A Nitrogen/Oxygen Dual-Doped Porous Carbon with High Catalytic Conversion Ability toward Polysulfides for Advanced Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
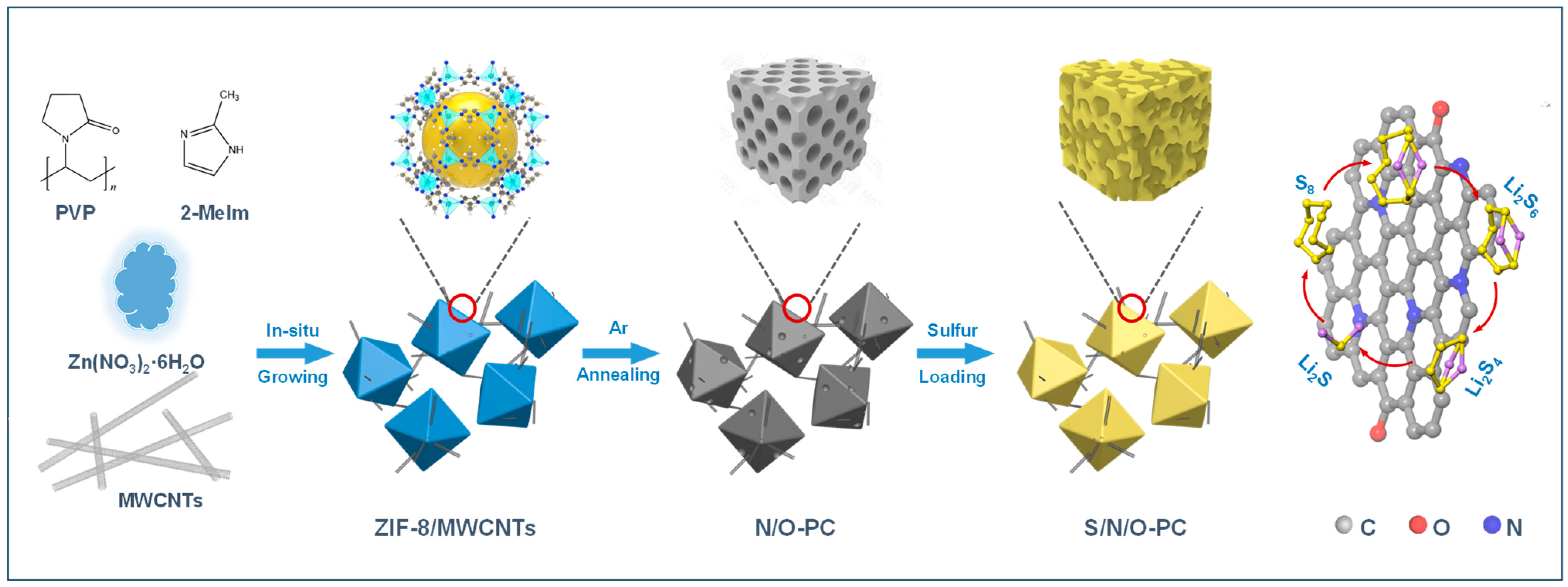
2.2. Synthesis of N/O-PC Composite
2.3. Fabrication of S/N/O-PC Composite
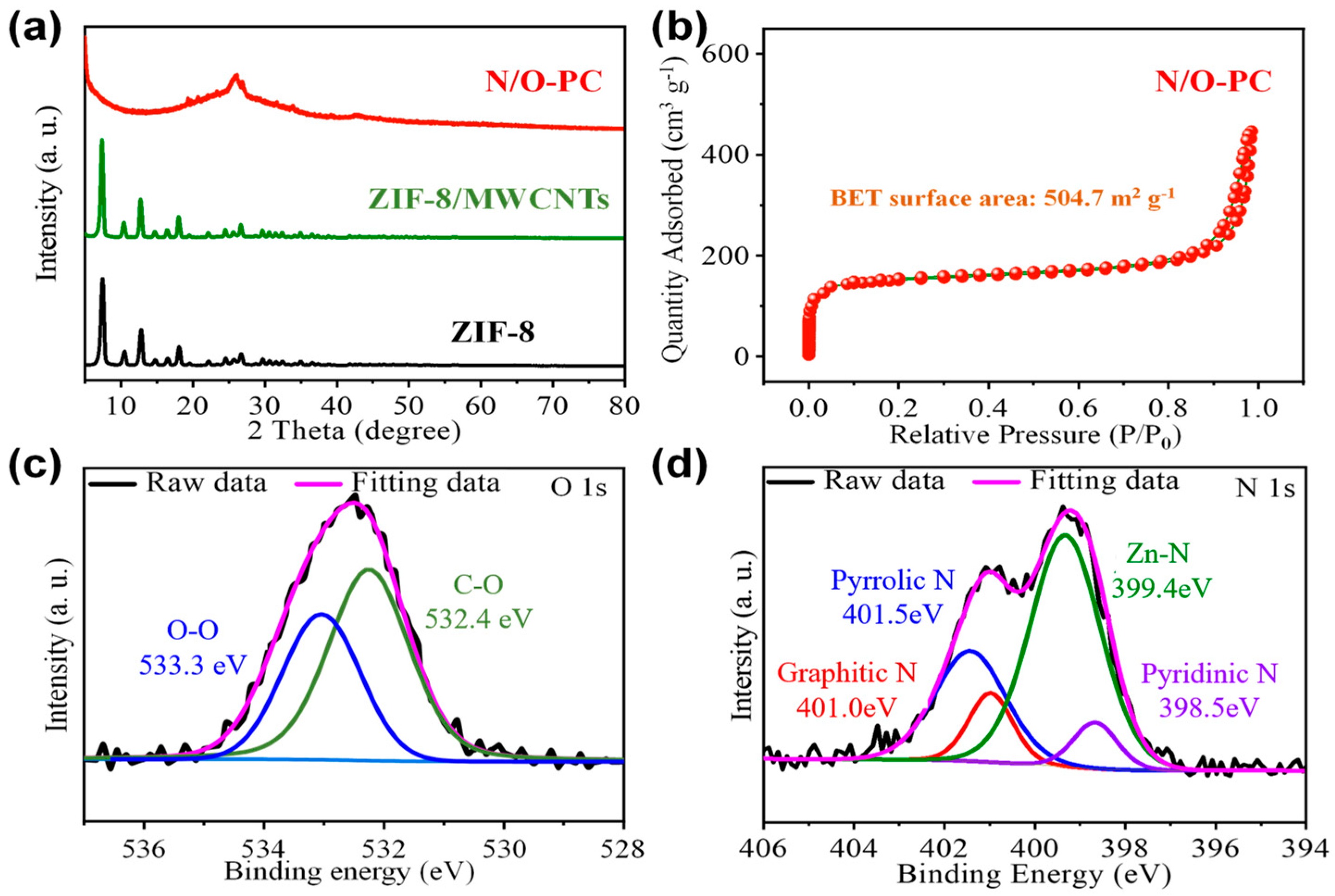
2.4. Materials Characterization
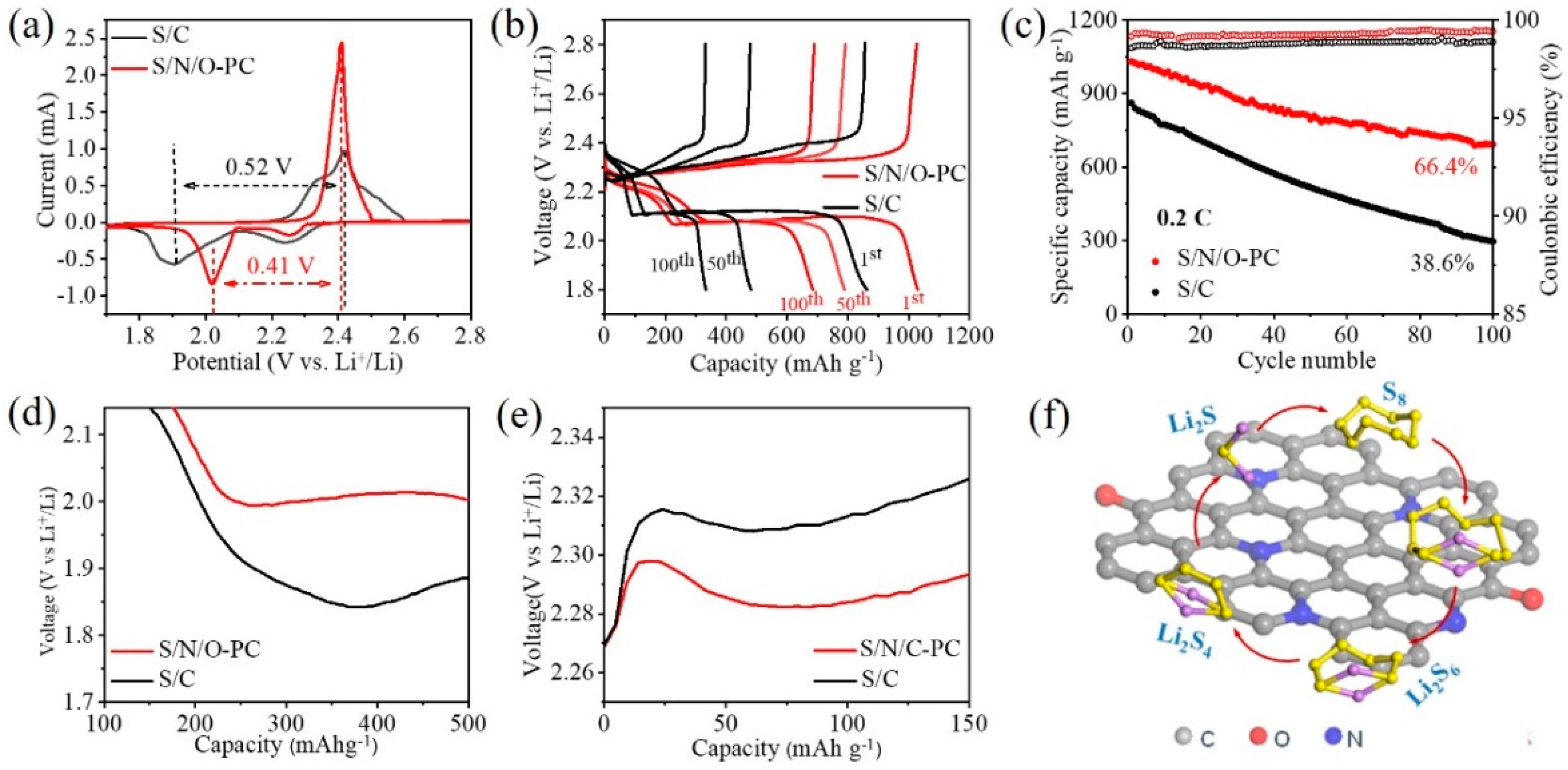
2.5. Electrochemical Measurement
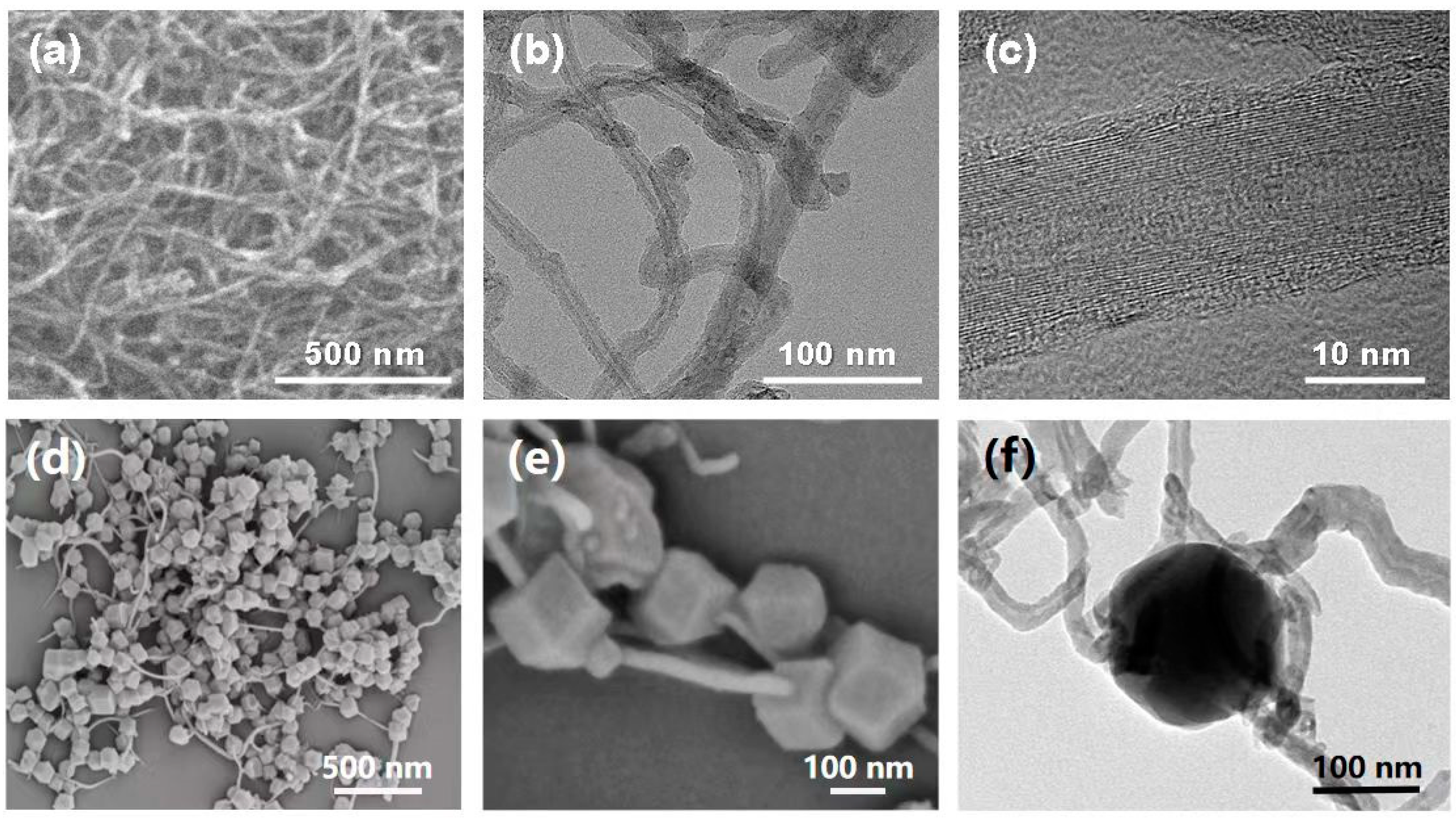
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Zhao, D.; Xu, Y.; Liu, S.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Wang, Z.; Wu, Y.; et al. A review on electrode materials of fast-charging lithium-ion batteries. Chem. Rec. 2022, 22, e202200127. [Google Scholar] [CrossRef] [PubMed]
- Ao, S.; Gouda, S.P.; Selvaraj, M.; Boddula, R.; Al-Qahtani, N.; Mohan, S.; Rokhum, S.L. Active sites engineered biomass-carbon as a catalyst for biodiesel production: Process optimization using RSM and life cycle assessment. Energy Convers. Manag. 2024, 300, 117956. [Google Scholar] [CrossRef]
- Ren, J.; Wang, Z.; Xu, P.; Wang, C.; Gao, F.; Zhao, D.; Liu, S.; Yang, H.; Di, W.; Niu, C.; et al. Porous Co2VO4 nanodisk as a high-energy and fast-charging anode for lithium-ion batteries. Nano Micro Lett. 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, Y.; Zhang, Y.; Li, D.; Huang, Z. Facile synthesis of sulfur@titanium carbide Mxene as high performance cathode for lithium-sulfur batteries. Nanophotonics 2020, 9, 2025–2032. [Google Scholar] [CrossRef]
- Baumann, A.E.; Han, X.; Butala, M.M.; Thoi, V.S. Lithium thiophosphate functionalized zirconium MOFs for Li-S batteries with enhanced rate capabilities. J. Am. Chem. Soc. 2019, 141, 17891–17899. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, Z.; Zhao, D.; Yang, J.; Zhang, Y.; Du, Y.; Wang, Y. Azo–branched covalent organic framework thin films as active separators for superior sodium–sulfur batteries. ACS Nano 2022, 16, 14178–14187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ge-Zhang, S.; Zhang, Z.; Tang, H.; Xu, Y.; Gao, F.; Xu, X.; Liu, S.; Zhou, J.; Wang, Z.; et al. Three–dimensional honeycomb–like carbon as sulfur host for sodium–sulfur batteries without the shuttle effect. ACS Appl. Mater. Interfaces 2022, 14, 54662–54669. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, R.; Wen, J.; Hu, X.; Li, Z.; Li, M.; Pan, F.; Xu, C. Separator engineering toward practical Li-S batteries: Targeted electrocatalytic sulfur conversion, lithium plating regulation, and thermal tolerance. Nano Energy 2022, 95, 106982. [Google Scholar] [CrossRef]
- Zhou, C.; Li, Z.; Xu, X.; Mai, L. Metal-organic frameworks enable broad strategies for lithium-sulfur batteries. Natl. Sci. Rev. 2021, 8, nwab055. [Google Scholar] [CrossRef]
- Cao, G.; Bi, D.; Zhao, J.; Zheng, J.; Wang, Z.; Lai, Q.; Liang, Y. Transformation of ZIF-8 nanoparticles into 3D nitrogen-doped hierarchically porous carbon for Li–S batteries. RSC Adv. 2020, 10, 17345–17352. [Google Scholar] [CrossRef]
- Du, X.-L.; You, Y.; Yan, Y.; Zhang, D.; Cong, H.-P.; Qin, H.; Zhang, C.; Cao, F.-F.; Jiang, K.-C.; Wang, Y.; et al. Conductive carbon network inside a sulfur-impregnated carbon sponge: A bioinspired high-performance cathode for li–s battery. ACS Appl. Mater. Interfaces 2016, 8, 22261–22269. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, Y.; Hori, K.; Mae, T.; Noda, S. High-energy-density Li-S battery with positive electrode of lithium polysulfides held by carbon nanotube sponge. Carbon 2021, 182, 32–41. [Google Scholar] [CrossRef]
- Gueon, D.; Yoon, J.; Hwang, J.T.; Moon, J.H. Microdomain sulfur-impregnated CeO2-coated CNT particles for high-performance Li-S batteries. Chem. Eng. J. 2020, 390, 124548. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.; Wen, X.; Ding, Y.; Zhou, C.; Zhang, Y.; Wei, H.; Hu, N. Graphene/Mxene fibers-enveloped sulfur cathodes for high-performance Li-S batteries. Electrochim. Acta 2021, 371, 137838. [Google Scholar] [CrossRef]
- Hong, X.; Liang, J.; Tang, X.; Yang, H.; Li, F. Hybrid graphene album with polysulfides adsorption layer for Li-S batteries. Chem. Eng. Sci. 2019, 194, 148–155. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, J.; Liang, X.; Li, Y.; Cui, J.; Chen, M. Tunable MOFs derivatives for stable and fast sulfur electrodes in Li-S batteries. Chem. Eng. J. 2022, 450, 138287. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Pan, H.; Zhang, J.; Xiang, S.; Cheng, Z.; Zhang, Z. Pore-space-partitioned MOF separator promotes high-sulfur-loading Li-S batteries with intensified rate capability and cycling life. J. Mater. Chem. A 2021, 47, 26929–26938. [Google Scholar] [CrossRef]
- Chenxi, Y.; Liping, T.; Bo, W.; Hulin, T.; Xinyue, L.; Jian, C.; Mengyao, G.; Naiqiang, L. NiCoSe4@CNFs derived from MOF compounds enabling robust polysulfide adsorption and catalysis in Li-S batteries. J. Alloys Compd. 2023, 957, 170449. [Google Scholar]
- Xiaohua, C.; Mi, Z.; Jin, Z.; Juan, W.; Zengbao, J.; Yong, L. Boosting electrochemical performance of Li-S batteries by cerium-based MOFs coated with polypyrrole. J. Alloys Compd. 2022, 901, 163649. [Google Scholar]
- Zhang, H.; Zhao, W.; Zou, M.; Wang, Y.; Chen, Y.; Xu, L.; Wu, H.; Cao, A. 3D, mutually embedded MOF@Carbon nanotube hybrid networks for high—performance lithium—sulfur batteries. Adv. Energy Mater. 2018, 819, 1614–6832. [Google Scholar] [CrossRef]
- Bi, T.; Zheng, W.; Zhou, Y.; Lin, Q.; Zhang, X. Three-dimensional N, S co-doped ultra-thin-walled hierarchical porous carbon as sulfur-loading host for high-performance lithiumsulfur battery. J. Energy Storage 2024, 93, 112428. [Google Scholar] [CrossRef]
- Haina, C.; Jingyu, S.; Menglei, W.; Yan, H.; Zixiong, S. A review in rational design of graphene toward advanced Li-S batteries. Nano Res. Energy 2023, 2, E9120054. [Google Scholar]
- Ye, H.; Li, Y. Towards practical lean-electrolyte Li-S batteries: Highly solvating electrolytes or sparingly solvating electrolytes? Nano Res. Energy 2022, 1, e9120012. [Google Scholar] [CrossRef]
- Liu, H.; Liu, F.; Qu, Z.; Chen, J.; Liu, H.; Tan, Y.; Guo, J.; Yan, Y.; Zhao, S.; Zhao, X.; et al. High sulfur loading and shuttle inhibition of advanced sulfur cathode enabled by graphene network skin and N, P, F-doped mesoporous carbon interfaces for ultra-stable lithium sulfur battery. Nano Res. Energy 2023, 2, E912049. [Google Scholar] [CrossRef]
- Xue, H.; Gong, H.; Yamauchi, Y.; Sasaki, T.; Ma, R. Photo-enhanced rechargeable high-energy-density metal batteries for solar energy conversion and storage. Nano Res. Energy 2022, 1, E9120007. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Chen, S.; Zhu, X.; Deng, Q.; Wang, J.; Zeng, Z.; Deng, S. Facile and low-temperature strategy to prepare hollow ZIF-8/CNT polyhedrons as high-performance lithium-sulfur cathodes. Chem. Eng. J. 2020, 404, 126579. [Google Scholar] [CrossRef]
- Jeon, Y.; Lee, J.; Jo, H.; Hong, H.; Lee, L.Y.S.; Piao, Y. Co/Co3O4-embedded n-doped hollow carbon composite derived from a bimetallic MOF/ZnO core-shell template as a sulfur host for li-s batteries. Chem. Eng. J. 2020, 407, 126967. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, D.; Zhou, W.; Xu, W.; Liang, X.; He, G.; Wu, Z.; Li, L. Nitrogen—doped hollow carbon polyhedrons with carbon nanotubes surface layers as effective sulfur hosts for high—rate, long—lifespan lithium–sulfur batteries. ChemElectroChem 2020, 724, 4990–4998. [Google Scholar] [CrossRef]
- Chen, P.; Wang, T.; Tang, F.; Chen, G.; Wang, C. Elaborate interface design of CoS2/Fe7S8/NG heterojunctions modified on a polypropylene separator for efficient lithium-sulfur batteries. Chem. Eng. J. 2022, 446, 136990. [Google Scholar] [CrossRef]
- Liu, G.; Zeng, Q.; Fan, Z.; Tian, S.; Li, X.; Lv, X.; Zhang, W.; Tao, K.; Xie, E.; Zhang, Z. Boosting sulfur catalytic kinetics by defect engineering of vanadium disulfide for high-performance lithium-sulfur batteries. Chem. Eng. J. 2022, 448, 137683. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M.; Kang, J.; Su, Q.; Du, G.; Xu, B. Nitrogen and oxygen dual-doped porous carbon derived from natural ficus microcarpas as host for high performance lithium-sulfur batteries. Mater. Res. Bull. 2019, 113, 70–76. [Google Scholar] [CrossRef]
- Yang, D.; Li, M.; Zheng, X.; Han, X.; Zhang, C.; Biendicho, J.J.; Llorca, J.; Wang, J.; Hao, H.; Li, J.; et al. Phase engineering of defective copper selenide toward robust lithium-sulfur batteries. ACS Nano 2022, 16, 11102–11114. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, B.; Sun, F.; Jiang, G.; Zheng, N.; Xu, C.; Li, Y. Core-shell polyhedrons of carbon nanotubes-grafted graphitic carbon@nitrogen doped carbon as efficient sulfur immobilizers for lithium-sulfur batteries. Appl. Surf. Sci. 2018, 450, 364–371. [Google Scholar] [CrossRef]
- Geng, M.; Yang, H.; Shang, C. The multi-functional effects of CuS as modifier to fabricate efficient interlayer for Li-S batteries. Adv. Sci. 2022, 9, 202204561. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-X.; Li, X.-T.; Zhao, C.-F.; Fu, Z.-H.; Zhang, Q.-S.; Hu, C. Promoted deposition of three-dimensional Li2S on catalytic Co phthalocyanine nanorods for stable high-loading lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2020, 1229, 32752–32763. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Xin, S.; Guo, Y.; Wan, L. Lithium-sulfur batteries: Electrochemistry, materials, and prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef] [PubMed]
- Uppugalla, S.; Pothu, R.; Boddula, R.; Desai, M.A.; Al-Qahtani, N. Nitrogen and sulfur co-doped activated carbon nanosheets for high-performance coin cell supercapacitor device with outstanding cycle stability. Emergent Mater. 2023, 6, 1167–1176. [Google Scholar] [CrossRef]
- Li, H.; Sun, L.; Zhao, Y.; Tan, T.; Zhang, Y. A novel CuS/graphene-coated separator for suppressing the shuttle effect of lithium/sulfur batteries. Appl. Surf. Sci. 2019, 466, 309–319. [Google Scholar] [CrossRef]
- Ma, F.; Srinivas, K.; Zhang, X.J.; Zhang, Z.H.; Wu, Y.; Liu, D.W.; Zhang, W.L.; Wu, Q.; Chen, Y.F. Mo2N quantum dots decorated n-doped graphene nanosheets as dual-functional interlayer for dendrite-free and shuttle-free lithium-sulfur batteries. Adv. Funct. Mater. 2022, 32, 2206113. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Li, J.; Wang, X.; Zhang, Y.; Guo, Y.; Ding, J.; Wang, L. Bifunctional separator with sandwich structure for high-performance lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 559, 13–20. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.; Cheng, H.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Zou, K.; Zhou, T.; Chen, Y.; Xiong, X.; Jing, W.; Dai, X.; Shi, M.; Li, N.; Sun, J.; Zhang, S.; et al. Defect engineering in a multiple confined geometry for robust lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2103981. [Google Scholar] [CrossRef]
- Wang, W.; Xi, K.; Li, B.; Li, H.; Liu, S.; Wang, J.; Zhao, H.; Li, H.; Abdelkader, A.M.; Gao, X.; et al. A Sustainable Multipurpose Separator Directed Against the Shuttle Effect of Polysulfides for High-Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2200160. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Z.; Yang, T.; Nie, C.; Xu, X.; Wang, Y.; Yang, J.; Dou, S.; Wang, N. Advances in high sulfur loading cathodes for practical lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2201585. [Google Scholar] [CrossRef]
- Yang, M.; Liu, P.; Qu, Z.; Sun, F.; Tian, Y.; Ye, X.; Wang, X.; Liu, X.; Li, H. Nitrogen-vacancy-regulated Mo2N quantum dots electrocatalyst enables fast polysulfides redox for high-energy-density lithium-sulfur batteries. Nano Energy 2022, 104, 107922. [Google Scholar] [CrossRef]
- Chen, S.; Li, D.; Liu, Y.; Huang, W. Morphology-dependent defect structures and photocatalytic performance of hydrogenated anatase TiO2 nanocrystals. J. Catal. 2016, 341, 126–135. [Google Scholar] [CrossRef]
- Fan, F.Y.; Chiang, Y.-M. Electrodeposition kinetics in Li-S batteries: Effects of low electrolyte/sulfur ratios and deposition surface composition. J. Electrochem. Soc. 2017, 164, A917–A922. [Google Scholar] [CrossRef]
- Yang, M.; Wang, X.; Wu, J.; Tian, Y.; Huang, X.; Liu, P.; Li, X.; Li, X.; Liu, X.; Li, H. Dual electrocatalytic heterostructures for efficient immobilization and conversion of polysulfides in li-s batteries. J. Mater. Chem. A 2021, 9, 18477–18487. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, X.; Yang, Y.; Yang, Z.; Wang, H.; Yu, N. A Nitrogen/Oxygen Dual-Doped Porous Carbon with High Catalytic Conversion Ability toward Polysulfides for Advanced Lithium–Sulfur Batteries. C 2024, 10, 67. https://doi.org/10.3390/c10030067
Shu X, Yang Y, Yang Z, Wang H, Yu N. A Nitrogen/Oxygen Dual-Doped Porous Carbon with High Catalytic Conversion Ability toward Polysulfides for Advanced Lithium–Sulfur Batteries. C. 2024; 10(3):67. https://doi.org/10.3390/c10030067
Chicago/Turabian StyleShu, Xiaoyan, Yuanjiang Yang, Zhongtang Yang, Honghui Wang, and Nengfei Yu. 2024. "A Nitrogen/Oxygen Dual-Doped Porous Carbon with High Catalytic Conversion Ability toward Polysulfides for Advanced Lithium–Sulfur Batteries" C 10, no. 3: 67. https://doi.org/10.3390/c10030067
APA StyleShu, X., Yang, Y., Yang, Z., Wang, H., & Yu, N. (2024). A Nitrogen/Oxygen Dual-Doped Porous Carbon with High Catalytic Conversion Ability toward Polysulfides for Advanced Lithium–Sulfur Batteries. C, 10(3), 67. https://doi.org/10.3390/c10030067






