Natural Yogurt Stabilized with Hydrocolloids from Butternut Squash (Cucurbita moschata) Seeds: Effect on Physicochemical, Rheological Properties and Sensory Perception
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Hydrocolloids from Butternut Squash Seeds
2.2.2. Production of Natural Yogurt
2.2.3. Physicochemical Properties and Proximal Composition of Yogurt
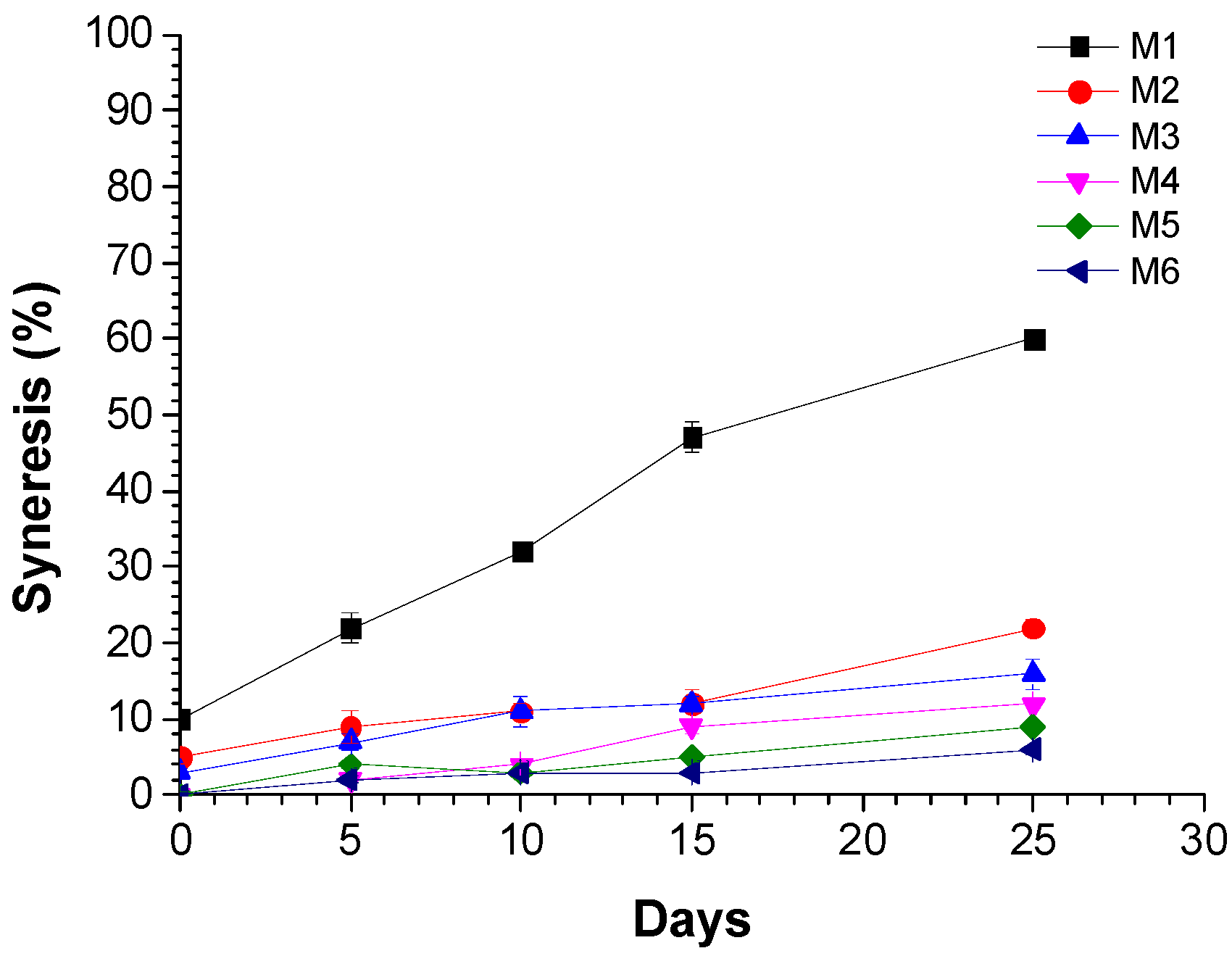
2.2.4. Syneresis Measurement
2.2.5. Color Parameters of Yogurt
2.2.6. Rheological Analysis
2.2.7. Sensorial Analysis
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Yogurt
3.2. Color Analysis
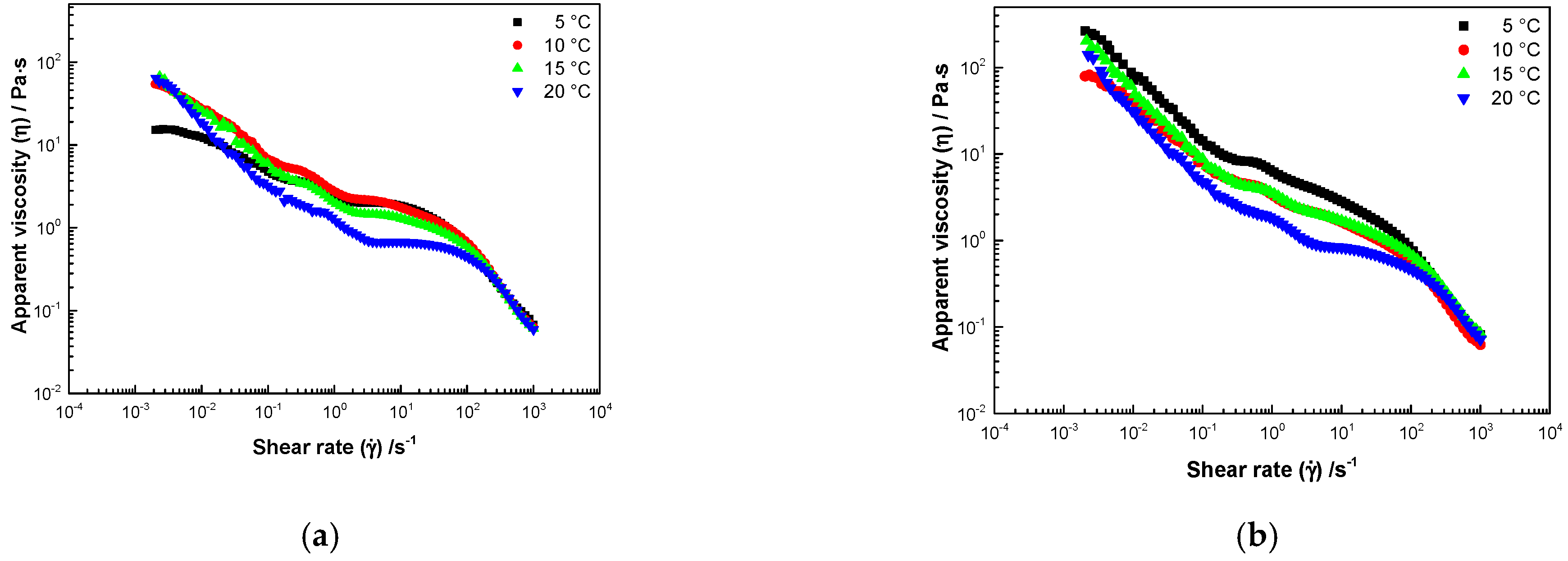
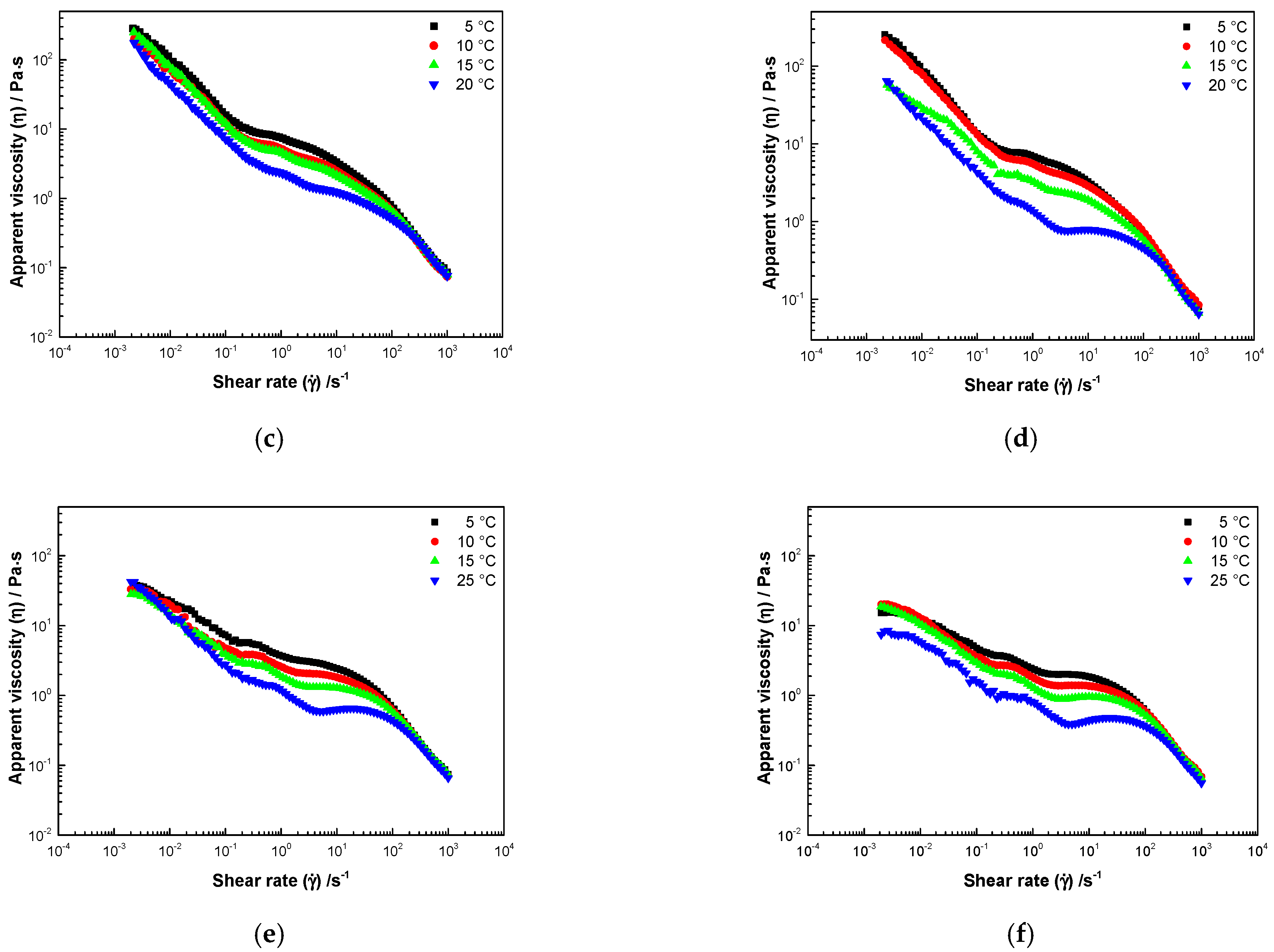
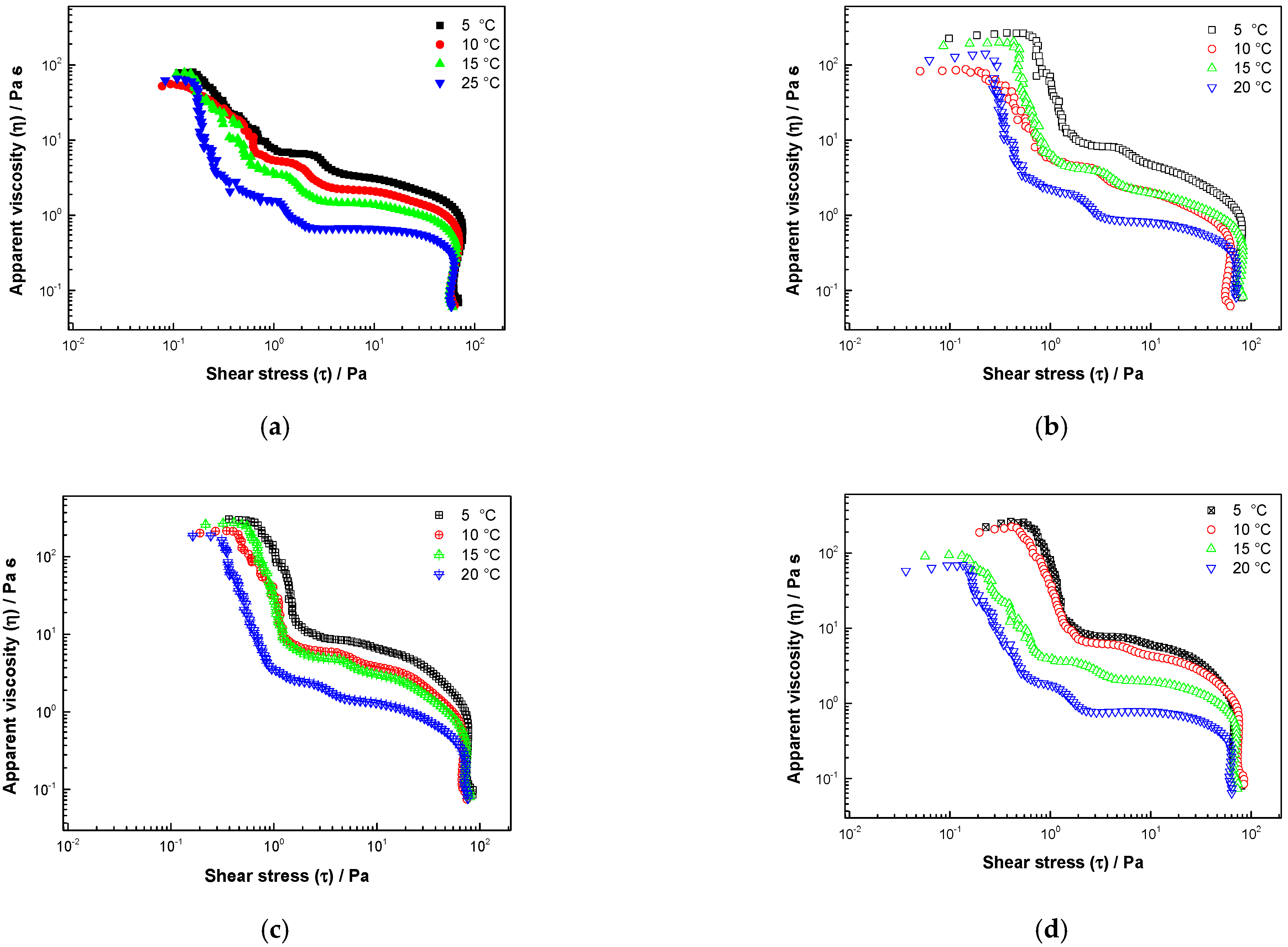
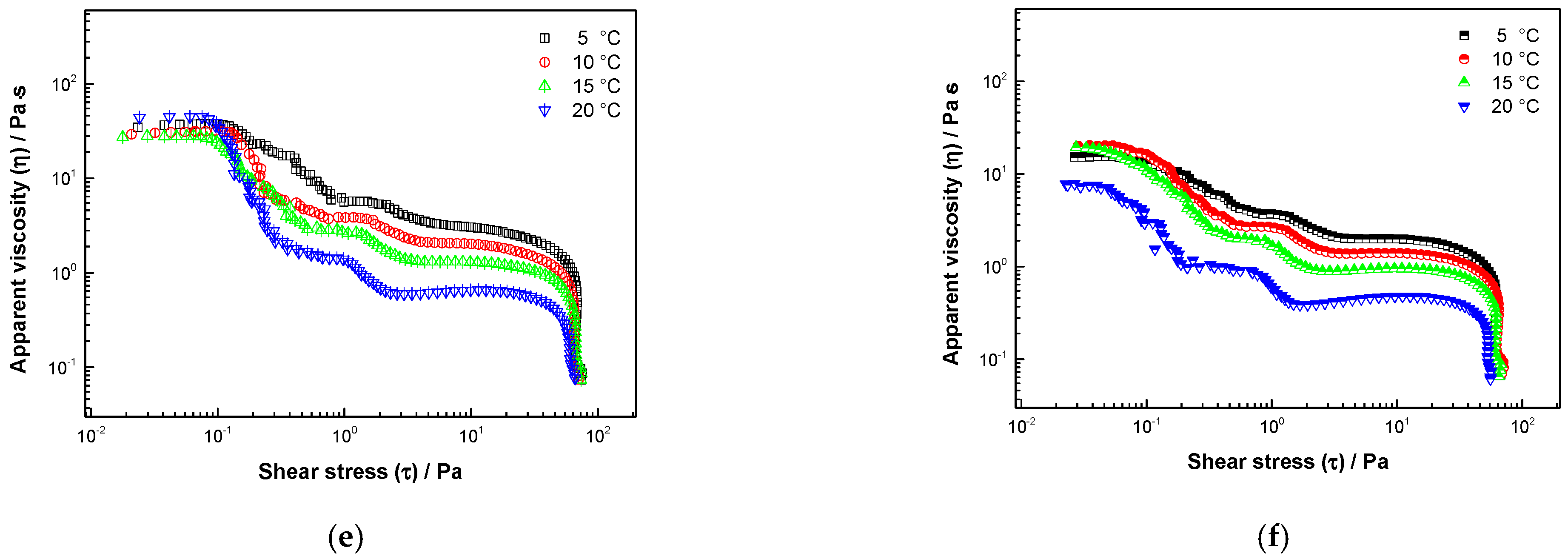
3.3. Rheological Properties
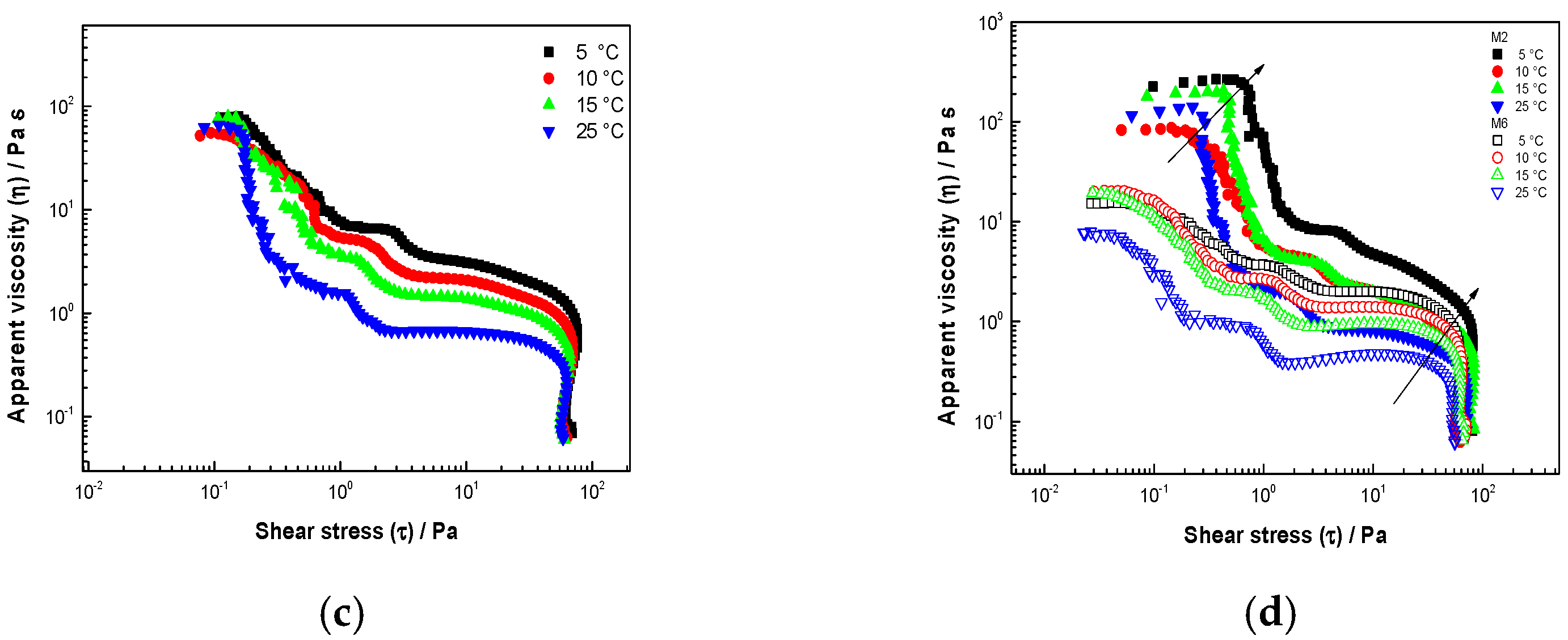
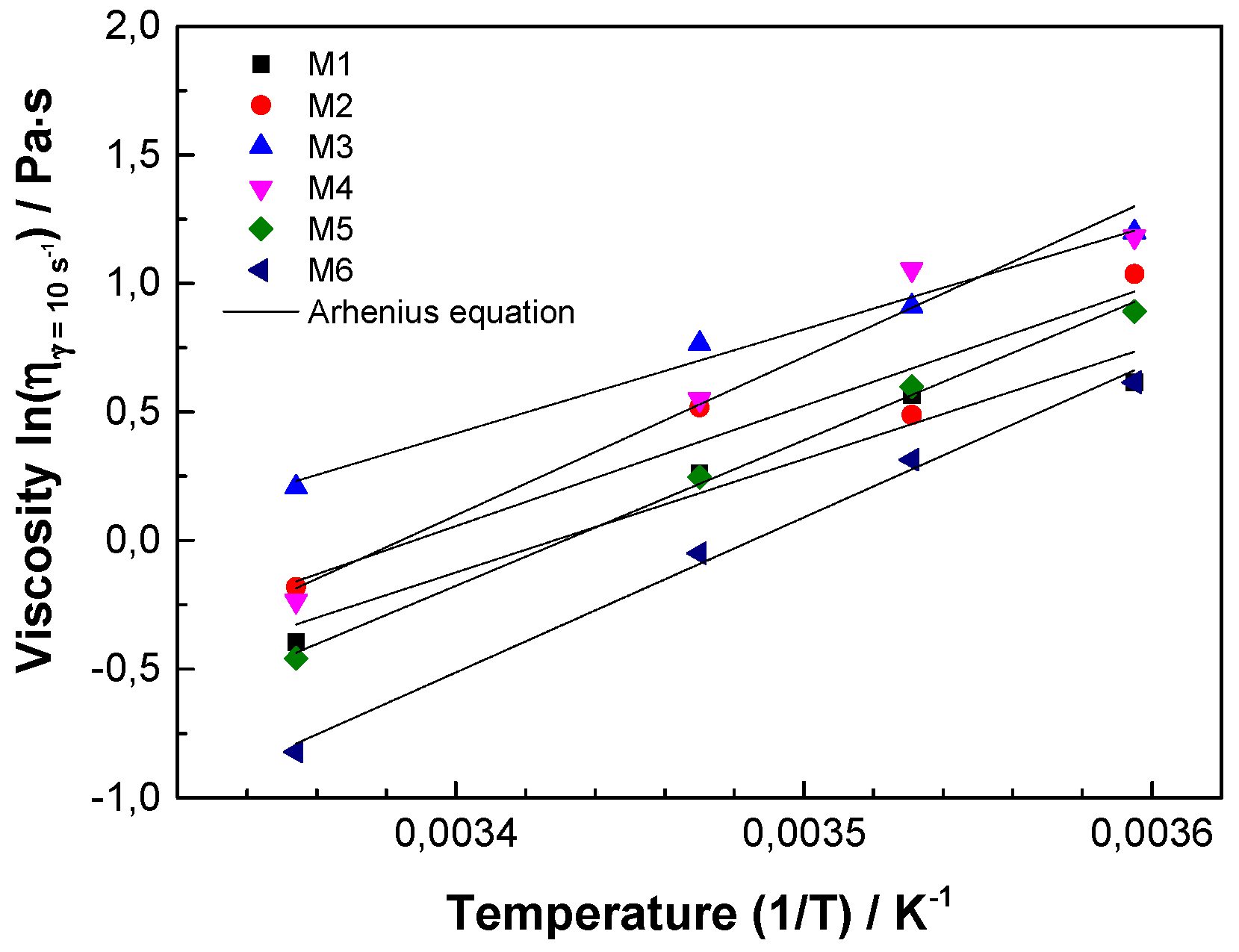
3.3.1. Steady Shear Rate
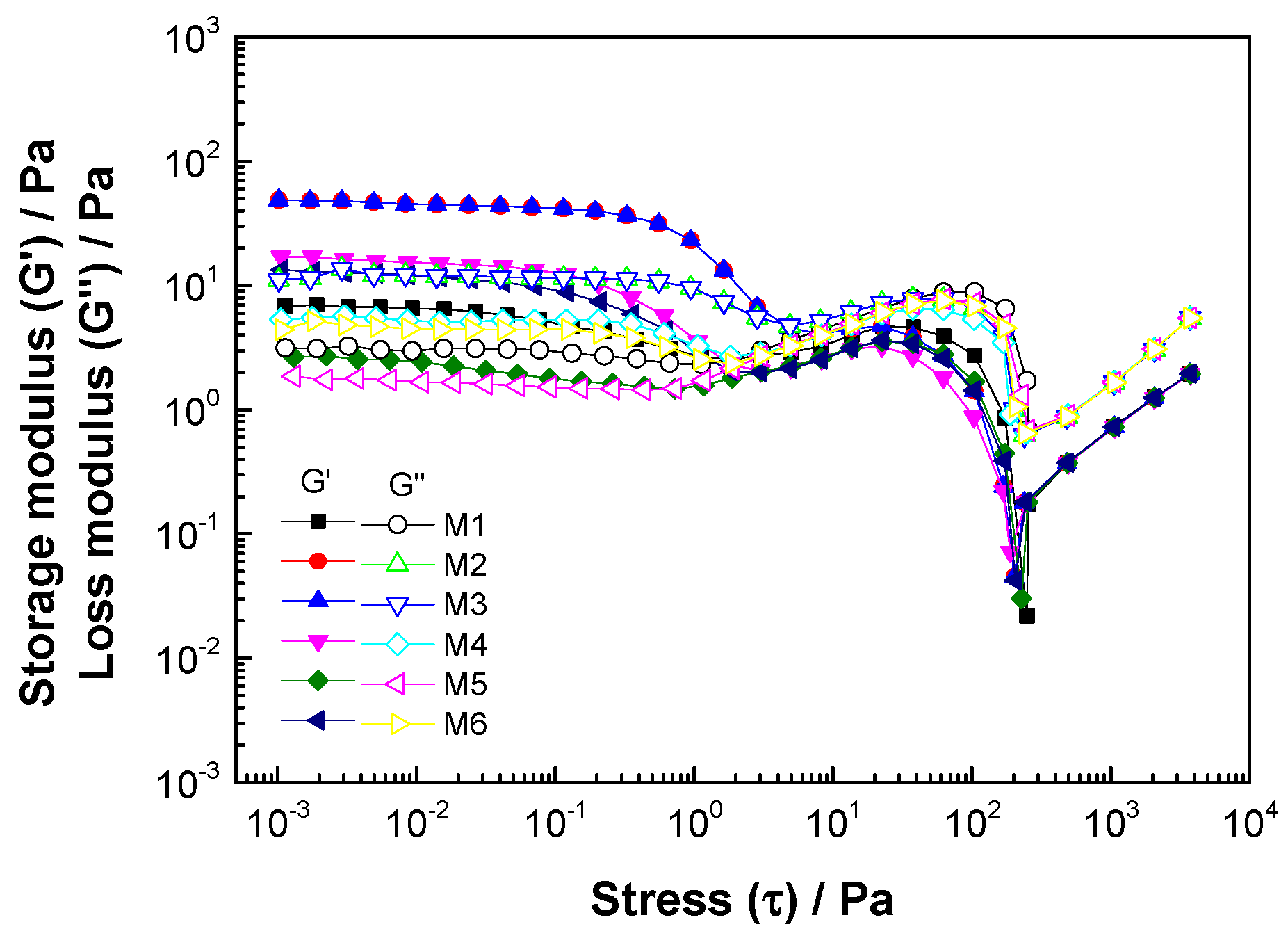
3.3.2. Stress Sweep
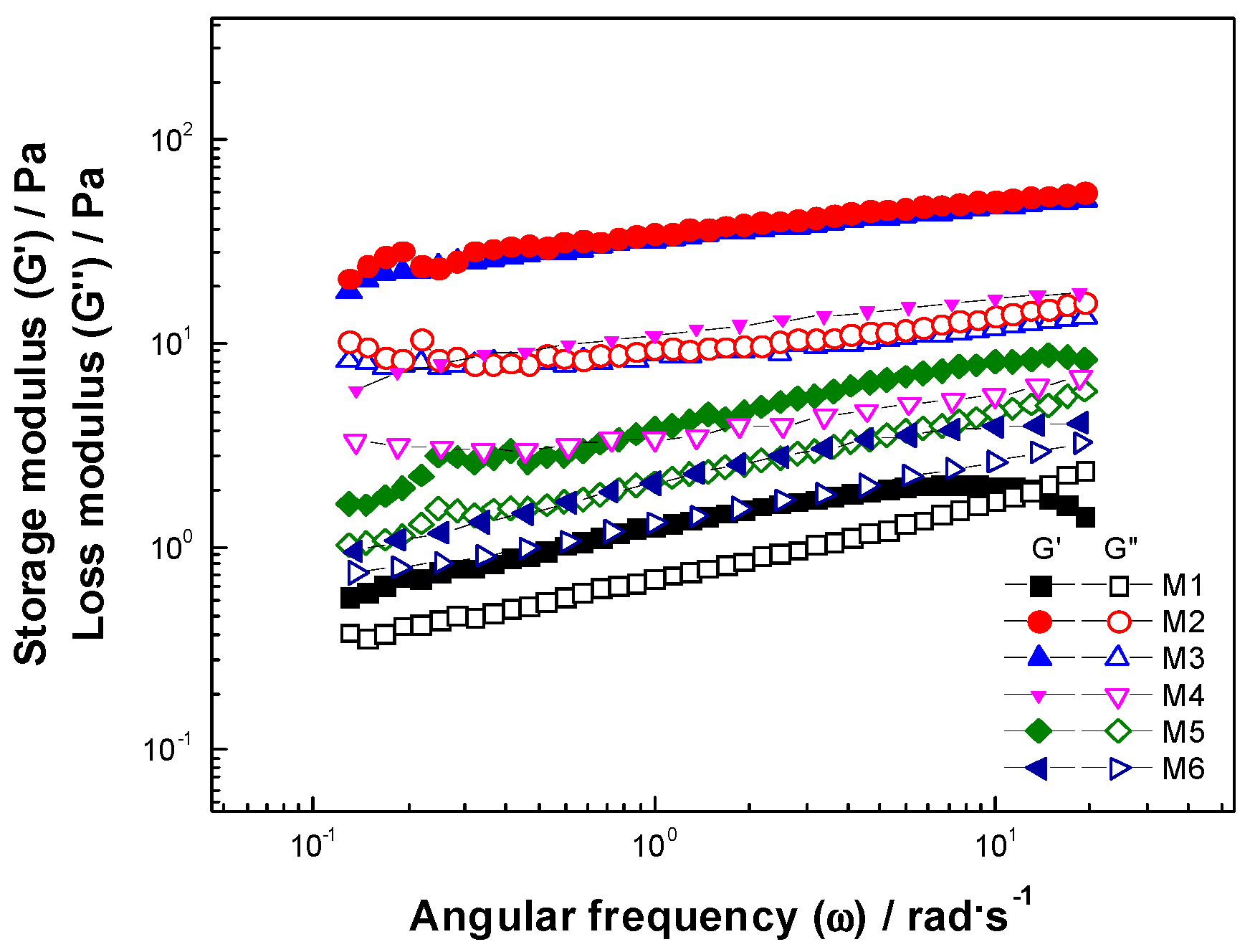
3.3.3. Frequency Sweep
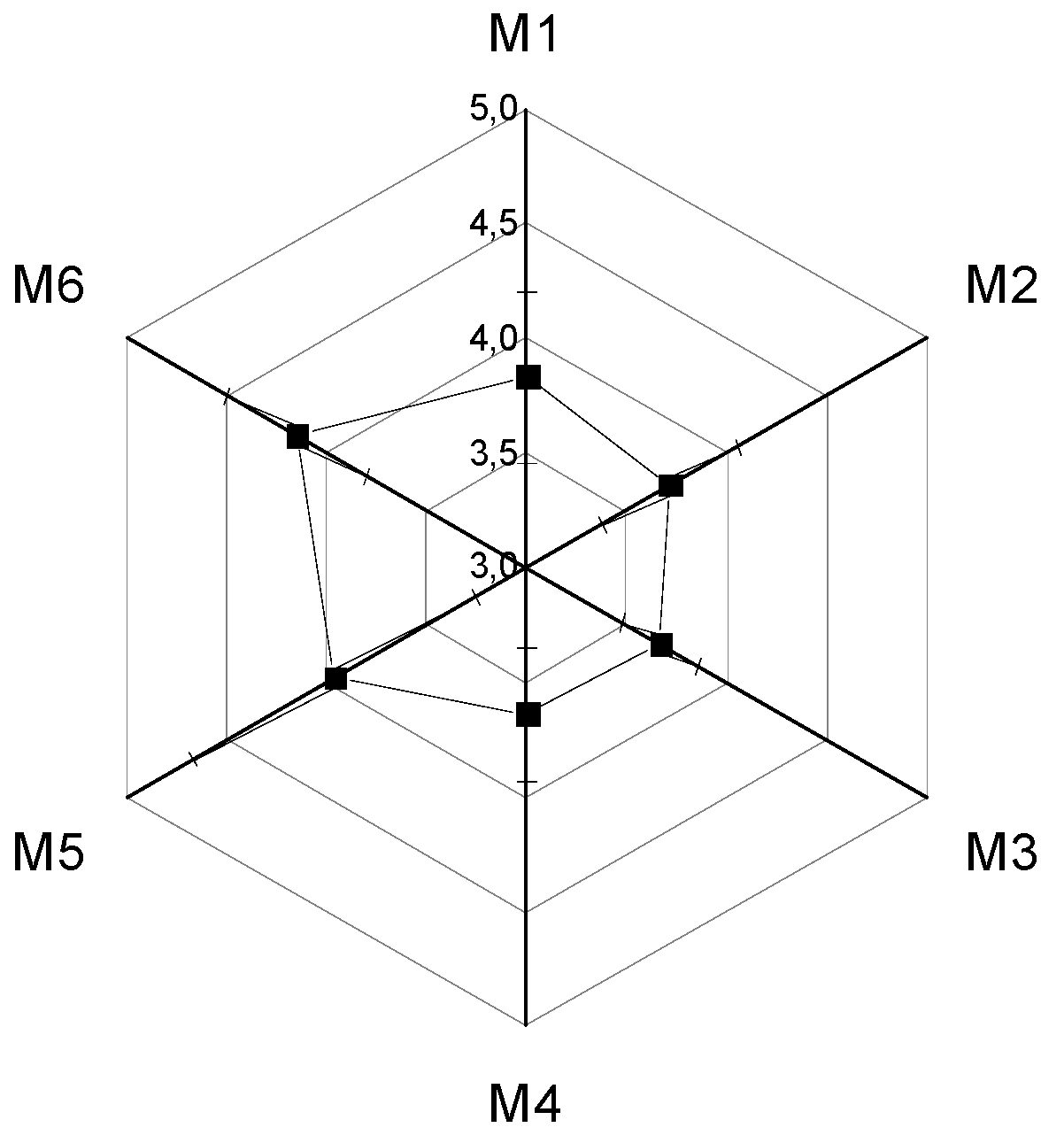
3.4. Sensory Analysis
3.5. Proximal Composition of a Selected Standardized Product
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
References
- Desobry-Banon, S.; Vetier, N.; Hardy, J. Health Benefits of Yogurt Consumption. A Review. Int. J. Food Prop. 1999, 2, 1–12. [Google Scholar] [CrossRef][Green Version]
- Shah, N.P. Health Benefits of Yogurt and Fermented Milks. In Manufacturing Yogurt and Fermented Milks; Blackwell Publishing: Ames, IA, USA, 2007; pp. 327–351. [Google Scholar]
- Andrade, R.D.; Arteaga, M.R.; Simanca, M.M. Efecto Del Salvado de Trigo En El Comportamiento Reológico Del Yogurt de Leche de Búfala. Inf. Tecnológica 2010, 21, 117–124. [Google Scholar] [CrossRef]
- Mendoza, R.; Trujillo, Y.; Duran, D. Evaluación Del Almidón de Ñame Espino (Dioscorea rotundata) Como Estabilizante En La Elaboración de Yogur Entero Tipo Batido—Dialnet. Rev. Fac. De Cienc. Básicas 2007, 5, 97–105. [Google Scholar]
- Lee, W.J.; Lucey, J.A. Formation and Physical Properties of Yogurt. Asian-Australas. J. Anim. Sci. 2010, 23, 1127–1136. [Google Scholar] [CrossRef]
- Sanchez, C.; Zuniga-Lopez, R.; Schmitt, C.; Despond, S.; Hardy, J. Microstructure of Acid-Induced Skim Milk-Locust Bean Gum-Xanthan Gels. Int. Dairy J. 2000, 10, 199–212. [Google Scholar] [CrossRef]
- Sigdel, A.; Ojha, P.; Karki, T.B. Phytochemicals and Syneresis of Osmo-Dried Mulberry Incorporated Yoghurt. Food Sci. Nutr. 2018, 6, 1045–1052. [Google Scholar] [CrossRef]
- Cárdenas Casanova, A.; Alvites Gutierrez, H.; Valladares Castillo, G.; Obregón Domínguez, J.; Vásquez-Villalobos, V. Optimization by Mixtures Design of Syneresis and Sensory Texture of Natural Smoothie Yogurt Using Three Types of Hydrocolloids. Agroind. Sci. 2013, 3, 35–40. [Google Scholar] [CrossRef][Green Version]
- Balestra, F.; Petracci, M. Technofunctional Ingredients for Meat Products. In Sustainable Meat Production and Processing; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–68. [Google Scholar]
- Fiszman, S.M.; Salvador, A. Effect of Gelatine on the Texture of Yoghurt and of Acid-Heat-Induced Milk Gels. Eur. Food Res. Technol. 1999, 208, 100–105. [Google Scholar] [CrossRef]
- Schmidt, K.A.; Smith, D.E. Milk Reactivity of Gum and Milk Protein Solutions. J. Dairy Sci. 1992, 75, 3290–3295. [Google Scholar] [CrossRef]
- Norton, I.T.; Spyropoulos, F.; Cox, P. Practical Food Rheology: An Interpretive Approach; Blackwell: Oxford, UK, 2011. [Google Scholar]
- Kirschweng, B.; Tátraaljai, D.; Földes, E.; Pukánszky, B. Natural Antioxidants as Stabilizers for Polymers. Polym. Degrad. Stab. 2017, 145, 25–40. [Google Scholar] [CrossRef]
- Kieserling, K.; Vu, T.M.; Drusch, S.; Schalow, S. Impact of Pectin-Rich Orange Fibre on Gel Characteristics and Sensory Properties in Lactic Acid Fermented Yoghurt. Food Hydrocoll. 2019, 94, 152–163. [Google Scholar] [CrossRef]
- McCann, T.H.; Fabre, F.; Day, L. Microstructure, Rheology and Storage Stability of Low-Fat Yoghurt Structured by Carrot Cell Wall Particles. Food Res. Int. 2011, 44, 884–892. [Google Scholar] [CrossRef]
- Wang, X.; Kristo, E.; LaPointe, G. The Effect of Apple Pomace on the Texture, Rheology and Microstructure of Set Type Yogurt. Food Hydrocoll. 2019, 91, 83–91. [Google Scholar] [CrossRef]
- Vinayashree, S.; Vasu, P. Biochemical, Nutritional and Functional Properties of Protein Isolate and Fractions from Pumpkin (Cucurbita Moschata Var. Kashi Harit) Seeds. Food Chem. 2021, 340, 128177. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Wang, A.; Yu, Z.; Xu, Y.; Yang, Y. Purification, Characterization and Bioactivity Determination of a Novel Polysaccharide from Pumpkin (Cucurbita Moschata) Seeds. Food Hydrocoll. 2017, 66, 357–364. [Google Scholar] [CrossRef]
- Caili, F.; Huan, S.; Quanhong, L. A Review on Pharmacological Activities and Utilization Technologies of Pumpkin. Plant Foods Hum. Nutr. 2006, 61, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Hermetter, A. Seeds and Oil of the Styrian Oil Pumpkin: Components and Biological Activities. Eur. J. Lipid Sci. Technol. 2007, 109, 1128–1140. [Google Scholar] [CrossRef]
- Sener, B.; Orhan, I.; Ozcelik, B.; Kartal, M.; Aslan, S.; Ozbilen, G. Antimicrobial and Antiviral Activities of Two Seed Oil Samples of Cucurbita Pepo L. and Their Fatty Acid Analysis. Nat. Prod. Commun. 2007. [Google Scholar] [CrossRef]
- Rezig, L.; Chibani, F.; Chouaibi, M.; Dalgalarrondo, M.; Hessini, K.; Guéguen, J.; Hamdi, S. Pumpkin (Cucurbita Maxima) Seed Proteins: Sequential Extraction Processing and Fraction Characterization. J. Agric. Food Chem. 2013, 61, 7715–7721. [Google Scholar] [CrossRef] [PubMed]
- Bučko, S.; Katona, J.; Popović, L.; Vaštag, Ž.; Petrović, L.; Vučiniće-Vasić, M. Investigation on Solubility, Interfacial and Emulsifying Properties of Pumpkin (Cucurbita Pepo) Seed Protein Isolate. LWT Food Sci. Technol. 2015, 64, 609–615. [Google Scholar] [CrossRef]
- Orgulloso-Bautista, S.; Ortega-Toro, R.; Alberto, L.; Zapateiro, G. Design and Application of Hydrocolloids from Butternut Squash (Cucurbita moschata) Epidermis as a Food Additive in Mayonnaise-Type Sauces. ACS Omega 2021. [Google Scholar] [CrossRef]
- Quintana Martínez, S.E.; Torregroza Fuentes, E.E.; García Zapateiro, L.A. Food Hydrocolloids from Butternut Squash (Cucurbita moschata) Peel: Rheological Properties and Their Use in Carica Papaya Jam. ACS Omega 2021, 6, 12114–12123. [Google Scholar] [CrossRef] [PubMed]
- Ladjevardi, Z.S.; Gharibzahedi, S.M.T.; Mousavi, M. Development of a Stable Low-Fat Yogurt Gel Using Functionality of Psyllium (Plantago Ovata Forsk) Husk Gum. Carbohydr. Polym. 2015, 125, 272–280. [Google Scholar] [CrossRef]
- Aziznia, S.; Khosrowshahi, A.; Madadlou, A.; Rahimi, J. Whey Protein Concentrate and Gum Tragacanth as Fat Replacers in Nonfat Yogurt: Chemical, Physical, and Microstructural Properties. J. Dairy Sci. 2008, 91, 2545–2552. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemist Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Santillán-Urquiza, E.; Méndez-Rojas, M.Á.; Vélez-Ruiz, J.F. Fortification of Yogurt with Nano and Micro Sized Calcium, Iron and Zinc, Effect on the Physicochemical and Rheological Properties. LWT 2017, 80, 462–469. [Google Scholar] [CrossRef]
- Säker Vásquez, W.; Zevallos, A.R. Influencia Del Cultivo Láctico, Gelatina y Sacarosa Sobre La Viscosidad, Sinéresis y Características Sensoriales En Leche Fermentada. Pueblo Cont. 2016, 23, 125–136. [Google Scholar]
- Quintana-Martinez, S.; Morales-Cano, A.; García-Zapateiro, L. Rheological Behaviour in the Interaction of Lecithin and Guar Gum for Oil-in-Water Emulsions. Czech J. Food Sci. 2018, 36, 73–80. [Google Scholar] [CrossRef]
- ICONTEC Analisis Sensorial. Guia General-GTC 165; ICONTEC Analisis Sensorial: Bogotá, Colombia, 1999. [Google Scholar]
- Aportela-Palacios, A.; Sosa-Morales, M.E.; Velez-Ruiz, J.F. Rheological and Physicochemical Behavior of Fortified Yogurt, With Fiber and Calcium. J. Texture Stud. 2005, 36, 333–349. [Google Scholar] [CrossRef]
- Raju, P.N.; Pal, D. Effect of Dietary Fibers on Physico-Chemical, Sensory and Textural Properties of Misti Dahi. J. Food Sci. Technol. 2014, 51, 3124–3133. [Google Scholar] [CrossRef]
- Marulanda, M.; Granados, C.; García-Zapateiro, L.A. Análisis Sensorial y Estimación Fisicoquímica de Vida Útil de Una Bebida Tipo Yogur a Base de Lactosuero Dulce Fermentada Con Estreptococcus Salivarius Ssp. Thermophilus y Lactobacillus Casei Ssp. Casei. Prod. Limpia 2016, 11, 94–102. [Google Scholar] [CrossRef]
- Granata, L.A.; Morr, C.V. Improved Acid, Flavor and Volatile Compound Production in a High Protein and Fiber Soymilk Yogurt-like Product. J. Food Sci. 1996, 61, 331–336. [Google Scholar] [CrossRef]
- Lucey, J.A. Formation and Physical Properties of Milk Protein Gels. J. Dairy Sci. 2002, 85, 281–294. [Google Scholar] [CrossRef]
- Molina Chew, I.S. Comparación de Tres Estabilizantes Comerciales Utilizados En La Elaboración de Yogurt de Leche Descremada de Vaca. Licenciatura Thesis, Universidad de San Carlos de Guatemala, de Guatemala, Guatemala, 2009. [Google Scholar]
- Macedo Ramírez, R.C.; Vélez-Ruíz, J.F. Propiedades Fisicoquímicas y de Flujo de Un Yogur Asentado Enriquecido Con Microcápsulas Que Contienen Ácidos Grasos Omega 3. Inf. Tecnol. 2015, 26, 87–96. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Yoghurt-Science and Technology; Woodhead Publishing: Sawston, UK, 1999. [Google Scholar]
- Ciron, C.I.E.; Gee, V.L.; Kelly, A.L.; Auty, M.A.E. Modifying the Microstructure of Low-Fat Yoghurt by Microfluidisation of Milk at Different Pressures to Enhance Rheological and Sensory Properties. Food Chem. 2012, 130, 510–519. [Google Scholar] [CrossRef]
- Truong, T.; Palmer, M.; Bansal, N.; Bhandari, B. Effect of Milk Fat Globule Size on Functionalities and Sensory Qualities of Dairy Products; Springer: Cham, Swizerland, 2016; pp. 47–67. [Google Scholar]
- Andoyo, R.; Guyomarc’h, F.; Burel, A.; Famelart, M.H. Spatial Arrangement of Casein Micelles and Whey Protein Aggregate Inacid Gels: Insight on Mechanisms. Food Hydrocoll. 2015, 51, 118–128. [Google Scholar] [CrossRef]
- Laiho, S.; Williams, R.P.W.; Poelman, A.; Appelqvist, I.; Logan, A. Effect of Whey Protein Phase Volume on the Tribology, Rheology and Sensory Properties of Fat-Free Stirred Yoghurts. Food Hydrocoll. 2017, 67, 166–177. [Google Scholar] [CrossRef]
- Hosseini-Parvar, S.H.; Matia-Merino, L.; Goh, K.K.T.; Razavi, S.M.A.; Mortazavi, S.A. Steady Shear Flow Behavior of Gum Extracted from Ocimum Basilicum L. Seed: Effect of Concentration and Temperature. J. Food Eng. 2010, 101, 236–243. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Zou, Z.; Shangguan, X.; Wang, H.; Bansal, N. Glycosylated Fish Gelatin Emulsion: Rheological, Tribological Properties and Its Application as Model Coffee Creamers. Food Hydrocoll. 2020, 102, 105552. [Google Scholar] [CrossRef]
- Quemada, D.; Berli, C. Energy of Interaction in Colloids and Its Implications in Rheological Modeling. Adv. Colloid Interface Sci. 2002, 98, 51–85. [Google Scholar] [CrossRef]
- Jaros, D.; Heidig, C.; Rohm, H. Enzymatic Modification Through Microbial Transglutaminase Enhances the Viscosity of Stirred Yogurt. J. Texture Stud. 2007, 38, 179–198. [Google Scholar] [CrossRef]
- Zhu, L.; Sun, N.; Papadopoulos, K.; De Kee, D. A Slotted Plate Device for Measuring Static Yield Stress. J. Rheol. 2001, 45, 1105–1122. [Google Scholar] [CrossRef]
- Ban, Q.; Liu, Z.; Yu, C.; Sun, X.; Jiang, Y.; Cheng, J.; Guo, M. Physiochemical, Rheological, Microstructural, and Antioxidant Properties of Yogurt Using Monk Fruit Extract as a Sweetener. J. Dairy Sci. 2020, 103, 10006–10014. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Li, J.; Zhang, T.; Zhu, T.; Cheng, R.; Wang, S.; Zhang, J. Salecan Stabilizes the Microstructure and Improves the Rheological Performance of Yogurt. Food Hydrocoll. 2018, 81, 474–480. [Google Scholar] [CrossRef]
- Pal, R. Effect of Droplet Size on the Rheology of Emulsions. AIChE J. 1996, 42, 3181–3190. [Google Scholar] [CrossRef]
- Shakeel, A.; Kirichek, A.; Chassagne, C. Yield Stress Measurements of Mud Sediments Using Different Rheological Methods and Geometries: An Evidence of Two-Step Yielding. Mar. Geol. 2020, 427, 106247. [Google Scholar] [CrossRef]
- Laity, P.R.; Holland, C. Thermo-Rheological Behaviour of Native Silk Feedstocks. Eur. Polym. J. 2017, 87, 519–534. [Google Scholar] [CrossRef]
- Garvín, A.; Ibarz, R.; Ibarz, A. Kinetic and Thermodynamic Compensation. A Current and Practical Review for Foods. Food Res. Int. 2017, 96, 132–153. [Google Scholar] [CrossRef]
- Crispín-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of Inulin and Agave Fructans Addition on the Rheological, Microstructural and Sensory Properties of Reduced-Fat Stirred Yogurt. LWT Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- Nguyen, P.T.M.; Kravchuk, O.; Bhandari, B.; Prakash, S. Effect of Different Hydrocolloids on Texture, Rheology, Tribology and Sensory Perception of Texture and Mouthfeel of Low-Fat Pot-Set Yoghurt. Food Hydrocoll. 2017, 72, 90–104. [Google Scholar] [CrossRef]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z. Okra Polysaccharide: Effect on the Texture and Microstructure of Set Yoghurt as a New Natural Stabilizer. Int. J. Biol. Macromol. 2019, 133, 117–126. [Google Scholar] [CrossRef]
- Aguirre-Mandujano, E.; Lobato-Calleros, C.; Beristain, C.I.; Garcia, H.S.; Vernon-Carter, E.J. Microstructure and Viscoelastic Properties of Low-Fat Yoghurt Structured by Monoglyceride Gels. LWT Food Sci. Technol. 2009, 42, 938–944. [Google Scholar] [CrossRef]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological Characterisation of Polymer Gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Kumar, A.; Sasmal, S. Rheological and Physico-Chemical Properties of Milk Gel Using Isolate of Pumpkin (Cucurbita moschata) Seeds: A New Source of Milk Clotting Peptidase. Food Hydrocoll. 2020, 106, 105866. [Google Scholar] [CrossRef]
- Harper, W.; Hall, C. Dairy Technology and Engineering, 2th ed.; AVI. Publishers: Lubumbashi, Zaire, 1981. [Google Scholar]
- Carpenter, R.P. Análisis Sensorial En El Desarrollo y Control de La Calidad de Alimentos; Editorial Acribia, S.A.: Zaragoza, Spain, 2002. [Google Scholar]
- Marcotte, M.; Hoshahili, A.R.T.; Ramaswamy, H.S. Rheological Properties of Selected Hydrocolloids as a Function of Concentration and Temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Lollo, P.C.B.; De Moura, C.S.; Morato, P.N.; Cruz, A.G.; Castro, W.; de Freitas Castro, W.; Betim, C.B.; Nisishima, L.; Faria, J.; de Assis, F.; et al. Probiotic Yogurt Offers Higher Immune-Protection than Probiotic Whey Beverage. Food Res. Int. 2013, 54, 118–124. [Google Scholar] [CrossRef]

| Sample Code | Xanthan Gum mg/100 g | Hydrocolloids mg/100 g |
|---|---|---|
| M1 | 0 | 0 |
| M2 | 1.00 | 0 |
| M3 | 0.75 | 0.25 |
| M4 | 0.50 | 0.50 |
| M5 | 0.25 | 0.75 |
| M6 | 0 | 1.00 |
| Sample Code | pH | Acidity % Acid Lactic | Density g/mL |
|---|---|---|---|
| M1 | 4.54 ± 0.01 a | 0.85 ± 0.005 a | 1.06 ± 0.00 a |
| M2 | 4.55 ± 0.01 a | 0.84 ± 0.005 a | 1.07 ± 0.00 a |
| M3 | 4.57 ± 0.01 a | 0.83 ± 0.005 a | 1.08 ± 0.00 a |
| M4 | 4.56 ± 0.05 a | 0.83 ± 0.005 a | 1.07 ± 0.00 a |
| M5 | 4.57 ± 0.01 b | 0.82 ± 0.005 a | 1.07 ± 0.00 a |
| M6 | 4.56 ± 0.05 a | 0.83 ± 0.005 a | 1.07 ± 0.00 a |
| Sample Code | L* | WI | |
|---|---|---|---|
| M1 | 62.81 1.94 ª | -- | 60.14 0.36 ª |
| M2 | 69.59 0.40 ª | 25.60 2.63 ª | 67.05 0.36 b |
| M3 | 70.11 ab | 58.20 6.20 b | 70.81 0.72 ab |
| M4 | 72.48 1.25 b | 61.13 7.75 b | 70.85 0.93 ab |
| M5 | 74.07 0.68 b | 79.15 10.51 c | 71.16 2.18 b |
| M6 | 76.11 0.50 b | 93.36 5.87 d | 73.88 0.38 b |
| Sample Code | Temp. °C | Pa·s | Pa·s | Pa | Pa |
|---|---|---|---|---|---|
| M1 | 5 | 79.20 a | 3.09 * | 0.15 b | 26.52 b |
| 10 | 51.12 a | 2.13 + | 0.14 b | 30.44 b | |
| 15 | 76.78 a | 1.48 + | 0.13 b | 28.93 b | |
| 25 | 66.20 a | 0.67 | 0.13 b | 29.72 b | |
| M2 | 5 | 256.3 b | 2.99 * | 0.66 a | 55.61 b |
| 10 | 84.83 b | 1.96 + | 0.23 a | 22.14 b | |
| 15 | 193.70 b | 1.68 +· | 0.37 a | 62.35 b | |
| 25 | 126.78 b | 0.87 + | 0.28 a | 40.96 b | |
| M3 | 5 | 299.50 c | 8.10 * | 0.61 a | 24.25 d |
| 10 | 211.04 c | 3.50 + | 0.43 a | 20.07 d | |
| 15 | 265.04 c | 3.06 +· | 0.52 a | 19.28 d | |
| 25 | 183.84 c | 1.30 | 0.28 a | 22.32 d | |
| M4 | 5 | 93.93 d | 8.15 * | 0.15 ab | 23.32 d |
| 10 | 220.07 d | 4.28 + | 0.48 ab | 24.09 d | |
| 15 | 90.97 d | 1.92 + | 0.14 ab | 23.06 d | |
| 25 | 66.28 d | 0.80 + | 0.15 ab | 23.03 d | |
| M5 | 5 | 37.52 e | 3.04 * | 0.12 b | 35.82 c |
| 10 | 30.75 e | 2.05 + | 0.12 b | 27.57 b | |
| 15 | 28.01 e | 1.33 + | 0.08 b | 19.42 b | |
| 25 | 29.30 e | 2.06 | 0.12 b | 26.87 b | |
| M6 | 5 | 14.40 f | 8.65 * | 0.05 ab | 27.69 b |
| 10 | 20.01 f | 1.42 + | 0.07 ab | 19.75 c | |
| 15 | 18.92 f | 1.38 + | 0.91 ab | 12.51 c | |
| 25 | 7.20 f | 0.05 | 0.05 ab | 11.07 c |
| Sample Code | Pa·s | Ea J/mol | R2 |
|---|---|---|---|
| M1 | 0.063 × 10−6 a | 528.55 a | 0.98 |
| M2 | 3.728 × 10−11 a | 561.05 b | 0.92 |
| M3 | 2.314 × 10−12 a | 485.09 c | 0.98 |
| M4 | 4.55 × 10−16 a | 734.03 d | 0.98 |
| M5 | 2.60 × 10−11 a | 679.55 e | 0.98 |
| M6 | 5.24 × 10−9 a | 723.26 f | 0.99 |
| Sample Code | Pa | Pa | Pa | Crossover Pa |
|---|---|---|---|---|
| M1 | 0.15 a | 6.359 a | 3.07 a | 2.57 cd |
| M2 | 0.15 a | 45.34 b | 11.60 b | 4.92 b |
| M3 | 0.25 ab | 53.74 c | 14.10 c | 6.49 a |
| M4 | 0.14 a | 15.39 d | 5.28 d | 3.09 c |
| M5 | 0.53 b | 2.152 e | 1.64 e | 1.47 a |
| M6 | 0.15 a | 11.65 f | 4.53 f | 2.48 d |
| Sample Code | Pa | Pa | |
|---|---|---|---|
| M1 | 1.25 a | 0.70 a | 0.56 a |
| M2 | 32.04 b | 8.97 a | 0.28 b |
| M3 | 34.35 c | 9.33 a | 0.28 b |
| M4 | 3.80 d | 2.08 a | 0.55 a |
| M5 | 10.88 e | 3.35 a | 0.30 b |
| M6 | 2.05 f | 1.30 a | 0.63 a |
| Parameters | Color | Odor | Flavor | Consistency |
|---|---|---|---|---|
| M1 | 4.10 ± 0.71 ab | 3.70 ± 0.70 ab | 4.16 ± 0.59 a | 3.36 ± 1.32 a |
| M2 | 4.00 ± 0.72 a | 3.76 ± 0.77 ab | 3.90 ± 0.99 a | 3.23 ± 1.07 a |
| M3 | 3.83 ± 0.87 a | 3.56 ± 0.72 a | 3.86 ± 1.10 a | 3.46 ± 0.93 a |
| M4 | 3.86 ± 0.86 a | 3.56 ± 0.81 a | 3.90 ± 0.92 a | 3.26 ± 0.98 a |
| M5 | 4.16 ± 0.74 ab | 3.96 ± 0.55 bc | 3.96 ± 0.96 a | 3.76 ± 0.93 a |
| M6 | 4.45 ± 0.72 ab | 4.26 ± 0.78 c | 4.26 ± 0.86 a | 3.63 ± 1.29 a |
| Parameters | M6 | Common Yogurt * | Probiotic Yogurt * |
|---|---|---|---|
| Moisture (%) | 79.58 ± 0.47 | 88.72 | 88.74 |
| Ash (%) | 0.56 ± 0.05 | 0.72 | 0.71 |
| Fat (%) | 4.68 ± 0.11 | 2.45 | 2.42 |
| Carbohydrates (%) | 8.18 ± 0.43 | 5.24 | 5.20 |
| Proteins (%) | 6.10 ± 0.18 | 2.90 | 2.92 |
| Fiber (%) | 0.89 ± 0.02 | -- | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Torres, S.A.; Quintana, S.E.; García-Zapateiro, L.A. Natural Yogurt Stabilized with Hydrocolloids from Butternut Squash (Cucurbita moschata) Seeds: Effect on Physicochemical, Rheological Properties and Sensory Perception. Fluids 2021, 6, 251. https://doi.org/10.3390/fluids6070251
Rojas-Torres SA, Quintana SE, García-Zapateiro LA. Natural Yogurt Stabilized with Hydrocolloids from Butternut Squash (Cucurbita moschata) Seeds: Effect on Physicochemical, Rheological Properties and Sensory Perception. Fluids. 2021; 6(7):251. https://doi.org/10.3390/fluids6070251
Chicago/Turabian StyleRojas-Torres, Sergio A., Somaris E. Quintana, and Luis Alberto García-Zapateiro. 2021. "Natural Yogurt Stabilized with Hydrocolloids from Butternut Squash (Cucurbita moschata) Seeds: Effect on Physicochemical, Rheological Properties and Sensory Perception" Fluids 6, no. 7: 251. https://doi.org/10.3390/fluids6070251
APA StyleRojas-Torres, S. A., Quintana, S. E., & García-Zapateiro, L. A. (2021). Natural Yogurt Stabilized with Hydrocolloids from Butternut Squash (Cucurbita moschata) Seeds: Effect on Physicochemical, Rheological Properties and Sensory Perception. Fluids, 6(7), 251. https://doi.org/10.3390/fluids6070251








