Abstract
In this study, we conducted a theoretical simulation to compare the effects of various factors on the atomic and electronic structures and the magnetic properties of copper and gadolinium ions bonded to carboxylated species of (111) diamond surfaces. It was experimentally found that in the temperature range above 120 K, the magnetic moments of chelated Gd3+ and Cu2+ equal 6.73 and 0.981 Bohr magnetons, respectively. In the temperature range from 12 to 2 K, these magnetic moments sharply decrease to 6.38 and 0.88 Bohr magnetons. Specifically, we examined the effects of the number of covalent adatom–diamond substrate bridges, coordination of water molecules, and shallow carbon-inherited spins in the substrate on the physical properties of the metal center. Our simulation predicted that increasing the number of bonds between the chelated metal ion and substrate while decreasing the number of coordinating water molecules corresponded to a decrease in the magnetic moment of metal ions in a metal–diamond system. This is due to the redistribution of the electron charge density in an asymmetric metal–diamond system. By comparing our theoretical results with experimental data, we proposed configurations involving one and, in a minor number of cases, two surface –COO− groups and maximum coordination of water molecules as the most realistic options for Cu- and Gd-complexes.
1. Introduction
Detonation nanodiamonds (DND) were discovered more than 50 years ago but began to be intensively studied only in the 1990s, mainly from the point of view of their structural and morphological characteristics and physical properties [1,2,3]. By the early 2000s, it was already clear that the study of chemical functional groups on the surface of nanosized DND particles and their targeted control promised extensive opportunities for the creation of a new class of nanomaterials. As a result, in the last 20 years, many research groups have focused on studying the surface of isolated DND particles specially functionalized with various atomic groups and molecules [4,5,6,7,8,9,10]. DNDs with an average size of 5 nm and a surface coated with oxygen-containing groups are currently an essential precursor for constructing smart hybrid particles of core–shell architecture and nanoplatforms for carrying isolated atomic functional groups of complex structure and metal atoms [11]. Nanostructures of this architecture based on 5 nm DND particles can be used both in nanobiomedicine for targeted delivery of drug molecules (and some necessary metal complexes) inside living organisms, and in catalysis to provide a catalytic effect through isolated metal clusters consisting of several metal atoms [12,13,14,15,16,17]. In the latter case, the nanodiamond particle plays only the role of a carrier of such a multinuclear metal complex or a cluster of several metal atoms. The above hybrid particles of complex architecture are easily realized in the case of carboxylated DND particles with a negative zeta potential and a degree of surface coverage with carboxyl groups of at least 50% [18]. Cations of several 3d and 4f metals can be incorporated into the surface of carboxylated DND particles through chelation, i.e., binding to surface carboxylate groups (–COO−) with the formation of chelate complexes of these metals. This binding of 3d- and 4f transition metal cations to the DND surface occurs by ion exchange of protons of carboxyl groups with cations when mixing an aqueous DND suspension with aqueous solutions of the corresponding metal salts [19,20]. The first experimental and theoretical studies performed more than ten years ago showed that some 3d metal ions, for example, copper ion Cu2+, are perfectly chelated by the surface of a carboxylated diamond by binding to two carboxylate groups located on the surface at specific sites of the diamond crystal lattice [21]. Such a complex has spin S = 1/2, associated with the magnetic moment of the doubly charged bound copper ion, and can be easily identified by magnetic methods, including electron paramagnetic resonance [19,21,22]. The copper ion Cu2+, bound to the surface by two carboxylate groups, is located at a distance of about 0.22–0.28 nm from the diamond surface [21,22]. The specific topology of the copper complex depends on the relative position of the carboxylate groups and the type of mobile functional groups or molecules that additionally coordinate the metal atom in the complex. A review of relevant data, mainly for copper, can be found in [18]. At the same time, the coordinating role of water molecules had never been considered theoretically. Nevertheless, when analyzing the possible magnitude of the elevation of the metal cation above the surface of the diamond, it was taken into account by correcting the distance obtained in the approximation of a “dry” complex formed without the participation of water molecules and/or acid groups.
A more interesting and practically important case is the gadolinium metal complex Gd3+ on the surface of a carboxylated diamond. It was previously shown using magnetic methods that Gd3+ ions chelated on the surface of DND particles in a concentration of up to ~2 wt.% cause an additional contribution to the paramagnetism of the particles, exceeding their intrinsic paramagnetism associated with the intrinsic paramagnetic defects of the diamond matrix by 15–20 times [20,23,24]. In this case, gadolinium is chelated on the surface of carboxylated particles in ionic form with spin S = 7/2 in an amount of up to 18 per nanoparticle [20,23,24]. In the work of Panich et al. [25], it was shown that Gd3+ ions fixed on the surface of DND particles accelerate the processes of nuclear spin–lattice relaxation of 13C and 1H nuclei located inside and on the surface of the diamond particle. The 13C nuclear spin–lattice relaxation data analysis revealed that Gd3+ ions bound in complexes are located at no more than 0.32 nm from the diamond surface [25]. Due to the large magnetic moment of the gadolinium ion, diamond particles with a large number of bound gadolinium ions are promising for use as a magnetic contrast agent in magnetic resonance imaging (MRI). The successful use of Gd3+- DND particles for MRI tasks has already been reported in the works of Panich et al. and Yano et al. [26,27]. Even though triply charged Gd3+ ions are successfully chelated by the surface of carboxylated DND and demonstrate a substantial decrease in the relaxation time of the nuclear magnetic resonance signal for protons of water molecules in layers adjacent to the DND particle (for contrast-enhanced MRI tasks), realistic models of binding of Gd3+ ions and other 4f transition metal ions to the diamond surface are still missing. This is mainly due to a lack of understanding about the actual number of carboxyl groups and other mobile agents (water molecules) that bind metal ions into a complex. Formally, complexes based on single carboxyl groups, as well as their pairs and triads, can bind triply charged metal ions. But it is a priori clear that the statistical probability of the appearance of complexes based on –COO− triads is smaller compared to the case of complexes based on –COO− pairs, and complexes based on single –COO− may not firmly hold triply charged Me3+ cations captured during the ion exchange reaction in an aqueous solution. The following facts support the importance of questions about the possible prevalence of complexes with two or only one –COO− group. Thus, Comet et al., using the Boehm titration method using reagents such as sodium bicarbonate, sodium carbonate, and sodium hydroxide, estimated the number of ionized carboxyl groups on the surface of DND particles to be 0.85 sites per square nanometer. Thus, a particle with a diameter of 5 nm has approximately six and a half dozen -–COO− groups [28]. Similar values for negatively charged –COO− were also found in a study by Shvidchenko et al. [29]. This is a tiny amount for the approximately 1250–1300 s-bonds sticking up on the surface of the diamond and saturated with various functional groups. Although there is no information in the literature about the specially treated and oxidized surface of DND particles with a negative zeta-potential of ~50 mV (in an aqueous suspension) and the maximum possible number of ionized carboxyl groups, we assume that this number can be about 100–130, i.e., approximately 8–10% of the total number of functional groups attached to surface sites.
This work aims to construct a realistic model of the surface of carboxylated DND particles that bind doubly and triply charged metal ions through chelation. The cases of chelate complexes with 1, 2, and 3 carboxyl groups and different numbers of coordinating water molecules will be considered in detail. A comparison will also be made of Gd3+ chelate complexes with Cu3+ chelate complexes in terms of their topology and the influence of the environment on the resulting effective magnetic moment of the chelated cation and the entire complex together with spin-polarized ligands.
Thus, the simulation of the effect of liquid media on the stability and physical properties of diamonds grafted by gadolinium is essential for developing this area. For this purpose, we simulated the impact of the coordination of gadolinium atoms on the diamond surface by different numbers of water molecules. For the sake of comprehensiveness, we considered the attachment of gadolinium atoms to the substrate by various numbers of –COO− groups. We also studied the effect of shallow subsurface defects with inherent carbon spins on gadolinium centers’ stability and magnetic properties. The impact of a liquid environment on the stability of copper atoms on the diamond surfaces has also been studied.
2. Computational Method
Theoretical modeling was carried out using the SIESTA pseudopotential code [30] employing the generalized gradient approximation (GGA-PBE) [31] for the exchange–correlation potential in a spin-polarized mode; van der Waals correction was also taken into account [32]. We note here that various research groups previously used the SIESTA code to model the interior and surface of diamonds [33,34,35]. A full optimization of the atomic positions was carried out, during which the electronic ground state was consistently found using norm-conserving pseudopotentials [36] for the cores with a double-ζ-plus polarization basis for the localized orbitals of non-hydrogen atoms and a double-ζ for hydrogen atoms. The forces and total energy levels were optimized with an accuracy of 0.04 eV Å−1 and 1.0 meV/cell, respectively. The densities of states plot was conducted with a 6 × 6 × 1 Monkhorst–Pack k-point grid for the Brillouin zone sampling [37]. The smearing by Gaussian with 0.1 eV half-width on half-height was used for the densities of the states plot.
3. Experimental Results
DND samples with the maximum achievable gadolinium ions Gd3+ and copper Cu2+ content were prepared. DND particles were chelated with metal ions by mixing aqueous suspensions of DND with solutions of the corresponding salts (nitrates) according to the method previously described in [19,20,22]. Direct chelation was carried out by mixing a certain (20 mL) volume of 0.5 wt.% 5 nm DND suspension with a negative zeta potential (a product taken from the Adamas Nanotechnology, Inc., Raleigh, NC, USA and two times diluted) and a certain volume of an aqueous solution of a metal salt prepared at a concentration of about 0.3 wt% (for copper nitrate trihydrate) or 0.2 wt% (for gadolinium nitrate hexahydrate). As copper (II) nitrate trihydrate Cu(NO3)2·3H2O and gadolinium (III) nitrate hexahydrate Gd(NO3)3·6H2O, we used high-purity products from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan). The progress of the reaction after mixing was determined by the change in color of the solution from black to gray [19]. Next, the solution settled, and the solid precipitate was separated by centrifugation. Each product obtained was not specially washed and was dried in a rotary evaporator at 50 °C. The manufactured products were given the names ND-Cu and ND-Gd. According to X-ray Photoelectron Spectroscopy data, the corresponding concentrations of metals in the ND-Cu and ND-Gd powders were 0.47 at% and 0.32 at%. The atomic concentrations of carbon, oxygen, and nitrogen were 83.74%, 13.14 at%, and 2.27 at% (ND-Cu), and 85.77 at%, 12.28 at%, and 1.63 at% (ND-Gd), respectively. A high oxygen content indicates the presence of oxygen-containing groups on the surface of DND particles with chelate complexes, particularly carboxyl and hydroxyl groups. The prepared samples were studied using a SQUID magnetometer at low temperatures (T = 2 K) according to the method previously described in Refs. [20,23,24]. A magnetometer MPMS-7 from Quantum Design (San Diego, CA, USA) was used for this study. In this case, the magnetization curves of the samples were especially carefully measured at T = 2 K in the field range of up to 70 kOe. The temperature dependences of the magnetizations of both samples were also studied to obtain information about the temperature dependence of the magnetic moment of each chelated metal ion (Cu2+ and Gd3+) under study. A description of sample preparation and container material parameters for precise magnetic measurements can be found in Ref. [24]. The measurement options used to measure magnetization at low temperatures are also described in Ref. [24]. Let us specifically note that the accuracy of temperature stabilization in the chamber with the sample was ±0.02 K. Magnetic measurements were carried out at the Tokyo Institute of Technology and Hosei University in Tokyo (Japan).
We especially emphasize that to obtain the magnetization from the studied subsystem of chelated metals, all contributions associated with the magnetization of the container material and the magnetization of the dry powder of carboxylated DND, that is, the nanodiamond adsorbent itself, were subtracted from the measured magnetization [23]. These contributions were measured separately using reference samples of pure carboxylated DND and the thin aluminum foil used to make the container. The procedures for subtracting magnetizations were carried out by processing the data on a computer in the Microsoft® Excel® 2016 MSO (version 2406 16.0.17726.20078) 32-bit program.
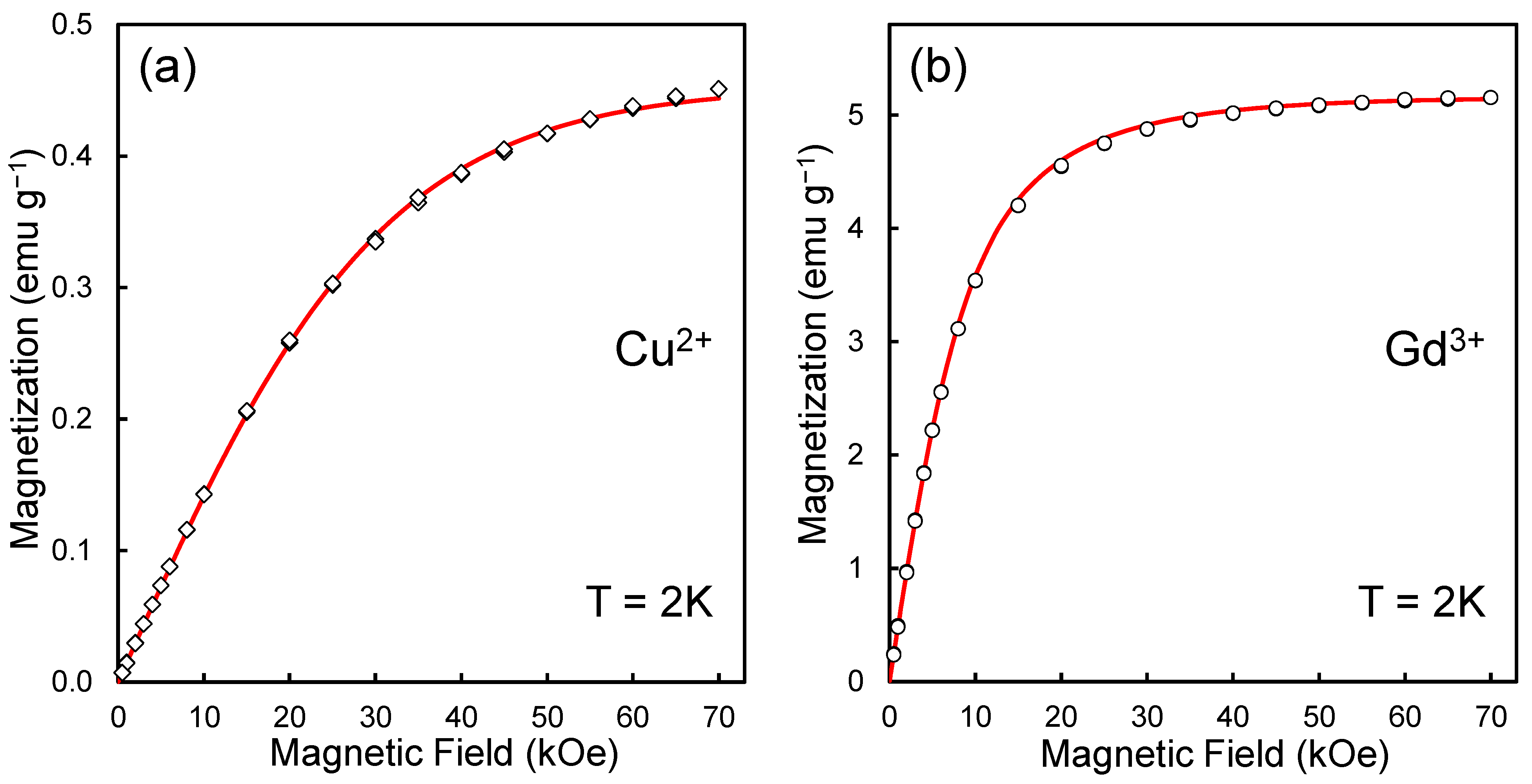
Figure 1 shows the dependences of the magnetization M of a system of paramagnetic metal ions on the applied magnetic field H, taken for samples ND-Cu and ND-Gd at a temperature of T = 2 K. The points (open diamonds and open circles) in Figure 1a,b correspond to the experimental values of the measured magnetization of ensembles of Cu2+ and Gd3+ ions in magnetic fields from 0 to 70 kOe. They were obtained by subtracting the magnetization of pure DND from the magnetization of DND with chelate metal complexes (taking into account corrections for partial contributions to magnetization from the diamagnetic diamond matrix, interior paramagnetic defects of DND, and paramagnetism of the container material). We previously analyzed similar M-H dependences demonstrating magnetization saturation in high fields in [23,24]. Fitting the obtained magnetic curves (M-H) for both samples to the analytical curves described by the Brillouin functions [38,39] for spins S = ½ (Cu2+) and S = 7/2 (Gd3+) allows us to determine the concentration of spin agents in g−1 units in both cases. Accordingly, for spins S = ½ and S = 7/2, we have concentrations of 4.90 × 1019 g−1 and 7.97 × 1019 g−1. Since the weight of one diamond particle with a diameter of 5 nm is about 2.32 × 10−19 g, these concentrations of transition metal spins correspond to about a dozen or even two dozen metal ions on the particle’s surface.
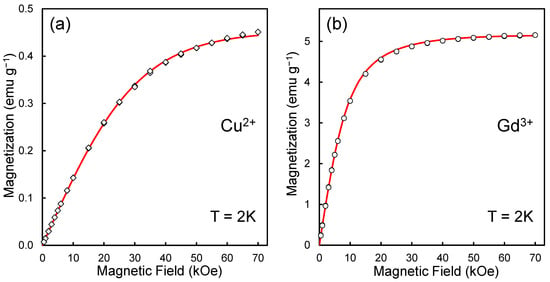
Figure 1.
Magnetization curves for ensembles of copper (a) and gadolinium ions (b) chelated on the surface of 5 nm carboxylated DND particles. The red lines are Brillouin function fits with magnetic moments 0.88 and 6.38 for Cu2+ and Gd3+ complexes. Temperature: T = 2 K. Experimental data were obtained using an MPMS-7 SQUID magnetometer (Quantum Design Inc., San Diego, CA, USA).
A more accurate analysis of the experimental M-H curves for both samples using the Brillouin function fitting method made it possible to discover that a better agreement between the experimental data and theoretical M-H curves is achieved at the following values of magnetic moments for structural elements, including Cu2+ and Gd3+ cations, namely at ~0.88 and ~6.38 Bohr magnetons (), and not at all for integer values 1 and 7. The corresponding Brillouin fitting curves plotted for the values J = 0.44 (Cu2+) and J = 3.19 (Gd3+) are shown in red in Figure 1a,b. Here, J is the quantum number of the total angular momentum of the multielectron shell of the metal atom [40]. Note that to determine the optimal fitting parameters (for example, J) to be substituted into the Brillouin formula, we used proven procedures previously applied in the analysis of the gadolinium ion in the Gd@C82 metallofullerene and described in detail in Ref. [41]. The magnetic moments obtained from the fitting procedures are related to the varied fitting parameters by the formula , where g is the Lande factor, L and S are, respectively, the quantum numbers of the orbital and spin angular momenta of the multielectron shell of the metal atom [40,42]. For the gadolinium ion (as well as the copper ion), the g-factor was chosen as equal to 2, and the orbital quantum number L was taken as zero [40,42]. If necessary, the values of magnetic moments μ can be easily recalculated into the commonly used values of effective magnetic moments using the standard formula [40].
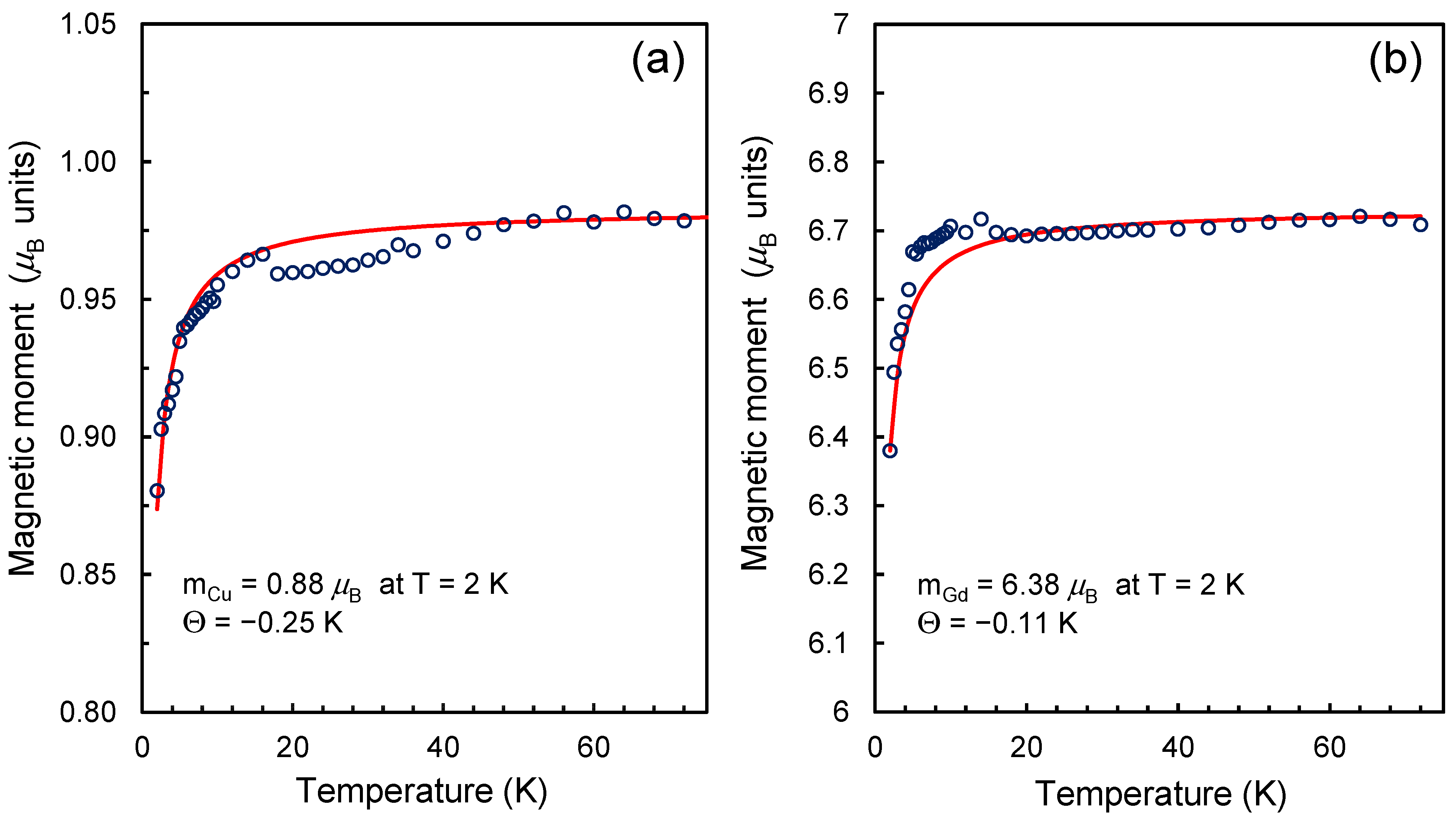
In the analyzed cases, the estimated magnetic moments of Cu2+ and Gd3+ are reduced by ~12 and ~8.9 percent compared to the theoretical values of the magnetic moments of these ions in an isolated state. This result is not surprising. For example, reduced values of the magnetic moment of the gadolinium ion were observed for the metallofullerene Gd@C82 and gadolinium systems on a carbon nanotube [43,44]. For both noted systems, the J- values found from fitting the magnetization curves with Brillouin functions were 3.38 (Gd@C82) and 3.2 (Gd@CNT). Non-integer values of 2J or 2S are also often obtained by fitting the magnetization curves of some organic compounds with Brillouin functions [45,46]. However, the reason for the deviation of 2J or 2S from integer values in each specific case may be unique, requiring additional research. In our case, the peculiarity is the fixation of the gadolinium ion on the surface through carboxylate groups. In this case, part of the electronic charge can be distributed over the molecular orbitals of carboxylate groups, which play the role of ligands. In our recent work, the data on the magnetization of Gd3+ ions on the DND surface were analyzed under other, simpler assumptions, using integer magnetic moments of the ions and weak exchange interaction (<0.25 K) between the spins of the 4f shell of Gd3+ and the spins on the surface of the diamond matrix [24]. At the same time, temperature measurements of the magnetizations of ND-Cu and ND-Gd show a decrease in the magnetic moments of Gd3+ and Cu2+ with decreasing temperature in the range of 2–75 K (Figure 2a,b). A noticeable drop (by no more than 6–10% of the value) in the magnetic moment in both cases occurs in the temperature range below 12 K. At the same time, in the high-temperature region of 120–150 K, magnetic moments of Gd3+ and Cu2+ are equal to 6.73 and 0.981 respectively. For the copper ion, the value of the magnetic moment in the high-temperature region is lower than the theoretical value by approximately only 2%, and for the gadolinium ion, it is 3.85% lower than the theoretical value of 7 . The absolute values of the decrease in the magnetic moment are 0.27 and ~0.02 for Gd3+ and Cu2+ ions. Thus, for both ions (Gd3+ and Cu2+), we have a reduced value of the magnetic moment both in the high-temperature region (T > 120 K) and at the lowest temperature of 2 K in the experiment. A suitable alternative to describing the reduced magnetic moments of Gd3+ and Cu2+ at T = 2 K can be a description that uses close to integer (within ±4%) values of high-temperature magnetic moments in units but takes into account the weak antiferromagnetic interaction between spin agents of different types in the system, in particular, the spins of the 3d and 4f shells of transition metal ions on the surface and the distributed spin-polarized density in the frame of –COO− ligands surrounding the metal ion, including dangling bonds in the diamond lattice near the metal complexes. However, within the framework of an experimental study, it is impossible to accurately establish the topological model of spin counterparts (localized or distributed within the ligands or even the underlying crystal lattice), with which the spins of the electron shells of 3d/4f metal ions weakly interact. We can only say with certainty that in the case of Gd3+ on DND, such spin counterparties are not the nearest neighboring Gd3+ cations on the particle surface since the observed effects do not depend on the concentration of metal ions in the system. For example, when the Gd3+ concentration decreases by 7–8 times, the temperature and field dependences for the magnetization of the system of chelated Gd3+ ions do not change qualitatively. Thus, to form a magnetic model of a gadolinium or copper chelate complex on the DND surface, theoretical studies that directly evaluate the magnetic moments of the complexes are required.
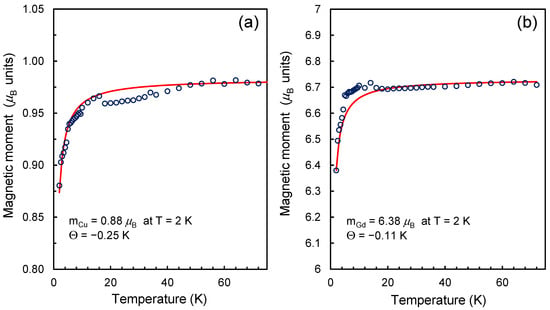
Figure 2.
Temperature dependences of the magnetic moment of a chelated metal cation in the range of 2–75 K for complexes of (a) copper and (b) gadolinium on the surface of carboxylated DND. The blue circles are the experimental points. The red lines show theoretical curves passing through the experimental points, in which the fitting parameters are the magnetic moment of the metal ion and the Weiss temperature (Θ) for a system of weakly interacting spin counterparts. The asymptotic behavior of the theoretical curves corresponds to magnetic moments of 0.981 and 6.73 in the temperature range of 120–150 K.
In conclusion of this section, we note that we obtained reduced values of the magnetic moments of Gd3+ and Cu2+ in the high-temperature region of 120–150 K. Possible reasons for this will be analyzed in the theoretical part of this work.
4. Theoretical Results and Discussions
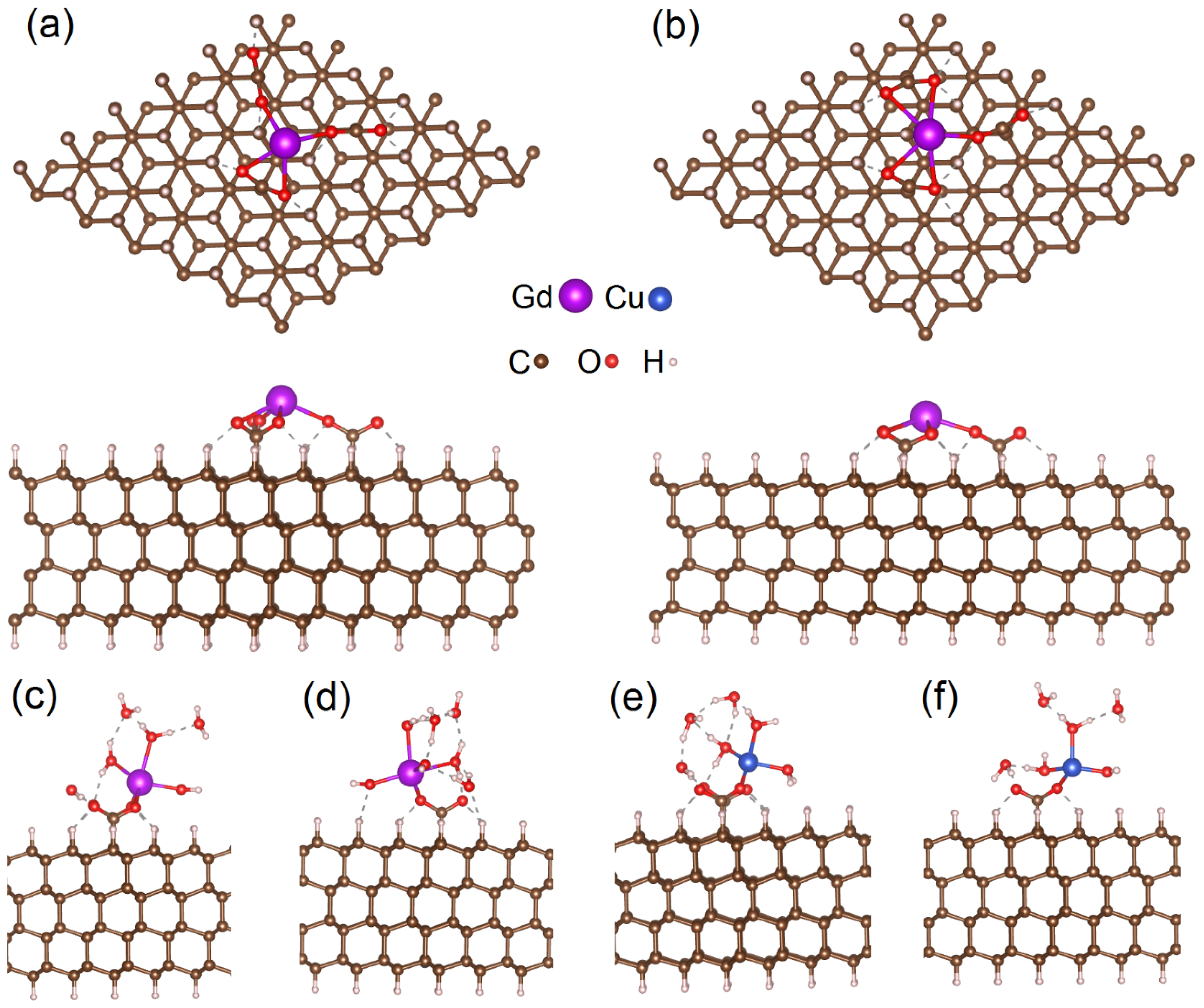
To simulate the interactions between gadolinium atoms and the surface of DNDs, we used a model slab (constructed from 6 × 6 × 4 supercell) corresponding to the (111) surface of the diamond. The slab consists of four layers of carbon, dangling bonds on the surface saturated by hydrogen atoms or –COO− groups (see Figure 3a,b). Our previous work studied the interaction of gadolinium atoms attached to the substrate by forming three –COO–Gd bonds [23,24]. These three bonds also keep the gadolinium atom in a III oxidation state (Gd3+). On the other hand, experimental data demonstrate that the amount of copper atoms attached to the same substrate is almost the same (see Section 1 and Section 3). Measurements also indicate that copper atoms on the surface of DND are in a II oxidation state (Cu2+); hence, these atoms cannot interact with three –COO− groups. Therefore, the interaction of gadolinium atoms with a smaller number of surface –COO− groups should also be studied. To simulate Gd3+ attached to the substrate via two and one –COO− groups, one and two hydroxyl groups (–OH), respectively, were attached to gadolinium atoms (see Figure 3c,d).
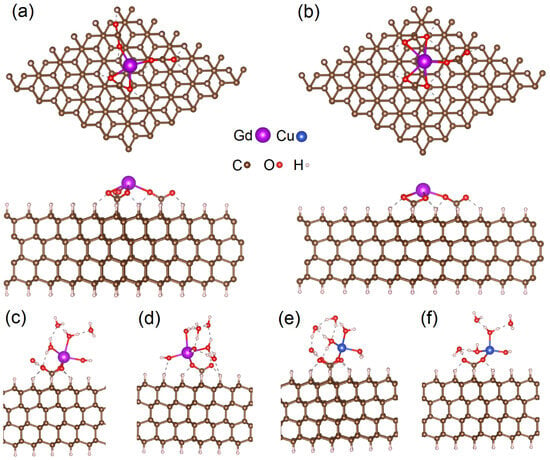
Figure 3.
Top and side views of optimized atomic structures of the supercell with Gd3+ center attached three –COO− groups located at different distances (a,b). The structures shown in panels (a,b) correspond to structures 1 and 3 in Table 1. The parts of optimized atomic structures of the part of supercells with metal atoms coordinated with water molecules (c–f). Panels (c,d) show Gd3+OH connected to the surface by two –COO− groups (9) and Gd3+(OH)2 connected to the surface by a single –COO− group (12), respectively. Panels (e,f) show Cu2+ connected to the surface by two –COO− groups (18) and Cu2+(OH) connected to the surface by a single –COO− group (20), respectively. Dashed lines indicate hydrogen bonds. Optimized atomic structures of other simulated configurations are shown in Figure 4 and Figure S1.
For the sake of comprehensiveness, we also simulated the possible effect of near-surface inherent carbon spin S = ½ on the stability of gadolinium adatom. Here, we mean the carbon spin (in the uppermost layer of the diamond lattice) underlying the metal atom. The layer of hydrogen atoms on the ‘bottom’ part of the H-terminated diamond slab simulates C—C bonds of the ‘bottom’ layer of carbon atoms with deeper layers in the bulk area. Therefore, removing one hydrogen atom from the ‘bottom’ side of the slab can be considered an adequate model for carbon-inherited spin in the outer shallow layers of DND particles [22,47]. In addition, the spin ½ of a dangling bond on a carbon atom is similar to that formed on a carbon atom near a vacancy [48]. The distance between the carbon atoms with the dangling bonds on the opposite side of the H-terminated slab and the surface layer is 0.618 nm. Thus, such simple modeling can consider the role of vacancies in the crystal in influencing surface metal complexes. We performed these simulations of carbon-inherited spins (VH, S = ½) for Gd-atoms coordinated by a maximal number of water molecules and attached to carbon substrates by three, two, and single –COO− groups (structures 6, 10, and 13, respectively). For all these three structures, only a tiny deviation in Gd–substrate distances (within 0.01 nm) from the values calculated for similar structures without carbon-inherited spin species was observed (5, 9, and 12, respectively). Thus, we can conclude that shallow carbon-inherited paramagnetic species do not have a valuable effect on the surfaces’ atomic structure of Gd–COO complexes.
The next step of our study is the simulation of the effect of water on the location and magnetic properties of copper adatoms by reducing the number of metal–substrate bonds and coordination. We simulated Cu2+ atom connected to the surface by two –COO− groups (structures 17 and 18, later shown in Figure 3e) and with one –COO− group (structures 19 and 20, later demonstrated in Figure 3f). In both cases, growing the number of coordinating water increases the Cu–substrate distance. Contrary to gadolinium, reducing the number of bonds with substrate does not have a visible effect on these distances.
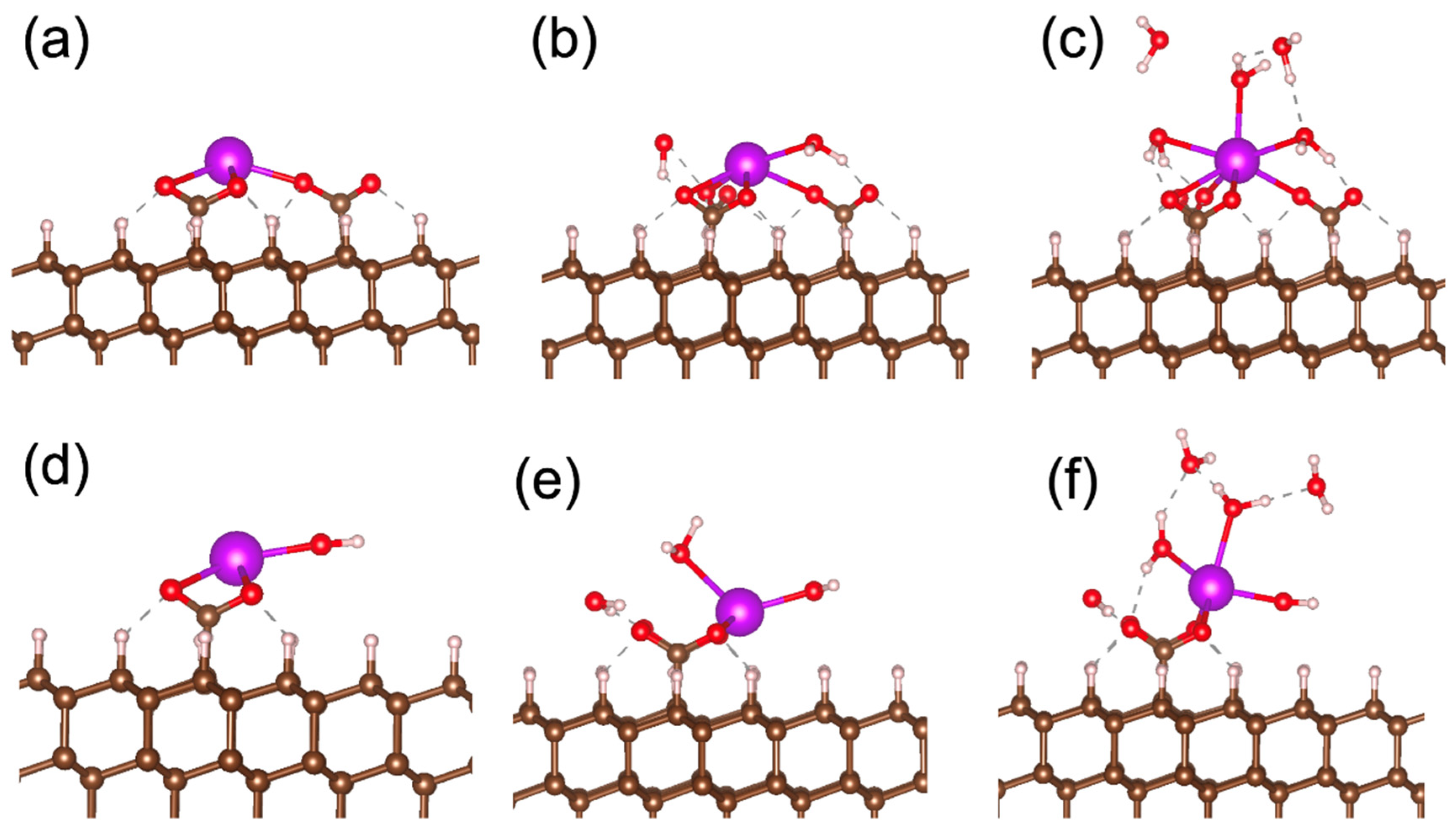
To check the stability of the water–Gd bonds, we calculated the binding energies for the attachment of water molecules to structures 3 and 7, as shown in Figure 4a,d. The binding energies are calculated by the following formula:
where E(substrate) and E(substrate + nH2O) are the total energies of the supercell before and after attachment of n molecules to the gadolinium center, and E(H2O) is the total energy of water molecules in the gaseous phase. The calculated binding energies for attaching two water molecules to the structures shown in Figure 4a and Figure 4d are −1.24 eV/H2O and −1.49 eV/H2O, respectively. Following the attachment of three water molecules to the structures shown in Figure 4b and Figure 4e, they are −1.01 eV/H2O and −0.69 eV/H2O, respectively. To estimate the stability of the water molecules on the metal centers and –COO− groups, we assume that non-covalent bonds between water and substrate groups are similar to hydrogen bonds in liquid water. Therefore, removing water from the substrate groups is similar to the vaporization of liquid. Since the measured enthalpy of water vaporization is about 0.43 eV/H2O [49], the structures shown in Figure 4b–f are stable at ambient conditions.
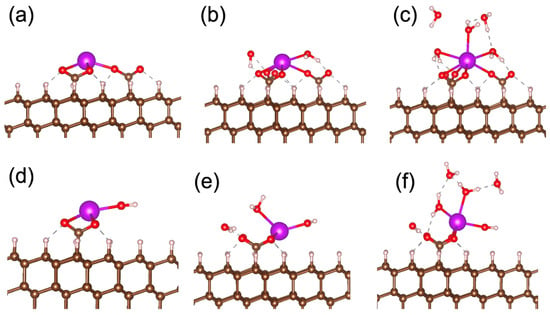
Figure 4.
Optimized atomic structures of gadolinium ion with or without coordinating water molecules located on (111) surface of diamond connected to the substrate by three (a–c) and two (d–f) –COO− bridges. Structures on panels (a–c) and (d–f) correspond to structures 3–5 and 7–9 in Table 1, respectively.
Thus, simulations of the nineteen different Gd or Cu attachment structures to diamond substrate demonstrate the insignificant effect of the changes in links between metal and substrate on metal–substrate distance. On the contrary, the impact of a varying number of metal–substrate bonds and coordinating water molecules is significant (see Table 1). The decrease in bonds with substrate leads to a gradual increase in the magnetic moment on Gd3+ from the values about 6.5 (structures 1, 3 and 4). On the contrary, increasing the number of coordinating water molecules provides a visible effect only in the structures with a single Gd–substrate bond (11–15). The simulated carbon-inherited spin in the shallow layer provides an insignificant decrease in magnetic moment on Gd3+ in structure 6 and an increase in magnetic moment by about 0.1 in structures 10 and 13. Since the formation of carbon-inherited spin does not visibly change structural properties, these effects on magnetic moments and gadolinium–substrate distance could be associated with a minor impact of the simulated inherent carbon spins on the electronic structure of surface layers [48].

Table 1.
The list of studied configurations, with corresponding numbers of atomic groups and molecules, magnetic moments on metal centers, and distances between the magnetic center and diamond substrate.
Note that, in our experiment, the magnetization per unit weight of carbon particles was measured, and the magnetic moment and concentration of elementary paramagnetic species associated with metal complexes were analyzed. Therefore, the magnetic moment of the whole supercell as an entire spin-polarized unit must also be considered. For almost all simulated Gd-containing systems, the total moment calculated for whole supercells is about 0.10~0.15 lower than that calculated for Gd3+. This result is in agreement with our experimental observation of magnetic moments below 7 /Gd3+ for gadolinium-grafted DNDs (see Section 3). Note that observed non-integer magnetic moments specified in units of Bohr magnetons are very unusual for non-metallic systems where values of whole moments of the system are integers. The calculations for model Gd3+(OH)3 (16) were carried out to rule out the influence of possible artifacts of the computational method. In this system, the calculated magnetic moment on the Gd3+ center and the whole system is about 7.4 and 7.0 , respectively. To check the influence of the used supercell, computational code, and possible correlation effect, additional calculations for structure 1 were carried out (see details in Supplementary Materials). The results of these tests demonstrate that the change in substrate thickness and type of the pseudopotential corresponds to insignificant changes in magnetic moment. On the contrary, calculations by DFT+U methods lead to an increase in the magnetic moment on gadolinium from about 6.4 to 7.2 . This result disagrees with the experimental data. Thus, computed non-integer values of magnetic moments in systems 1–15 cannot be caused by artifacts of the computational method. Our experiments provide values of magnetic moments per Gd3+ on DND in order about 6.7 . Measured numbers can be associated with the structures 8–12. Thus, we cannot draw a more precise conclusion about the prevailing configuration of Gd–COO structures from this list of structures 8–12. Additionally, we note that the distances from the Gd3+ ion to the crystalline diamond surface obtained for structures 8–12 with calculated magnetic moments that are in good agreement with the magnetization experiment actually do not contradict the data on the Gd3+– surface distances obtained in Ref. [50] by 13C nuclear magnetic resonance spectroscopy.
A similar pattern was observed in the robust attachment of Cu2+ to the diamond substrate (systems 17–20). However, the effect of decreasing the number of copper–substrate bonds and increasing the number of coordinating water molecules is more significant (see Table 1). For structure 20, shown in Figure 3f, the overall magnetic moment of the whole supercell is pretty close to one (0.885 ). Because the experimentally obtained magnetic moment for the Cu-grafted DNDs is equal to 0.98 /Cu2+, we can firmly conclude that structures similar to 20 shown in Figure 3f are the most abundant for discussed systems. Since our previous and present experimental studies give approximately the same number of Gd3+ centers on the surface of DND (with an accuracy of a factor of 1.5) as for Cu2+ [20,22,23], we may also conclude that the attachment of a gadolinium atom to the diamond surface preferably occurs through a single Gd–substrate bond (structures 11–15).
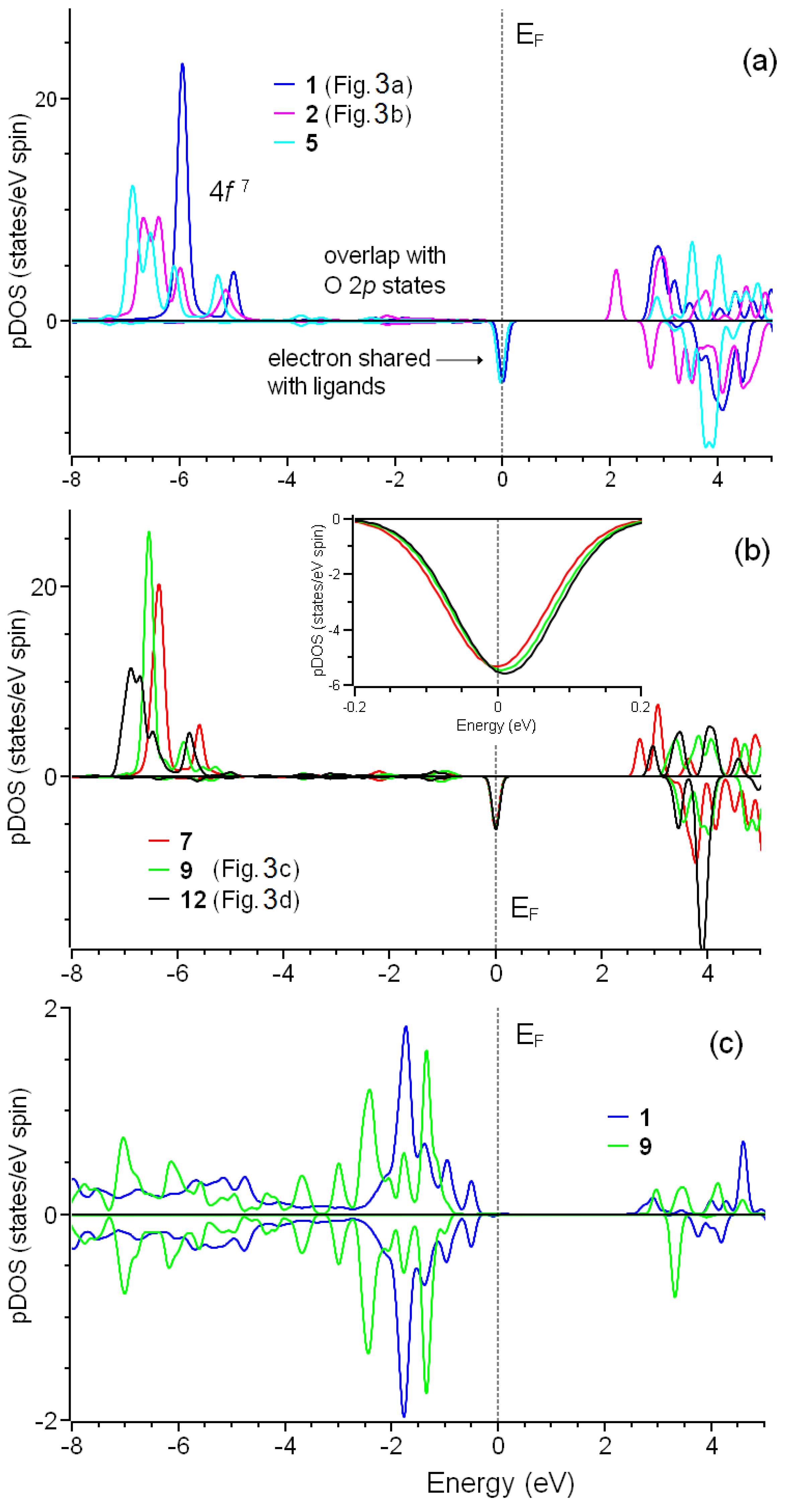
The purpose of this study was to clarify the surprising deviation in magnetic moments integer numbers in studied non-metallic systems from and unveil the nature of the difference in experimentally observed magnetic properties of Gd- and Cu-grafted DNDs. Since the formation of COO–metal groups on the surface does not provide visible changes in the electronic structure of the substrate (see Figure S2), we considered only partial densities of states of metal ions and nearest oxygen atoms (Figure 5 and Figure 6). First, we checked the simplest case: systems 1 and 3 shown (Figure 3a,b). As shown in Figure 5a, both systems’ electronic structures almost coincide. Note that in this case, Fermi energy is defined by achieving the number of electrons in valence bands in the process of integration of electron densities. Coordinating by water and decreasing the number of –COO groups attached to gadolinium do not lead to valuable changes in the electronic structure of Gd3+ (Figure 5a,b). The electron configuration of Gd3+ is 4f7 5d0 6s0. The occupied part of the 4f shell has energy levels lower than those of 2p orbitals of –COO ligands (Figure 5a,b vs. Figure 5c). This reciprocal location of Gd 4f and O 2p bands is caused by a strong crystal field and makes the electronic structure of this system different from standard transitional metal oxides [51] and explains the inadequacy of DFT+U methods for simulated systems (see Figure S3 and corresponding discussions). The gadolinium atom has a high-spin configuration since all seven electrons have spins with the same polarization (↑). However, density-of-states spectra of gadolinium have one very unusual feature: a peak at the Fermi level. Analysis of Mulliken populations [52] of the orbitals demonstrates the presence of half of the electrons with opposite spin in 4f orbitals.
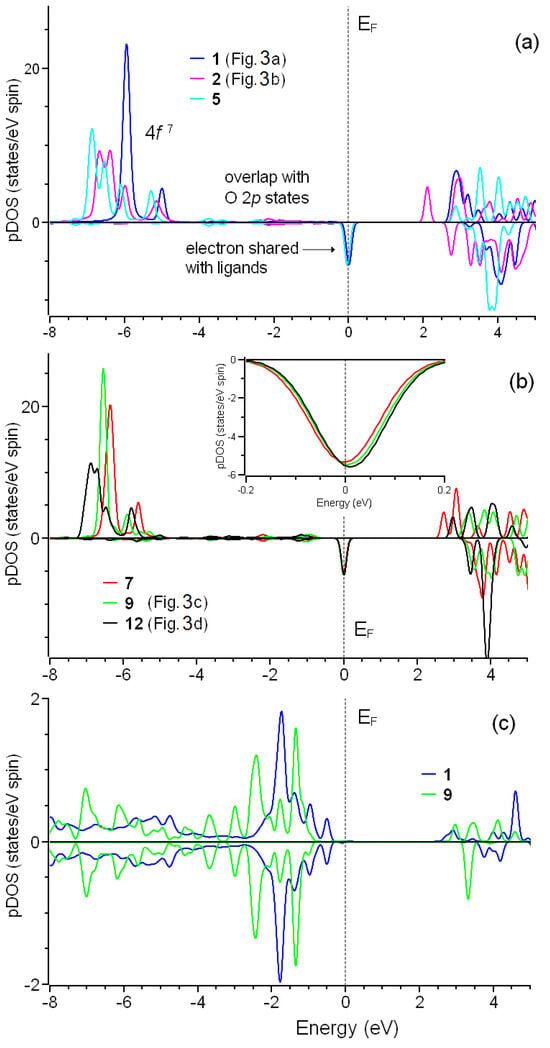
Figure 5.
Partial spin resolved densities of states for different configurations of Gd3+ connected to the diamond surface by three (a) or smaller number (b) –COO− groups. The inset in panel (b) shows the same densities of states in a narrower range of energy levels. Panel (c) shows partial densities of states for selected configurations.
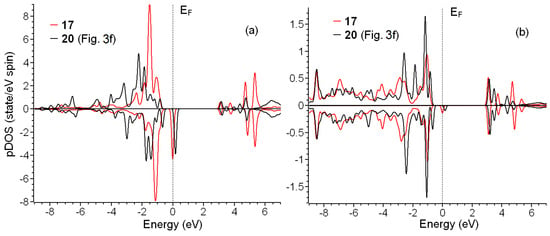
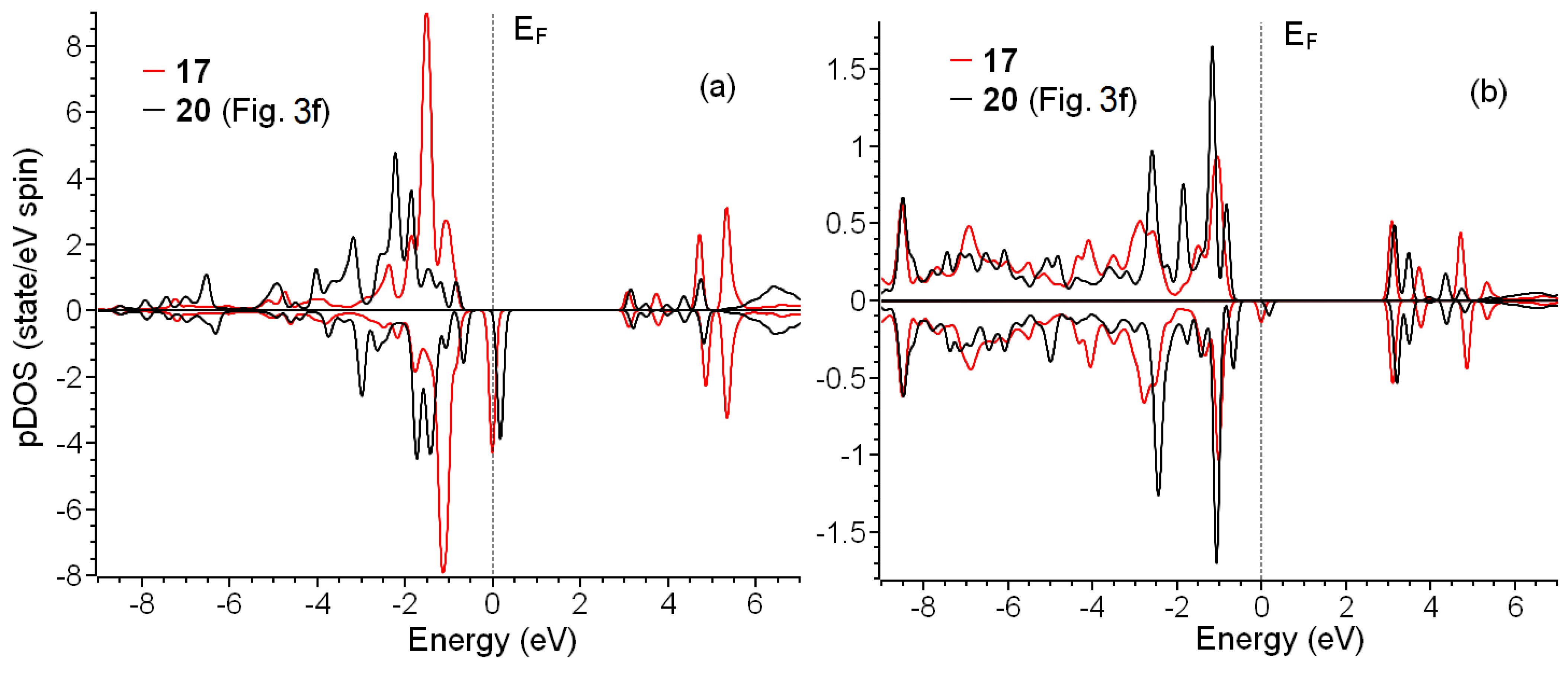
Figure 6.
Partial spin resolved densities of states for oxygen in C–O–Cu bridges (a) and copper atoms (b) in configurations 17 and 20.
In the case of adsorption of copper, the picture is partially different. The electronic configuration of Cu2+ is 3d9 4s0. For structure 17, connected to the substrate by two –COO− groups and coordinated by two coordination water molecules, we observed the peak on Fermi level similar to that discussed above for Gd3+ and half of the electron in Milliken populations (see Figure 5b). In contrast to the systems with gadolinium, reducing the number of copper–substrate bonds and increasing the number of coordinating water molecules lead to qualitative changes in electronic structure. The essential difference in electronic structures of 17 and 20 is the peak shift above the Fermi level (see red curve in Figure 6b). Thus, the electronic structure of configuration 20 can be discussed as typical for non-metallic systems. Note that the magnetic moment of system 20 is the closest to 1.0 (see Table 1). Note that for Gd-containing systems, a similar shift in the peak to the area above Fermi level (see inset in Figure 5b) with a simultaneous approach of the magnetic moment of the system to 7.0 also take place. However, the magnitude of this shift is small and does not provide qualitative changes in electronic structure.
As discussed in the previous paragraph, changes in the electronic structure of Cu2+ centers also provide us with an answer about the nature of the above peak on the Fermi level. The electronic structure of copper in system 20 (red lines in Figure 6b) corresponds to the 3d9 4s0 electron configuration. The peak above the Fermi level corresponds to a single unoccupied 3d orbital. Since the electron configuration of the copper atom is 3d10, 4s1, the formation of two bonds corresponds to leaving one electron from the 4s shell and one from the 3d shell. The shift in the peak on the Fermi level (blue line in Figure 6b) can be described as a partial electron return from the metal–oxygen bond back to metal. Thus, decreasing metal–substrate bonds reduces the quantity of electrons “withdrawn” by metals from bonds and increases magnetic moments. The effect of coordination by water on magnetic moments provides a clue about the cause of the return of the electron on the metal ion. The absence or lack of coordination of metal ions significantly affects the crystal field and stimulates the “withdrawal” of the electrons from bonds. After the coordination of the metal ion by five or more water molecules, this previously utmost ion turns to the inside of some media. However, the asymmetry of these media (water above and bonds with oxygen atoms below) still affects the electronic structure. Described changes in electron distribution also lead to the weakening of metal–substrate bonds. Thus, the more abundant single metal–substrate bond proposed in the previous paragraph is more stable due to the minimal “withdrawal” of the electron participating in this bond formation.
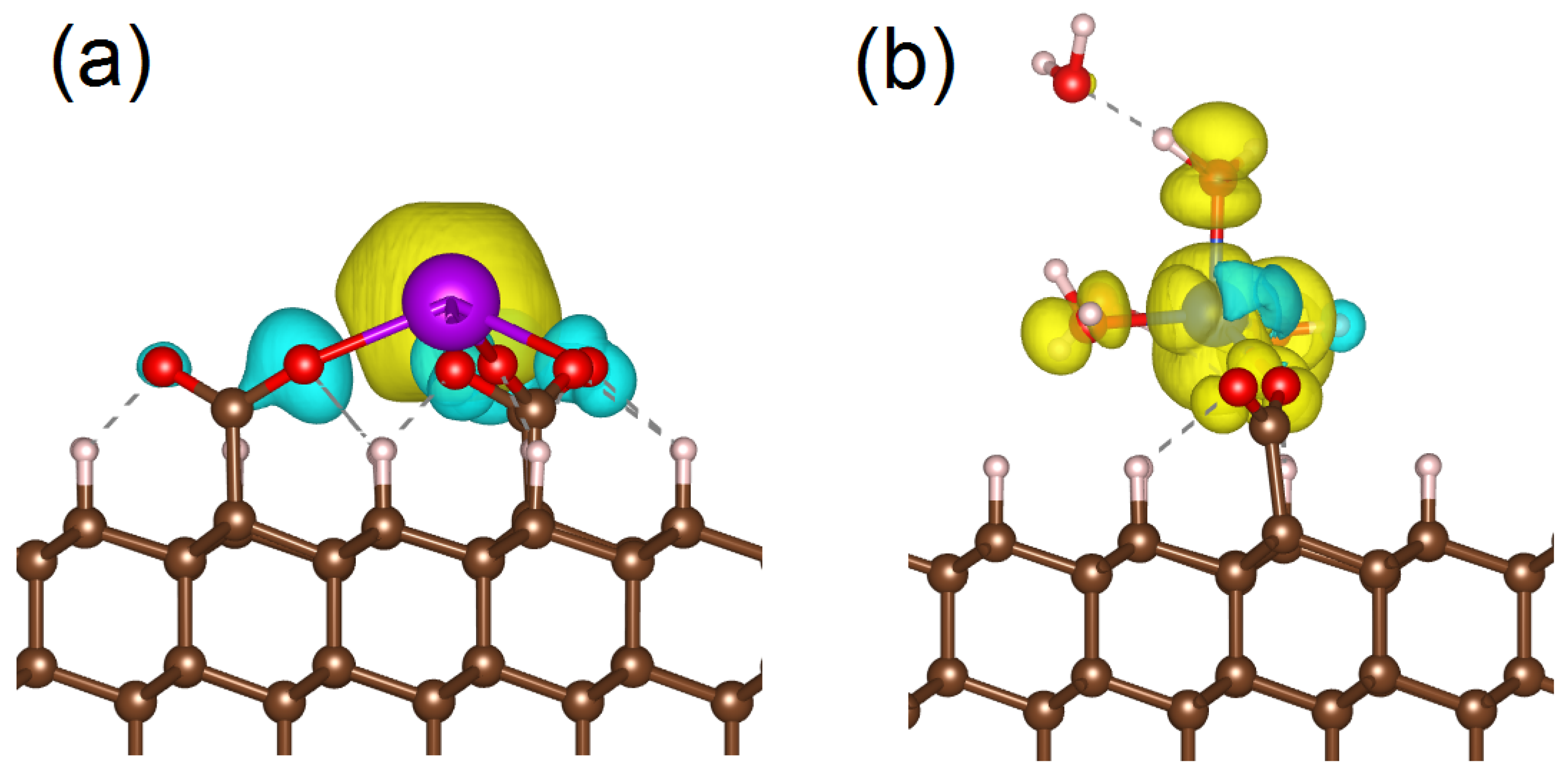
The last step of our work involves revealing the nature of experimentally detected and theoretically simulated differences in magnetic properties of gadolinium and copper on the substrate. For this purpose, we checked the effect of the formation of the appearance and disappearance of the peak on the Fermi level on the electronic structure of oxygen atoms connected to metals by firm bonds. Electron configuration of these oxygen atoms is 2s2 2p8. Removal of part of the electron from the 2p shell induces magnetic moment on oxygen atoms. These magnetic moments on ligands are oriented antiparallel to the magnetic moment on the metal center (see Figure 7a). These antiparallel magnetic moments on ligands decrease the overall magnetic moment of the supercell (see Table 1). In structure 20, copper forms a standard bond with –COO group and magnetic moments induced on ligands by hybridization between Cu 3d and O 2p orbitals are oriented parallel in the same direction to magnetic moment on metal ion (see Figure 7b). Therefore, a magnetic moment close to 1.0 /cell is distributed between the metal center and surrounding oxygen atoms. The described effect of hybridization O 2p and 3d or 4f orbitals of metals for charge redistribution and magnetic properties is the key to understanding the difference between the magnetic properties of copper and gadolinium on diamonds’ surfaces. Occupied 3d orbitals of copper have the same energy levels as occupied O 2p orbitals (see Figure 6). On the contrary, occupied 4f orbitals of gadolinium are located below oxygen bands (see Figure 5). Thus, decreases in metal–substrate bonds and additional coordinating of metal ions affect the redistribution of the charge more significantly in copper–oxygen bonds than in gadolinium–oxygen bonds.
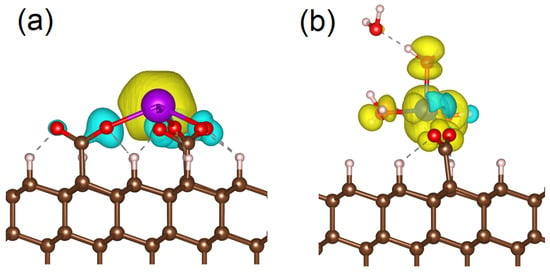
Figure 7.
Spin-polarized charge density for Gd3+ center connected to the diamond substrate by three –COO− groups (a) and Cu2+ connected to the substrate by one –COO− group and additionally coordinated by water molecules (b). Yellow clouds correspond to spin–up, and cyan clouds correspond to spin–down. The red balls are oxygen atoms, the white balls are hydrogen atoms and the brown balls are carbon atoms.
To prove the effect of symmetry on the magnetic properties of gadolinium on the diamond, we took the optimized atomic structures of configurations 5, 9, and 12, shifted the gadolinium–water complex away from the surface at 0.01 nm, and performed calculations of electronic structure and magnetic moments in these systems. A decrease in the magnetic moments by values of about 0.1 per metal–substrate bond was observed for all three studied systems. This decrease in the magnetic moment is caused by the enhancement of the ‘withdrawal’ of the electron from Gd–O bonds discussed above. The simulated minor changes in metal–substrate distances can also be considered a model of the contribution of thermal motion to the magnetic properties of the system. The larger number of metal–substrate bonds could correspond to more significant changes in the values of magnetic moments with increasing temperature.
5. Conclusions
The results of systematic experimental and theoretical studies of partially carboxylated hydrogenated (111) surface of diamonds by Gd3+ and Cu2+ ions demonstrate that attachment of metal ions by the formation of a robust bond with a single –COO− surface group is a more probable scenario. The attachment of the metal ions by two bridges to the substrate cannot be decisively ruled out, especially for gadolinium. Calculated values of the binding between metal ions and coordinating water molecules demonstrate the stability of the coordination of metal centers at ambient conditions. In other words, water molecules should coordinate metal centers even in dried samples. Magnetic measurements demonstrate visible a deviation in the magnetic moment of copper, especially for gadolinium centers, from integer numerical values. Theoretical simulations explain this phenomenon as the effect of the asymmetry of a metal atom’s environment on the redistribution of the charge in firm metal–COO bonds, which causes the shift in the electron density to the metal ion and reduction in the magnetic moment of the system. An increase in the number of water molecules attached to the metal center decreases the asymmetry of the environment of the metal center. It leads to the rise in magnetic moments on metal centers. Additional simulations of the effects of shallow carbon-inherited paramagnetic species demonstrate the negligible impact of this type of defect on magnetic properties and the atomic and electronic structure of metal ions on the surface.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/c10030063/s1, Figure S1: Optimized atomic structure for selected studied configurations (see Table 1 in the manuscript); Figure S2: Total densities of states for selected structures (see Table 1 in the manuscript); Figure S3: Optimized atomic structure of the model slab corresponding to structure 1 (a), and partial densities of states for gadolinium atom calculated by different methods. Description of technical details of DFT+U; calculations are as follows. Refs. [53,54] are cited in Supplementary Materials file.
Author Contributions
Conceptualization, D.W.B. and V.Y.O.; methodology, V.Y.O. and A.S.; software, D.W.B. and K.T.; validation, D.W.B., V.Y.O. and K.T.; formal analysis, V.Y.O., A.S. and K.T.; investigation, D.W.B., V.Y.O. and A.S.; resources, A.S. and K.T.; data curation, V.Y.O. and K.T.; writing—original draft preparation, D.W.B. and V.Y.O.; writing—review and editing, D.W.B., V.Y.O., A.S. and K.T.; visualization, D.W.B. and V.Y.O.; funding acquisition, K.T., A.S. and V.Y.O. All authors have read and agreed to the published version of the manuscript.
Funding
D.W.B. and A.S. acknowledge the support of the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (grant No. BR21881954). V.Y.O. acknowledges the support from Ioffe Institute program FFUG-2024-0019. K.T. acknowledges the support of JSPS KAKENHI (Grant Nos. 19K05410, 22K05056).
Data Availability Statement
Data are available on request due to restrictions, e.g., privacy or ethics.
Acknowledgments
V.Y.O. thanks colleagues from the Ioffe Institute for discussing problems associated with nanodiamonds.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Greiner, N.R.; Phillips, D.S.; Johnson, J.D.; Volk, F. Diamonds in detonation soot. Nature 1988, 333, 440–442. [Google Scholar] [CrossRef]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Danilenko, V.V. Specific features of synthesis of detonation nanodiamonds. Combust. Explos. Shock Waves 2005, 41, 577–588. [Google Scholar] [CrossRef]
- Reina, G.; Zhao, L.; Bianco, A.; Komatsu, N. Chemical functionalization of nanodiamonds: Opportunities and challenges ahead. Angew. Chem. Int. Ed. 2019, 58, 17918–17929. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A.; Lang, D. Functionality is key: Recent progress in the surface modification of nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Zheng, W.W.; Hsieh, Y.H.; Chiu, Y.C.; Cai, S.J.; Cheng, C.L.; Chen, C. Organic functionalization of ultradispersed nanodiamond: Synthesis and applications. J. Mater. Chem. 2009, 19, 8432–8441. [Google Scholar] [CrossRef]
- Meinhardt, T.; Lang, D.; Dill, H.; Krueger, A. Pushing the functionality of diamond nanoparticles to new horizons: Orthogonally functionalized nanodiamond using click chemistry. Adv. Funct. Mater. 2011, 21, 494–500. [Google Scholar] [CrossRef]
- Zou, Y.; Nishikawa, M.; Komatsu, N. Organic chemistry for nanodiamond: Controlled functionalization, quantitative characterization and structure-property relationships. Carbon Rep. 2022, 1, 70–78. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, Z.; Margrave, J.L.; Khabashesku, V.N. Functionalization of nanoscale diamond powder: Fluoro-, alkyl-, amino-, and amino acid-nanodiamond derivatives. Chem. Mater. 2004, 16, 3924–3930. [Google Scholar] [CrossRef]
- Barras, A.; Lyskawa, J.; Szunerits, S.; Woisel, P.; Boukherroub, R. Direct functionalization of nanodiamond particles using dopamine derivatives. Langmuir 2011, 27, 12451–12457. [Google Scholar] [CrossRef]
- Mayerhoefer, E.; Krueger, A. Surface control of nanodiamond: From homogeneous termination to complex functional architectures for biomedical applications. Acc. Chem. Res. 2022, 55, 3594–3604. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.H.; Patel, D.; Wairkar, S. Surface functionalization of nanodiamonds for biomedical applications. Mater. Sci. Eng. C 2020, 113, 110996. [Google Scholar] [CrossRef]
- Komatsu, N. Chemical functionalization of nanodiamond for nanobiomedicine. In Synthesis and Applications of Nanocarbons; Arnault, J.-C., Eder, D., Eds.; Jonh Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 229–246. [Google Scholar] [CrossRef]
- Shenderova, O.A.; McGuire, G.E. Science and engineering of nanodiamond particle surfaces for biological applications. Biointerphases 2015, 10, 030802. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A. The Chemistry of Nanodiamonds. In Nanodiamond; (Nanoscience, series), Williams, O.A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2014; Chapter 3; pp. 49–88. [Google Scholar] [CrossRef]
- Man, H.; Sasine, J.; Chow, E.K.; Ho, D. Nanodiamonds for drug delivery and diagnostics. In Nanodiamond (Nanoscience Series); Williams, O.A., Ed.; Royal Society of Chemistry: Cambridge, UK, 2014; Chapter 7; pp. 151–169. [Google Scholar] [CrossRef]
- Turova, O.V.; Starodubtseva, E.V.; Vinogradov, M.G.; Sokolov, V.I.; Abramova, N.V.; Vul, A.Y.; Alexenskiy, A.E. Palladium supported on detonation nanodiamond as a highly effective catalyst of the C=C and C≡C bond hydrogenation. Catal. Commun. 2011, 12, 577–579. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Osipov, V.Y. Transition metal atoms grafted on the nanodiamonds surface: Identification and guest–host spin-spin interactions. Mendeleev Commun. 2022, 32, 143–151. [Google Scholar] [CrossRef]
- Osipov, V.Y.; Romanov, N.M.; Suvorkova, I.E.; Osipova, E.V.; Tsuji, T.; Ishiguro, Y.; Takai, K. Magnetic resonance tracking of copper ion fixation on the surface of carboxylated nanodiamonds from viewpoint of changes in carbon-inherited paramagnetism. Mendeleev Commun. 2022, 32, 132–135. [Google Scholar] [CrossRef]
- Osipov, V.Y.; Aleksenskiy, A.E.; Takai, K.; Vul’, A.Y. Magnetic studies of a detonation nanodiamond with the surface modified by gadolinium ions. Phys. Solid State 2015, 57, 2314–2319. [Google Scholar] [CrossRef]
- Gridnev, I.D.; Osipov, V.Y.; Aleksenskii, A.E.; Vul’, A.Y.; Enoki, T. Combined experimental and DFT study of the chemical binding of copper ions on the surface of nanodiamonds. Bull. Chem. Soc. Jpn. 2014, 87, 693–704. [Google Scholar] [CrossRef]
- Shames, A.I.; Osipov, V.Y.; Aleksenskiy, A.E.; Ōsawa, E.; Vul, A.Y. Locating inherent unpaired orbital spins in detonation nanodiamonds through the targeted surface decoration by paramagnetic probes. Diam. Relat. Mater. 2011, 20, 318–321. [Google Scholar] [CrossRef]
- Osipov, V.Y.; Boukhvalov, D.W.; Takai, K. Gadolinium ion bonding on the surface of carboxylated detonation nanodiamond in terms of magnetochemistry and density functional theory. Mendeleev Commun. 2020, 30, 436–438. [Google Scholar] [CrossRef]
- Osipov, V.Y.; Boukhvalov, D.W.; Takai, K. Isolated spin-7/2 species of gadolinium (III) chelate complexes on the surface of 5-nm diamond particles. Nanomaterials 2023, 13, 1995. [Google Scholar] [CrossRef] [PubMed]
- Panich, A.M.; Shames, A.I.; Sergeev, N.A.; Osipov, V.Y.; Alexenskiy, A.E.; Vul’, A.Y. Magnetic resonance study of gadolinium-grafted nanodiamonds. J. Phys. Chem. C 2016, 120, 19804–19811. [Google Scholar] [CrossRef]
- Panich, A.M.; Salti, M.; Goren, S.D.; Yudina, E.B.; Aleksenskii, A.E.; Vul’, A.Y.; Shames, A.I. Gd (III)-grafted detonation nanodiamonds for MRI contrast enhancement. J. Phys. Chem. C 2019, 123, 2627–2631. [Google Scholar] [CrossRef]
- Yano, K.; Matsumoto, T.; Okamoto, Y.; Bito, K.; Kurokawa, N.; Hasebe, T.; Hotta, A. Gadolinium-complexed carboxylated nanodiamond particles for magnetic resonance imaging of the lymphatic system. ACS Appl. Nano Mater. 2021, 4, 1702–1711. [Google Scholar] [CrossRef]
- Comet, M.; Pichot, V.; Siegert, B.; Britz, F.; Spitzer, D. Detonation nanodiamonds for doping Kevlar. J. Nanosci. Nanotechnol. 2010, 10, 4286–4292. [Google Scholar] [CrossRef] [PubMed]
- Shvidchenko, A.V.; Zhukov, A.N.; Dideikin, A.T.; Baidakova, M.V.; Shestakov, M.S.; Shnitov, V.V.; Vul’, A.Y. Electrosurface properties of single-crystalline detonation nanodiamond particles obtained by air annealing of their agglomerates. Colloid J. 2016, 78, 235–241. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; Garsia, A.; Junquera, J.; Orejon, P.; Sanchez-Portal, D. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter 2002, 14, 2745. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van der Waals density functional for general geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef]
- Tromer, R.M.; Felix, L.C.; Woellner, C.F.; Galvao, D.S. A DFT investigation of the electronic, optical, and thermoelectric properties of pentadiamond. Chem. Phys. Lett. 2021, 763, 138210. [Google Scholar] [CrossRef]
- Gali, A.; Fyta, M.; Kaxiras, E. Ab initio supercell calculations on nitrogen-vacancy center in diamond: Electronic structure and hyperfine tensors. Phys. Rev. B 2008, 77, 155206. [Google Scholar] [CrossRef]
- Yndurain, F. First-principles calculations of the diamond (110) surface: A Mott insulator. Phys. Rev. B 2007, 75, 195443. [Google Scholar] [CrossRef]
- Troullier, O.N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Bates, L.F. Modern Magnetism, 2nd ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 1–454. ISBN 978-1-316-60186-0. [Google Scholar]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005; pp. 1–680. ISBN 0-471-41526-X. [Google Scholar]
- Getzlaff, M. Fundamentals of Magnetism; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–386. ISBN 978-3-540-31150-8. [Google Scholar]
- Huang, H.; Yang, S.; Zhang, X. Magnetic properties of heavy rare-earth metallofullerenes M@C82 (M= Gd, Tb, Dy, Ho, and Er). J. Phys. Chem. B 2000, 104, 1473–1482. [Google Scholar] [CrossRef]
- Yosida, K. Theory of Magnetism: Edition en Anglais, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 122, pp. 7–12. ISBN 3-540-60651-3. [Google Scholar]
- Funasaka, H.; Sakurai, K.; Oda, Y.; Yamamoto, K.; Takahashi, T. Magnetic properties of Gd@C82 metallofullerene. Chem. Phys. Lett. 1995, 232, 273–277. [Google Scholar] [CrossRef]
- Marangon, I.; Ménard-Moyon, C.; Kolosnjaj-Tabi, J.; Béoutis, M.L.; Lartigue, L.; Alloyeau, D.; Pach, E.; Ballesteros, B.; Autret, G.; Ninjbadgar, T.; et al. Covalent functionalization of multi-walled carbon nanotubes with a gadolinium chelate for efficient T1-weighted magnetic resonance imaging. Adv. Funct. Mater. 2014, 24, 7173–7186. [Google Scholar] [CrossRef]
- Murata, H.; Takahashi, M.; Namba, K.; Takahashi, N.; Nishide, H. A high-spin and durable polyradical: Poly(4-diphenylaminium-1,2-phenylenevinylene). J. Org. Chem. 2004, 69, 631–638. [Google Scholar] [CrossRef]
- Sugawara, T.; Bandow, S.; Kimura, K.; Iwamura, H.; Itoh, K. Magnetic behavior of nonet tetracarbene as a model for one-dimensional organic ferromagnets. J. Am. Chem. Soc. 1986, 108, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Shames, A.I.; Panich, A.M.; Kempiński, W.; Alexenskii, A.E.; Baidakova, M.V.; Dideikin, A.T.; Osipov, V.Y.; Siklitski, V.I.; Osawa, E.; Ozawa, M.; et al. Defects and impurities in nanodiamonds: EPR, NMR and TEM study. J. Phys. Chem. Solids 2002, 63, 1993–2001. [Google Scholar] [CrossRef]
- Boukhvalov, D.W.; Osipov, V.Y.; Takai, K. Long range interactions and related carbon-carbon bond reconstruction between interior and surface defects in nanodiamonds. Phys. Chem. Chem. Phys. 2021, 23, 14592–14600. [Google Scholar] [CrossRef]
- CRC Handbook of Chemistry and Physics, 102nd, ed.; Rumble, J., Ed.; Tailor & Fransis, CRC Press: Boca Raton, FL, USA, 2021; pp. 1–1604. ISBN 9780367712600. [Google Scholar]
- Panich, A.M.; Sergeev, N.A. Towards determination of distances between nanoparticles and grafted paramagnetic ions by NMR relaxation. Appl. Magn. Reson. 2018, 49, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Long, O.L.; Gautam, G.S.; Carter, E.A. Evaluating optimal U for 3d transition-metal oxides within the SCAN+U framework. Phys. Rev. Mater. 2020, 4, 045401. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic population analysis on LCAO–MO molecular wave functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Giannozzi, G.P.; Andreussi, O.; Brumme, T.; Bunau, O.; Buongiorno Nardelli, M.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Cococcioni, M.; et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys.: Condens. Matter 2017, 29, 465901. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).