Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water
Abstract
:1. Introduction
2. Results and Discussion
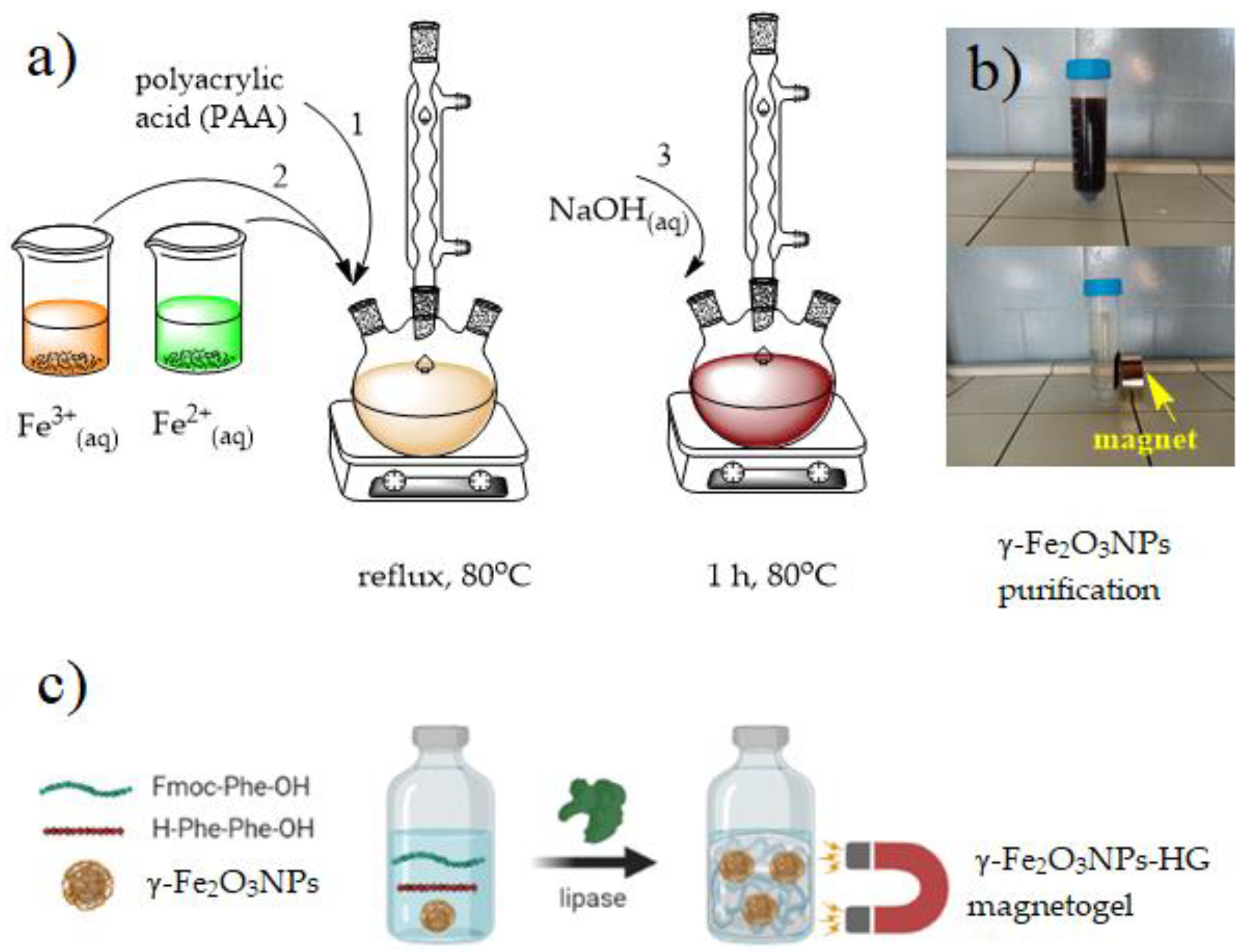
2.1. Preparation of γ-Fe2O3NPs and γ-Fe2O3NPs@HG Magnetogel
2.2. Raman, FT-IR/ATR, and XPS Characterization of γ-Fe2O3NPs
2.3. DSL and UV-Vis Characterization of γ-Fe2O3NPs
2.4. FESEM-EDS Characterization of γ-Fe2O3NPs and γ-Fe2O3NPs@HG Magnetogel
2.5. Rheological Studies and Swelling Ability
2.6. Magnetogels Application in the Removal of Metallic Cations
2.6.1. Co(II) Removal Studies
2.6.2. Ni(II) Removal Studies
2.6.3. Cr(III) Removal Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of γ-Fe2O3NPs@HG Magnetogels
4.3. UV-Vis, FT-IR/ATR and Raman Spectroscopies and Dynamic Light Scattering (DLS)
4.4. X-ray Photoelectron Spectroscopy (XPS)
4.5. Electron Microscopy Studies
4.6. Rheology Measurements
4.7. Swelling Test
4.8. Adsorption Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mondal, B.; Bairagi, D.; Nandi, N.; Hansda, B.; Das, K.S.; Edwards-Gayle, C.J.C.; Castelletto, V.; Hamley, I.W.; Banerjee, A. Peptide-Based Gel in Environmental Remediation: Removal of Toxic Organic Dyes and Hazardous Pb2+ and Cd2+ Ions from Wastewater and Oil Spill Recovery. Langmuir 2020, 36, 12942–12953. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, D.; Xiang, C.; Zhang, F.; Liu, L.; Zhou, X.; Zhang, H. Facile Synthesis of Boron Organic Polymers for Efficient Removal and Separation of Methylene Blue, Rhodamine B, and Rhodamine 6G. ACS Sustain. Chem. Eng. 2018, 6, 16777–16787. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Ghfar, A.A.; García-Peñas, A.; Naushad, M.; Stadler, F.J. Fabrication and Characterization of Xanthan Gum-Cl-Poly(Acrylamide-Co-Alginic Acid) Hydrogel for Adsorption of Cadmium Ions from Aqueous Medium. Gels 2022, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Mahey, S.; Kumar, R.; Sharma, M.; Kumar, V.; Bhardwaj, R. A Critical Review on Toxicity of Cobalt and Its Bioremediation Strategies. SN Appl. Sci. 2020, 2, 1279. [Google Scholar] [CrossRef]
- Purushotham, D.; Rashid, M.; Lone, M.A.; Rao, A.N.; Ahmed, S.; Nagaiah, E.; Dar, F.A. Environmental Impact Assessment of Air and Heavy Metal Concentration in Groundwater of Maheshwaram Watershed, Ranga Reddy District, Andhra Pradesh. J. Geol. Soc. India 2013, 81, 385–396. [Google Scholar] [CrossRef]
- Fratoddi, I.; Cerra, S.; Salamone, T.A.; Fioravanti, R.; Sciubba, F.; Zampetti, E.; Macagnano, A.; Generosi, A.; Paci, B.; Scaramuzzo, F.A.; et al. Functionalized Gold Nanoparticles as an Active Layer for Mercury Vapor Detection at Room Temperature. ACS Appl. Nano Mater. 2021, 4, 2930–2940. [Google Scholar] [CrossRef]
- Cerra, S.; Salamone, T.A.; Bearzotti, A.; Hajareh Haghighi, F.; Mercurio, M.; Marsotto, M.; Battocchio, C.; Fioravanti, R.; Diociaiuti, M.; Fratoddi, I. Thiol-Functionalized Palladium Nanoparticles Networks: Synthesis, Characterization, and Room Temperature (Toxic) Vapor Detection. Part. Part. Syst. Charact. 2023, 40, 2200189. [Google Scholar] [CrossRef]
- Dakova, I.; Vasileva, P.; Karadjova, I. Cr(III) Ion-Imprinted Hydrogel Membrane for Chromium Speciation Analysis in Water Samples. Gels 2022, 8, 757. [Google Scholar] [CrossRef]
- Chowdhury, A.; Khan, A.A.; Kumari, S.; Hussain, S. Superadsorbent Ni–Co–S/SDS Nanocomposites for Ultrahigh Removal of Cationic, Anionic Organic Dyes and Toxic Metal Ions: Kinetics, Isotherm and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2019, 7, 4165–4176. [Google Scholar] [CrossRef]
- Maiti, D.; Mukhopadhyay, S.; Devi, P.S. Evaluation of Mechanism on Selective, Rapid, and Superior Adsorption of Congo Red by Reusable Mesoporous α-Fe2O3 Nanorods. ACS Sustain. Chem. Eng. 2017, 5, 11255–11267. [Google Scholar] [CrossRef]
- Ramalingam, B.; Parandhaman, T.; Choudhary, P.; Das, S.K. Biomaterial Functionalized Graphene-Magnetite Nanocomposite: A Novel Approach for Simultaneous Removal of Anionic Dyes and Heavy-Metal Ions. ACS Sustain. Chem. Eng. 2018, 6, 6328–6341. [Google Scholar] [CrossRef]
- Minju, N.; Jobin, G.; Savithri, S.; Ananthakumar, S. Double-Silicate Derived Hybrid Foams for High-Capacity Adsorption of Textile Dye Effluent: Statistical Optimization and Adsorption Studies. Langmuir 2019, 35, 9382–9395. [Google Scholar] [CrossRef]
- Ray, S.; Das, A.K.; Banerjee, A. PH-Responsive, Bolaamphiphile-Based Smart Metallo-Hydrogels as Potential Dye-Adsorbing Agents, Water Purifier, and Vitamin B12 Carrier. Chem. Mater. 2007, 19, 1633–1639. [Google Scholar] [CrossRef]
- Fortunato, A.; Mba, M. A Peptide-Based Hydrogel for Adsorption of Dyes and Pharmaceuticals in Water Remediation. Gels 2022, 8, 672. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Godiya, C.B.; Ruotolo, L.A.M.; Cai, W. Functional Biobased Hydrogels for the Removal of Aqueous Hazardous Pollutants: Current Status, Challenges, and Future Perspectives. J. Mater. Chem. A 2020, 8, 21585–21612. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying Low-Molecular Weight Supramolecular Gelators in an Environmental Setting–Self-Assembled Gels as Smart Materials for Pollutant Removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef] [Green Version]
- Chronopoulou, L.; Margheritelli, S.; Toumia, Y.; Paradossi, G.; Bordi, F.; Sennato, S.; Palocci, C. Biosynthesis and Characterization of Cross-Linked Fmoc Peptide-Based Hydrogels for Drug Delivery Applications. Gels 2015, 1, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Boni, R.; Regan, L. Modulating the Viscoelastic Properties of Covalently Crosslinked Protein Hydrogels. Gels 2023, 9, 481. [Google Scholar] [CrossRef]
- Chen, H.; Feng, R.; Xia, T.; Wen, Z.; Li, Q.; Qiu, X.; Huang, B.; Li, Y. Progress in Surface Modification of Titanium Implants by Hydrogel Coatings. Gels 2023, 9, 423. [Google Scholar] [CrossRef] [PubMed]
- Hajareh Haghighi, F.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Bianymotlagh, R.; Palocci, C.; Romano Spica, V.; Fratoddi, I. Surface Modification of TiO2 Nanoparticles with Organic Molecules and Their Biological Applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef] [PubMed]
- Ningrum, E.O.; Gotoh, T.; Ciptonugroho, W.; Karisma, A.D.; Agustiani, E.; Safitri, Z.M.; Dzaky, M.A. Novel Thermosensitive-Co-Zwitterionic Sulfobetaine Gels for Metal Ion Removal: Synthesis and Characterization. Gels 2021, 7, 273. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Chronopoulou, L.; Hajareh Haghighi, F.; Fratoddi, I.; Palocci, C. Peptide-Based Hydrogels: New Materials for Biosensing and Biomedical Applications. Materials 2022, 15, 5871. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Ferreira, P.M.T.; Martins, J.A.; Coutinho, P.J.G.; Castanheira, E.M.S. Magnetogels: Prospects and Main Challenges in Biomedical Applications. Pharmaceutics 2018, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Salahuddin, B.; Aziz, S.; Gao, S.; Hossain, M.S.A.; Billah, M.; Zhu, Z.; Amiralian, N. Magnetic Hydrogel Composite for Wastewater Treatment. Polymers 2022, 14, 5074. [Google Scholar] [CrossRef]
- Veloso, S.R.S.; Andrade, R.G.D.; Castanheira, E.M.S. Review on the Advancements of Magnetic Gels: Towards Multifunctional Magnetic Liposome-Hydrogel Composites for Biomedical Applications. Adv. Colloid Interface Sci. 2021, 288, 102351. [Google Scholar] [CrossRef]
- Gang, F.; Jiang, L.; Xiao, Y.; Zhang, J.; Sun, X. Multi-Functional Magnetic Hydrogel: Design Strategies and Applications. Nano Sel. 2021, 2, 2291–2307. [Google Scholar] [CrossRef]
- Milakin, K.A.; Taboubi, O.; Acharya, U.; Lhotka, M.; Pokorný, V.; Konefał, M.; Kočková, O.; Hromádková, J.; Hodan, J.; Bober, P. Polypyrrole-Barium Ferrite Magnetic Cryogels for Water Purification. Gels 2023, 9, 92. [Google Scholar] [CrossRef]
- Gonçalves, A.; Almeida, F.V.; Borges, J.P.; Soares, P.I.P. Incorporation of Dual-Stimuli Responsive Microgels in Nanofibrous Membranes for Cancer Treatment by Magnetic Hyperthermia. Gels 2021, 7, 28. [Google Scholar] [CrossRef]
- Häring, M.; Schiller, J.; Mayr, J.; Grijalvo, S.; Eritja, R.; Díaz, D.D. Magnetic Gel Composites for Hyperthermia Cancer Therapy. Gels 2015, 1, 135–161. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Mora, V.; Soares, P.I.P.; Echeverria, C.; Hernández, R.; Mijangos, C. Composite Chitosan/Agarose Ferrogels for Potential Applications in Magnetic Hyperthermia. Gels 2015, 1, 69–80. [Google Scholar] [CrossRef] [Green Version]
- De Melo, F.M.; Grasseschi, D.; Brandão, B.B.N.S.; Fu, Y.; Toma, H.E. Superparamagnetic Maghemite-Based CdTe Quantum Dots as Efficient Hybrid Nanoprobes for Water-Bath Magnetic Particle Inspection. ACS Appl. Nano Mater. 2018, 1, 2858–2868. [Google Scholar] [CrossRef]
- Ali, A.F.; Atwa, S.M.; El-Giar, E.M. 6—Development of Magnetic Nanoparticles for Fluoride and Organic Matter Removal from Drinking Water. In Water Purification; Grumezescu, A.M.B.T.-W.P., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 209–262. ISBN 978-0-12-804300-4. [Google Scholar]
- Kunduru, K.R.; Nazarkovsky, M.; Farah, S.; Pawar, R.P.; Basu, A.; Domb, A.J. 2—Nanotechnology for Water Purification: Applications of Nanotechnology Methods in Wastewater Treatment. In Water Purification; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 33–74. ISBN 978-0-12-804300-4. [Google Scholar]
- Asadi, S.; Eris, S.; Azizian, S. Alginate-Based Hydrogel Beads as a Biocompatible and Efficient Adsorbent for Dye Removal from Aqueous Solutions. ACS Omega 2018, 3, 15140–15148. [Google Scholar] [CrossRef]
- Amiralian, N.; Mustapic, M.; Hossain, M.S.A.; Wang, C.; Konarova, M.; Tang, J.; Na, J.; Khan, A.; Rowan, A. Magnetic Nanocellulose: A Potential Material for Removal of Dye from Water. J. Hazard. Mater. 2020, 394, 122571. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Actis, D.G.; Gonzalez, J.S.; Zélis, P.M.; Alvarez, V.A. Effect of PAA-Coated Magnetic Nanoparticles on the Performance of PVA-Based Hydrogels Developed to Be Used as Environmental Remediation Devices. J. Nanoparticle Res. 2019, 21, 64. [Google Scholar] [CrossRef]
- Chełminiak, D.; Ziegler-Borowska, M.; Kaczmarek, H. Synthesis of Magnetite Nanoparticles Coated with Poly(Acrylic Acid) by Photopolymerization. Mater. Lett. 2016, 164, 464–467. [Google Scholar] [CrossRef]
- Liang, Y.-Y.; Zhang, L.-M.; Jiang, W.; Li, W. Embedding Magnetic Nanoparticles into Polysaccharide-Based Hydrogels for Magnetically Assisted Bioseparation. ChemPhysChem 2007, 8, 2367–2372. [Google Scholar] [CrossRef]
- Sang, J.; Wu, R.; Guo, P.; Du, J.; Xu, S.; Wang, J. Affinity-Tuned Peroxidase-like Activity of Hydrogel-Supported Fe3O4 Nanozyme through Alteration of Crosslinking Concentration. J. Appl. Polym. Sci. 2016, 133, 43065. [Google Scholar] [CrossRef]
- Witt, M.U.; Hinrichs, S.; Möller, N.; Backes, S.; Fischer, B.; von Klitzing, R. Distribution of CoFe2O4 Nanoparticles Inside PNIPAM-Based Microgels of Different Cross-Linker Distributions. J. Phys. Chem. B 2019, 123, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Binaymotlagh, R.; Del Giudice, A.; Mignardi, S.; Amato, F.; Marrani, A.G.; Sivori, F.; Cavallo, I.; Di Domenico, E.G.; Palocci, C.; Chronopoulou, L. Green In Situ Synthesis of Silver Nanoparticles-Peptide Hydrogel Composites: Investigation of Their Antibacterial Activities. Gels 2022, 8, 700. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.; Kim, J.H.; Yang, H.-M.; Lee, J.W.; Kim, J.-D. Formation Pathways of Magnetite Nanoparticles by Coprecipitation Method. J. Phys. Chem. C 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2003; Volume 529. [Google Scholar]
- Rebodos, R.L.; Vikesland, P.J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef]
- Cerra, S.; Carlini, L.; Salamone, T.A.; Hajareh Haghighi, F.; Mercurio, M.; Pennacchi, B.; Sappino, C.; Battocchio, C.; Nottola, S.; Matassa, R.; et al. Noble Metal Nanoparticles Networks Stabilized by Rod-Like Organometallic Bifunctional Thiols. ChemistrySelect 2023, 8, e202300874. [Google Scholar] [CrossRef]
- Gutiérrez, L.; de la Cueva, L.; Moros, M.; Mazarío, E.; de Bernardo, S.; de la Fuente, J.M.; Morales, M.P.; Salas, G. Aggregation Effects on the Magnetic Properties of Iron Oxide Colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [Green Version]
- Harris, L.A.; Goff, J.D.; Carmichael, A.Y.; Riffle, J.S.; Harburn, J.J.; St Pierre, T.G.; Saunders, M. Magnetite Nanoparticle Dispersions Stabilized with Triblock Copolymers. Chem. Mater. 2003, 15, 1367–1377. [Google Scholar] [CrossRef]
- Pardoe, H.; Chua-Anusorn, W.; Pierre, T.G.S.; Dobson, J. Structural and Magnetic Properties of Nanoscale Iron Oxide Particles Synthesized in the Presence of Dextran or Polyvinyl Alcohol. J. Magn. Magn. Mater. 2001, 225, 41–46. [Google Scholar] [CrossRef]
- Di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic Hyperthermia Efficiency in the Cellular Environment for Different Nanoparticle Designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef]
- Nahar, Y.; Rahman, M.A.; Hossain, M.K.; Sharafat, M.K.; Karim, M.R.; Elaissari, A.; Ochiai, B.; Ahmad, H.; Rahman, M.M. A Facile One-Pot Synthesis of Poly(Acrylic Acid)-Functionalized Magnetic Iron Oxide Nanoparticles for Suppressing Reactive Oxygen Species Generation and Adsorption of Biocatalyst. Mater. Res. Express 2020, 7, 16102. [Google Scholar] [CrossRef]
- Rutnakornpituk, M.; Puangsin, N.; Theamdee, P.; Rutnakornpituk, B.; Wichai, U. Poly (Acrylic Acid)-Grafted Magnetic Nanoparticle for Conjugation with Folic Acid. Polymer 2011, 52, 987–995. [Google Scholar] [CrossRef]
- Jain, N.; Wang, Y.; Jones, S.K.; Hawkett, B.S.; Warr, G.G. Optimized Steric Stabilization of Aqueous Ferrofluids and Magnetic Nanoparticles. Langmuir 2010, 26, 4465–4472. [Google Scholar] [CrossRef]
- Lin, C.-L.; Lee, C.-F.; Chiu, W.-Y. Preparation and Properties of Poly(Acrylic Acid) Oligomer Stabilized Superparamagnetic Ferrofluid. J. Colloid Interface Sci. 2005, 291, 411–420. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Lorenzoni, S.; Masci, G.; Dentini, M.; Togna, A.R.; Togna, G.; Bordi, F.; Palocci, C. Lipase-Supported Synthesis of Peptidic Hydrogels. Soft Matter 2010, 6, 2525–2532. [Google Scholar] [CrossRef]
- Aaron, M.J.; Heather, C. Allen Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar]
- Yadav, B.S.; Singh, R.; Vishwakarma, A.K.; Kumar, N. Facile Synthesis of Substantially Magnetic Hollow Nanospheres of Maghemite (γ-Fe2O3) Originated from Magnetite (Fe3O4) via Solvothermal Method. J. Supercond. Nov. Magn. 2020, 33, 2199–2208. [Google Scholar] [CrossRef]
- Chamritski, I.; Burns, G. Infrared-and Raman-Active Phonons of Magnetite, Maghemite, and Hematite: A Computer Simulation and Spectroscopic Study. J. Phys. Chem. B 2005, 109, 4965–4968. [Google Scholar] [CrossRef]
- Kirwan, L.J.; Fawell, P.D.; van Bronswijk, W. In Situ FTIR-ATR Examination of Poly(Acrylic Acid) Adsorbed onto Hematite at Low PH. Langmuir 2003, 19, 5802–5807. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman Spectroscopy to Unravel the Magnetic Properties of Iron Oxide Nanocrystals for Bio-Related Applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Murli, C.; Song, Y. Pressure-Induced Polymerization of Acrylic Acid: A Raman Spectroscopic Study. J. Phys. Chem. B 2010, 114, 9744–9750. [Google Scholar] [CrossRef] [PubMed]
- de Faria, D.L.A.; Lopes, F.N. Heated Goethite and Natural Hematite: Can Raman Spectroscopy Be Used to Differentiate Them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Guo, C.; Hu, Y.; Qian, H.; Ning, J.; Xu, S. Magnetite (Fe3O4) Tetrakaidecahedral Microcrystals: Synthesis, Characterization, and Micro-Raman Study. Mater. Charact. 2011, 62, 148–151. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman Study of Magnetite (Fe3O4): Laser-induced Thermal Effects and Oxidation. J. Raman Spectrosc. 2003, 34, 845–852. [Google Scholar] [CrossRef]
- Slavov, L.; Abrashev, M.V.; Merodiiska, T.; Gelev, C.; Vandenberghe, R.E.; Markova-Deneva, I.; Nedkov, I. Raman Spectroscopy Investigation of Magnetite Nanoparticles in Ferrofluids. J. Magn. Magn. Mater. 2010, 322, 1904–1911. [Google Scholar] [CrossRef] [Green Version]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Gupta, R.P.; Sen, S.K. Calculation of Multiplet Structure of Core $p$-Vacancy Levels. Phys. Rev. B 1974, 10, 71–77. [Google Scholar] [CrossRef]
- Gupta, R.P.; Sen, S.K. Calculation of Multiplet Structure of Core $p$ -Vacancy Levels. II. Phys. Rev. B 1975, 12, 15–19. [Google Scholar] [CrossRef]
- Dong, J.; Ozaki, Y.; Nakashima, K. Infrared, Raman, and Near-Infrared Spectroscopic Evidence for the Coexistence of Various Hydrogen-Bond Forms in Poly(Acrylic Acid). Macromolecules 1997, 30, 1111–1117. [Google Scholar] [CrossRef]
- Sharif, S.M.; Golestani Fard, F.; Khatibi, E.; Sarpoolaky, H. Dispersion and Stability of Carbon Black Nanoparticles, Studied by Ultraviolet–Visible Spectroscopy. J. Taiwan Inst. Chem. Eng. 2009, 40, 524–527. [Google Scholar] [CrossRef]
- Safaei, Y.; Aminzare, M.; Golestani-Fard, F.; Khorasanizadeh, F.; Salahi, E. Suspension Stability of Titania Nanoparticles Studied by UV-VIS Spectroscopy Method. Iran. J. Mater. Sci. Eng. 2012, 9, 62–68. [Google Scholar]
- Salzmann, C.G.; Chu, B.T.T.; Tobias, G.; Llewellyn, S.A.; Green, M.L.H. Quantitative Assessment of Carbon Nanotube Dispersions by Raman Spectroscopy. Carbon 2007, 45, 907–912. [Google Scholar] [CrossRef]
- Horia, F.; Easawi, K.; Khalil, R.; Abdallah, S.; El-Mansy, M.; Negm, S. Optical and Thermophysical Characterization of Fe3O4 Nanoparticle. IOP Conf. Ser. Mater. Sci. Eng. 2020, 956, 12016. [Google Scholar] [CrossRef]
- Jung, H.; Schimpf, A.M. Photochemical Reduction of Nanocrystalline Maghemite to Magnetite. Nanoscale 2021, 13, 17465–17472. [Google Scholar] [CrossRef]
- Sutherland, T.I.; Sparks, C.J.; Joseph, J.M.; Wang, Z.; Whitaker, G.; Sham, T.K.; Wren, J.C. Effect of Ferrous Ion Concentration on the Kinetics of Radiation-Induced Iron-Oxide Nanoparticle Formation and Growth. Phys. Chem. Chem. Phys. 2017, 19, 695–708. [Google Scholar] [CrossRef]
- Heinrich, C.A.; Seward, T.M. A Spectrophotometric Study of Aqueous Iron(II) Chloride Complexing from 25 to 200 °C. Geochim. Cosmochim. Acta 1990, 54, 2207–2221. [Google Scholar] [CrossRef]
- Zhao, R.; Pan, P. A Spectrophotometric Study of Fe(II)-Chloride Complexes in Aqueous Solutions from 10 to 100 °C. Can. J. Chem. 2001, 79, 131–144. [Google Scholar] [CrossRef]
- Po, H.N.; Sutin, N. Stability Constant of the Monochloro Complex of Iron(II). Inorg. Chem. 1968, 7, 621–624. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Sharma, S.; Singh, K.; Kumar, A. Magnetoresponsive Biocomposite Hydrogels Comprising Gelatin and Valine Based Magnetic Ionic Liquid Surfactant as Controlled Release Nanocarrier for Drug Delivery. Mater. Adv. 2022, 3, 484–492. [Google Scholar] [CrossRef]
- Gils, P.S.; Ray, D.; Sahoo, P.K. Designing of Silver Nanoparticles in Gum Arabic Based Semi-IPN Hydrogel. Int. J. Biol. Macromol. 2010, 46, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, K.; Krishna Rao, K.S.V.; Zo, S.; Soo Han, S.; Rao, K.M. Synthesis of Novel Tamarind Gum-Co-Poly(Acrylamidoglycolic Acid)-Based PH Responsive Semi-IPN Hydrogels and Their Ag Nanocomposites for Controlled Release of Chemotherapeutics and Inactivation of Multi-Drug-Resistant Bacteria. Gels 2021, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Jayaramudu, T.; Raghavendra, G.M.; Varaprasad, K.; Sadiku, R.; Ramam, K.; Raju, K.M. Iota-Carrageenan-Based Biodegradable Ag0 Nanocomposite Hydrogels for the Inactivation of Bacteria. Carbohydr. Polym. 2013, 95, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Veloso, S.R.S.; Martins, J.A.; Hilliou, L.O.; Amorim, C.; Amaral, V.S.; Almeida, B.G.; Jervis, P.J.; Moreira, R.; Pereira, D.M.; Coutinho, P.J.G.; et al. Dehydropeptide-Based Plasmonic Magnetogels: A Supramolecular Composite Nanosystem for Multimodal Cancer Therapy. J. Mater. Chem. B 2020, 8, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Gallo, J.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Hilliou, L.; Ferreira, P.M.T.; Bañobre-López, M.; Martins, J.A. Magnetic Dehydrodipeptide-Based Self-Assembled Hydrogels for Theragnostic Applications. Nanomaterials 2019, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Nagireddy, N.R.; Yallapu, M.M.; Kokkarachedu, V.; Sakey, R.; Kanikireddy, V.; Pattayil Alias, J.; Konduru, M.R. Preparation and Characterization of Magnetic Nanoparticles Embedded in Hydrogels for Protein Purification and Metal Extraction. J. Polym. Res. 2011, 18, 2285–2294. [Google Scholar] [CrossRef]
- Jørgensen, C.K.; De Verdier, C.-H.; Glomset, J.; Sörensen, N.A. Studies of Absorption Spectra. IV. Some New Transition Group Bands of Low Intensity. Acta Chem. Scand. 1954, 8, 1502–1512. [Google Scholar] [CrossRef]
- Nonkumwong, J.; Ananta, S.; Srisombat, L. Effective Removal of Lead(Ii) from Wastewater by Amine-Functionalized Magnesium Ferrite Nanoparticles. RSC Adv. 2016, 6, 47382–47393. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-Responsive Hydrogels: From Strategic Design to Biomedical Applications. J. Control. Release 2021, 335, 541–556. [Google Scholar] [CrossRef]
- Marey, A.; Ahmed, D.F. Batch Adsorption Studies of Natural Composite Hydrogel for Removal of Co(II) Ions. J. Appl. Membr. Sci. Technol. 2022, 26, 13–18. [Google Scholar] [CrossRef]
- Low, K.S.; Lee, C.K.; Liew, S.C. Sorption of Cadmium and Lead from Aqueous Solutions by Spent Grain. Process Biochem. 2000, 36, 59–64. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A Comparison of Chemisorption Kinetic Models Applied to Pollutant Removal on Various Sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Hu, L.; Hu, S.; Liu, Y. Optimized Synthesis of Novel Hydrogel for the Adsorption of Copper and Cobalt Ions in Wastewater. RSC Adv. 2019, 9, 16058–16068. [Google Scholar] [CrossRef]
- Lawrance, G.A. Leaving Groups on Inert Metal Complexes with Inherent or Induced Lability. In Advances in Inorganic Chemistry; Academic Press: Cambridge, MA, USA, 1989; Volume 34, pp. 145–194. ISBN 0898-8838. [Google Scholar]
- Facchi, D.P.; Cazetta, A.L.; Canesin, E.A.; Almeida, V.C.; Bonafé, E.G.; Kipper, M.J.; Martins, A.F. New Magnetic Chitosan/Alginate/Fe3O4@SiO2 Hydrogel Composites Applied for Removal of Pb(II) Ions from Aqueous Systems. Chem. Eng. J. 2018, 337, 595–608. [Google Scholar] [CrossRef]
- Yao, G.; Bi, W.; Liu, H. PH-Responsive Magnetic Graphene Oxide/Poly(NVI-Co-AA) Hydrogel as an Easily Recyclable Adsorbent for Cationic and Anionic Dyes. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 588, 124393. [Google Scholar] [CrossRef]
- Van Berkum, S.; Biewenga, P.D.; Verkleij, S.P.; van Zon, J.H.B.A.; Boere, K.W.M.; Pal, A.; Philipse, A.P.; Erné, B.H. Swelling Enhanced Remanent Magnetization of Hydrogels Cross-Linked with Magnetic Nanoparticles. Langmuir 2015, 31, 442–450. [Google Scholar] [CrossRef]
- Saadli, M.; Braunmiller, D.L.; Mourran, A.; Crassous, J.J. Thermally and Magnetically Programmable Hydrogel Microactuators. Small 2023, 19, 2207035. [Google Scholar] [CrossRef]
- Liu, W.; Migdisov, A.; Williams-Jones, A. The Stability of Aqueous Nickel(II) Chloride Complexes in Hydrothermal Solutions: Results of UV–Visible Spectroscopic Experiments. Geochim. Cosmochim. Acta 2012, 94, 276–290. [Google Scholar] [CrossRef]
- Radoń, M.; Drabik, G. Spin States and Other Ligand–Field States of Aqua Complexes Revisited with Multireference Ab Initio Calculations Including Solvation Effects. J. Chem. Theory Comput. 2018, 14, 4010–4027. [Google Scholar] [CrossRef]
- Staszak, K.; Kruszelnicka, I.; Ginter-Kramarczyk, D.; Góra, W.; Baraniak, M.; Lota, G.; Regel-Rosocka, M. Advances in the Removal of Cr(III) from Spent Industrial Effluents-A Review. Materials 2023, 16, 378. [Google Scholar] [CrossRef]
- Amato, F.; Motta, A.; Giaccari, L.; Di Pasquale, R.; Scaramuzzo, F.A.; Zanoni, R.; Marrani, A.G. One-Pot Carboxyl Enrichment Fosters Water-Dispersibility of Reduced Graphene Oxide: A Combined Experimental and Theoretical Assessment. Nanoscale Adv. 2023, 5, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Marrani, A.G.; Motta, A.; Palmieri, V.; Perini, G.; Papi, M.; Dalchiele, E.A.; Schrebler, R.; Zanoni, R. A Comparative Experimental and Theoretical Study of the Mechanism of Graphene Oxide Mild Reduction by Ascorbic Acid and N-Acetyl Cysteine for Biomedical Applications. Mater. Adv. 2020, 1, 2745–2754. [Google Scholar] [CrossRef]
- Dalalibera, A.; Vilela, P.B.; Vieira, T.; Becegato, V.A.; Paulino, A.T. Removal and Selective Separation of Synthetic Dyes from Water Using a Polyacrylic Acid-Based Hydrogel: Characterization, Isotherm, Kinetic, and Thermodynamic Data. J. Environ. Chem. Eng. 2020, 8, 104465. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; Zhang, C.; Yu, Q.; Zhao, Z.; Niu, X.; Sun, X.; Liu, Y.; Ma, L.; Li, Z. Enhanced Adsorptive Removal of Anionic and Cationic Dyes from Single or Mixed Dye Solutions Using MOF PCN-222. RSC Adv. 2017, 7, 16273–16281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, F.; Zhao, X.; Cao, J.; Liu, S.; Zhang, Y.; Yuan, Z.; Huang, X.; De Hoop, C.F.; Peng, X.; et al. Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions. Gels 2023, 9, 40. [Google Scholar] [CrossRef]
- Aljar, M.A.A.; Rashdan, S.; Almutawah, A.; El-Fattah, A.A. Synthesis and Characterization of Biodegradable Poly(Vinyl Alcohol)-Chitosan/Cellulose Hydrogel Beads for Efficient Removal of Pb(II), Cd(II), Zn(II), and Co(II) from Water. Gels 2023, 9, 328. [Google Scholar] [CrossRef]







| Heavy Metal | Sources | Main Organ and System Affected | Permitted Amounts (μg) |
|---|---|---|---|
| Lead (Pb) | Lead-based batteries, solder, alloys, cable sheathing pigments, rust inhibitors, ammunition, glazes, plastic stabilizers | Bones, liver, kidneys, brain, lungs, spleen, immunological system, hematological system, cardiovascular system, reproductive system | 10 |
| Arsenic (As) | Electronics and glass production | Skin, lungs, brain, kidneys, metabolic system, cardiovascular system, immunological system, endocrine system | 10 |
| Copper (Cu) | Corroded plumbing systems, electronic and cables industry | Liver, brain, kidneys, cornea, gastrointestinal system, lungs, immunological system, hematological system | 2000 |
| Zinc (Zn) | Brass coating, rubber products, some cosmetics and aerosol deodorants | Stomach cramps, skin irritations, vomiting, nausea, anemia, convulsions | 3000 |
| Chromium (Cr) | Steel and pulp mills, tanneries | Skin, lungs, kidneys, liver, brain, pancreas, tastes, gastrointestinal system, reproductive system | 50 |
| Cadmium (Cd) | Batteries, paints, steel industry, plastic industries, metal refineries, corroded galvanized pipes | Bones, liver, kidneys, lungs, testes, brain, immunological system, cardiovascular system | 3 |
| Mercury (Hg) | Electrolytic production of caustic soda and chlorine, electrical appliances, runoff from landfills and agriculture, industrial and control instruments, laboratory apparatus, refineries | Brain, lungs, kidneys, liver, immunological system, cardiovascular system, endocrine and reproductive system | 6 |
| Nickel (Ni) | Nickel alloy production, stainless steel | Skin, gastrointestinal distress, lung, pulmonary fibrosis, kidney | 70 |
| 1 Cobalt (Co) | Cement industries, polishing disc used in diamond polishing, mobile batteries, televisions (TVs), liquid crystal display TVs, computer monitors | High concentrations cause vomiting, nausea, vision problems, thyroid gland damage | N/A |
| PAA | PAA-Stabilized γ-Fe2O3NPs | Peak Assignment |
|---|---|---|
| 1699 | 1705 | –C=O (free COOH) |
| - | 1556 | –COO− (asymmetric) |
| 1446 | 1444 | –CH2 scissor |
| 1402 | 1408 | –COO− (symmetric) |
| 1236 | - | –C–O |
| Samples | Swelling Degree (q) |
|---|---|
| HG | 62.18 ± 0.35 |
| γ-Fe2O3NPs@HG (10 mg/mL) | 73.82 ± 0.99 |
| γ-Fe2O3NPs@HG (20 mg/mL) | 81.28 ± 0.22 |
| γ-Fe2O3NPs@HG (30 mg/mL) | 88.24 ± 0.31 |
| Adsorbent | Removal% (RE%) | Experimental qe (mg g−1) |
|---|---|---|
| HG | 20.4 ± 0.3 | 1680 ± 34 |
| γ-Fe2O3NPs@HG | 20.7 ± 0.4 | 1703 ± 42 |
| γ-Fe2O3NPs@HG + magnet | 25.7 ± 0.6 | 2111 ± 72 |
| Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|
| Adsorbent | k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 |
| HG | 0.0036 | 1867 | 0.9057 | 0.0005 | 1958 | 0.9722 |
| γ-Fe2O3NPs@HG | 0.0036 | 1611 | 0.9363 | 0.0005 | 1895 | 0.9872 |
| γ-Fe2O3NPs@HG + magnet | 0.0032 | 1486 | 0.9667 | 0.0004 | 2285 | 0.9834 |
| Adsorbent | Removal% (RE%) | Experimental qe (mg g−1) |
|---|---|---|
| HG | 18.6 ± 0.1 | 1399 ± 12 |
| γ-Fe2O3NPs@HG | 25.9 ± 0.4 | 1945 ± 41 |
| γ-Fe2O3NPs@HG + magnet | 23.7 ± 0.4 | 1758 ± 39 |
| Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|
| Adsorbent | k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 |
| HG | 0.0115 | 1948 | 0.8548 | 0.000005 | 1785 | 0.9735 |
| γ-Fe2O3NPs@HG | 0.0161 | 2998 | 0.8247 | 0.000007 | 2325 | 0.9954 |
| γ-Fe2O3NPs@HG + magnet | 0.0092 | 613 | 0.9457 | 0.000036 | 1851 | 0.9993 |
| Adsorbent | Removal% (RE%) | Experimental qe (mg g−1) |
|---|---|---|
| HG | 13.2 ± 0.1 | 127 ± 6 |
| γ-Fe2O3NPs@HG | 15.5 ± 0.1 | 149 ± 8 |
| γ-Fe2O3NPs@HG + magnet | 14.7 ± 0.1 | 142 ± 5 |
| Pseudo-First Order | Pseudo-Second Order | |||||
|---|---|---|---|---|---|---|
| Adsorbent | k1 (min−1) | qe (mg g−1) | R2 | k2 (g mg−1 min−1) | qe (mg g−1) | R2 |
| HG | 0.0105 | 248 | 0.8355 | - | 27 | 0.0040 |
| γ-Fe2O3NPs@HG | 0.0101 | 184 | 0.9052 | 0.00003 | 212 | 0.9538 |
| γ-Fe2O3NPs@HG + magnet | 0.0112 | 134 | 0.9197 | 0.0001 | 163 | 0.9794 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajareh Haghighi, F.; Binaymotlagh, R.; Chronopoulou, L.; Cerra, S.; Marrani, A.G.; Amato, F.; Palocci, C.; Fratoddi, I. Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water. Gels 2023, 9, 621. https://doi.org/10.3390/gels9080621
Hajareh Haghighi F, Binaymotlagh R, Chronopoulou L, Cerra S, Marrani AG, Amato F, Palocci C, Fratoddi I. Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water. Gels. 2023; 9(8):621. https://doi.org/10.3390/gels9080621
Chicago/Turabian StyleHajareh Haghighi, Farid, Roya Binaymotlagh, Laura Chronopoulou, Sara Cerra, Andrea Giacomo Marrani, Francesco Amato, Cleofe Palocci, and Ilaria Fratoddi. 2023. "Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water" Gels 9, no. 8: 621. https://doi.org/10.3390/gels9080621
APA StyleHajareh Haghighi, F., Binaymotlagh, R., Chronopoulou, L., Cerra, S., Marrani, A. G., Amato, F., Palocci, C., & Fratoddi, I. (2023). Self-Assembling Peptide-Based Magnetogels for the Removal of Heavy Metals from Water. Gels, 9(8), 621. https://doi.org/10.3390/gels9080621














