Experimental Comparison of Methods to Evaluate Heat Generated by Magnetic Nanofluids Exposed to Alternating Magnetic Fields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Nanoparticles
2.1.1. Synthesis of C26
2.1.2. Synthesis of C12
2.1.3. Synthesis of C5
2.2. Nanofluid
2.2.1. TEM Analysis for Shape and Size
2.2.2. Hydrodynamic Radius
2.2.3. Magnetic Properties
2.2.4. ICP-MS Characterization
2.3. Temperature vs. Time Characterization
2.3.1. Nanofluid Heating
2.3.2. SLP Obtained by Exponential Function Analysis
2.3.3. SLP Obtained by Initial Slope Method
2.3.4. SLP Obtained by Quasi-Adiabatic Calculation
3. Results
3.1. Magneto-Fluid Characterization
3.1.1. Size Analysis from TEM Image
3.1.2. Size Analysis: Hydrodynamic Diameter
3.1.3. Magnetic Properties
3.1.4. ICP-MS Analysis
3.2. Temperature vs. TIME Characterization
3.2.1. Evaluation of the SLP by Means of the Exponential Method and Initial Slope
3.2.2. Evaluation of the SLP by Selection of Quasi-Adiabatic Heating Interval
4. Discussion
4.1. Size Characterization
4.2. Thermal Characterization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jordan, A.; Scholz, R.; Wust, P.; Fahling, H.; Roland, F. Magnetic Fluid Hyperthermia (MFH): Cancer Treatment with AC Magnetic Field Induced Excitation of Biocompatible Superparamagnetic Nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Krause, J.; Wlodarczyk, W.; Sander, B.; Vogl, T.; Felix, R. Effects of Magnetic Fluid Hyperthermia (MFH) on C3H Mammary Carcinoma In Vivo. Int. J. Hyperth. 1997, 13, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Gneveckow, U.; Jordan, A.; Scholz, R.; Brüss, V.; Waldöfner, N.; Ricke, J.; Feussner, A.; Hildebrandt, B.; Rau, B.; Wust, P. Description and Characterization of the Novel Hyperthermia- and Thermoablation-System MFH 300F for Clinical Magnetic Fluid Hyperthermia. Med. Phys. 2004, 31, 1444–1451. [Google Scholar] [CrossRef]
- Goya, G.F.; Asín, L.; Ibarra, M.R. Cell Death Induced by AC Magnetic Fields and Magnetic Nanoparticles: Current State and Perspectives. Int. J. Hyperth. 2013, 29, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Baronzio, G. A Brief Overview of Hyperthermia in Cancer Treatment. J. Integr. Oncol. 2014, 3, 115. [Google Scholar] [CrossRef] [Green Version]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of High Amplitude Alternating Magnetic Fields for Heat Induction of Nanoparticles Localized in Cancer. Clin. Cancer Res. 2005, 11, 7093s–7103s. [Google Scholar] [CrossRef] [Green Version]
- Rosensweig, R.E. Heating Magnetic Fluid with Alternating Magnetic Field. J. Magn. Magn. Mater. 2002, 252, 370. [Google Scholar] [CrossRef]
- Dewhirst, M.; Stauffer, P.R.; Das, S.; Craciunescu, O.I.; Vujaskovic, Z.; Gunderson, L.; Tepper, J. Hyperthermia. In Clinical Radiation Oncology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–398. ISBN 978-0-323-67246-7. [Google Scholar]
- Fortin, J.P.; Gazeau, F.; Wilhelm, C. Intracellular Heating of Living Cells through Neel Relaxation of Magnetic Nanoparticles. Eur. Biophys. J. EBJ 2008, 37, 223–228. [Google Scholar] [CrossRef]
- Borkamo, E.D.; Fluge, O.; Mella, O.; Akslen, L.A.; Bruland, O.; Dahl, O. Hyperthermia Improves the Antitumour Effect of Metronomic Cyclophosphamide in a Rat Transplantable Brain Tumour. Radiother. Oncol. 2008, 86, 435–442. [Google Scholar] [CrossRef]
- Moroz, P.; Jones, S.K.; Gray, B.N. Magnetically Mediated Hyperthermia: Current Status and Future Directions. Int. J. Hyperth. 2002, 18, 267–284. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The Cellular and Molecular Basis of Hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Gerweck, L.E. Hyperthermia in Cancer Therapy: The Biological Basis and Unresolved Questions. Cancer Res. 1985, 45, 3408–3414. [Google Scholar] [PubMed]
- Dutz, S.; Hergt, R. Magnetic Nanoparticle Heating and Heat Transfer on a Microscale: Basic Principles, Realities and Physical Limitations of Hyperthermia for Tumour Therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Bian, L.; Ma, L.-J.; Tang, R.-Z.; Xun, S.; He, Y.-W. Hyperthermia-Induced Apoptosis in Tca8113 Cells Is Inhibited by Heat Shock Protein 27 through Blocking Phospholipid Scramblase 3 Phosphorylation. Int. J. Hyperth. 2010, 26, 523–537. [Google Scholar] [CrossRef]
- Nishikawa, H.; Kimura, T.; Kita, R.; Osaki, Y. Radiofrequency Ablation for Hepatocellular Carcinoma. Int. J. Hyperth. 2013, 29, 558–568. [Google Scholar] [CrossRef]
- Hergt, R.; Andrä, W. Magnetic Hyperthermia and Thermoablation. In Magnetism in Medicine: A Handbook, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; 550p. [Google Scholar]
- Kozissnik, B.; Bohorquez, A.C.; Dobson, J.; Rinaldi, C. Magnetic Fluid Hyperthermia: Advances, Challenges, and Opportunity. Int. J. Hyperth. 2013, 29, 706–714. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical Nanomagnetics: A Spin Through Possibilities in Imaging, Diagnostics, and Therapy. Magn. IEEE Trans. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [Green Version]
- Giordano, M.A.; Gutierrez, G.; Rinaldi, C. Fundamental Solutions to the Bioheat Equation and Their Application to Magnetic Fluid Hyperthermia. Int. J. Hyperth. 2010, 26, 475–484. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Maier-Hauff, K.; Johannsen, M.; Wust, P.; Nadobny, J.; Schirra, H.; Schmidt, H.; Deger, S.; Loening, S.; et al. Presentation of a New Magnetic Field Therapy System for the Treatment of Human Solid Tumors with Magnetic Fluid Hyperthermia. J. Magn. Magn. Mater. 2001, 225, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Short, J.G.; Turner, P.F. Physical Hyperthermia and Cancer Therapy. Proc. IEEE 1980, 68, 133–142. [Google Scholar] [CrossRef]
- Brezovich, I.A.; Atkinson, W.J.; Lilly, M.B. Local Hyperthermia with Interstitial Techniques. Cancer Res. 1984, 44, 4752s–4756s. [Google Scholar]
- Arriortua, O.K.; Garaio, E.; Herrero de la Parte, B.; Insausti, M.; Lezama, L.; Plazaola, F.; García, J.A.; Aizpurua, J.M.; Sagartzazu, M.; Irazola, M.; et al. Antitumor Magnetic Hyperthermia Induced by RGD-Functionalized Fe 3 O 4 Nanoparticles, in an Experimental Model of Colorectal Liver Metastases. Beilstein J. Nanotechnol. 2016, 7, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- Herrero de la Parte, B.; Rodrigo, I.; Gutiérrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarría-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; García-Alonso, I. Proposal of New Safety Limits for In Vivo Experiments of Magnetic Hyperthermia Antitumor Therapy. Cancers 2022, 14, 3084. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jang, J.; Choi, J.; Moon, S.H.; Noh, S.; Kim, J.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-Coupled Magnetic Nanoparticles for Efficient Heat Induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic Fluid Hyperthermia: Focus on Superparamagnetic Iron Oxide Nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Zeisberger, M.; Dutz, S.; Müller, R.; Hergt, R.; Matoussevitch, N.; Bönnemann, H. Metallic Cobalt Nanoparticles for Heating Applications. J. Magn. Magn. Mater. 2007, 311, 224–227. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic Particle Hyperthermia—Biophysical Limitations of a Visionary Tumour Therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Dutz, S.H.R. Magnetic Nanoparticles for Biomedical Heating Applications. Z. Phys. Chem. 2005, 220, 145. [Google Scholar] [CrossRef]
- Roca, A.G.; Costo, R.; Rebolledo, A.F.; Veintemillas-Verdaguer, S.; Tartaj, P.; González-Carreño, T.; Morales, M.P.; Serna, C.J. Progress in the Preparation of Magnetic Nanoparticles for Applications in Biomedicine. J. Phys. D Appl. Phys. 2009, 42, 224002. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S. Superparamagnetic Nanoparticles as Targeted Probes for Diagnostic and Therapeutic Applications. Dalton Trans. 2009, 29, 5583–5591. [Google Scholar] [CrossRef] [Green Version]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic Nanoparticles: Synthesis, Functionalization, and Applications in Bioimaging and Magnetic Energy Storage. Chem. Soc. Rev. 2009, 38, 2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2003, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204. [Google Scholar] [CrossRef]
- Borgohain, C.; Senapati, K.K.; Mishra, D.; Sarma, K.C.; Phukan, P. A New CoFe2O4–Cr2O3–SiO2 Fluorescent Magnetic Nanocomposite. Nanoscale 2010, 2, 2250. [Google Scholar] [CrossRef]
- Baldi, G.; Bonacchi, D.; Franchini, M.C.; Gentili, D.; Lorenzi, G.; Ricci, A.; Ravagli, C. Synthesis and Coating of Cobalt Ferrite Nanoparticles: A First Step toward the Obtainment of New Magnetic Nanocarriers. Langmuir ACS J. Surf. Colloids 2007, 23, 4026–4028. [Google Scholar] [CrossRef]
- Goya, G.F.; Grazu, V.; Ibarra, M.R. Magnetic nanoparticles for cancer therapy. Curr. Nanosci. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Pal, R. Fundamental Rheology of Disperse Systems Based on Single-Particle Mechanics. Fluids 2016, 1, 40. [Google Scholar] [CrossRef] [Green Version]
- Darwish, M.S.A.; Kim, H.; Bui, M.P.; Le, T.-A.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. The Heating Efficiency and Imaging Performance of Magnesium Iron Oxide@tetramethyl Ammonium Hydroxide Nanoparticles for Biomedical Applications. Nanomaterials 2021, 11, 1096. [Google Scholar] [CrossRef]
- Goya, G.F.; Berquó, T.S.; Fonseca, F.C.; Morales, M.P. Static and Dynamic Magnetic Properties of Spherical Magnetite Nanoparticles. J. Appl. Phys. 2003, 94, 3520–3528. [Google Scholar] [CrossRef] [Green Version]
- Natividad, E.; Castro, M.; Mediano, A. Adiabatic Magnetothermia Makes Possible the Study of the Temperature Dependence of the Heat Dissipated by Magnetic Nanoparticles under Alternating Magnetic Fields. Appl. Phys. Lett. 2011, 98, 243119. [Google Scholar] [CrossRef] [Green Version]
- Natividad, E.; Castro, M.; Mediano, A. Adiabatic vs. Non-Adiabatic Determination of Specific Absorption Rate of Ferrofluids. J. Magn. Magn. Mater. 2009, 321, 1497–1500. [Google Scholar] [CrossRef]
- Andreu, I.; Natividad, E. Accuracy of Available Methods for Quantifying the Heat Power Generation of Nanoparticles for Magnetic Hyperthermia. Int. J. Hyperth. 2013, 29, 739–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soetaert, F.; Kandala, S.K.; Bakuzis, A.; Ivkov, R. Experimental Estimation and Analysis of Variance of the Measured Loss Power of Magnetic Nanoparticles. Sci. Rep. 2017, 7, 6661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the Reliable Measurement of Specific Absorption Rates and Intrinsic Loss Parameters in Magnetic Hyperthermia Materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Ranoo, S.; Philip, J. Uncertainties in the Estimation of Specific Absorption Rate during Radiofrequency Alternating Magnetic Field Induced Non-Adiabatic Heating of Ferrofluids. J. Phys. D Appl. Phys. 2017, 50, 455005. [Google Scholar] [CrossRef]
- Bordelon, D.E.; Cornejo, C.; Grüttner, C.; Westphal, F.; DeWeese, T.L.; Ivkov, R. Magnetic Nanoparticle Heating Efficiency Reveals Magneto-Structural Differences When Characterized with Wide Ranging and High Amplitude Alternating Magnetic Fields. J. Appl. Phys. 2011, 109, 124904. [Google Scholar] [CrossRef]
- Pavel, M.; Stancu, A. Ferromagnetic Nanoparticles Dose Based on Tumor Size in Magnetic Fluid Hyperthermia Cancer Therapy. Magn. IEEE Trans. 2009, 45, 5251–5254. [Google Scholar] [CrossRef]
- Dennis, C.L.; Ivkov, R. Physics of Heat Generation Using Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estradé, S.; Peiró, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013, 3, 1652. [Google Scholar] [CrossRef] [Green Version]
- Urtizberea, A.; Natividad, E.; Arizaga, A.; Castro, M.; Mediano, A. Specific Absorption Rates and Magnetic Properties of Ferrofluids with Interaction Effects at Low Concentrations. J. Phys. Chem. C 2010, 114, 4916–4922. [Google Scholar] [CrossRef]
- De la Presa, P.; Luengo, Y.; Multigner, M.; Costo, R.; Morales, M.P.; Rivero, G.; Hernando, A. Study of Heating Efficiency as a Function of Concentration, Size, and Applied Field in γ-Fe2O3 Nanoparticles. J. Phys. Chem. C 2012, 116, 25602–25610. [Google Scholar] [CrossRef]
- Tomitaka, A.; Ueda, K.; Yamada, T.; Takemura, Y. Heat Dissipation and Magnetic Properties of Surface-Coated Fe3O4 Nanoparticles for Biomedical Applications. J. Magn. Magn. Mater. 2012, 324, 3437–3442. [Google Scholar] [CrossRef]
- Branquinho, L.C.; Carrião, M.S.; Costa, A.S.; Zufelato, N.; Sousa, M.H.; Miotto, R.; Ivkov, R.; Bakuzis, A.F. Effect of Magnetic Dipolar Interactions on Nanoparticle Heating Efficiency: Implications for Cancer Hyperthermia. Sci. Rep. 2013, 3, 2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, E.; Mirhabibi, A.; Edrissi, M. Synthesis and Rheological Properties of an Iron Oxide Ferrofluid. J. Magn. Magn. Mater. 2008, 320, 2635–2639. [Google Scholar] [CrossRef]
- ImageJ. Available online: http://imagej.nih.gov/ij/ (accessed on 3 October 2014).
- Ruggiero, M.R.; Crich, S.G.; Sieni, E.; Sgarbossa, P.; Forzan, M.; Cavallari, E.; Stefania, R.; Dughiero, F.; Aime, S. Magnetic Hyperthermia Efficiency and 1H-NMR Relaxation Properties of Iron Oxide/Paclitaxel-Loaded PLGA Nanoparticles. Nanotechnology 2016, 27, 285104. [Google Scholar] [CrossRef] [Green Version]
- Spizzo, F.; Sgarbossa, P.; Sieni, E.; Semenzato, A.; Dughiero, F.; Forzan, M.; Bertani, R.; Del Bianco, L. Synthesis of Ferrofluids Made of Iron Oxide Nanoflowers: Interplay between Carrier Fluid and Magnetic Properties. Nanomaterials 2017, 7, 373. [Google Scholar] [CrossRef] [Green Version]
- Di Barba, P.D.; Dughiero, F.; Sieni, E. Magnetic Field Synthesis in the Design of Inductors for Magnetic Fluid Hyperthermia. Magn. IEEE Trans. 2010, 46, 2931–2934. [Google Scholar] [CrossRef]
- Bertani, R.; Ceretta, F.; Barba, P.D.; Dughiero, F.; Forzan, M.; Michelin, R.A.; Sgarbossa, P.; Sieni, E.; Spizzo, F. Optimal Inductor Design for Nanofluid Heating Characterisation. Eng. Comput. 2015, 32, 1870–1892. [Google Scholar] [CrossRef]
- Van de Hulst, H.C. Light Scattering by Small Particles; Dover Publications: New York, NY, USA, 1981; ISBN 978-0-486-64228-4. [Google Scholar]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley-VCH: Weinheim, Germany, 2004; ISBN 978-0-471-29340-8. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; IEEE/Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-471-47741-9. [Google Scholar]
- Coey, J.M.D. Noncollinear Spin Arrangement in Ultrafine Ferrimagnetic Crystallites. Phys. Rev. Lett. 1971, 27, 1140–1142. [Google Scholar] [CrossRef] [Green Version]
- Coduri, M.; Masala, P.; Del Bianco, L.; Spizzo, F.; Ceresoli, D.; Castellano, C.; Cappelli, S.; Oliva, C.; Checchia, S.; Allieta, M.; et al. Local Structure and Magnetism of Fe2O3 Maghemite Nanocrystals: The Role of Crystal Dimension. Nanomaterials 2020, 10, 867. [Google Scholar] [CrossRef]
- Morales, M.P.; Veintemillas-Verdaguer, S.; Montero, M.I.; Serna, C.J.; Roig, A.; Casas, L.; Martínez, B.; Sandiumenge, F. Surface and Internal Spin Canting in γ-Fe2O3 Nanoparticles. Chem. Mater. 1999, 11, 3058–3064. [Google Scholar] [CrossRef]
- Parker, F.T.; Foster, M.W.; Margulies, D.T.; Berkowitz, A.E. Spin Canting, Surface Magnetization, and Finite-Size Effects in γ-Fe2O3 Particles. Phys. Rev. B 1993, 47, 7885–7891. [Google Scholar] [CrossRef]
- Zhen, G.; Muir, B.W.; Moffat, B.A.; Harbour, P.; Murray, K.S.; Moubaraki, B.; Suzuki, K.; Madsen, I.; Agron-Olshina, N.; Waddington, L.; et al. Comparative Study of the Magnetic Behavior of Spherical and Cubic Superparamagnetic Iron Oxide Nanoparticles. J. Phys. Chem. C 2011, 115, 327–334. [Google Scholar] [CrossRef]
- Luigjes, B.; Woudenberg, S.M.C.; de Groot, R.; Meeldijk, J.D.; Torres Galvis, H.M.; de Jong, K.P.; Philipse, A.P.; Erné, B.H. Diverging Geometric and Magnetic Size Distributions of Iron Oxide Nanocrystals. J. Phys. Chem. C 2011, 115, 14598–14605. [Google Scholar] [CrossRef]
- Del Bianco, L.; Spizzo, F.; Barucca, G.; Ruggiero, M.R.; Geninatti Crich, S.; Forzan, M.; Sieni, E.; Sgarbossa, P. Mechanism of Magnetic Heating in Mn-Doped Magnetite Nanoparticles and the Role of Intertwined Structural and Magnetic Properties. Nanoscale 2019, 11, 10896–10910. [Google Scholar] [CrossRef]
- Yang, L.; Ma, L.; Xin, J.; Li, A.; Sun, C.; Wei, R.; Ren, B.W.; Chen, Z.; Lin, H.; Gao, J. Composition Tunable Manganese Ferrite Nanoparticles for Optimized T2 Contrast Ability. Chem. Mater. 2017, 29, 3038–3047. [Google Scholar] [CrossRef]
- Giri, J.; Pradhan, P.; Sriharsha, T.; Bahadur, D. Preparation and Investigation of Potentiality of Different Soft Ferrites for Hyperthermia Applications. J. Appl. Phys. 2005, 97, 10Q916. [Google Scholar] [CrossRef] [Green Version]
- Dormann, J.L.; Fiorani, D.; Tronc, E. Magnetic Relaxation in Fine-Particle Systems. In Advances in Chemical Physics; Prigogine, I., Rice, S.A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 283–494. ISBN 978-0-470-14157-1. [Google Scholar]
- Blanco-Mantecón, M.; O’Grady, K. Interaction and Size Effects in Magnetic Nanoparticles. J. Magn. Magn. Mater. 2006, 296, 124–133. [Google Scholar] [CrossRef]
- Burrows, F.; Parker, C.; Evans, R.F.L.; Hancock, Y.; Hovorka, O.; Chantrell, R.W. Energy Losses in Interacting Fine-Particle Magnetic Composites. J. Phys. D Appl. Phys. 2010, 43, 474010. [Google Scholar] [CrossRef] [Green Version]
- Ovejero, J.G.; Spizzo, F.; Morales, M.P.; Del Bianco, L. Nanoparticles for Magnetic Heating: When Two (or More) Is Better Than One. Materials 2021, 14, 6416. [Google Scholar] [CrossRef]
- El-Hilo, M.; O’Grady, K.; Chantrell, R.W. Susceptibility Phenomena in a Fine Particle System. J. Magn. Magn. Mater. 1992, 114, 295–306. [Google Scholar] [CrossRef]
- Binns, C.; Maher, M.J.; Pankhurst, Q.A.; Kechrakos, D.; Trohidou, K.N. Magnetic Behavior of Nanostructured Films Assembled from Preformed Fe Clusters Embedded in Ag. Phys. Rev. B 2002, 66, 184413. [Google Scholar] [CrossRef] [Green Version]
- Coral, D.F.; Mendoza Zélis, P.; Marciello, M.; Morales, M.d.P.; Craievich, A.; Sánchez, F.H.; Fernández van Raap, M.B. Effect of Nanoclustering and Dipolar Interactions in Heat Generation for Magnetic Hyperthermia. Langmuir 2016, 32, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Del Bianco, L.; Lesci, I.G.; Fracasso, G.; Barucca, G.; Spizzo, F.; Tamisari, M.; Scotti, R.; Ciocca, L. Synthesis of Nanogranular Fe3O4/Biomimetic Hydroxyapatite for Potential Applications in Nanomedicine: Structural and Magnetic Characterization. Mater. Res. Express 2015, 2, 065002. [Google Scholar] [CrossRef]
- Bedanta, S.; Kleemann, W. Supermagnetism. J. Phys. D Appl. Phys. 2009, 42, 013001. [Google Scholar] [CrossRef]
- Dormann, J.L.; Cherkaoui, R.; Spinu, L.; Noguès, M.; Lucari, F.; D’Orazio, F.; Fiorani, D.; Garcia, A.; Tronc, E.; Jolivet, J.P. From Pure Superparamagnetic Regime to Glass Collective State of Magnetic Moments in γ-Fe2O3 Nanoparticle Assemblies. J. Magn. Magn. Mater. 1998, 187, L139–L144. [Google Scholar] [CrossRef]
- Del Bianco, L.; Spizzo, F.; Barucca, G.; Marangoni, G.; Sgarbossa, P. Glassy Magnetic Behavior and Correlation Length in Nanogranular Fe-Oxide and Au/Fe-Oxide Samples. Materials 2019, 12, 3958. [Google Scholar] [CrossRef] [Green Version]
- Ring, H.L.; Sharma, A.; Ivkov, R.; Bischof, J.C. The Impact of Data Selection and Fitting on SAR Estimation for Magnetic Nanoparticle Heating. Int. J. Hyperth. 2020, 37, 100–107. [Google Scholar] [CrossRef]
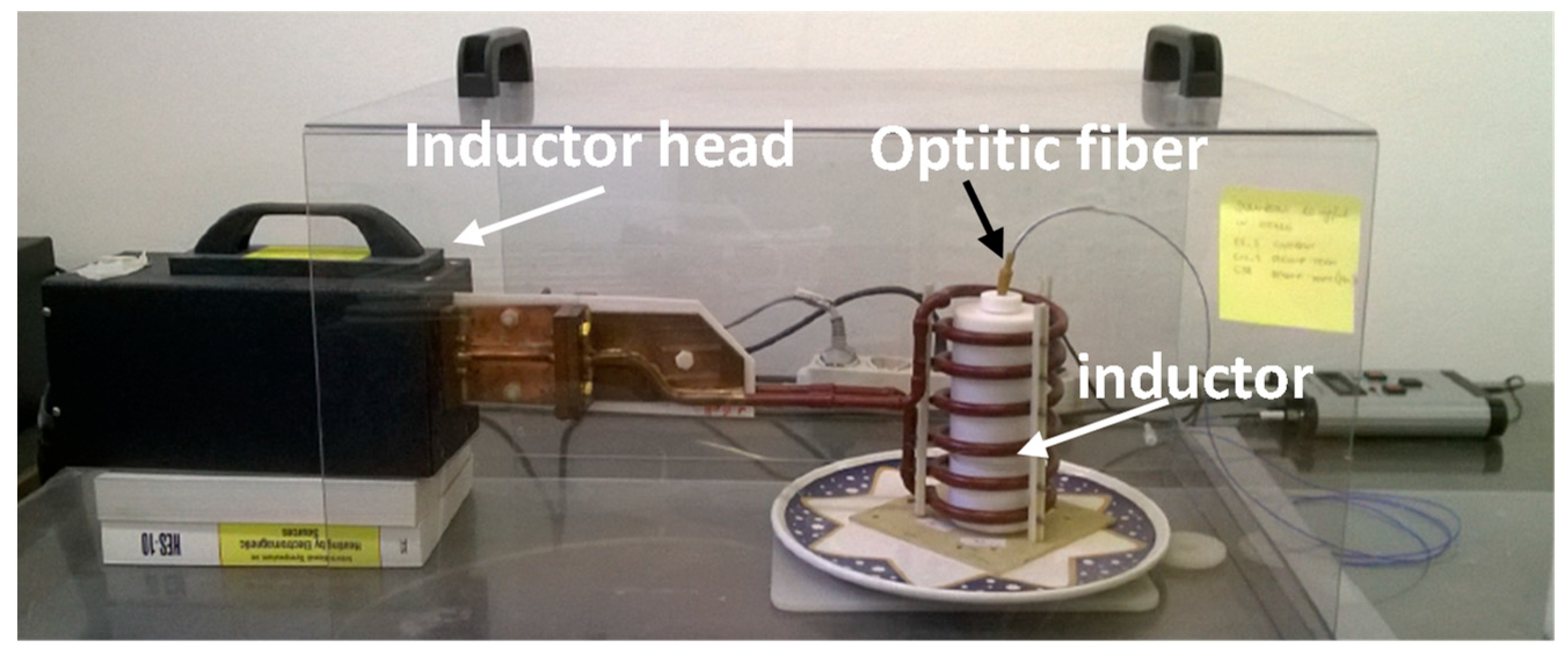








| Magnetic Core Diameter | Hydrodynamic Diameter * | ||||
|---|---|---|---|---|---|
| Average [nm] | Std [nm] | Average [nm] | Std [nm] | PDI | |
| C26 | 29.2 | ±10.2 | 109(100%) | ±8 | 0.43 |
| C12 | 8.8 | ±3.7 | 132(97%) 486 (3%) | ±15 ±94 | 0.51 |
| C5 | 9.5 | ±3.1 | 132 (95%) 610 (5%) | ±28 ±198 | 0.46 |
| MS at T = 5 K (emu/g) ±2% | MS at T = 300 K (emu/g) ±2% | |
|---|---|---|
| C26 | 86 | 72 |
| C12 | 73 | 65 |
| C5 | 80 | 69 |
| Fe Fe/10 mg NP [mg] | Mn Mn/10 mg NP [mg] | Fe3O4 /10 mg NP [mg] | MnxFe3−xO4 /10 mg NP [mg] | |
|---|---|---|---|---|
| C26 | 5.54 ± 0.28 | 0.86 ± 0.04 | - | 8.85 |
| C12 | 5.76 ± 0.29 | - | 7.96 | - |
| C5 | 7.07 ± 0.35 | - | 9.77 | - |
| NP | C5o#1 * | C5o#2 * | C5o3 * | Average | C5#1 | C5#2 | C5#3 | Average |
|---|---|---|---|---|---|---|---|---|
| SLP (A) [W/g] | 5.41 | 5.85 | 4.85 | 5.37 ± 0.50 | 8.35 | 9.54 | 9.07 | 8.99 ± 0.60 |
| SLP (B) [W/g] | 3.69 | 4.48 | 3.95 | 4.04 ± 0.40 | 13.26 | 12.56 | 13.26 | 13.03 ± 0.40 |
| QA-SLP [W/g] | 3.80 | 4.52 | 3.79 | 4.04 ± 0.42 | 14.50 | 12.57 | 13.17 | 13.42 ± 0.99 |
| σ(QA-SLP) [W/g] | 0.13 | 0.10 | 0.28 | 0.11 | 0.38 | 0.28 | 0.37 | 0.20 |
| NP | C12#1 | C12#2 | C12#3 | Average | C26#1 | C26#2 | C26#3 | average |
| SLP (A) [W/g] | 12.87 | 11.33 | 9.58 | 11.26 ± 1.65 | 84.44 | 75.68 | 59.19 | 73.10 ± 12.82 |
| SLP (B) [W/g] | 11.16 | 13.26 | 14.65 | 13.03 ± 1.75 | 96.25 | 106.01 | 101.83 | 101.37 ± 4.90 |
| QA-SLP [W/g] | 11.56 | 13.85 | 14.50 | 13.30 ± 1.54 | 97.50 | 106.43 | 104.21 | 102.71 ± 4.65 |
| σ(QA-SLP) [W/g] | 0.77 | 0.64 | 0.70 | 0.41 | 4.85 | 0.95 | 1.88 | 1.76 |
| C5o * | C5 | C12 | C26 | |
|---|---|---|---|---|
| SLP (A)-SLP (B) | 0.0231 | 6.37 × 10−4 | 0.2734 | 0.0234 |
| SLP (B)-QA-SLP | 0.9925 | 0.5643 | 0.8459 | 0.7466 |
| SLP (A)-QA-SLP | 0.0241 | 0.0027 | 0.1920 | 0.0198 |
| C5 Octane | C5 Water | C12 Water | C26 Water | ||||||
|---|---|---|---|---|---|---|---|---|---|
| f [kHz] | Field [Oe] | SLP [W/gNP] | σ (SLP) | SLP [W/gNP] | σ (SLP) | SLP [W/gNP] | σ (SLP) | SLP [W/gNP] | σ (SLP) |
| 177 | 113 | 1.99 | 0.09 | 5.19 | 0.39 | 3.52 | 0.12 | 33.27 | 0.73 |
| 177 | 170 | 4.36 | 0.73 | 12.85 | 0.31 | 12.67 | 1.01 | 102.43 | 1.48 |
| 177 | 227 | 5.86 | 0.24 | 14.92 | 0.21 | 31.24 | 0.59 | 132.89 | 6.15 |
| Linear fit | 0.066 (R2 = 0.981) | 0.025 (R2 = 0.987) | 0.104 (R2 = 0.868) | 0.551 (R2 = 0.967) | |||||
| 215 | 113 | 3.37 | 0.12 | 8.62 | 0.17 | 5.19 | 0.18 | 42.30 | 3.02 |
| 215 | 132 | 4.67 | 0.19 | 10.6 | 0.24 | 8.39 | 0.44 | 73.98 | 0.86 |
| 215 | 263 | 7.85 | 0.72 | 16.82 | 0.31 | 46.83 | 0.24 | 192.81 | 3.87 |
| Linear fit | 0.068 (R2 = 0.99) | 0.031 (R2 = 0.99) | 0.141 (R2 = 0.863) | 0.657 (R2 = 0.965) | |||||
| 245 | 56 | 2.33 | 0.86 | 3.22 | 0.37 | 1.73 | 0.12 | 2.28 | 0.21 |
| 245 | 113 | 3.52 | 0.12 | 10.62 | 0.52 | 5.59 | 0.16 | 44.22 | 0.64 |
| 245 | 170 | 6.14 | 0.22 | 15.38 | 0.35 | 17.65 | 1.05 | 126.76 | 2.46 |
| Linear fit | 0.089 (R2 = 0.99) | 0.035 (R2 = 0.99) | 0.088 (R2 = 0.898) | 0.595 (R2 = 0.88) | |||||
| Method | Advantages | Disadvantages |
|---|---|---|
| SLP(A) |
|
|
| SLP(B) |
|
|
| QA-SLP |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sieni, E.; Geninatti Crich, S.; Ruggiero, M.R.; Del Bianco, L.; Spizzo, F.; Bertani, R.; Mozzon, M.; Barozzi, M.; Forzan, M.; Sgarbossa, P. Experimental Comparison of Methods to Evaluate Heat Generated by Magnetic Nanofluids Exposed to Alternating Magnetic Fields. Fluids 2023, 8, 83. https://doi.org/10.3390/fluids8030083
Sieni E, Geninatti Crich S, Ruggiero MR, Del Bianco L, Spizzo F, Bertani R, Mozzon M, Barozzi M, Forzan M, Sgarbossa P. Experimental Comparison of Methods to Evaluate Heat Generated by Magnetic Nanofluids Exposed to Alternating Magnetic Fields. Fluids. 2023; 8(3):83. https://doi.org/10.3390/fluids8030083
Chicago/Turabian StyleSieni, Elisabetta, Simonetta Geninatti Crich, Maria Rosaria Ruggiero, Lucia Del Bianco, Federico Spizzo, Roberta Bertani, Mirto Mozzon, Marco Barozzi, Michele Forzan, and Paolo Sgarbossa. 2023. "Experimental Comparison of Methods to Evaluate Heat Generated by Magnetic Nanofluids Exposed to Alternating Magnetic Fields" Fluids 8, no. 3: 83. https://doi.org/10.3390/fluids8030083
APA StyleSieni, E., Geninatti Crich, S., Ruggiero, M. R., Del Bianco, L., Spizzo, F., Bertani, R., Mozzon, M., Barozzi, M., Forzan, M., & Sgarbossa, P. (2023). Experimental Comparison of Methods to Evaluate Heat Generated by Magnetic Nanofluids Exposed to Alternating Magnetic Fields. Fluids, 8(3), 83. https://doi.org/10.3390/fluids8030083











