM | 0.5 | - | 95.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senarat, S.; Tuntarawongsa, S.; Lertsuphotvanit, N.; Rojviriya, C.; Phaechamud, T.; Chantadee, T. Levofloxacin HCl-Loaded Eudragit L-Based Solvent Exchange-Induced In Situ Forming Gel Using Monopropylene Glycol as a Solvent for Periodontitis Treatment. Gels 2023, 9, 583. https://doi.org/10.3390/gels9070583
Senarat S, Tuntarawongsa S, Lertsuphotvanit N, Rojviriya C, Phaechamud T, Chantadee T. Levofloxacin HCl-Loaded Eudragit L-Based Solvent Exchange-Induced In Situ Forming Gel Using Monopropylene Glycol as a Solvent for Periodontitis Treatment. Gels. 2023; 9(7):583. https://doi.org/10.3390/gels9070583
Chicago/Turabian StyleSenarat, Setthapong, Sarun Tuntarawongsa, Nutdanai Lertsuphotvanit, Catleya Rojviriya, Thawatchai Phaechamud, and Takron Chantadee. 2023. "Levofloxacin HCl-Loaded Eudragit L-Based Solvent Exchange-Induced In Situ Forming Gel Using Monopropylene Glycol as a Solvent for Periodontitis Treatment" Gels 9, no. 7: 583. https://doi.org/10.3390/gels9070583