From Free Tissue Transfer to Hydrogels: A Brief Review of the Application of the Periosteum in Bone Regeneration
Abstract
:1. Introduction
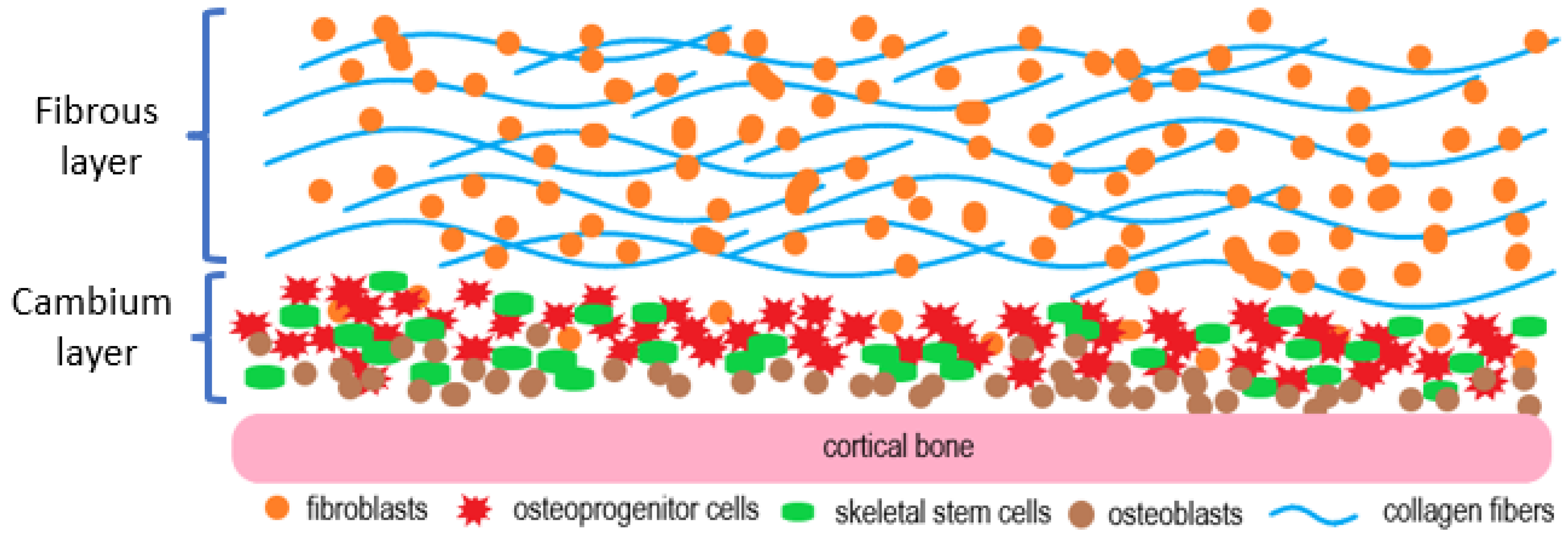
2. The Histology of the Periosteum
3. Early, Contentious Studies of the Periosteum
4. Periosteum-Induced Intramembranous and Endochondral Ossification
5. The Periosteum Contains Skeletal Stem Cells That Undergo Chondrogenic and Osteogenic Differentiation in Response to BMP-2
6. The Procurement of the Cambium Layer
7. Periosteal Cell Isolation, Expansion, and Characterization
8. In Vivo and Ex Vivo Periosteal Bioreactor Systems
9. Hydrogel-Based Artificial Periostea for Bone Regeneration
10. Summary and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Maruf, D.S.A.; Ghosh, Y.A.; Xin, H.; Cheng, K.; Mukherjee, P.; Crook, J.M.; Wallace, G.G.; Klein, T.J.; Clark, J.R. Hydrogel: A Potential Material for Bone Tissue Engineering Repairing the Segmental Mandibular Defect. Polymers 2022, 14, 4186. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Al Maruf, D.S.A.; Parthasarathi, K.; Cheng, K.; Mukherjee, P.; McKenzie, D.R.; Crook, J.M.; Wallace, G.G.; Clark, J.R. Current and future perspectives on biomaterials for segmental mandibular defect repair. Int. J. Polym. Mater. Polym. Biomater. 2023, 72, 725–737. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.L.; Chen, X. 3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies. Front. Bioeng. Biotechnol. 2022, 10, 824156. [Google Scholar] [CrossRef] [PubMed]
- Lo Sicco, C.; Tasso, R.; Reverberi, D.; Cilli, M.; Pfeffer, U.; Cancedda, R. Identification of a New Cell Population Constitutively Circulating in Healthy Conditions and Endowed with a Homing Ability toward Injured Sites. Sci. Rep. 2015, 5, 16574. [Google Scholar] [CrossRef] [PubMed]
- Lo Sicco, C.; Tasso, R. Harnessing Endogenous Cellular Mechanisms for Bone Repair. Front. Bioeng. Biotechnol. 2017, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Accorona, R.; Gazzini, L.; Grigolato, R.; Fazio, E.; Nitro, L.; Abousiam, M.; Giorgetti, G.; Pignataro, L.; Capaccio, P.; Calabrese, L. Free Periosteal Flaps with Scaffold: An Overlooked Armamentarium for Maxillary and Mandibular Reconstruction. Cancers 2021, 13, 4373. [Google Scholar] [CrossRef]
- Kelley, P.; Klebuc, M.; Hollier, L. Complex midface reconstruction: Maximizing contour and bone graft survival utilizing periosteal free flaps. J. Craniofac. Surg. 2003, 14, 779–782. [Google Scholar] [CrossRef]
- Hurrell, M.J.L.; Low, T.H.; Ch’ng, S.; Clark, J.R. Fascio-cutaneous and fascio-periosteal free flaps for treatment of intermediate stage osteoradionecrosis of the jaws. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 136, 128–135. [Google Scholar] [CrossRef]
- Knothe, U.R.; Springfield, D.S. A novel surgical procedure for bridging of massive bone defects. World J. Surg. Oncol. 2005, 3, 7. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Ma, J.; Pan, J. Spontaneous regeneration of bone after removal of a vascularised fibular bone graft from a mandibular segmental defect: A case report. Br. J. Oral Maxillofac. Surg. 2015, 53, 650–651. [Google Scholar] [CrossRef]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and Applications in Craniofacial Bone Regeneration. J. Dent. Res. 2013, 93, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Sittinger, M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003, 9, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Romanazzo, S.; Tomaskovic-Crook, E.; Mitchell, T.C.; Hung, J.C.; Wise, S.G.; Cheng, K.; Al Maruf, D.S.A.; Stokan, M.J.; Manzie, T.G.H.; et al. Ex Vivo Preservation of Ovine Periosteum Using a Perfusion Bioreactor System. Cells 2023, 12, 1724. [Google Scholar] [CrossRef]
- Li, N.; Song, J.; Zhu, G.; Li, X.; Liu, L.; Shi, X.; Wang, Y. Periosteum tissue engineering—A review. Biomater. Sci. 2016, 4, 1554–1561. [Google Scholar] [CrossRef]
- He, X.; Li, W.; Liu, K.; Wen, W.; Lu, L.; Liu, M.; Zhou, C.; Luo, B. Anisotropic and robust hydrogels combined osteogenic and angiogenic activity as artificial periosteum. Compos. Part B Eng. 2022, 233, 109627. [Google Scholar] [CrossRef]
- Dwek, J.R. The periosteum: What is it, where is it, and what mimics it in its absence? Skelet. Radiol. 2010, 39, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, N.; Yang, M.; Sun, T.; Zhang, J.; Zhao, Y.; Huo, N.; Li, Z. Periosteum and development of the tissue-engineered periosteum for guided bone regeneration. J. Orthop. Transl. 2022, 33, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Schell, H.; Duda, G.N.; Peters, A.; Tsitsilonis, S.; Johnson, K.A.; Schmidt-Bleek, K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017, 4, 5. [Google Scholar] [CrossRef]
- McBride, S.H.; Evans, S.F.; Knothe Tate, M.L. Anisotropic mechanical properties of ovine femoral periosteum and the effects of cryopreservation. J. Biomech. 2011, 44, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, X.; Bikle, D.D. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J. Cell Physiol. 2017, 232, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.M.; Van Sickle, D.C.; Kunishima, D.H.; Jackson, D.W. Cambium cell stimulation from surgical release of the periosteum. J. Orthop. Res. 2003, 21, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, T.; Kusuhara, H.; Ueda, Y.; Morotomi, T.; Isogai, N. Influence of Periosteum Location on the Bone and Cartilage in Tissue-Engineered Phalanx. J. Hand Surg. Am. 2020, 45, 62.e1–62.e10. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.R.; Milz, S.; Knothe Tate, M.L. Periosteal thickness and cellularity in mid-diaphyseal cross-sections from human femora and tibiae of aged donors. J. Anat. 2014, 224, 142–149. [Google Scholar] [CrossRef]
- Burman, M.; UMANSKY, M. An experimental study of free periosteal transplants, wrapped around tendon: With a review of the literature. J. Bone Jt. Surg. JBJS 1930, 12, 579–594. [Google Scholar]
- Ritsilä, V.; Alhopuro, S.; Rintala, A. Bone formation with free periosteum: An experimental study. Scand. J. Plast. Reconstr. Surg. 1972, 6, 51–56. [Google Scholar]
- EYRE-BROOK, A.L. The periosteum: Its function reassessed. Clin. Orthop. Relat. Res. (1976–2007) 1984, 189, 300–307. [Google Scholar] [CrossRef]
- Kolodny, A. The periosteal blood supply and healing of fractures: Experimental study. J. Bone Jt. Surg. JBJS 1923, 5, 698–711. [Google Scholar]
- URIST, M.R.; McLEAN, F.C. Osteogenetic potency and new-bone formation by induction in transplants to the anterior chamber of the eye. J. Bone Jt. Surg. JBJS 1952, 34, 443–475. [Google Scholar] [CrossRef]
- Colnot, C.; Zhang, X.; Tate, M.L.K. Current insights on the regenerative potential of the periosteum: Molecular, cellular, and endogenous engineering approaches. J. Orthop. Res. 2012, 30, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A. Periostin in bone biology. Periostin 2019, 1132, 43–47. [Google Scholar]
- Li, Z.; Pan, J.; Ma, J.; Zhang, Z.; Bai, Y. Microarray gene expression of periosteum in spontaneous bone regeneration of mandibular segmental defects. Sci. Rep. 2017, 7, 13535. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, C.; Xue, M.; Zhang, X. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone 2011, 48, 524–532. [Google Scholar] [CrossRef]
- Mi, M.; Jin, H.; Wang, B.; Yukata, K.; Sheu, T.-j.; Ke, Q.H.; Tong, P.; Im, H.-J.; Xiao, G.; Chen, D. Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene 2013, 512, 211–218. [Google Scholar] [CrossRef]
- Poussa, M.; Ritsilä, V. The osteogenic capacity of free periosteal and osteoperiosteal grafts. A comparative study in growing rabbits. Acta Orthop. Scand. 1979, 50, 491–499. [Google Scholar] [CrossRef]
- Brownlow, H.C.; Reed, A.; Joyner, C.; Simpson, A.H. Anatomical effects of periosteal elevation. J. Orthop. Res. 2000, 18, 500–502. [Google Scholar] [CrossRef]
- Patro, B.P.; Rath, M.; Mohapatra, D.; Kumar Patra, S.; Chandra Sahu, M.; Das, G.; Sahoo, J. Traumatized periosteum: Its histology, viability, and clinical significance. Orthop. Rev. 2022, 14, 30044. [Google Scholar] [CrossRef]
- Chang, H.; Knothe Tate, M.L. Concise review: The periosteum: Tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl. Med. 2012, 1, 480–491. [Google Scholar] [CrossRef] [PubMed]
- O’Driscoll, S.W.; Recklies, A.D.; Poole, A.R. Chondrogenesis in periosteal explants. An organ culture model for in vitro study. J. Bone Jt. Surg. Am. 1994, 76, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- O’driscoll, S.W.; Meisami, B.; Miura, Y.; Fitzsimmons, J.S. Viability of periosteal tissue obtained postmortem. Cell Transplant. 1999, 8, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.V.; Suaid, F.F.; Ruiz, K.G.; Salmon, C.R.; Paparotto, T.; Nociti Jr, F.H.; Sallum, E.A.; Casati, M.Z. Periosteum-derived cells as an alternative to bone marrow cells for bone tissue engineering around dental implants. A histomorphometric study in beagle dogs. J. Periodontol. 2010, 81, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Srisa-Art, M.; Bonzani, I.C.; Williams, A.; Stevens, M.M.; Demello, A.J.; Edel, J.B. Identification of rare progenitor cells from human periosteal tissue using droplet microfluidics. Analyst 2009, 134, 2239–2245. [Google Scholar] [CrossRef]
- Samee, M.; Kasugai, S.; Kondo, H.; Ohya, K.; Shimokawa, H.; Kuroda, S. Bone morphogenetic protein-2 (BMP-2) and vascular endothelial growth factor (VEGF) transfection to human periosteal cells enhances osteoblast differentiation and bone formation. J. Pharmacol. Sci. 2008, 108, 18–31. [Google Scholar] [CrossRef]
- Stich, S.; Loch, A.; Leinhase, I.; Neumann, K.; Kaps, C.; Sittinger, M.; Ringe, J. Human periosteum-derived progenitor cells express distinct chemokine receptors and migrate upon stimulation with CCL2, CCL25, CXCL8, CXCL12, and CXCL13. Eur. J. Cell Biol. 2008, 87, 365–376. [Google Scholar] [CrossRef]
- Ogita, M.; Rached, M.T.; Dworakowski, E.; Bilezikian, J.P.; Kousteni, S. Differentiation and proliferation of periosteal osteoblast progenitors are differentially regulated by estrogens and intermittent parathyroid hormone administration. Endocrinology 2008, 149, 5713–5723. [Google Scholar] [CrossRef]
- Roberts, S.J.; Chen, Y.; Moesen, M.; Schrooten, J.; Luyten, F.P. Enhancement of osteogenic gene expression for the differentiation of human periosteal derived cells. Stem Cell Res. 2011, 7, 137–144. [Google Scholar] [CrossRef]
- Demol, J.; Eyckmans, J.; Roberts, S.; Luyten, F.; Van Oosterwyck, H. Does tranexamic acid stabilised fibrin support the osteogenic differentiation of human periosteum derived cells. Eur. Cells Mater. 2011, 21, 272–285. [Google Scholar] [CrossRef]
- Eyckmans, J.; Luyten, F.P. Species specificity of ectopic bone formation using periosteum-derived mesenchymal progenitor cells. Tissue Eng. 2006, 12, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.; Schäfer, F.; Munz, A.; Friedrich, B.; Klein, C.; Hoffmann, J.; Bühring, H.-J.; Reinert, S. LNGFR induction during osteogenesis of human jaw periosteum-derived cells. Cell. Physiol. Biochem. 2009, 24, 283–290. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2001, 44, 85–95. [Google Scholar] [CrossRef]
- Hayashi, O.; Katsube, Y.; Hirose, M.; Ohgushi, H.; Ito, H. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif. Tissue Int. 2008, 82, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Solchaga, L.A.; Cassiede, P.; Caplan, A.I. Different response to osteo-inductive agents in bone marrow-and periosteum-derived cell preparations. Acta Orthop. Scand. 1998, 69, 426–432. [Google Scholar] [CrossRef]
- Kubo, K.; Att, W.; Yamada, M.; Ohmi, K.; Tsukimura, N.; Suzuki, T.; Maeda, H.; Ogawa, T. Microtopography of titanium suppresses osteoblastic differentiation but enhances chondroblastic differentiation of rat femoral periosteum-derived cells. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 87, 380–391. [Google Scholar] [CrossRef]
- Izumi, T.; Scully, S.P.; Heydemann, A.; Bolander, M.E. Transforming growth factor β1 stimulates type II collagen expression in cultured periosteum-derived cells. J. Bone Miner. Res. 1992, 7, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ballock, R.T.; Heydemann, A.; Izumi, T.; Reddi, A.H. Regulation of the expression of the type-II collagen gene in periosteum-derived cells by three members of the transforming growth factor–β superfamily. J. Orthop. Res. 1997, 15, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Gelse, K.; Frank, S.; von der Mark, K.; Aigner, T.; Schneider, H. Transgene-activated mesenchymal cells for articular cartilage repair: A comparison of primary bone marrow-, perichondrium/periosteum-and fat-derived cells. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2006, 8, 112–125. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Halfon, S.; Abramov, N.; Grinblat, B.; Ginis, I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011, 20, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.O.; Yue, R.; Murphy, M.M.; Peyer, J.G.; Morrison, S.J. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014, 15, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hu, L.; Cao, Z.; Liu, X.; Pan, J. Periosteal skeletal stem cells and their response to bone injury. Front. Cell Dev. Biol. 2022, 10, 812094. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Wang, L.; Li, T.; Miranda, M.; Spagnoli, A. Role of Prx1-expressing skeletal cells and Prx1-expression in fracture repair. Bone 2020, 139, 115521. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.E.; Hoffman, S.; Kern, M.J. Opposing roles of two isoforms of the Prx1 homeobox gene in chondrogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2005, 233, 811–821. [Google Scholar] [CrossRef]
- Gao, B.; Deng, R.; Chai, Y.; Chen, H.; Hu, B.; Wang, X.; Zhu, S.; Cao, Y.; Ni, S.; Wan, M. Macrophage-lineage TRAP+ cells recruit periosteum-derived cells for periosteal osteogenesis and regeneration. J. Clin. Investig. 2019, 129, 2578–2594. [Google Scholar] [CrossRef]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Holt, G.E.; Halpern, J.L.; Dovan, T.T.; Hamming, D.; Schwartz, H.S. Evolution of an in vivo bioreactor. J. Orthop. Res. 2005, 23, 916–923. [Google Scholar] [CrossRef]
- Van Gastel, N.; Torrekens, S.; Roberts, S.J.; Moermans, K.; Schrooten, J.; Carmeliet, P.; Luttun, A.; Luyten, F.P.; Carmeliet, G. Engineering vascularized bone: Osteogenic and proangiogenic potential of murine periosteal cells. Stem Cells 2012, 30, 2460–2471. [Google Scholar] [CrossRef]
- Tatara, A.M.; Shah, S.R.; Demian, N.; Ho, T.; Shum, J.; van den Beucken, J.J.J.P.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. Reconstruction of large mandibular defects using autologous tissues generated from in vivo bioreactors. Acta Biomater. 2016, 45, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-L.; Tremp, M.; Ho, C.-K.; Sun, Y.; Liu, K.; Li, Q. Prefabrication of a functional bone graft with a pedicled periosteal flap as an in vivo bioreactor. Sci. Rep. 2017, 7, 18038. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, Y.A.; Gupta, R.; Al Maruf, D.A.; Cheng, K.; Mukherjee, P.; Clark, J.R. Surgical technique: A novel pedicled periosteal scapular flap to facilitate bone growth in an Ovine model. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 1497–1520. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Marolt, D.; Bhumiratana, S.; Fröhlich, M.; Guo, X.E.; Vunjak-Novakovic, G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol. Bioeng. 2011, 108, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Dua, R.; Jones, H.; Noble, P.C. Designing and validation of an automated ex-vivo bioreactor system for long term culture of bone. Bone Rep. 2021, 14, 101074. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, S.H.; Porter, B.D.; García, A.J.; Guldberg, R.E. Effects of Medium Perfusion Rate on Cell-Seeded Three-Dimensional Bone Constructs In Vitro. Tissue Eng. 2003, 9, 1197–1203. [Google Scholar] [CrossRef]
- Won, N.; Castillo-Prado, J.; Tan, X.; Ford, J.; Heath, D.; Mazilescu, L.I.; Selzner, M.; Rogers, I.M. Ex Vivo Perfusion Using a Mathematical Modeled, Controlled Gas Exchange Self-Contained Bioreactor Can Maintain a Mouse Kidney for Seven Days. Cells 2022, 11, 1822. [Google Scholar] [CrossRef]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Gao, Y.; Yang, L. Cell sheet technology: A promising strategy in regenerative medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef]
- Okuda, K.; Kawase, T.; Nagata, M.; Yamamiya, K.; Nakata, K.; Wolff, L.F.; Yoshie, H. Tissue-engineered cultured periosteum sheet application to treat infrabony defects: Case series and 5-year results. Int. J. Periodontics Restor. Dent. 2013, 33, 281–287. [Google Scholar] [CrossRef]
- Fu, T.-S.; Wang, Y.-C.; Chen, C.-H.; Chang, C.-W.; Lin, T.-Y.; Wong, C.-B.; Chen, D.W.-C.; Su, C.-Y. Engineered periosteum-bone biomimetic bone graft enhances posterolateral spine fusion in a rabbit model. Spine J. 2019, 19, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Spizzirri, U.G.; Curcio, M.; Nicoletta, F.P.; Iemma, F. Injectable Hydrogels for Cancer Therapy over the Last Decade. Pharmaceutics 2019, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhao, X.; Ma, P.X.; Guo, B.; Du, Y.; Han, X. pH-responsive injectable hydrogels with mucosal adhesiveness based on chitosan-grafted-dihydrocaffeic acid and oxidized pullulan for localized drug delivery. J. Colloid Interface Sci. 2019, 536, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.T.; Chen, C.-H.; Chen, J.-P. Intratumoral delivery of doxorubicin on folate-conjugated graphene oxide by in-situ forming thermo-sensitive hydrogel for breast cancer therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Song, L.; Chen, L.; Zhang, W.; Chen, Y.; Zang, F.; Chen, H.; Ma, M.; Gu, N.; Zhang, Y. Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents post-operative recurrence in a breast cancer model. Acta Biomater. 2018, 74, 302–311. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-p.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Xin, H.; Naficy, S. Drug Delivery Based on Stimuli-Responsive Injectable Hydrogels for Breast Cancer Therapy: A Review. Gels 2022, 8, 45. [Google Scholar] [CrossRef]
- Cimen, Z.; Babadag, S.; Odabas, S.; Altuntas, S.; Demirel, G.; Demirel, G.B. Injectable and Self-Healable pH-Responsive Gelatin–PEG/Laponite Hybrid Hydrogels as Long-Acting Implants for Local Cancer Treatment. ACS Appl. Polym. Mater. 2021, 3, 3504–3518. [Google Scholar] [CrossRef]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing: 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4034. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Q.; Xu, S.; Zheng, Q.; Cao, X. Preparation and Properties of 3D Printed Alginate–Chitosan Polyion Complex Hydrogels for Tissue Engineering. Polymers 2018, 10, 664. [Google Scholar] [CrossRef]
- Park, N.; Kim, J. Hydrogel-based artificial muscles: Overview and recent progress. Adv. Intell. Syst. 2020, 2, 1900135. [Google Scholar] [CrossRef]
- Oveissi, F.; Fletcher, D.F.; Dehghani, F.; Naficy, S. Tough hydrogels for soft artificial muscles. Mater. Des. 2021, 203, 109609. [Google Scholar] [CrossRef]
- Dou, C.; Li, Z.; Luo, Y.; Gong, J.; Li, Q.; Zhang, J.; Zhang, Q.; Qiao, C. Bio-based poly (γ-glutamic acid)-gelatin double-network hydrogel with high strength for wound healing. Int. J. Biol. Macromol. 2022, 202, 438–452. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Z.; Liu, F.; Zhao, L.; Ling, Q.; Gu, H. Multifunctional Self-Healing Dual Network Hydrogels Constructed via Host-Guest Interaction and Dynamic Covalent Bond as Wearable Strain Sensors for Monitoring Human and Organ Motions. ACS Appl. Mater. Interfaces 2021, 13, 14612–14622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xue, W.; Dai, Y.; Wu, L.; Liao, B.; Zeng, W.; Tao, X. Double network hydrogel sensors with high sensitivity in large strain range. Macromol. Mater. Eng. 2021, 306, 2100486. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.; You, J.; Tomaskovic-Crook, E.; Yue, Z.; Talaei, A.; Sutton, G.; Crook, J.; Wallace, G. Electro-compacted collagen for corneal epithelial tissue engineering. J. Biomed. Mater. Res. A 2023, 111, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Le, P.; Fernandes-Cunha, G.M.; Heilshorn, S.C.; Myung, D. Bio-orthogonally crosslinked hyaluronate-collagen hydrogel for suture-free corneal defect repair. Biomaterials 2020, 255, 120176. [Google Scholar] [CrossRef]
- Means, A.K.; Shrode, C.S.; Whitney, L.V.; Ehrhardt, D.A.; Grunlan, M.A. Double network hydrogels that mimic the modulus, strength, and lubricity of cartilage. Biomacromolecules 2019, 20, 2034–2042. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, J.; Koshut, W.J.; Watt, J.; Riboh, J.C.; Gall, K.; Wiley, B.J. A synthetic hydrogel composite with the mechanical behavior and durability of cartilage. Adv. Funct. Mater. 2020, 30, 2003451. [Google Scholar] [CrossRef]
- Jeong, C.H.; Yune, J.H.; Kwon, H.C.; Shin, D.-M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; Kim, E.K. In vitro toxicity assessment of crosslinking agents used in hyaluronic acid dermal filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef]
- Takigawa, T.; Endo, Y. Effects of glutaraldehyde exposure on human health. J. Occup. Health 2006, 48, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.K.; White, J.F.; Glattauer, V.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Variation in Hydrogel Formation and Network Structure for Telo-, Atelo- and Methacrylated Collagens. Polymers 2022, 14, 1775. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-based biomaterials for bone tissue engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Y.; Tian, Y.; Yang, Y.; Wang, J.; Guo, H.; Banitaba, S.N.; Khademolqorani, S.; Li, J.A. The Collagen-Based Scaffolds for Bone Regeneration: A Journey through Electrospun Composites Integrated with Organic and Inorganic Additives. Processes 2023, 11, 2105. [Google Scholar] [CrossRef]
- Dinescu, S.; Albu Kaya, M.; Chitoiu, L.; Ignat, S.; Kaya, D.A.; Costache, M. Collagen-Based Hydrogels and Their Applications for Tissue Engineering and Regenerative Medicine. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1643–1664. [Google Scholar]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Minamide, A.; Yoshida, M.; Kawakami, M.; Yamasaki, S.; Kojima, H.; Hashizume, H.; Boden, S.D. The Use of Cultured Bone Marrow Cells in Type I Collagen Gel and Porous Hydroxyapatite for Posterolateral Lumbar Spine Fusion. Spine 2005, 30, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, G.; Molino, G.; Fiorilli, S.; Vitale-Brovarone, C. Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: A hybrid formulation for 3D printing of bone scaffolds. J. Eur. Ceram. Soc. 2020, 40, 3689–3697. [Google Scholar] [CrossRef]
- Wang, L.; Stegemann, J.P. Glyoxal crosslinking of cell-seeded chitosan/collagen hydrogels for bone regeneration. Acta Biomater. 2011, 7, 2410–2417. [Google Scholar] [CrossRef]
- Jin, G.Z.; Kim, H.W. Efficacy of collagen and alginate hydrogels for the prevention of rat chondrocyte dedifferentiation. J. Tissue Eng. 2018, 9, 2041731418802438. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Li, M.; Song, P.; Lei, H.; Gui, X.; Zhou, C.; Liu, L. Biomimetic Methacrylated Gelatin Hydrogel Loaded With Bone Marrow Mesenchymal Stem Cells for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770049. [Google Scholar] [CrossRef]
- Noshadi, I.; Hong, S.; Sullivan, K.E.; Shirzaei Sani, E.; Portillo-Lara, R.; Tamayol, A.; Shin, S.R.; Gao, A.E.; Stoppel, W.L.; Black, L.D., III; et al. In vitro and in vivo analysis of visible light crosslinkable gelatin methacryloyl (GelMA) hydrogels. Biomater. Sci. 2017, 5, 2093–2105. [Google Scholar] [CrossRef] [PubMed]
- Goto, R.; Nishida, E.; Kobayashi, S.; Aino, M.; Ohno, T.; Iwamura, Y.; Kikuchi, T.; Hayashi, J.I.; Yamamoto, G.; Asakura, M.; et al. Gelatin Methacryloyl-Riboflavin (GelMA-RF) Hydrogels for Bone Regeneration. Int. J. Mol. Sci. 2021, 22, 1635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Liang, Z.; Lan, W.; Wei, Y.; Hu, Y.; Wang, L.; Lei, Q.; Huang, D. Injectable antibacterial Ag-HA/ GelMA hydrogel for bone tissue engineering. Front. Bioeng. Biotechnol. 2023, 11, 1219460. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, Y.; Chen, F.; Liu, J.; Wang, T.; Luo, Y.; Jia, S.; Wang, P.; Tan, S.; Lu, B.; et al. The hydroxyapatite microtubes enhanced GelMA hydrogel scaffold with inner “pipeline framework” structure for bone tissue regeneration. Compos. Part B Eng. 2022, 228, 109396. [Google Scholar] [CrossRef]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Allen, N.B.; Abar, B.; Johnson, L.; Burbano, J.; Danilkowicz, R.M.; Adams, S.B. 3D-bioprinted GelMA-gelatin-hydroxyapatite osteoblast-laden composite hydrogels for bone tissue engineering. Bioprinting 2022, 26, e00196. [Google Scholar] [CrossRef]
- Tavares, M.T.; Gaspar, V.M.; Monteiro, M.V.; Farinha, J.P.S.; Baleizão, C.; Mano, J.F. GelMA/bioactive silica nanocomposite bioinks for stem cell osteogenic differentiation. Biofabrication 2021, 13, 035012. [Google Scholar] [CrossRef]
- Gao, J.; Li, M.; Cheng, J.; Liu, X.; Liu, Z.; Liu, J.; Tang, P. 3D-Printed GelMA/PEGDA/F127DA Scaffolds for Bone Regeneration. J. Funct. Biomater. 2023, 14, 96. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Benoit, D.S. Emerging ideas: Engineering the periosteum: Revitalizing allografts by mimicking autograft healing. Clin. Orthop. Relat. Res. 2013, 471, 721–726. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Xie, C.; Zhang, X.; Benoit, D.S. The effect of mesenchymal stem cells delivered via hydrogel-based tissue engineered periosteum on bone allograft healing. Biomaterials 2013, 34, 8887–8898. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Benoit, D.S. Emulating native periosteum cell population and subsequent paracrine factor production to promote tissue engineered periosteum-mediated allograft healing. Biomaterials 2015, 52, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bi, W.; Sun, Y.; Wang, L.; Yu, X.; Cheng, R.; Yu, Y.; Cui, W. Biomimetic organic-inorganic hybrid hydrogel electrospinning periosteum for accelerating bone regeneration. Mater. Sci. Eng. C 2020, 110, 110670. [Google Scholar] [CrossRef] [PubMed]
- D’Elía, N.L.; Silva, R.R.; Sartuqui, J.; Ercoli, D.; Ruso, J.; Messina, P.; Mestres, G. Development and characterisation of bilayered periosteum-inspired composite membranes based on sodium alginate-hydroxyapatite nanoparticles. J. Colloid Interface Sci. 2020, 572, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Nuzzo, M.; Yang, X.; Yang, Y.; Zhang, X. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials 2018, 182, 279–288. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Q.; Liu, G. A Review of 3D Printed Bone Implants. Micromachines 2022, 13, 528. [Google Scholar] [CrossRef]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 045008. [Google Scholar] [CrossRef] [PubMed]
- Kruse, H.V.; Lewin, W.T.; Suchowerska, N.; Al Maruf, D.S.A.; Cheng, K.; Clark, J.R.; McKenzie, D.R. Plasma immersion ion-implanted 3D-printed PEEK bone implants: In vivo sheep study shows strong osseointegration. Plasma Process. Polym. 2022, 19, 2100244. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The Application of Polycaprolactone in Three-Dimensional Printing Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Liu, C.; Qin, W.; Wang, Y.; Ma, J.; Liu, J.; Wu, S.; Zhao, H. 3D Printed Gelatin/Sodium Alginate Hydrogel Scaffolds Doped with Nano-Attapulgite for Bone Tissue Repair. Int. J. Nanomed. 2021, 16, 8417–8432. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, Z.; Zhang, X.; Xu, Z.; Zhang, Y.; He, B.; Yang, R.; Zhang, Q.; Yang, Q.; Liu, W. 3D-printed, bi-layer, biomimetic artificial periosteum for boosting bone regeneration. Bio-Des. Manuf. 2022, 5, 540–555. [Google Scholar] [CrossRef]
- Tajvar, S.; Hadjizadeh, A.; Samandari, S.S. Scaffold degradation in bone tissue engineering: An overview. Int. Biodeterior. Biodegrad. 2023, 180, 105599. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Huang, J.; Yuan, N.; Chen, C.; Lin, D. Research Advances in Mechanical Properties and Applications of Dual Network Hydrogels. Int. J. Mol. Sci. 2022, 23, 15757. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Takehisa, T. Nanocomposite Hydrogels: A Unique Organic-Inorganic Network Structure with Extraordinary Mechanical, Optical, and Swelling/De-swelling Properties. Adv. Mater. 2002, 14, 1120–1124. [Google Scholar] [CrossRef]
- Ito, K. Slide-ring materials using topological supramolecular architecture. Curr. Opin. Solid State Mater. Sci. 2010, 14, 28–34. [Google Scholar] [CrossRef]
- Xin, H. Double-Network Tough Hydrogels: A Brief Review on Achievements and Challenges. Gels 2022, 8, 247. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Xuanhe, Z.; Illeperuma, W.R.K.; Chaudhuri, O.; Kyu Hwan, O.H.; Mooney, D.J.; Vlassak, J.J.; Zhigang, S.U.O. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef]
- Naficy, S.; Kawakami, S.; Sadegholvaad, S.; Wakisaka, M.; Spinks, G.M. Mechanical properties of interpenetrating polymer network hydrogels based on hybrid ionically and covalently crosslinked networks. J. Appl. Polym. Sci. 2013, 130, 2504–2513. [Google Scholar] [CrossRef]
- Xin, H.; Oveissi, F.; Naficy, S.; Spinks, G.M. A Sequential Debonding Fracture Model for Hydrogen-Bonded Hydrogels. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1287–1293. [Google Scholar] [CrossRef]
- Oveissi, F.; Spinks, G.M.; Naficy, S. Bond Reformation, Self-Recovery, and Toughness in Hydrogen-Bonded Hydrogels. ACS Appl. Polym. Mater. 2020, 2, 5798–5807. [Google Scholar] [CrossRef]
- Xin, H.; Brown, H.R.; Spinks, G.M. Molecular weight distribution of network strands in double network hydrogels estimated by mechanical testing. Polymer 2014, 55, 3037–3044. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, P.; Li, Q.; Li, J.; Li, X.; Liu, X.; Kang, Y.; Ren, L. Engineering biomimetic periosteum with β-TCP scaffolds to promote bone formation in calvarial defects of rats. Stem Cell Res. Ther. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, S.; Zhao, Y.; Lai, C.; Wu, H. Enhanced osteogenesis by a biomimic pseudo-periosteum-involved tissue engineering strategy. Adv. Healthc. Mater. 2013, 2, 1229–1235. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xin, H.; Tomaskovic-Crook, E.; Al Maruf, D.S.A.; Cheng, K.; Wykes, J.; Manzie, T.G.H.; Wise, S.G.; Crook, J.M.; Clark, J.R. From Free Tissue Transfer to Hydrogels: A Brief Review of the Application of the Periosteum in Bone Regeneration. Gels 2023, 9, 768. https://doi.org/10.3390/gels9090768
Xin H, Tomaskovic-Crook E, Al Maruf DSA, Cheng K, Wykes J, Manzie TGH, Wise SG, Crook JM, Clark JR. From Free Tissue Transfer to Hydrogels: A Brief Review of the Application of the Periosteum in Bone Regeneration. Gels. 2023; 9(9):768. https://doi.org/10.3390/gels9090768
Chicago/Turabian StyleXin, Hai, Eva Tomaskovic-Crook, D S Abdullah Al Maruf, Kai Cheng, James Wykes, Timothy G. H. Manzie, Steven G. Wise, Jeremy M. Crook, and Jonathan R. Clark. 2023. "From Free Tissue Transfer to Hydrogels: A Brief Review of the Application of the Periosteum in Bone Regeneration" Gels 9, no. 9: 768. https://doi.org/10.3390/gels9090768
APA StyleXin, H., Tomaskovic-Crook, E., Al Maruf, D. S. A., Cheng, K., Wykes, J., Manzie, T. G. H., Wise, S. G., Crook, J. M., & Clark, J. R. (2023). From Free Tissue Transfer to Hydrogels: A Brief Review of the Application of the Periosteum in Bone Regeneration. Gels, 9(9), 768. https://doi.org/10.3390/gels9090768








