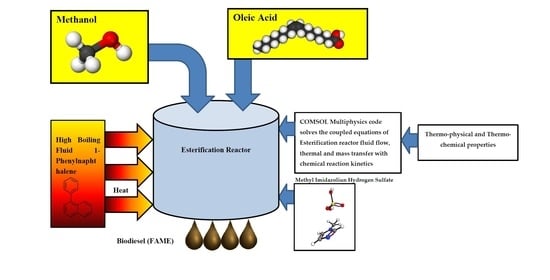
Thermal Hydraulics and Thermochemical Design of Fatty Acid Methyl Ester (Biodiesel) Esterification Reactor by Heating with High Boiling Point Phenyl-Naphthalene Liquid
Abstract
:1. Introduction
1.1. Review of the Biodiesel Production by Applying Ionic Liquid
Numerical Simulations of Biodiesel Production
1.2. Advantages of Applying High Boiling Liquid Heat Carriers
- (1)
- By using high boiling point liquids operating at atmospheric pressure, it is possible to construct heating plants that are very easy to run and are reliable in operation.
- (2)
- It is possible to control the heating temperature.
- (3)
- The great qualities of high boiling temperature liquids make them more suitable and safer. This is because water coming into contact with liquid metal may cause a steam explosion hazard.
- (4)
- It eliminates the need of using heavy forgings for pressure vessels and piping.
- (5)
- Heat carrier compatibility with low-cost materials and virtually no corrosion potential enables the usage of plain carbon steel and aluminum alloys.
Critical Heat Flux of Water
2. Materials and Methods
2.1. Thermophysical Properties of Methylimidazolium Hydrogen Sulfate
2.2. Multiphysics Analyses of the Esterification Reactor
2.2.1. Model Kinetics
2.2.2. Fluid Flow and Continuity Equations
2.2.3. Heat Transfer Equation
2.2.4. Diffusion Transport Equation
2.3. Thermo-Physical Properties of 1-Phenylnaphthalene
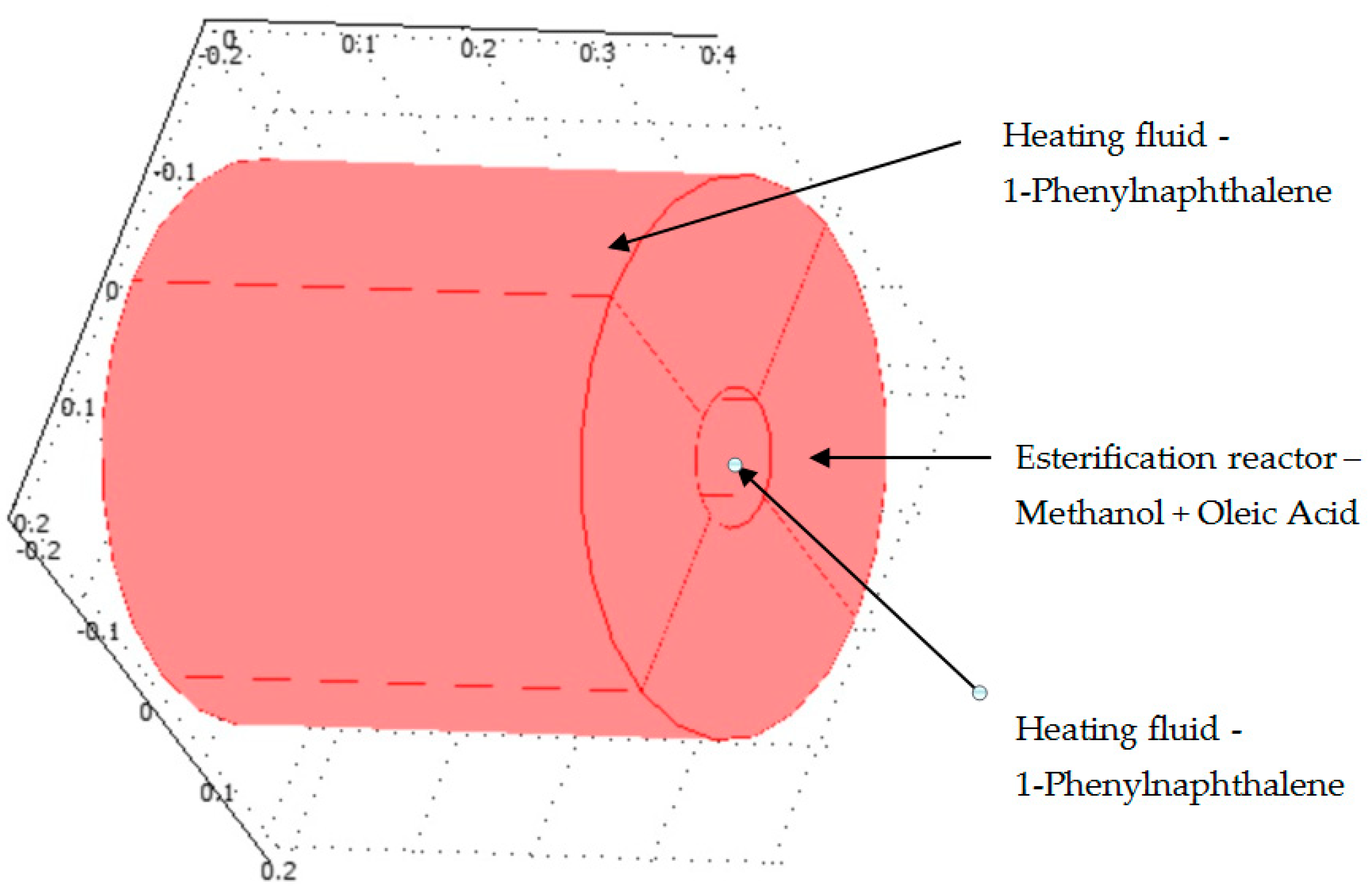
2.4. Calculation of Convective Heat Transfer Inside the Reactor Hole
2.5. Boundary Conditions
3. Results
Numerical Model Convergence and Validation
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
Nomenclature
| A | frequency factor in [1/s] |
| C | concentration |
| cp | specific heat in [J/(kg·K)] |
| D | diffusion coefficient in [m2/s] |
| E | activation energy in [J/mole] |
| Hf | enthalpy of formation in [J/mole] |
| h | convective coefficient in [W/(m2·K)] |
| k | thermal conductivity in [W/(m·K)] |
| p | pressure in [Pa] |
| atmospheric pressure in [Pa] | |
| Pr | Prandtl number |
| R | reaction rate on [mole/(m3·s)] |
| gas constant (8.3143 J/(mole·K)) | |
| Re | Reynolds number |
| inner radius [m] | |
| outer radius [m] | |
| Nu | Nusselt number |
| T | temperature in [K] |
| X | conversion |
| velocity vector in [m/s] | |
| Subscripts | |
| FAME | fatty acid methyl ester |
| in | inlet, inner radius |
| Met | methanol |
| OA | oleic acid |
| Out | outlet, outer |
| P | phenylnaphthalene liquid |
| Greek letters | |
| η | viscosity in [Pa·s] |
| ν | velocity of Phenylnaphthalene liquid in [m/s] |
| ρ | density in [kg/m3] |
| Abbreviation | |
| FAME | fatty acid methyl ester |
| IL | ionic liquid |
References
- Fang, Z.; Smith, R.L., Jr.; Qi, X. Productions of Biofuels and Chemicals with Ionic Liquid; Springer: Heidelberg, Germany, 2014. [Google Scholar]
- Tran, A.T.; Tomlin, J.; Lam, P.H.; Stinger, B.L.; Miller, A.D.; Walczyk, D.J.; Cruz, O.; Vaden, T.D.; Yu, L. Conductivity, Viscosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids. ChemEngineering 2019, 3, 81. Available online: https://www.mdpi.com/2305-7084/3/4/81 (accessed on 7 January 2022). [CrossRef] [Green Version]
- Wu, B.; Reddy, R.G.; Rogers, R.D. Novel Ionic Liquid Thermal Storage for Solar Thermal Electric Power Systems. In Proceedings of the Solar Forum 2001, Solar Energy: The Power to Choose, Washington, DC, USA, 21–25 April 2001. [Google Scholar]
- Roman, F.F. Biodiesel Production Through Esterification Applying Ionic Liquid as Catalyst. Master’s Thesis, Escola Superior de Tecnologia e Gestão do Instituto Politécnico de Bragança, Braganca, Portugal, 2018. [Google Scholar]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Kuzmina, O.; Hallet, J.P. Application Purification and Recovery of Ionic Liquid; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Lucu, R.; Melero, J.A. Advances in Biodiesel Production Processes and Technologies; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Hamdy Makhlouf, A.S.; Aliofkhazraei, M. Handbook of Material Failure Analysis with Case Studies from the Chemicals, Concrete and Power Industries; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ha, S.; Lan, M.; Lee, S.; Hwang, S.; Koo, Y. Lipase-catalyzed biodiesel production from soybean oil in ionic liquids. Enzym. Microbiol. Technol. 2007, 41, 480–483. [Google Scholar] [CrossRef]
- Lozano, P.; De Tiego, T.; Iborra, J.; Vaultier, M. Use of Ionic Liquids for Implementing a Process for the Preparation of Biodiesel. European Patent EP 2189535 A1, 21 November 2008. [Google Scholar]
- Elsheikh, Y.A.; Man, Z.; Bustam, M.A.; Yusup, S.; Wilfred, C.D. Brønsted imidazolium ionic liquids: Synthesis and comparison of their catalytic activities as pre-catalyst for biodiesel production through two stage process. Energy Convers. Manag. 2011, 52, 804–809. [Google Scholar] [CrossRef]
- Fauzi, A.H.M.; Amin, N.A.S. Optimization of oleic acid esterification catalyzed by ionic liquid for green biodiesel synthesis. Energy Convers. Manag. 2013, 76, 818–827. [Google Scholar] [CrossRef]
- Mohiuddin, A.K.M.; Adeyemi, N. Numerical Simulation of Biodiesel Production Using Waste Cooking Oil. In Proceedings of the ASME 2013 International Mechanical Engineering Congress and Exposition IMECE2013, San Diego, CA, USA, 15–21 November 2013. [Google Scholar]
- Mekala, S.J. CFD Studies of Reactive Flow with Thermal and Mass Diffusional effects in a Supercritical Packed Bed Catalytic Reactor. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2016. [Google Scholar]
- Chechetkin, A.V. High Temperature Heat Carriers, Pergamon Press Book; The Macmillam Company: New Yok, NY, USA, 1963. [Google Scholar]
- Shirvan, K.; Forest, E. Design of an Organic Simplified Nuclear Reactor. Nucl. Eng. Technol. 2016, 48, 893–905. [Google Scholar] [CrossRef] [Green Version]
- Davidy, A. CFD Design of Hydrogenation Reactor for Transformation of Levulinic Acid to γ-Valerolactone (GVL) by using High Boiling Point Organic Fluids. ChemEngineering 2019, 3, 32. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, G.F. Chapter 7: Pool Boiling. In Two Phase Flow and Heat Transfer; Butterworth, D., Hewitt, G.F., Eds.; Oxford University Press: Oxford, UK, 1977. [Google Scholar]
- McCabe, W.L.; Smith, J.C. Unit Operations of Chemical Engineering, 2nd ed.; MacGraw-Hill, Inc.: New York, NY, USA, 1967. [Google Scholar]
- Marcus, Y. Ionic Liquid Properties from molten salt to RTIL; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Zhao, H. Innovative Applications of ionic liquids as “green” engineering liquids. Chem. Eng. Commun. 2006, 193, 1660–1677. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Structure Evolution of Synthetic Amino Acids-Derived Basic Ionic Liquids for Catalytic Production of Biodiesel. ACS Sustain. Chem. Eng. 2017, 5, 1237–1247. [Google Scholar] [CrossRef]
- COMSOL. Multiphysics—Modeling Guide, Version 4.3b; COMSOL AB: Stockholm, Sweden, 2013. [Google Scholar]
- Cheméo, High Quality Chemical Properties Web Site. Available online: https://www.chemeo.com/ (accessed on 5 February 2022).
- Standard Enthalpy of Formation. Available online: https://en.wikipedia.org/wiki/Standard_enthalpy_of_formation (accessed on 5 February 2022).
- McFarlane, J.; Luo, H.; Garland, M.; Steele, W.V. Evaluation of Phenylnaphthalenes as Heat Transfer Fluids for High Temperature Energy Applications. Sep. Sci. Technol. 2010, 45, 1908–1920. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Moran, M.J.; Shapiro, H.N.; Boettner, D.D.; Bailey, M.B. Fundamentals of Engineering Thermodynamics, 7th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gräsvik, J. Ionic liquids in bio-Refining Synthesis and Applications. Ph.D. Thesis, Umeå University, Umea, Sweden, 2013. [Google Scholar]










| Material Property | Value |
|---|---|
| ρ | 1367 (kg/m3) |
| Cp | 1280 (J/(kg·°C)) |
| k | 0.2 (w/(m·°C)) |
| η | 0.0367 (Pa·s) |
| Material Property | Value |
|---|---|
| ρ | 358 (kg/m3) |
| Cp | 2323 (J/(kg·°C)) |
| k | 0.077 (W/(m·°C)) |
| η | 0.00011 (Pa·s) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidy, A. Thermal Hydraulics and Thermochemical Design of Fatty Acid Methyl Ester (Biodiesel) Esterification Reactor by Heating with High Boiling Point Phenyl-Naphthalene Liquid. Fluids 2022, 7, 93. https://doi.org/10.3390/fluids7030093
Davidy A. Thermal Hydraulics and Thermochemical Design of Fatty Acid Methyl Ester (Biodiesel) Esterification Reactor by Heating with High Boiling Point Phenyl-Naphthalene Liquid. Fluids. 2022; 7(3):93. https://doi.org/10.3390/fluids7030093
Chicago/Turabian StyleDavidy, Alon. 2022. "Thermal Hydraulics and Thermochemical Design of Fatty Acid Methyl Ester (Biodiesel) Esterification Reactor by Heating with High Boiling Point Phenyl-Naphthalene Liquid" Fluids 7, no. 3: 93. https://doi.org/10.3390/fluids7030093
APA StyleDavidy, A. (2022). Thermal Hydraulics and Thermochemical Design of Fatty Acid Methyl Ester (Biodiesel) Esterification Reactor by Heating with High Boiling Point Phenyl-Naphthalene Liquid. Fluids, 7(3), 93. https://doi.org/10.3390/fluids7030093