Sinus Hemodynamics in Representative Stenotic Native Bicuspid and Tricuspid Aortic Valves: An In-Vitro Study
Abstract
1. Introduction
2. Materials and Methods
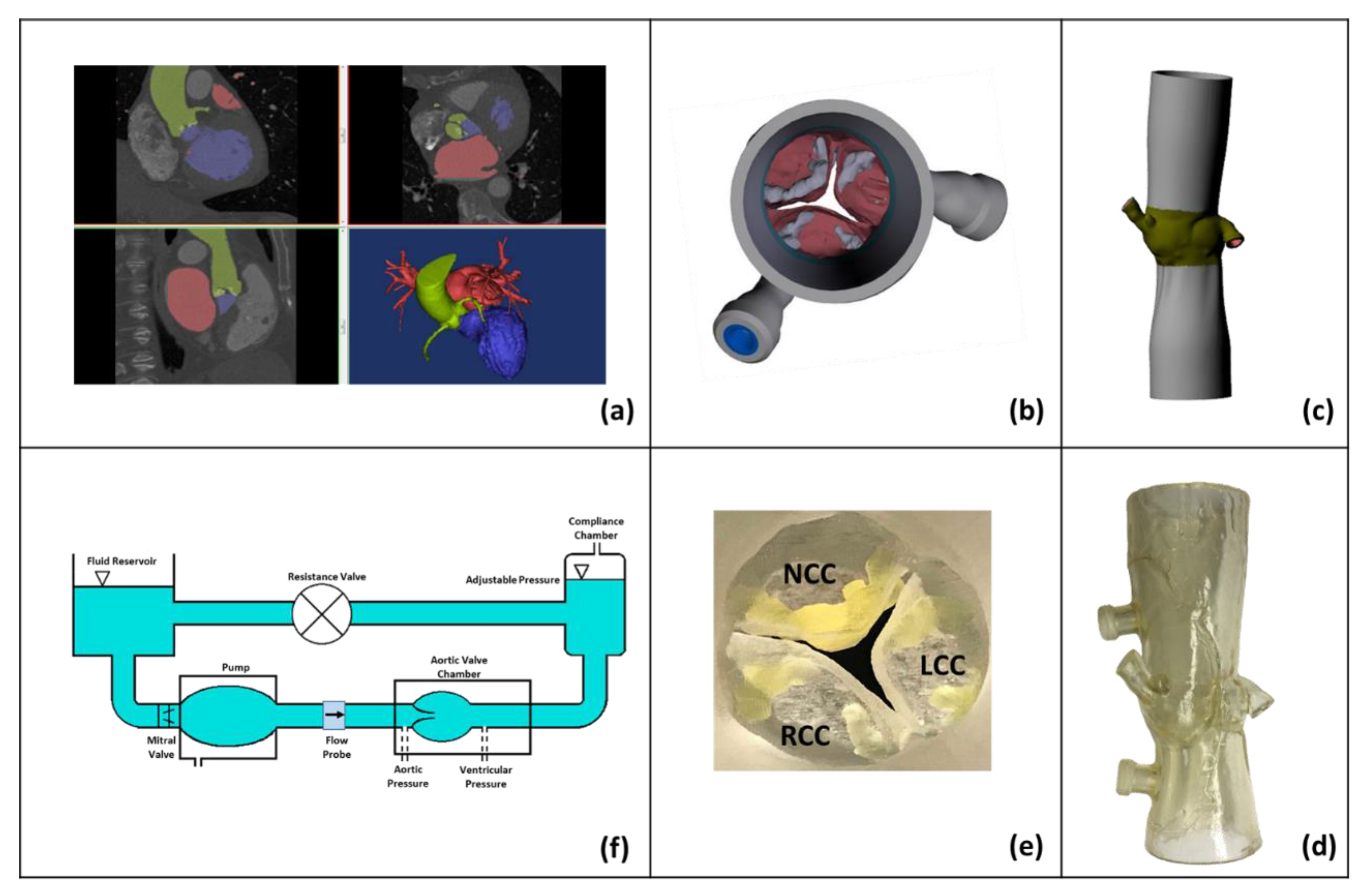
2.1. Patient-Specific Aortic Root Modeling
2.2. Hemodynamic Assessment
2.3. Particle Image Velocimetry (PIV)
2.4. Sinus Vorticity and Shear Stress Dynamics
2.5. Sinus Washout
3. Results
3.1. Hemodynamic Parameters
3.2. Flow Velocity Fields
3.3. Shear Stress Distribution
3.4. Sinus Washout
4. Discussion
4.1. Flow Velocity and Vorticity Fields and Hemodynamics
4.2. Shear Stress Distribution
4.3. Sinus Washout
4.4. Comparison between Idealized and Anatomical Sinus Geometries
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Verma, S.; Siu, S.C. Aortic dilatation in patients with bicuspid aortic valve. N. Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.; Wang, Z.; Nishimura, R.A.; Greason, K.L.; Yoon, S.H.; Makkar, R.R.; Holmes, D.R., Jr. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: S ystematic review and meta analyses of observational studies. Catheter. Cardiovasc. Interv. 2018, 91, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ahn, J.-M.; Hayashida, K.; Watanabe, Y.; Shirai, S.; Kao, H.-L.; Yin, W.-H.; Lee, M.K.-Y.; Tay, E.; Araki, M. Clinical outcomes following transcatheter aortic valve replacement in Asian population. JACC Cardiovasc. Interv. 2016, 9, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Brennan, J.M.; Brindis, R.; Carroll, J.; Edwards, F.; Grover, F.; Shahian, D.; Tuzcu, E.M.; Peterson, E.D.; Rumsfeld, J.S. Outcomes following transcatheter aortic valve replacement in the United States. Jama 2013, 310, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Dollery, J.; Lilly, S.M.; Crestanello, J.; Dasi, L.P. Impact of Patient Morphologies on Sinus Flow Stasis in Transcatheter Aortic Valve Replacement: An in-vitro study. J. Thorac. Cardiovasc. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Dollery, J.; Lilly, S.M.; Crestanello, J.; Dasi, L.P. Implantation Depth and Rotational Orientation Effect on Valve-in-Valve Hemodynamics and Sinus Flow. Ann. Thorac. Surg. 2018, 106, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Moore, B.L.; Maureira, P.; Dollery, J.; Crestanello, J.A.; Dasi, L.P. Aortic sinus flow stasis likely in valve-in-valve transcatheter aortic valve implantation. J. Thorac. Cardiovasc. Surg. 2017, 154, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.V.; Otto, C.M. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation 2005, 111, 3316–3326. [Google Scholar] [CrossRef] [PubMed]
- Wallby, L.; Janerot-Sjöberg, B.; Steffensen, T.; Broqvist, M. T lymphocyte infiltration in non-rheumatic aortic stenosis: A comparative descriptive study between tricuspid and bicuspid aortic valves. Heart 2002, 88, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, F.; Le, T.B.; Gilmanov, A. Fluid mechanics of heart valves and their replacements. Ann. Rev. Fluid Mech. 2016, 48, 259–283. [Google Scholar] [CrossRef]
- Moore, B.L.; Dasi, L.P. Coronary flow impacts aortic leaflet mechanics and aortic sinus hemodynamics. Ann. Biomed. Eng. 2015, 43, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Crestanello, J.; Dasi, L.P. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2016, 374, 1591. [Google Scholar] [PubMed]
- Toninato, R.; Salmon, J.; Susin, F.M.; Ducci, A.; Burriesci, G. Physiological vortices in the sinuses of Valsalva: An in vitro approach for bio-prosthetic valves. J. Biomech. 2016, 49, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Dasi, L.P. Spatiotemporal complexity of the aortic sinus vortex. Exp. Fluids 2014, 55, 1770. [Google Scholar] [CrossRef] [PubMed]
- Peacock, J.A. An in vitro study of the onset of turbulence in the sinus of Valsalva. Circ. Res. 1990, 67, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Kheradvar, A.; Groves, E.M.; Falahatpisheh, A.; Mofrad, M.K.; Alavi, S.H.; Tranquillo, R.; Dasi, L.P.; Simmons, C.A.; Grande-Allen, K.J.; Goergen, C.J. Emerging trends in heart valve engineering: Part IV. Computational modeling and experimental studies. Ann. Biomed. Eng. 2015, 43, 2314–2333. [Google Scholar] [CrossRef] [PubMed]
- Maragiannis, D.; Jackson, M.S.; Igo, S.R.; Schutt, R.C.; Connell, P.; Grande-Allen, J.; Barker, C.M.; Chang, S.M.; Reardon, M.J.; Zoghbi, W.A. Replicating patient-specific severe aortic valve stenosis with functional 3D modeling. Circ. Cardiovasc. Imaging 2015, 8, e003626. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Yousefi, A.; Lilly, S.; Maureira, P.; Crestanello, J.; Dasi, L.P. An In-Vitro Evaluation of Turbulence after Transcatheter Aortic Valve Implantation. J. Thorac. Cardiovasc. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Dollery, J.; Lilly, S.M.; Crestanello, J.A.; Dasi, L.P. Effect of severe bioprosthetic valve tissue ingrowth and inflow calcification on valve-in-valve performance. J. Biomech. 2018, 74, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Heim, F.; Dasi, L.P. Stented valve dynamic behavior induced by polyester fiber leaflet material in transcatheter aortic valve devices. J. Mech. Behav. Biomed. Mater. 2018, 86, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Hatoum, H.; Moore, B.L.; Dasi, L.P. On the Significance of Systolic Flow Waveform on Aortic Valve Energy Loss. Ann. Biomed. Eng. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Raghav, V.; Lerakis, S.; Yoganathan, A.P. High transcatheter valve replacement may reduce washout in the aortic sinuses: An in-vitro study. J. Heart Valve Disease 2015, 24, 22–29. [Google Scholar]
- Gustafson, K.E.; Sethian, J.A. Vortex Methods and Vortex Motion; SIAM: Philadelphia, PA, USA, 1991; ISBN 978-0-89871-258-2. [Google Scholar]
- Reynolds, O. On the resistance encountered by vortex rings, and the relation between the vortex rings and streamlines of a disk. Nature 1876, 14, 477–479. [Google Scholar]
- Hope, M.D.; Hope, T.A.; Meadows, A.K.; Ordovas, K.G.; Urbania, T.H.; Alley, M.T.; Higgins, C.B. Bicuspid aortic valve: Four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 2010, 255, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Rajamannan, N.M.; Sucosky, P. Computational assessment of bicuspid aortic valve wall-shear stress: Implications for calcific aortic valve disease. Biomech. Model. Mechanobiol. 2012, 11, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Dasi, L.P.; Hatoum, H.; Kheradvar, A.; Zareian, R.; Alavi, S.H.; Sun, W.; Martin, C.; Pham, T.; Wang, Q.; Midha, P.A. On the mechanics of transcatheter aortic valve replacement. Ann. Biomed. Eng. 2017, 45, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.H.; Liu, X.; Pekkan, K. Characterizaton of the vessel geometry, flow mechanics and wall shear stress in the great arteries of wildtype prenatal mouse. PLoS ONE 2014, 9, e86878. [Google Scholar] [CrossRef] [PubMed]
- Traub, O.; Berk, B.C. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Berk, B.C.; ABE, J.I.; Min, W.; Surapisitchat, J.; Yan, C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann. N. Y. Acad. Sci. 2001, 947, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.-D.; Kouchi, Y.; Onuki, Y.; Shi, Q.; Yoshida, H.; Kaplan, S.; Viggers, R.F.; Ghali, R.; Sauvage, L.R. Effect of differential shear stress on platelet aggregation, surface thrombosis, and endothelialization of bilateral carotid-femoral grafts in the dog. J. Vasc. Surg. 1995, 22, 382–392. [Google Scholar] [CrossRef]
- Cunningham, K.S.; Gotlieb, A.I. The role of shear stress in the pathogenesis of atherosclerosis. Lab. Investig. 2005, 85, 9. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.N.; Dawn, C.; Biswas, A.; Mattar, C.N.Z.; Yap, C.H. Characterization of the in vivo wall shear stress environment of human fetus umbilical arteries and veins. Biomech. Model. Mechanobiol. 2017, 16, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Casa, L.D.; Deaton, D.H.; Ku, D.N. Role of high shear rate in thrombosis. J. Vasc. Surg. 2015, 61, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Bark, D.L., Jr.; Para, A.N.; Ku, D.N. Correlation of thrombosis growth rate to pathological wall shear rate during platelet accumulation. Biotechnol. Bioeng. 2012, 109, 2642–2650. [Google Scholar] [CrossRef] [PubMed]
- Kolář, V. Vortex identification: New requirements and limitations. Int. J. Heat Fluid Flow 2007, 28, 638–652. [Google Scholar] [CrossRef]
- Fedak, P.W.; Verma, S.; David, T.E.; Leask, R.L.; Weisel, R.D.; Butany, J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation 2002, 106, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Saikrishnan, N.; Yap, C.-H.; Milligan, N.C.; Vasilyev, N.V.; Yoganathan, A.P. In vitro characterization of bicuspid aortic valve hemodynamics using particle image velocimetry. Ann. Biomed. Eng. 2012, 40, 1760–1775. [Google Scholar] [CrossRef] [PubMed]
- Yoganathan, A.; Woo, Y.; Sung, H.; Williams, F.; Franch, R.; Jones, M. In vitro hemodynamic characteristics of tissue bioprostheses in the aortic position. J. Thorac. Cardiovasc. Surg. 1986, 92, 198–209. [Google Scholar] [PubMed]
- Morshed, K.N.; Bark, D., Jr.; Forleo, M.; Dasi, L.P. Theory to predict shear stress on cells in turbulent blood flow. PLoS ONE 2014, 9, e105357. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.M.; Dasi, L.; Aidun, C.; Yoganathan, A. Highly resolved pulsatile flows through prosthetic heart valves using the entropic lattice-Boltzmann method. J. Fluid Mech. 2014, 754, 122–160. [Google Scholar] [CrossRef]
- Dasi, L.P.; Morshed, K.N.; Forleo, M. Phenomenology of Hemolysis in Turbulent Flows. In Proceedings of the ASME 2013 Summer Bioengineering Conference, Sunriver, OR, USA, 26–29 June 2013. [Google Scholar]
- Hedayat, M.; Asgharzadeh, H.; Borazjani, I. Platelet activation of mechanical versus bioprosthetic heart valves during systole. J. Biomech. 2017, 56, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Yun, B.M.; Aidun, C.K.; Yoganathan, A.P. Blood damage through a bileaflet mechanical heart valve: A quantitative computational study using a multiscale suspension flow solver. J. Biomech. Eng. 2014, 136, 101009. [Google Scholar] [CrossRef] [PubMed]
- Khalili, F.; Gamage, P.; Mansy, H.A. The Influence of the Aortic Root Geometry on Flow Characteristics of a Bileaflet Mechanical Heart Valve. arXiv, 2018; arXiv:1803.03362. [Google Scholar]
- Giersiepen, M.; Wurzinger, L.; Opitz, R.; Reul, H. Estimation of shear stress-related blood damage in heart valve prostheses-in vitro comparison of 25 aortic valves. Int. J. Artif. Organs 1990, 13, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, H.; Giersiepen, M.; Hasenkam, J.; Reul, H.; Paulsen, P.; Rovsing, P.; Westphal, D. Two-dimensional color-mapping of turbulent shear stress distribution downstream of two aortic bioprosthetic valves in vitro. J. Biomech. 1992, 25, 437–440. [Google Scholar] [CrossRef]
- Hanle, D.; Harrison, E.; Yoganathan, A.; Corcoran, W. Turbulence downstream from the Ionescu-Shiley bioprosthesis in steady and pulsatile flow. Med. Biol. Eng. Comput. 1987, 25, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Schoephoerster, R.T.; Chandran, K.B. Velocity and turbulence measurements past mitrial valve prostheses in a model left ventricle. J. Biomech. 1991, 24, 549–562. [Google Scholar] [CrossRef]
- Jones, S.A. A relationship between Reynolds stresses and viscous dissipation: Implications to red cell damage. Ann. Biomed. Eng. 1995, 23, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Dasi, L.P.; Simon, H.A.; Sucosky, P.; Yoganathan, A.P. Fluid mechanics of artificial heart valves. Clin. Exp. Pharmacol. Physiol. 2009, 36, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Williams, A. Release of serotonin from human platelets by acoustic microstreaming. J. Acoust. Soc. Am. 1974, 56, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.; Hochmuth, R.; Joist, J.; Sutera, S. Shear-induced aggregation and lysis of platelets. ASAIO J. 1976, 22, 285–290. [Google Scholar]
- Ramstack, J.; Zuckerman, L.; Mockros, L. Shear-induced activation of platelets. J. Biomech. 1979, 12, 113–125. [Google Scholar] [CrossRef]



| Stenosed Valves | BAV | TrAV |
|---|---|---|
| CO (L/min) | 4.2 | 5.15 |
| HR (BPM) | 81 | 81 |
| Pressures (mmHg) | 98/68 | 100/61 |
| ∆P (mmHg) | 76.25 ± 0.98 | 49.47 ± 2.62 |
| EOA (cm2) | 0.55 ± 0.01 | 1.12 ± 0.06 |
| Qmax (L/min) | 25.4 ± 0.48 | 37.62 ± 1.92 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatoum, H.; Dasi, L.P. Sinus Hemodynamics in Representative Stenotic Native Bicuspid and Tricuspid Aortic Valves: An In-Vitro Study. Fluids 2018, 3, 56. https://doi.org/10.3390/fluids3030056
Hatoum H, Dasi LP. Sinus Hemodynamics in Representative Stenotic Native Bicuspid and Tricuspid Aortic Valves: An In-Vitro Study. Fluids. 2018; 3(3):56. https://doi.org/10.3390/fluids3030056
Chicago/Turabian StyleHatoum, Hoda, and Lakshmi Prasad Dasi. 2018. "Sinus Hemodynamics in Representative Stenotic Native Bicuspid and Tricuspid Aortic Valves: An In-Vitro Study" Fluids 3, no. 3: 56. https://doi.org/10.3390/fluids3030056
APA StyleHatoum, H., & Dasi, L. P. (2018). Sinus Hemodynamics in Representative Stenotic Native Bicuspid and Tricuspid Aortic Valves: An In-Vitro Study. Fluids, 3(3), 56. https://doi.org/10.3390/fluids3030056





