Highly Stretchable, Self-Healing, Injectable and pH Responsive Hydrogel from Multiple Hydrogen Bonding and Boron-Carbohydrate Interactions
Abstract
:1. Introduction
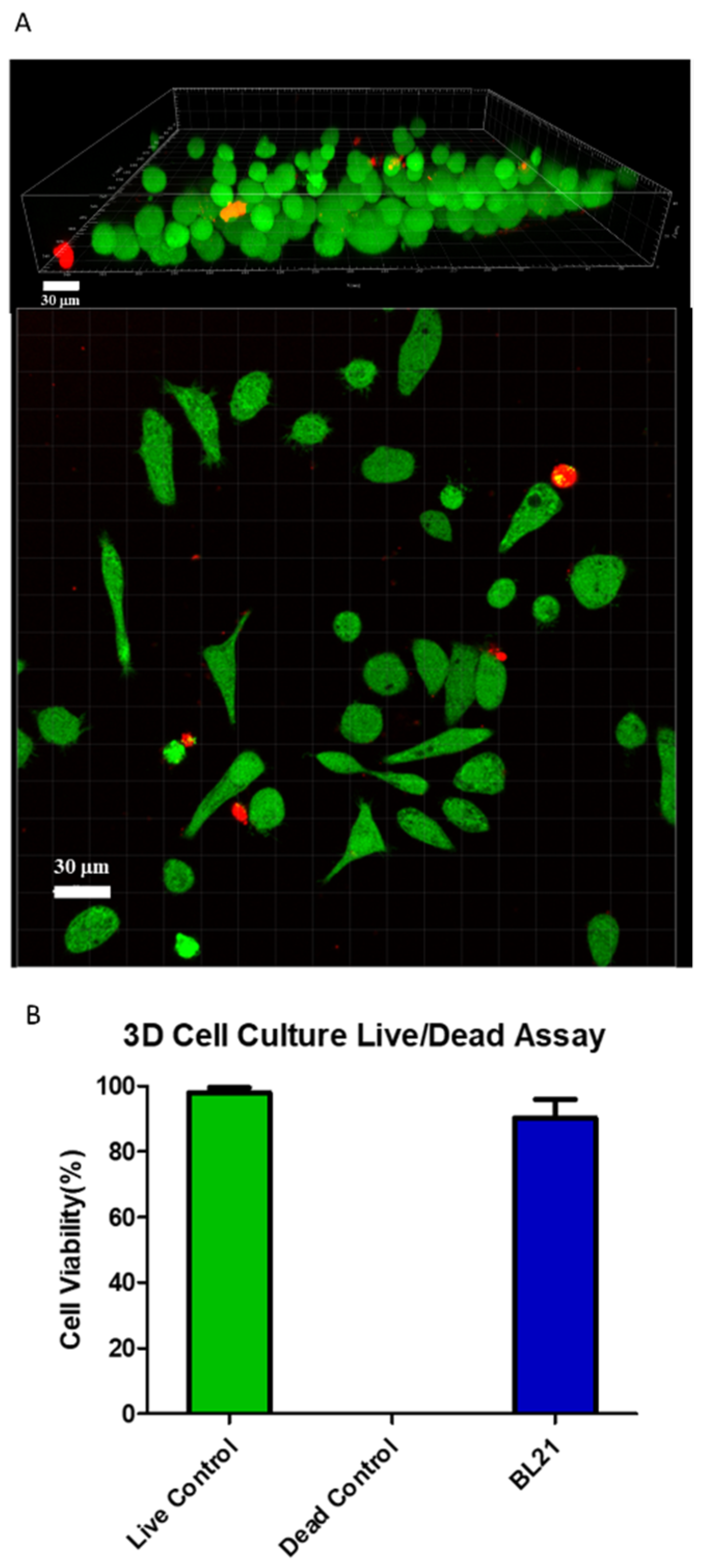
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Poly(LAEMA-st-DMA) (PLD) Preparation
4.3. Hydrogel Fabrication and Characterization
4.4. Characterization
4.4.1. The 1H Nuclear Magnetic Resonance (NMR)
4.4.2. Gel Permeation Chromatography (GPC)
4.4.3. Rheometer
4.5. Responsiveness of Hydrogel
4.6. Cell Culture
4.7. Cytotoxicity of PLD
4.8. The 3D Live/Dead Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-driven Biomedical Application Diversity. Signal Transduct. Target Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 604. [Google Scholar] [CrossRef]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Diaz-Prado, S.; Rey-Rico, A. Hydrogel-Based Localized Nonviral Gene Delivery in Regenerative Medicine Approaches-An Overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E. Hydrogels for Cell Delivery. Gels 2018, 4, 58. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y. Rational Design of Smart Hydrogels for Biomedical Applications. Front. Chem. 2021, 8, 615665. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in Drug Delivery: Progress and Challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.H.; Zhou, J.; Xu, F.; Zrinyi, M.; Dussault, P.H.; Osada, Y.; Chen, Y.M. Self-Healing Gels Based on Constitutional Dynamic Chemistry and Their Potential Applications. Chem. Soc. Rev. 2014, 43, 8114–8131. [Google Scholar] [CrossRef]
- Yang, X.; Guo, M.; Wu, Y.; Xue, S.; Xia, Y.; Zhang, R.; Wang, H.; Guo, Q. A Facile Approach for Polymer Hydrogels with Enhanced Strength, Self-Healing and Multi-responsive Shape Memory Properties. Mater. Res. Express 2019, 6, 125340. [Google Scholar] [CrossRef]
- Taylor, D.L.; In Het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Zeng, Y.; Wu, Y.; Wei, Y.; Tao, L. Self-healing hydrogel with a double dynamic network comprising imine and borate ester linkages. Chem. Mater. 2019, 31, 5576–5583. [Google Scholar] [CrossRef]
- Aeridou, E.; Diaz, D.D.; Aleman, C.; Perez-Madrigal, M. Advanced functional hydrogel biomaterials based on dynamic B-O bonds and polysaccharide building blocks. Biomacromolecules 2020, 21, 3984–3996. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Liu, G.; Wei, Y.; Wu, Y.; Tao, L. Antibacterial self-healing hydrogel via the Ugi reaction. ACS Appl. Polym. Mater. 2020, 2, 404–410. [Google Scholar] [CrossRef]
- He, X.; Zeng, Y.; Liu, G.; Tian, Y.; Wei, Y.; Zhao, L.; Yang, L.; Tao, L. Magnetic self-healing hydrogel from difunctional polymers prepared via the Kabachnik-Fields reaction. ACS Macro Lett. 2022, 11, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Fu, S.; Zhou, S.; Li, M.; Li, K.; Sun, W.; Zhai, Y. Advances in Hydrogels based on Dynamic Covalent Bonding and Prospects for its Biomedical Application. Eur. Polym. J. 2020, 139, 110024. [Google Scholar] [CrossRef]
- Picchioni, F.; Muljana, H. Hydrogels Based on Dynamic Covalent and Non Covalent Bonds: A Chemistry Perspective. Gels 2018, 4, 21. [Google Scholar] [CrossRef]
- Perera, M.M.; Ayres, N. Dynamic Covalent Bonds in Self-healing, Shape Memory, and Controllable Stiffness Hydrogels. Polym. Chem. 2020, 11, 1410–1423. [Google Scholar] [CrossRef]
- Parhi, R. Cross-linked Hydrogel for Pharmaceutical Applications: A Review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef]
- Lee, J.H. Injectable Hydrogels Delivering Therapeutic Agents for Disease Treatment and Tissue Engineering. Biomater. Res. 2018, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Y.; Chen, Q.; Fu, L.; Tao, L.; Wei, Y. Injectable and Self-Healing Chitosan Hydrogel Based on Imine Bonds: Design and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 2198. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, R.; Rana, N.K.; Koch, B. The Consequence of Imine Bond Origination: Fabrication of Rapid Self-Healing Chitosan Hydrogel as a Drug Delivery Candidate for Water-Soluble Drug. Eur. Polym. J. 2022, 180, 111605. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Yang, H.; Peng, K.; Zhang, X. Injectable Self-Healing Hydrogels Formed via Thiol/Disulfide Exchange of Thiol Functionalized F127 and Dithiolane Modified PEG. J. Mater. Chem. B 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Q.; Chen, M.; Guan, Y.; Zhang, Y. PHEMA Hydrogel Films Crosslinked with Dynamic Disulfide Bonds: Synthesis, Swelling-induced Mechanical Instability and Self-Healing. Polym. Chem. 2019, 10, 4844–4851. [Google Scholar] [CrossRef]
- Figueiredo, T.; Jing, J.; Jeacomine, I.; Olsson, J.; Gerfaud, T.; Boiteau, J.-G.; Rome, C.; Harris, C.; Auzély-Velty, R. Injectable Self-Healing Hydrogels Based on Boronate Ester Formation between Hyaluronic Acid Partners Modified with Benzoxaborin Derivatives and Saccharides. Biomacromolecules 2020, 21, 230–239. [Google Scholar] [CrossRef]
- An, H.; Bo, Y.; Chen, D.; Wang, Y.; Wang, H.; He, Y.; Qin, J. Cellulose-based Self-Healing Hydrogel Through Boronic Ester Bonds with Excellent Biocompatibility and Conductivity. RSC Adv. 2020, 10, 11300–11310. [Google Scholar] [CrossRef]
- Gosecki, M.; Gosecka, M. Boronic Acid Esters and Anhydrates as Dynamic Cross-Links in Vitrimers. Polymers 2022, 14, 842. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Cheng, Q.; Wang, W.; Wu, M.; Diaz-Dussan, D.; Kumar, P.; Narain, R. Multi-Responsive, Injectable, and Self-Healing Hydrogels based on Benzoxaborole-Tannic Acid Complexation. Polym. Chem. 2021, 12, 5623. [Google Scholar] [CrossRef]
- Macro Dufort, B.; Tibbitt, M.W. Design of moldable hydrogels for biomedical applications using dynamic covalent boronic esters. Mater. Today Chem. 2019, 12, 16–33. [Google Scholar] [CrossRef]
- Zhang, W.; Kuss, M.; Yan, Y.; Shi, W. Dynamic Alginate Hydrogel as an Antioxidative Bioink for Bioprinting. Gels 2023, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, W.; Wu, D.; Zeng, H.; Hall, D.G.; Narain, R. Multiresponsive and Self-Healing Hydrogel via Formation of Polymer-Nanogel Interfacial Dynamic Benzoxaborole Esters at Physiological pH. ACS Appl. Mater. Interfaces 2019, 11, 44742–44750. [Google Scholar] [CrossRef]
- Chirani, N.; Yahia, L.; Gritsch, L.; Motta, F.L.; Chirani, S.; Fare, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 13. [Google Scholar]
- Mellati, A.; Akhtari, J. Injectable Hydrogels: A Review of Injectability Mechanisms and Biomedical Applications. Res. Mol. Med. 2018, 4, 1–20. [Google Scholar] [CrossRef]
- Tu, Y.; Chen, N.; Li, C.; Liu, H.; Zhu, R.; Chen, S.; Xiao, Q.; Liu, J.; Ramakrishna, S.; He, L. Advances in Injectable Self-Healing Biomedical Hydrogels. Acta Biomater. 2019, 90, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, P.; Diba, M.; Mooney, D.J.; Leeuwenburgh, S.C.G. Self-Healing Injectable Hydrogels for Tissue Regeneration. Chem. Rev. 2023, 123, 834–873. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A Comprehensive Review on Cell-laden Hydrogels, Bioink Formulations, and Future Perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Clark, E.R.; Clark, E.L. Microscopic Observations on the Growth of Blood Capillaries in the Living Mammal. Am. J. Anat. 1939, 64, 251–301. [Google Scholar] [CrossRef]
- Chiu, L.L.; Montgomery, M.; Liang, Y.; Liu, H.; Radisic, M. Perfusable Branching MIcrovessel Bed for Vascularization of Engineered Tissues. Proc. Natl. Acad. Sci. USA 2012, 109, E3414–E3423. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional Human Vascular Network Generated in Photocrosslinkable Gelatin Methacrylate Hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef]
- Tseng, T.-C.; Hsieh, F.-Y.; Theato, P.; Wei, Y.; Hsu, S.-H. Glucose-Sensitive Self-Healing Hydrogel as Sacrificial Materials to Fabricate Vascularized Constructs. Biomaterials 2017, 133, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, W.; Diaz-Dussan, D.; Peng, Y.-Y.; Chen, Y.; Narain, R.; Hall, D.G. In Situ Forming, Dual-Crosslink Network, Self-Healing Hydrogel Enabled by a Bioorthogonal Nopodiol-Benzoxcaborolate Click Reaction with a Wide pH Range. Chem. Mater. 2019, 31, 4092–4102. [Google Scholar] [CrossRef]
- Tanpichai, S.; Phoothong, F.; Boonmahitthisud, A. Superabsorbent Cellulose-based Hydrogels Cross-liked with Borax. Sci. Rep. 2022, 12, 8920. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lei, T.; Wu, Q. Facile Preparation of Mouldable Polyvinyl Alcohol-Borax Hydrogels Reinforced by Well-Dispersed Cellulose Nanoparticles: Physical, Viscoelastic and Mechanical Properties. Cellulose 2013, 20, 2947–2958. [Google Scholar] [CrossRef]
- Han, J.; Lei, T.; Wu, Q. High-water-content Mouldable Polyvinyl Alcohol-borax Hydrogels Reinforced by Well-Dispersed Cellulose Nanoparticles: Dynamic Rheological Properties and Hydrogel Formation Mechanism. Carbohydr. Polym. 2014, 102, 306–316. [Google Scholar] [CrossRef]
- Sringam, J.; Trongsatitkul, T.; Suppakarn, N. Effects of Borax and Montmorillonite Contents on Mechanical Properties of Cassava Starch-based Composite Hydrogels. AIP Conf. Proc. 2020, 2279, 07005. [Google Scholar]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Borax Cross-linked Guar Gum Hydrogels as Potential Adsorbents for Water Purification. Carbohydr. Polym. 2017, 168, 274–281. [Google Scholar] [CrossRef]
- Itous, T.; Kitai, H.; Shimazu, A.; Miyazaki, T.; Tashiro, K. Clarification of Cross-linkage Structure in Boric Acid Doped Poly(vinyl alcohol) and its Model Compound as studied by an Organized Combination of X-ray Single-Crystal Structure Analysis, Raman Spectroscopy, and Density Functional Theoretical Calculation. J. Phys. Chem. N 2014, 118, 6032–6037. [Google Scholar] [CrossRef]
- Geng, S.; Haque, M.M.-U.; Oksman, K. Crosslinked Poly(vinyl acetate) (PVAc) Reinforced with Cellulose Nanocrystals (CNC): Structure and Mechanical Properties. Compos. Sci. Technol. 2016, 126, 35–42. [Google Scholar] [CrossRef]
- Lu, B.; Lin, G.; Jiang, X.; Cheng, J.; Lu, Q.; Song, J.; Chen, C.; Huang, B. One-Pot Assembly of Microfibrillated Cellulose Reinforced PVA-Borax Hydrogels with Self-Healing and pH-Responsive Properties. ACS Sustain. Chem. Eng. 2017, 5, 948–956. [Google Scholar] [CrossRef]
- Lin, H.L.; Yu, T.L.; Cheng, C.H. Reentrant Behaviour of Poly(vinyl alcohol)-borax Semidilute Aqueous Solutions. Colloid Polym. Sci. 2000, 278, 187–194. [Google Scholar] [CrossRef]
- Diaz-Dussan, D.; Peng, Y.-Y.; Rashed, F.B.; Macdonald, D.; Weinfeld, M.; Kumar, P.; Narain, R. Optimized Carbohydrate-based Nanogel Formulation to Sensitize Hypoxic Tumors. Mol. Pharm. 2023, 20, 3100–3114. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-Y.; Diaz-Dussan, D.; Kumar, P.; Narain, R. Acid Degradable Cationic Galactose-Based Hyperbranched Polymers as Nanotherapeutic Vehicles for Epidermal Growth Factor Receptor (EGFR) Knockdown in Cervical Carcinoma. Biomacromolecules 2018, 19, 4052–4058. [Google Scholar] [CrossRef]
- Peng, Y.-Y.; Diaz-Dussan, D.; Kumar, P.; Narain, R. Tumor Microenvironment-Regulated Redox Responsive Cationic Galactose-Based Hyperbranched Polymers for siRNA Delivery. Bioconjugate Chem. 2019, 30, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.-Y.; Hu, H.; Diaz-Dussan, D.; Zhao, J.; Hao, X.; Narain, R. Glycopolymer-Cell-Penetrating Peptide (CPP) Conjugates for Efficient Epidermal Growth Factor Receptor (EGFR) Silencing. ACS Macro Lett. 2022, 11, 580–587. [Google Scholar] [CrossRef]
- Bevington, J.C.; Harris, D.O. Reactivities of acrylate and methacrylates. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 799–802. [Google Scholar] [CrossRef]
- Yesilyurt, V.; Webber, M.; Appel, E.A.; Godwin, C.; Langer, R.; Anderson, D.G. Injectable Self-Healing Glucose-responsive Hydrogels with pH-Regulated Mechanical Properties. Adv. Mater. 2016, 28, 86–91. [Google Scholar] [CrossRef]
- Deng, G.; Li, F.; Yu, H.; Liu, F.; Liu, C.; Sun, W.; Jiang, H.; Chen, Y. Dynamic Hydrogels with an Environmental Adaptive Self-Healing Ability and Dual Responsive Sol-Gel Transitions. ACS Macro Lett. 2012, 1, 275–279. [Google Scholar] [CrossRef]
- Deng, Z.; Li, S.; Jiang, X.; Narain, R. Well Defined Galactose-Containing Multi-Functional Copolymers and Glyconanopaticles for Biomolecular Recognition Processes. Biomacromolecules 2009, 42, 6393–6405. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Chen, Y.; Zhao, Y.; Zhang, Q.; Zheng, X.; Chen, L.; Zhang, L. Strong and Rapidly Self-Healing Hydrogels: Potential Hemostatic Materials. Adv. Healthc. Mater. 2016, 5, 2813–2822. [Google Scholar] [CrossRef]

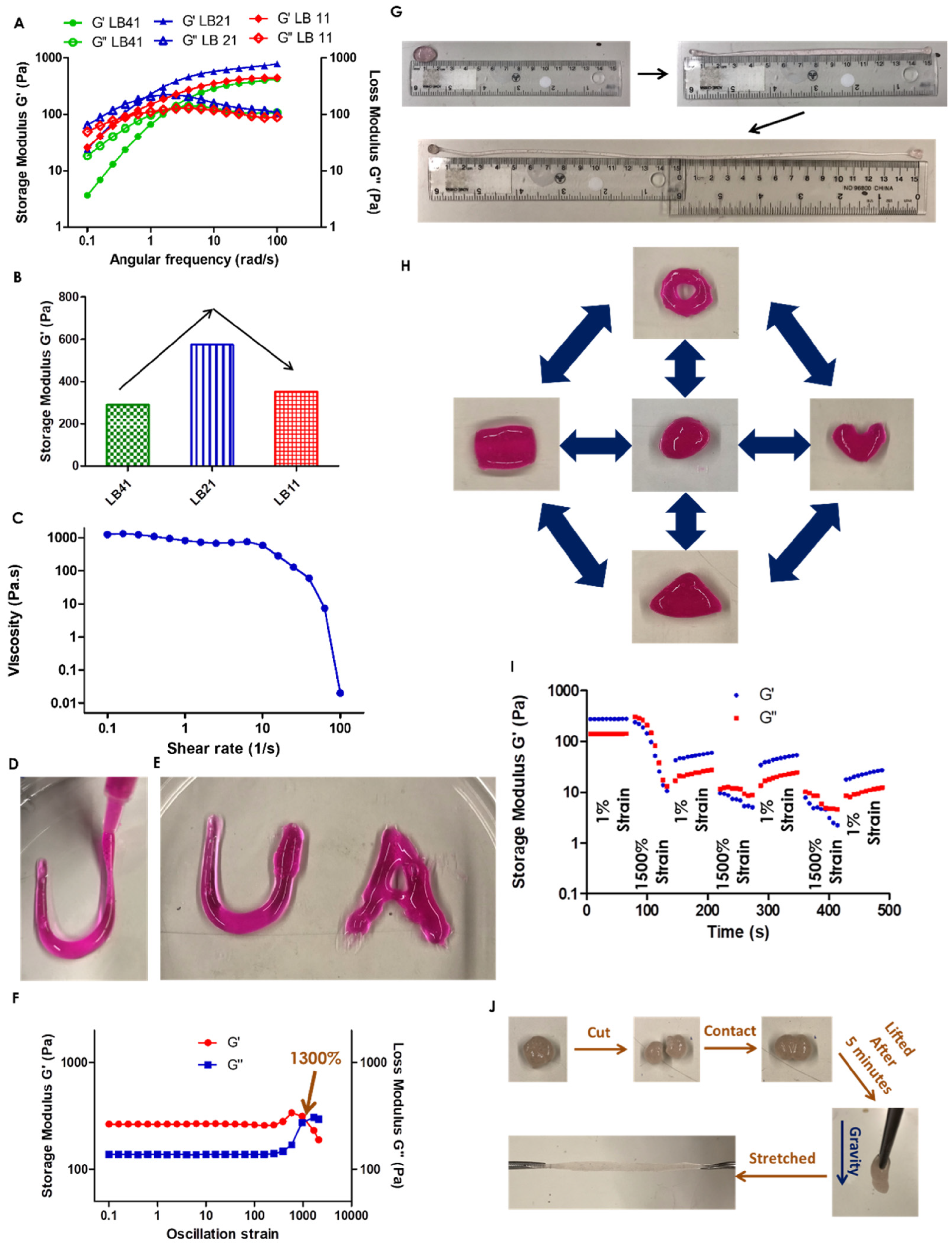

| Hydrogel | 10 wt% PLD (mL) | 10 wt% Borax (μL) |
|---|---|---|
| LB 41 | 0.3 | 2.63 |
| LB 21 | 0.3 | 5.25 |
| LB 11 | 0.3 | 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.-Y.; Cheng, Q.; Wu, M.; Wang, W.; Zhao, J.; Diaz-Dussan, D.; McKay, M.; Zeng, H.; Ummartyotin, S.; Narain, R. Highly Stretchable, Self-Healing, Injectable and pH Responsive Hydrogel from Multiple Hydrogen Bonding and Boron-Carbohydrate Interactions. Gels 2023, 9, 709. https://doi.org/10.3390/gels9090709
Peng Y-Y, Cheng Q, Wu M, Wang W, Zhao J, Diaz-Dussan D, McKay M, Zeng H, Ummartyotin S, Narain R. Highly Stretchable, Self-Healing, Injectable and pH Responsive Hydrogel from Multiple Hydrogen Bonding and Boron-Carbohydrate Interactions. Gels. 2023; 9(9):709. https://doi.org/10.3390/gels9090709
Chicago/Turabian StylePeng, Yi-Yang, Qiuli Cheng, Meng Wu, Wenda Wang, Jianyang Zhao, Diana Diaz-Dussan, Michelle McKay, Hongbo Zeng, Sarute Ummartyotin, and Ravin Narain. 2023. "Highly Stretchable, Self-Healing, Injectable and pH Responsive Hydrogel from Multiple Hydrogen Bonding and Boron-Carbohydrate Interactions" Gels 9, no. 9: 709. https://doi.org/10.3390/gels9090709





