Non-Electroneutrality Generated by Bacteriorhodopsin-Incorporated Membranes Enhances the Conductivity of a Gelatin Memory Device
Abstract
:1. Introduction
2. Results and Discussion
2.1. Addition of BR Membranes to Gelatin Devices Significantly Enhances Electron Conductivity
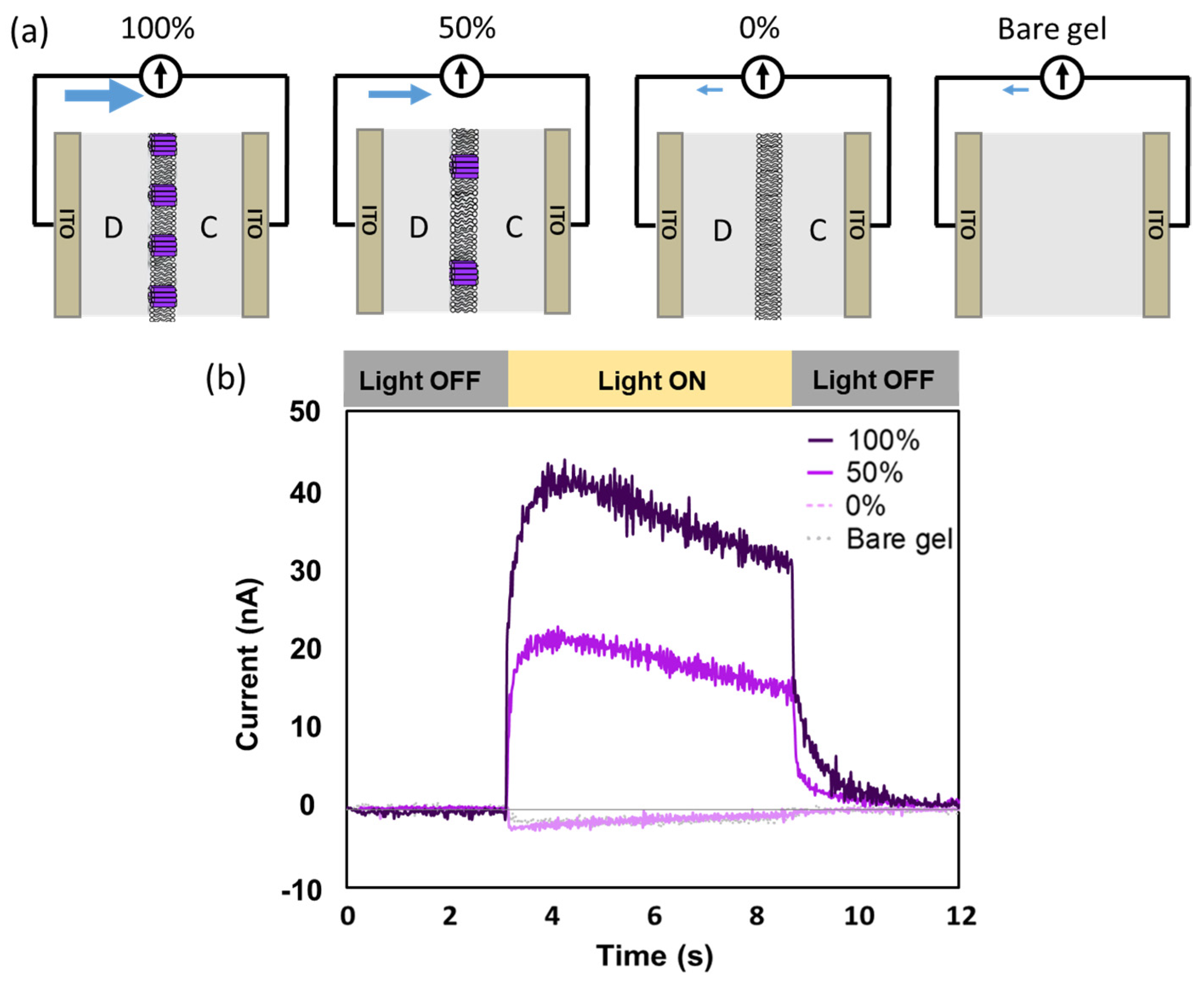
2.2. Net Proton Flow toward the Deposited Side of the BR-Incorporated Membrane Revealed by Photocurrent Measurements
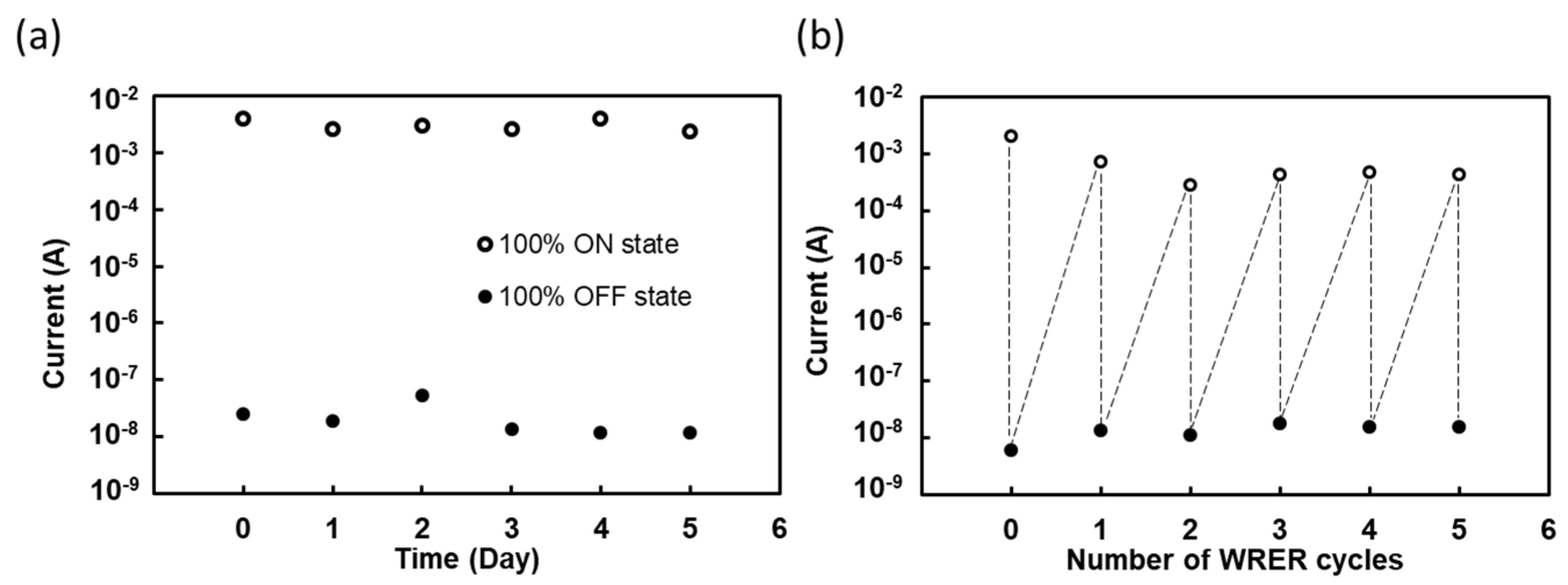
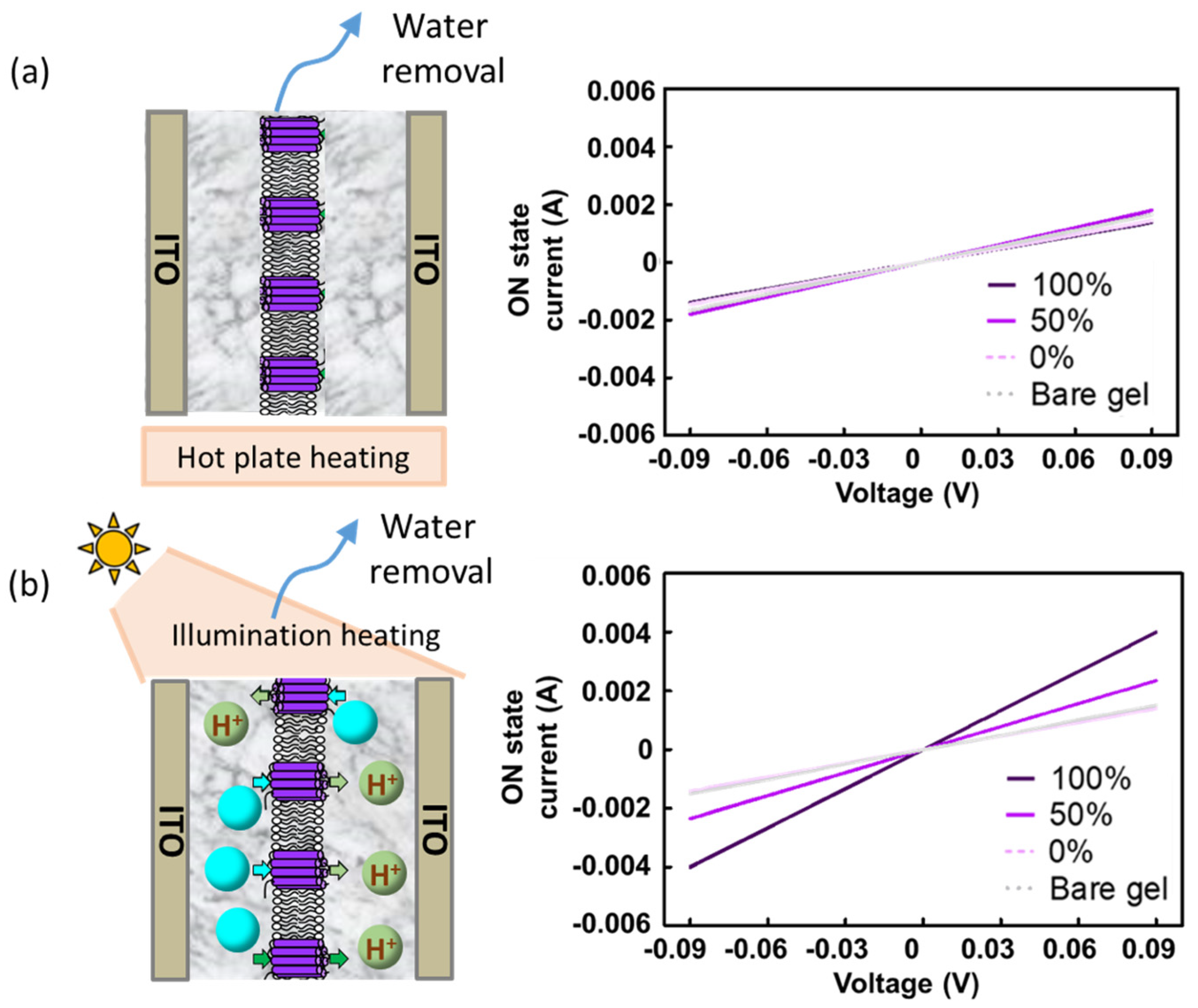
2.3. The Proton Pumping Enhances the Conductivity of the Gelatin Device in the ON State
2.4. The Increased Conductivity Is Not Due to the pH Change
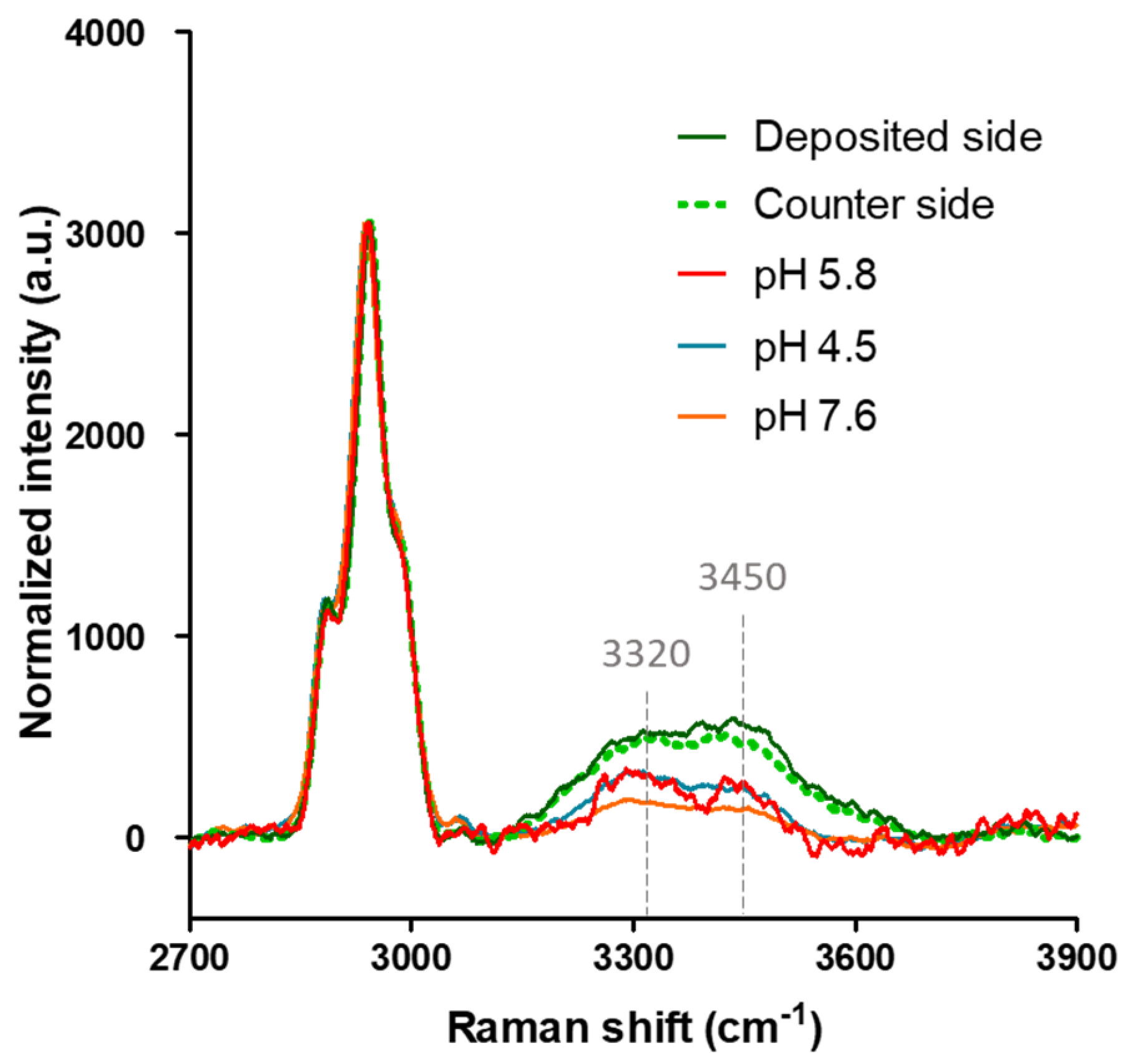
2.5. Raman Spectroscopy Analysis Shows Increased Amounts of Bound Water and a More Disordered Gelatin Structure in the ON State Gelatin Devices
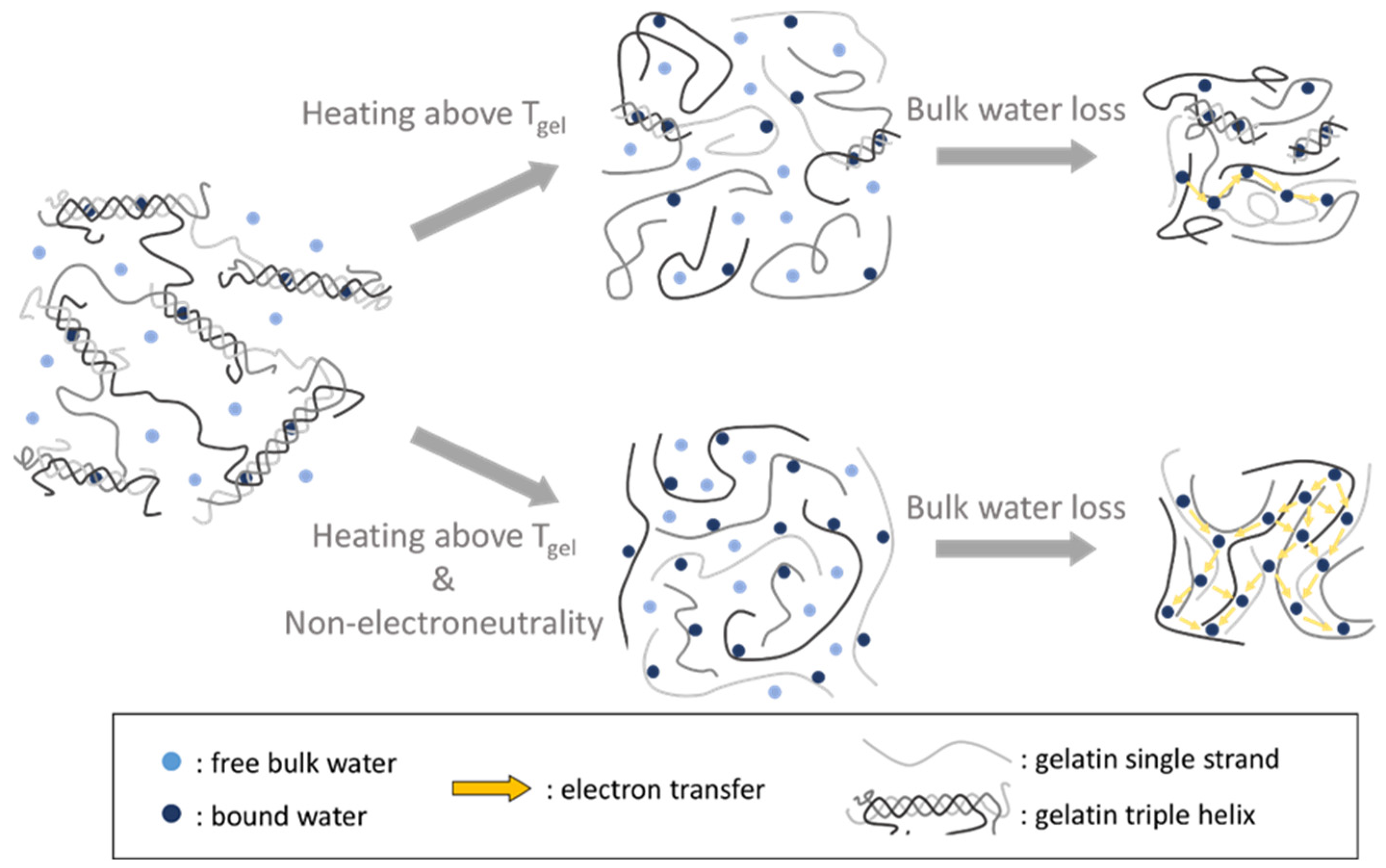
2.6. Proposed Mechanism of How Non-Electroneutrality Influences the Gelatin-Bound Water Network in the ON State
3. Conclusions
4. Materials and Methods
4.1. BR-Incorporated Liposome Preparation
4.2. Preparation of Gelatin Solution
4.3. Fabrication of BR-Incorporated Gelatin Memory Devices
4.4. Electrical Performance Measurements
4.5. Raman Spectroscopy to Examine the Bound Water Content and Gelatin Secondary Structure
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, P.; Mathew, L.; Syal, P. Increasing trend of wearables and multimodal interface for human activity monitoring: A review. Biosens. Bioelectron. 2017, 90, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Son, D.; Lee, J.; Qiao, S.; Ghaffari, R.; Kim, J.; Lee, J.E.; Song, C.; Kim, S.J.; Lee, D.J.; Jun, S.W. Multifunctional wearable devices for diagnosis and therapy of movement disorders. Nat. Nanotechnol. 2014, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Chiu, U.T.; Chao, L. Electron transfer through protein-bound water and its bioelectronic application. Biosens. Bioelectron. 2019, 136, 16–22. [Google Scholar] [CrossRef] [PubMed]
- de Wolf, F.A. Chapter V Collagen and gelatin. In Progress in Biotechnology; Aalbersberg, W.Y., Hamer, R.J., Jasperse, P., de Jongh, H.H.J., de Kruif, C.G., Walstra, P., de Wolf, F.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 23, pp. 133–218. [Google Scholar]
- Nishino, T.; Hayashi, N.; Bui, P.T. Direct measurement of electron transfer through a hydrogen bond between single molecules. J. Am. Chem. Soc. 2013, 135, 4592–4595. [Google Scholar] [CrossRef]
- Meidanshahi, R.V.; Mazinani, S.K.; Mujica, V.; Tarakeshwar, P. Electronic transport across hydrogen bonds in organic electronics. Int. J. Nanotechnol. 2015, 12, 297–312. [Google Scholar] [CrossRef]
- Medhi, C.; Mitchell, J.B.; Price, S.L.; Tabor, A.B. Electrostatic factors in DNA intercalation. Biopolym. Orig. Res. Biomol. 1999, 52, 84–93. [Google Scholar] [CrossRef]
- Cocco, S.; Marko, J.; Monasson, R.; Sarkar, A.; Yan, J. Force-extension behavior of folding polymers. Eur. Phys. J. E 2003, 10, 249–263. [Google Scholar] [CrossRef]
- Baler, K.; Martin, O.A.; Carignano, M.A.; Ameer, G.A.; Vila, J.A.; Szleifer, I. Electrostatic unfolding and interactions of albumin driven by pH changes: A molecular dynamics study. J. Phys. Chem. B 2014, 118, 921–930. [Google Scholar] [CrossRef]
- Stigter, D. An electrostatic model of B-DNA for its stability against unwinding. Biophys. Chem. 1998, 75, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wickstrand, C.; Dods, R.; Royant, A.; Neutze, R. Bacteriorhodopsin: Would the real structural intermediates please stand up? Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 536–553. [Google Scholar] [CrossRef] [Green Version]
- Kimura, Y.; Vassylyev, D.G.; Miyazawa, A.; Kidera, A.; Matsushima, M.; Mitsuoka, K.; Murata, K.; Hirai, T.; Fujiyoshi, Y. Surface of bacteriorhodopsin revealed by high-resolution electron crystallography. Nature 1997, 389, 206–211. [Google Scholar] [CrossRef]
- Jin, Y.; Honig, T.; Ron, I.; Friedman, N.; Sheves, M.; Cahen, D. Bacteriorhodopsin as an electronic conduction medium for biomolecular electronics. Chem. Soc. Rev. 2008, 37, 2422–2432. [Google Scholar] [CrossRef]
- He, J.A.; Samuelson, L.; Li, L.; Kumar, J.; Tripathy, S.K. Bacteriorhodopsin Thin-Film Assemblies—Immobilization, Properties, and Applications. Adv. Mater. 1999, 11, 435–446. [Google Scholar] [CrossRef]
- Hampp, N. Bacteriorhodopsin as a photochromic retinal protein for optical memories. Chem. Rev. 2000, 100, 1755–1776. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.-K.; Yen, C.-W.; El-Sayed, M.A. Bacteriorhodopsin-based photo-electrochemical cell. Biosens. Bioelectron. 2010, 26, 620–626. [Google Scholar] [CrossRef]
- Rao, S.; Lu, S.; Guo, Z.; Li, Y.; Chen, D.; Xiang, Y. A Light-Powered Bio-Capacitor with Nanochannel Modulation. Adv. Mater. 2014, 26, 5846–5850. [Google Scholar] [CrossRef]
- Lozier, R.H.; Bogomolni, R.A.; Stoeckenius, W. Bacteriorhodopsin: A light-driven proton pump in Halobacterium Halobium. Biophys. J. 1975, 15, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.; Henderson, R. Molecular mechanism of vectorial proton translocation by bacteriorhodopsin. Nature 2000, 406, 653–657. [Google Scholar] [CrossRef]
- Lanyi, J.K. Proton transfers in the bacteriorhodopsin photocycle. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 1012–1018. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K. Advanced Semiconductor Memories: Architectures, Designs, and Applications; Wiley-IEEE Press: Hoboken, NJ, USA, 2009; p. 672. [Google Scholar]
- Ling, Q.-D.; Liaw, D.-J.; Zhu, C.; Chan, D.S.-H.; Kang, E.-T.; Neoh, K.-G. Polymer electronic memories: Materials, devices and mechanisms. Prog. Polym. Sci. 2008, 33, 917–978. [Google Scholar] [CrossRef]
- Hosseini, N.R.; Lee, J.S. Biocompatible and Flexible Chitosan-Based Resistive Switching Memory with Magnesium Electrodes. Adv. Funct. Mater. 2015, 25, 5586–5592. [Google Scholar] [CrossRef]
- Raeis-Hosseini, N.; Lee, J.-S. Controlling the resistive switching behavior in starch-based flexible biomemristors. ACS Appl. Mater. Interfaces 2016, 8, 7326–7332. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Heremans, P.; Gelinck, G.H.; Muller, R.; Baeg, K.-J.; Kim, D.-Y.; Noh, Y.-Y. Polymer and organic nonvolatile memory devices. Chem. Mater. 2010, 23, 341–358. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, N.; Li, S.; Lu, S.; Xiang, Y. A novel light-driven pH-biosensor based on bacteriorhodopsin. Nano Energy 2019, 66, 104129. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.-R.; Zhao, L.-Y.; Li, G.-P.; Shen, H.-Y.; Li, Y.-T.; Ren, T.-L. Review on bacteriorhodopsin-based self-powered bio-photoelectric sensors. Mater. Sci. Semicond. Process. 2023, 162, 107501. [Google Scholar] [CrossRef]
- Xue, H.; Li, X.; Wang, K.; Cui, W.; Zhao, J.; Fei, J.; Li, J. Solvent-tunable dipeptide-based nanostructures with enhanced optical-to-electrical transduction. Chem. Commun. 2019, 55, 13136–13139. [Google Scholar] [CrossRef]
- Unal, M.; Yang, S.; Akkus, O. Molecular spectroscopic identification of the water compartments in bone. Bone 2014, 67, 228–236. [Google Scholar] [CrossRef]
- Leikin, S.; Parsegian, V.; Yang, W.-H.; Walrafen, G. Raman spectral evidence for hydration forces between collagen triple helices. Proc. Natl. Acad. Sci. USA 1997, 94, 11312–11317. [Google Scholar] [CrossRef] [PubMed]
- Cavatorta, F.; Fontana, M.P.; Vecli, A. Raman spectroscopy of protein–water interactions in aqueous solutions. J. Chem. Phys. 1976, 65, 3635–3640. [Google Scholar] [CrossRef]
- Devitt, G.; Howard, K.; Mudher, A.; Mahajan, S. Raman Spectroscopy: An emerging tool in neurodegenerative disease research and diagnosis. ACS Chem. Neurosci. 2018, 9, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Gullekson, C.; Lucas, L.; Hewitt, K.; Kreplak, L. Surface-sensitive Raman spectroscopy of collagen I fibrils. Biophys. J. 2011, 100, 1837–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefèvre, T.; Rousseau, M.-E.; Pézolet, M. Protein secondary structure and orientation in silk as revealed by Raman spectromicroscopy. Biophys. J. 2007, 92, 2885–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehring, K.A.; Crane, N.J.; Smukler, A.R.; McHugh, J.B.; Roessler, B.J.; Morris, M.D. Identifying chemical changes in subchondral bone taken from murine knee joints using Raman spectroscopy. Appl. Spectrosc. 2006, 60, 1134–1141. [Google Scholar] [CrossRef]
- Dong, R.; Yan, X.; Pang, X.; Liu, S. Temperature-dependent Raman spectra of collagen and DNA. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 557–561. [Google Scholar] [CrossRef]
- Fu, H.-Y.; Lin, Y.-C.; Chang, Y.-N.; Tseng, H.; Huang, C.-C.; Liu, K.-C.; Huang, C.-S.; Su, C.-W.; Weng, R.R.; Lee, Y.-Y. A novel six-rhodopsin system in a single archaeon. J. Bacteriol. 2010, 192, 5866–5873. [Google Scholar] [CrossRef] [Green Version]



| BR Density Ratio in the DOPC Membrane | OFF State Capacitance (μF/cm2) | ON State Conductivity (×10−5 S/cm) |
|---|---|---|
| 100% | 41 ± 2 | 6.9 ± 0.9 |
| 50% | 42 ± 1 | 4.9 ± 0.3 |
| 0% | 43.2 ± 9.1 | 2.7 ± 0.5 |
| Bare gel (no membrane) | 42.2 ± 3.2 | 3.4 ± 0.6 |
| pH Value | ON State Conductivity (×10−5 S/cm) |
|---|---|
| 5.8/5.8 | 3.4 ± 0.6 |
| 4.5/4.5 | 3.2 ± 0.5 |
| 4.5/7.6 | 3.7 ± 0.7 |
| 7.6/7.6 | 3.3 ± 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, U.-T.; Lee, B.-F.; Ko, L.-N.; Yang, C.-S.; Chao, L. Non-Electroneutrality Generated by Bacteriorhodopsin-Incorporated Membranes Enhances the Conductivity of a Gelatin Memory Device. Gels 2023, 9, 635. https://doi.org/10.3390/gels9080635
Chiu U-T, Lee B-F, Ko L-N, Yang C-S, Chao L. Non-Electroneutrality Generated by Bacteriorhodopsin-Incorporated Membranes Enhances the Conductivity of a Gelatin Memory Device. Gels. 2023; 9(8):635. https://doi.org/10.3390/gels9080635
Chicago/Turabian StyleChiu, U-Ting, Bo-Fan Lee, Ling-Ning Ko, Chii-Shen Yang, and Ling Chao. 2023. "Non-Electroneutrality Generated by Bacteriorhodopsin-Incorporated Membranes Enhances the Conductivity of a Gelatin Memory Device" Gels 9, no. 8: 635. https://doi.org/10.3390/gels9080635





