Polydopamine-Functionalized Bacterial Cellulose as Hydrogel Scaffolds for Skin Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
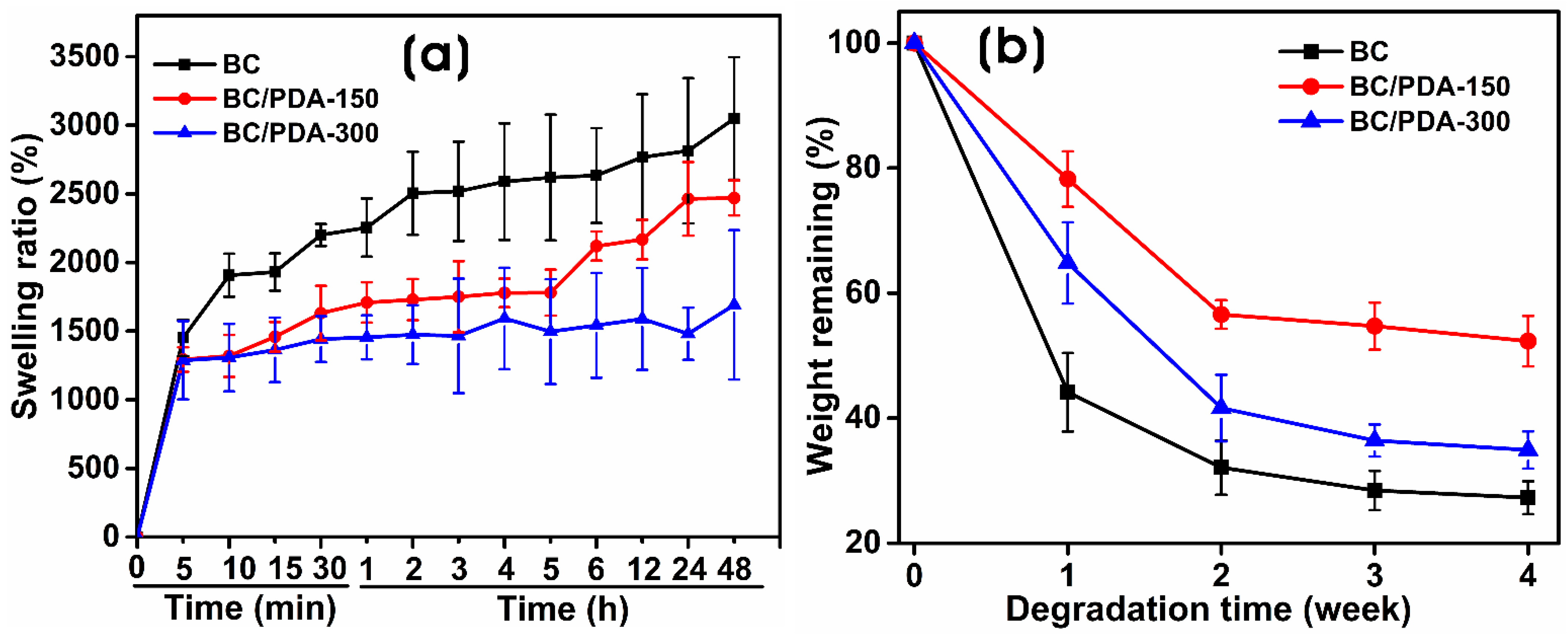
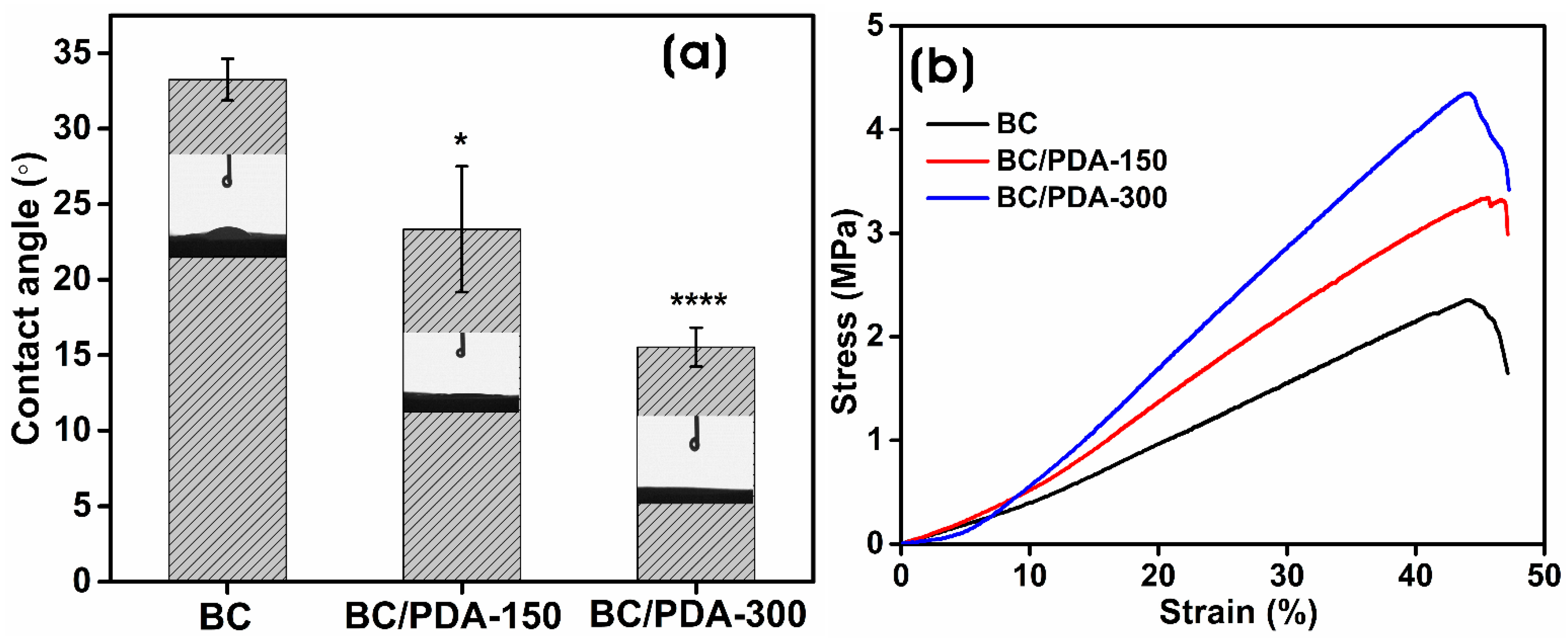
2.1. Characterization of BC and BC/PDA Hydrogels
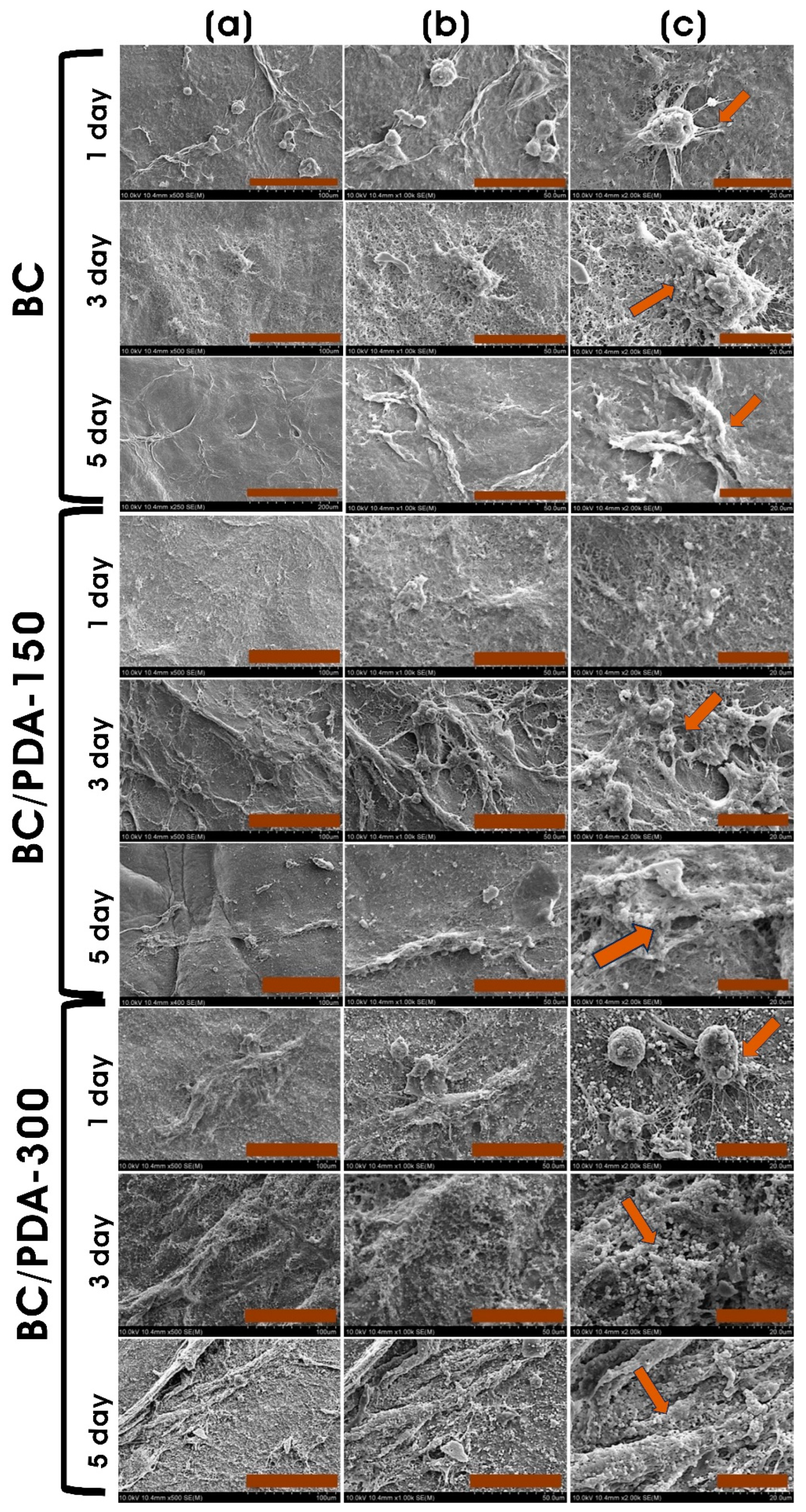
2.2. In Vitro Cytocompatibility Assay
3. Conclusions
4. Experimental
4.1. Materials
4.2. Preparation of Bacterial Cellulose (BC) Hydrogels
4.3. Preparation of Polydopamine-Functionalized BC (BC/PDA) Hydrogels
4.4. Characterization of BC and BC/PDA Hydrogels
4.4.1. Mechanical Properties
4.4.2. Surface Wettability
4.4.3. Swelling Ratio
4.4.4. In Vitro Degradation
4.5. In Vitro Cytocompatibility Evaluation of Hydrogels
4.5.1. Adherent Cell Culture
4.5.2. PrestoBlue Assay
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar]
- Luneva, O.; Olekhnovich, R.; Uspenskaya, M. Bilayer Hydrogels for Wound Dressing and Tissue Engineering. Polymers 2022, 14, 3135. [Google Scholar] [CrossRef]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Charruyer, A.; Ghadially, R. Stem cells and tissue-engineered skin. Ski. Pharmacol. Physiol. 2009, 22, 55–62. [Google Scholar]
- Stojic, M.; López, V.; Montero, A.; Quílez, C.; de Aranda Izuzquiza, G.; Vojtova, L.; Luis Jorcano, J.; Velasco, D. 3-Skin tissue engineering. In Biomaterials for Skin Repair and Regeneration; García-Gareta, E., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 59–99. [Google Scholar]
- Prost-Squarcioni, C.; Fraitag, S.; Heller, M.; Boehm, N. Functional histology of dermis. Proc. Ann. Dermatol. Venereol. 2008, 135, 1S5–1S20. [Google Scholar]
- Ashcroft, G.S.; Mills, S.J.; Ashworth, J.J. Ageing and wound healing. Biogerontology 2002, 3, 337–345. [Google Scholar] [PubMed]
- Vig, K.; Chaudhari, A.; Tripathi, S.; Dixit, S.; Sahu, R.; Pillai, S.; Dennis, V.A.; Singh, S.R. Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 2017, 18, 789. [Google Scholar] [CrossRef] [Green Version]
- Tai, C.; Xie, Z.; Li, Y.; Feng, Y.; Xie, Y.; Yang, H.; Wang, L.; Wang, B. Human skin dermis-derived fibroblasts are a kind of functional mesenchymal stromal cells: Judgements from surface markers, biological characteristics, to therapeutic efficacy. Cell Biosci. 2022, 12, 105. [Google Scholar]
- Nilforoushzadeh, M.A.; Ashtiani, H.R.A.; Jaffary, F.; Jahangiri, F.; Nikkhah, N.; Mahmoudbeyk, M.; Fard, M.; Ansari, Z.; Zare, S. Dermal fibroblast cells: Biology and function in skin regeneration. J. Ski. Stem Cell 2017, 4, e69080. [Google Scholar]
- Clark, R.A.; Ghosh, K.; Tonnesen, M.G. Tissue engineering for cutaneous wounds. J. Investig. Dermatol. 2007, 127, 1018–1029. [Google Scholar] [CrossRef] [Green Version]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [PubMed] [Green Version]
- Ouyang, Y.; Zhao, Y.; Zheng, X.; Zhang, Y.; Zhao, J.; Wang, S.; Gu, Y. Rapidly degrading and mussel-inspired multifunctional carboxymethyl chitosan/montmorillonite hydrogel for wound hemostasis. Int. J. Biol. Macromol. 2023, 242, 124960. [Google Scholar] [CrossRef]
- Morris, A.H.; Stamer, D.; Kyriakides, T. The host response to naturally-derived extracellular matrix biomaterials. In Proceedings of the Seminars in Immunology, Madrid, Spain, 29 June–1 July 2017; pp. 72–91. [Google Scholar]
- Bacakova, L.; Novotná, K.; Parizek, M. Polysaccharides as cell carriers for tissue engineering: The use of cellulose in vascular wall reconstruction. Physiol. Res. 2014, 63, S29. [Google Scholar]
- Lévesque, S.G.; Shoichet, M.S. Synthesis of cell-adhesive dextran hydrogels and macroporous scaffolds. Biomaterials 2006, 27, 5277–5285. [Google Scholar] [CrossRef]
- Wei, C.; Feng, Y.; Che, D.; Zhang, J.; Zhou, X.; Shi, Y.; Wang, L. Biomaterials in skin tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 993–1011. [Google Scholar] [CrossRef]
- Tarrahi, R.; Khataee, A.; Karimi, A.; Yoon, Y. The latest achievements in plant cellulose-based biomaterials for tissue engineering focusing on skin repair. Chemosphere 2022, 288, 132529. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [PubMed]
- Naomi, R.; Bt Hj Idrus, R.; Fauzi, M.B. Plant- vs. Bacterial-derived cellulose for wound healing: A review. Int. J. Environ. Res. Public Health 2020, 17, 6803. [Google Scholar] [PubMed]
- Luo, H.; Gan, D.; Gama, M.; Tu, J.; Yao, F.; Zhang, Q.; Ao, H.; Yang, Z.; Li, J.; Wan, Y. Interpenetrated nano- and submicro-fibrous biomimetic scaffolds towards enhanced mechanical and biological performances. Mater. Sci. Eng. C 2020, 108, 110416. [Google Scholar]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.-T.; Buehler, M.J. Polydopamine and eumelanin: From structure–property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef]
- Ball, V. Polydopamine nanomaterials: Recent advances in synthesis methods and applications. Front. Bioeng. Biotechnol. 2018, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- García-Mayorga, J.C.; Rosu, H.-C.; Jasso-Salcedo, A.B.; Escobar-Barrios, V.A. Kinetic study of polydopamine sphere synthesis using TRIS: Relationship between synthesis conditions and final properties. RSC Adv. 2023, 13, 5081–5095. [Google Scholar]
- Jiang, Q.; Derami, H.G.; Ghim, D.; Cao, S.; Jun, Y.-S.; Singamaneni, S. Polydopamine-filled bacterial nanocellulose as a biodegradable interfacial photothermal evaporator for highly efficient solar steam generation. J. Mater. Chem. A 2017, 5, 18397–18402. [Google Scholar]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Gracio, J.J.d.A.; Toniazzo, V.; Ruch, D. Dopamine−melanin film deposition depends on the used oxidant and buffer solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [PubMed]
- Hong, G.; Cheng, H.; Meng, Y.; Lin, J.; Chen, Z.; Zhang, S.; Song, W. Mussel-inspired polydopamine as a green, efficient, and stable platform to functionalize bamboo fiber with amino-terminated alkyl for high performance poly (butylene succinate) composites. Polymers 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Guo, R.; Lan, J.; Jiang, S.; Lin, S. Microwave-assisted deposition of silver nanoparticles on bamboo pulp fabric through dopamine functionalization. Appl. Surf. Sci. 2016, 386, 151–159. [Google Scholar]
- Cox, H.J.; Li, J.; Saini, P.; Paterson, J.R.; Sharples, G.J.; Badyal, J.P.S. Bioinspired and eco-friendly high efficacy cinnamaldehyde antibacterial surfaces. J. Mater. Chem. B 2021, 9, 2918–2930. [Google Scholar] [CrossRef] [PubMed]
- Moraes, P.R.F.d.S.; Saska, S.; Barud, H.; Lima, L.R.d.; Martins, V.d.C.A.; Plepis, A.M.d.G.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial cellulose/collagen hydrogel for wound healing. Mater. Res. 2016, 19, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Janpetch, N.; Vanichvattanadecha, C.; Rujiravanit, R. Photocatalytic disinfection of water by bacterial cellulose/N–F co-doped TiO2 under fluorescent light. Cellulose 2015, 22, 3321–3335. [Google Scholar]
- Sajjad, W.; Khan, T.; Ul-Islam, M.; Khan, R.; Hussain, Z.; Khalid, A.; Wahid, F. Development of modified montmorillonite-bacterial cellulose nanocomposites as a novel substitute for burn skin and tissue regeneration. Carbohydr. Polym. 2019, 206, 548–556. [Google Scholar]
- Ren, L.; He, G.; Zhou, Y.; Dai, J.; Miao, W.; Ouyang, C.; Liu, J.; Chen, G. A hydrogel based on nanocellulose/polydopamine/gelatin used for the treatment of MRSA infected wounds with broad-spectrum antibacterial and antioxidant properties and tissue suitability. Biomater. Sci. 2022, 10, 3174–3187. [Google Scholar] [CrossRef] [PubMed]
- Okyere, D.T. Understanding the Chemical Structures of Polydopamine. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2021. [Google Scholar]
- Wang, X.; Jin, B.; Lin, X. In-situ FTIR spectroelectrochemical study of dopamine at a glassy carbon electrode in a neutral solution. Anal. Sci. 2002, 18, 931–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-inspired polydopamine: A biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Aleshina, L.; Gladysheva, E.; Budaeva, V.; Mironova, G.; Skiba, E.; Sakovich, G. X-ray Diffraction Data on the Bacterial Nanocellulose Synthesized by Komagataeibacter xylinus B-12429 and B-12431 Microbial Producers in Miscanthus- and Oat Hull-Derived Enzymatic Hydrolyzates. Crystallogr. Rep. 2022, 67, 391–397. [Google Scholar] [CrossRef]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and characterization of bacterial cellulose produced by Komagatacibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Huo, M.; Zhai, X.; Li, F.; Zhong, C. Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomater. Nanotechnol. 2017, 7, 1847980417707172. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Catchmark, J.M.; Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Betlej, I.; Rybak, K.; Nowacka, M.; Antczak, A.; Borysiak, S.; Krochmal-Marczak, B.; Lipska, K.; Boruszewski, P. Structural properties of bacterial cellulose film obtained on a substrate containing sweet potato waste. Crystals 2022, 12, 1191. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Li, W.; Liu, Y.; Wang, J.; Wang, S. High-performance multilayer composite membranes with mussel-inspired polydopamine as a versatile molecular bridge for CO2 separation. ACS Appl. Mater. Interfaces 2015, 7, 15481–15493. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-h. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Tolabi, H.; Bakhtiary, N.; Sayadi, S.; Tamaddon, M.; Ghorbani, F.; Boccaccini, A.R.; Liu, C. A critical review on polydopamine surface-modified scaffolds in musculoskeletal regeneration. Front. Bioeng. Biotechnol. 2022, 10, 1008360. [Google Scholar] [PubMed]
- Wang, Z.; Yang, H.-C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-inspired surface engineering for water-remediation materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymer 2010, 51, 3472–3485. [Google Scholar]
- Ge, J.; Gu, K.; Sun, K.; Wang, X.; Yao, S.; Mo, X.; Long, S.; Lan, T.; Qin, C. Preparation and swelling behaviors of high-strength hemicellulose-g-polydopamine composite hydrogels. Materials 2021, 14, 186. [Google Scholar] [PubMed]
- Subramani, R.; Izquierdo-Alvarez, A.; Bhattacharya, P.; Meerts, M.; Moldenaers, P.; Ramon, H.; Van Oosterwyck, H. The influence of swelling on elastic properties of polyacrylamide hydrogels. Front. Mater. 2020, 7, 212. [Google Scholar]
- Kacvinská, K.; Pavliňáková, V.; Poláček, P.; Michlovská, L.; Blahnová, V.H.; Filová, E.; Knoz, M.; Lipový, B.; Holoubek, J.; Faldyna, M. Accelular nanofibrous bilayer scaffold intrapenetrated with polydopamine network and implemented into a full-thickness wound of a white-pig model affects inflammation and healing process. J. Nanobiotechnology 2023, 21, 80. [Google Scholar]
- Gorgieva, S.; Kokol, V. Preparation, characterization, and in vitro enzymatic degradation of chitosan-gelatine hydrogel scaffolds as potential biomaterials. J. Biomed. Mater. Res. Part A 2012, 100, 1655–1667. [Google Scholar] [CrossRef]
- Ye, J.; Yang, G.; Zhang, J.; Xiao, Z.; He, L.; Zhang, H.; Liu, Q. Preparation and characterization of gelatin-polysaccharide composite hydrogels for tissue engineering. PeerJ 2021, 9, e11022. [Google Scholar] [CrossRef]
- Chen, X.; Yang, W.; Zhang, J.; Zhang, L.; Shen, H.; Shi, D. Alkalinity triggered the degradation of polydopamine nanoparticles. Polym. Bull. 2021, 78, 4439–4452. [Google Scholar] [CrossRef]
- Jin, L.; Yuan, F.; Chen, C.; Wu, J.; Gong, R.; Yuan, G.; Zeng, H.; Pei, J.; Chen, T. Degradation products of polydopamine restrained inflammatory response of LPS-stimulated macrophages through mediation TLR-4-MYD88 dependent signaling pathways by antioxidant. Inflammation 2019, 42, 658–671. [Google Scholar] [CrossRef]
- Alsberg, E.; Kong, H.; Hirano, Y.; Smith, M.; Albeiruti, A.; Mooney, D. Regulating bone formation via controlled scaffold degradation. J. Dent. Res. 2003, 82, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Dhote, V.; Skaalure, S.; Akalp, U.; Roberts, J.; Bryant, S.J.; Vernerey, F.J. On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage. J. Mech. Behav. Biomed. Mater. 2013, 19, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isopencu, G.; Deleanu, I.; Busuioc, C.; Oprea, O.; Surdu, V.-A.; Bacalum, M.; Stoica, R.; Stoica-Guzun, A. Bacterial Cellulose—Carboxymethylcellulose Composite Loaded with Turmeric Extract for Antimicrobial Wound Dressing Applications. Int. J. Mol. Sci. 2023, 24, 1719. [Google Scholar] [CrossRef]
- Arena, J.T.; McCloskey, B.; Freeman, B.D.; McCutcheon, J.R. Surface modification of thin film composite membrane support layers with polydopamine: Enabling use of reverse osmosis membranes in pressure retarded osmosis. J. Membr. Sci. 2011, 375, 55–62. [Google Scholar]
- Jiang, J.-H.; Zhu, L.-P.; Li, X.-L.; Xu, Y.-Y.; Zhu, B.-K. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J. Membr. Sci. 2010, 364, 194–202. [Google Scholar] [CrossRef]
- Matai, I.; Garg, M.; Rana, K.; Singh, S. Polydopamine functionalized hydrogel beads as magnetically separable antibacterial materials. RSC Adv. 2019, 9, 13444–13457. [Google Scholar] [CrossRef]
- Sanbhal, N.; Saitaer, X.; Peerzada, M.; Habboush, A.; Wang, F.; Wang, L. One-step surface functionalized hydrophilic polypropylene meshes for hernia repair using bio-inspired polydopamine. Fibers 2019, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Chen, C.; Hu, Y.; Wei, F.; Cui, J.; Zhao, Y.; Xu, X.; Chen, X.; Sun, D. Three-dimensional bacterial cellulose/polydopamine/TiO2 nanocomposite membrane with enhanced adsorption and photocatalytic degradation for dyes under ultraviolet-visible irradiation. J. Colloid Interface Sci. 2020, 562, 21–28. [Google Scholar] [CrossRef]
- Kang, D.; Shin, Y.E.; Jo, H.J.; Ko, H.; Shin, H.S. Mechanical Properties of Poly (dopamine)-Coated Graphene Oxide and Poly (vinyl alcohol) Composite Fibers Coated with Reduced Graphene Oxide and Their Use for Piezoresistive Sensing. Part. Part. Syst. Charact. 2017, 34, 1600382. [Google Scholar] [CrossRef] [Green Version]
- Luzak, B.; Siarkiewicz, P.; Boncler, M. An evaluation of a new high-sensitivity PrestoBlue assay for measuring cell viability and drug cytotoxicity using EA.hy926 endothelial cells. Toxicol. Vitr. 2022, 83, 105407. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Xie, C.; Liu, J.; Zheng, Y.; Wang, J.; Lu, X. Self-adhesive hydrogels for tissue engineering. J. Mater. Chem. B 2021, 9, 8739–8767. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.; Ribeiro, A.; Gemini-Piperni, S.; Silva, R.; Saraiva, A.; Leite, P.; Perez, G.; Oliveira, S.; Araujo, J.; Archanjo, B. TiO2 nanotubes enriched with calcium, phosphorous and zinc: Promising bio-selective functional surfaces for osseointegrated titanium implants. RSC Adv. 2017, 7, 49720–49738. [Google Scholar] [CrossRef] [Green Version]
- Holder, C.F.; Schaak, R.E. Tutorial on powder X-ray diffraction for characterizing nanoscale materials. ACS Nano 2019, 13, 7359–7365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [PubMed] [Green Version]
- González-Henríquez, C.; Sarabia-Vallejos, M. Electrospinning deposition of hydrogel fibers used as scaffold for biomembranes. Thermal stability of DPPC corroborated by ellipsometry. Chem. Phys. Lipids 2015, 190, 51–60. [Google Scholar] [CrossRef]
- Liu, Q.; Zuo, Q.; Guo, R.; Hong, A.; Li, C.; Zhang, Y.; He, L.; Xue, W. Fabrication and characterization of carboxymethyl chitosan/poly (vinyl alcohol) hydrogels containing alginate microspheres for protein delivery. J. Bioact. Compat. Polym. 2015, 30, 397–411. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. Degradation behavior of biopolymer-based membranes for skin tissue regeneration. Procedia Eng. 2013, 59, 285–291. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Zo, S.M.; Han, S.S. Novel biomimetic chitin-glucan polysaccharide nano/microfibrous fungal-scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2020, 149, 724–731. [Google Scholar] [CrossRef]
- Grønlien, K.G.; Pedersen, M.E.; Rønning, S.B.; Solberg, N.T.; Tønnesen, H.H. Tuning of 2D cultured human fibroblast behavior using lumichrome photocrosslinked collagen hydrogels. Mater. Today Commun. 2022, 31, 103635. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanan, K.B.; Bhaskar, R.; Sudhakar, K.; Nam, D.H.; Han, S.S. Polydopamine-Functionalized Bacterial Cellulose as Hydrogel Scaffolds for Skin Tissue Engineering. Gels 2023, 9, 656. https://doi.org/10.3390/gels9080656
Narayanan KB, Bhaskar R, Sudhakar K, Nam DH, Han SS. Polydopamine-Functionalized Bacterial Cellulose as Hydrogel Scaffolds for Skin Tissue Engineering. Gels. 2023; 9(8):656. https://doi.org/10.3390/gels9080656
Chicago/Turabian StyleNarayanan, Kannan Badri, Rakesh Bhaskar, Kuncham Sudhakar, Dong Hyun Nam, and Sung Soo Han. 2023. "Polydopamine-Functionalized Bacterial Cellulose as Hydrogel Scaffolds for Skin Tissue Engineering" Gels 9, no. 8: 656. https://doi.org/10.3390/gels9080656




