Research Progress in Graphene-Based Adsorbents for Wastewater Treatment: Preparation, Adsorption Properties and Mechanisms for Inorganic and Organic Pollutants
Abstract
1. Introduction
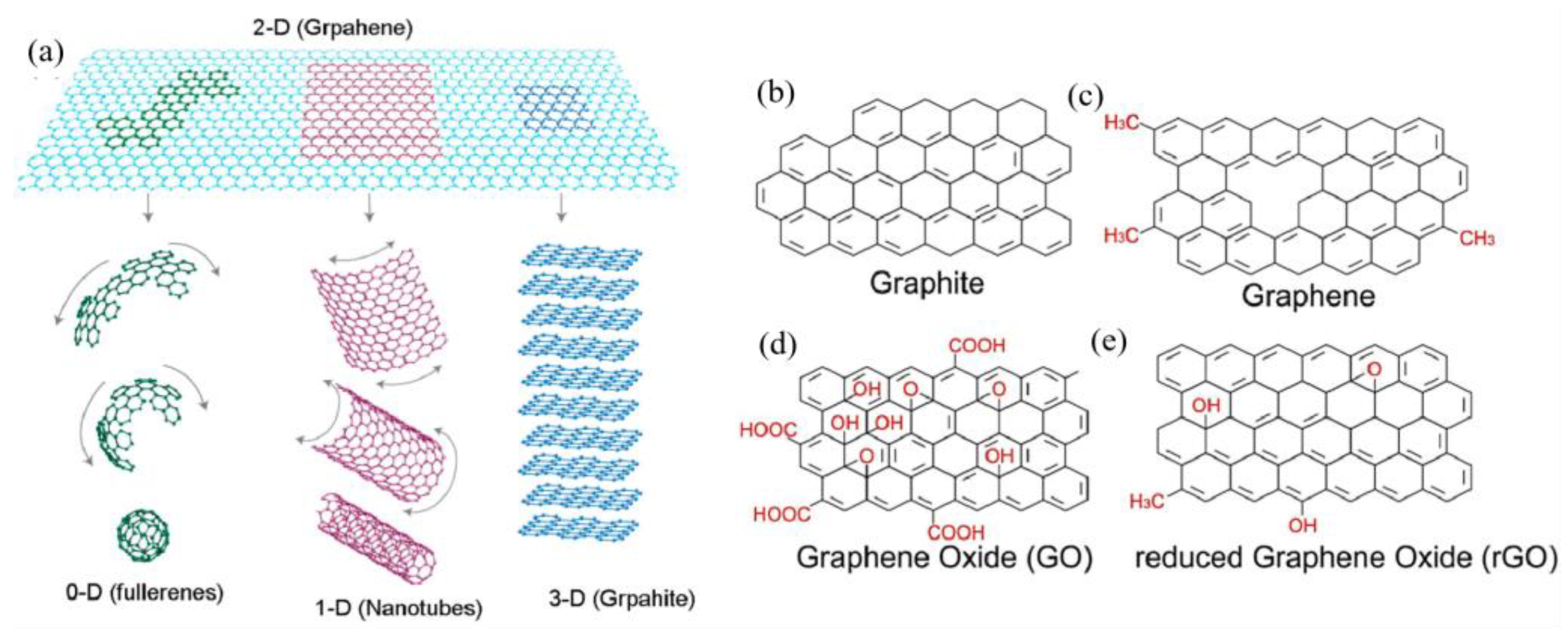
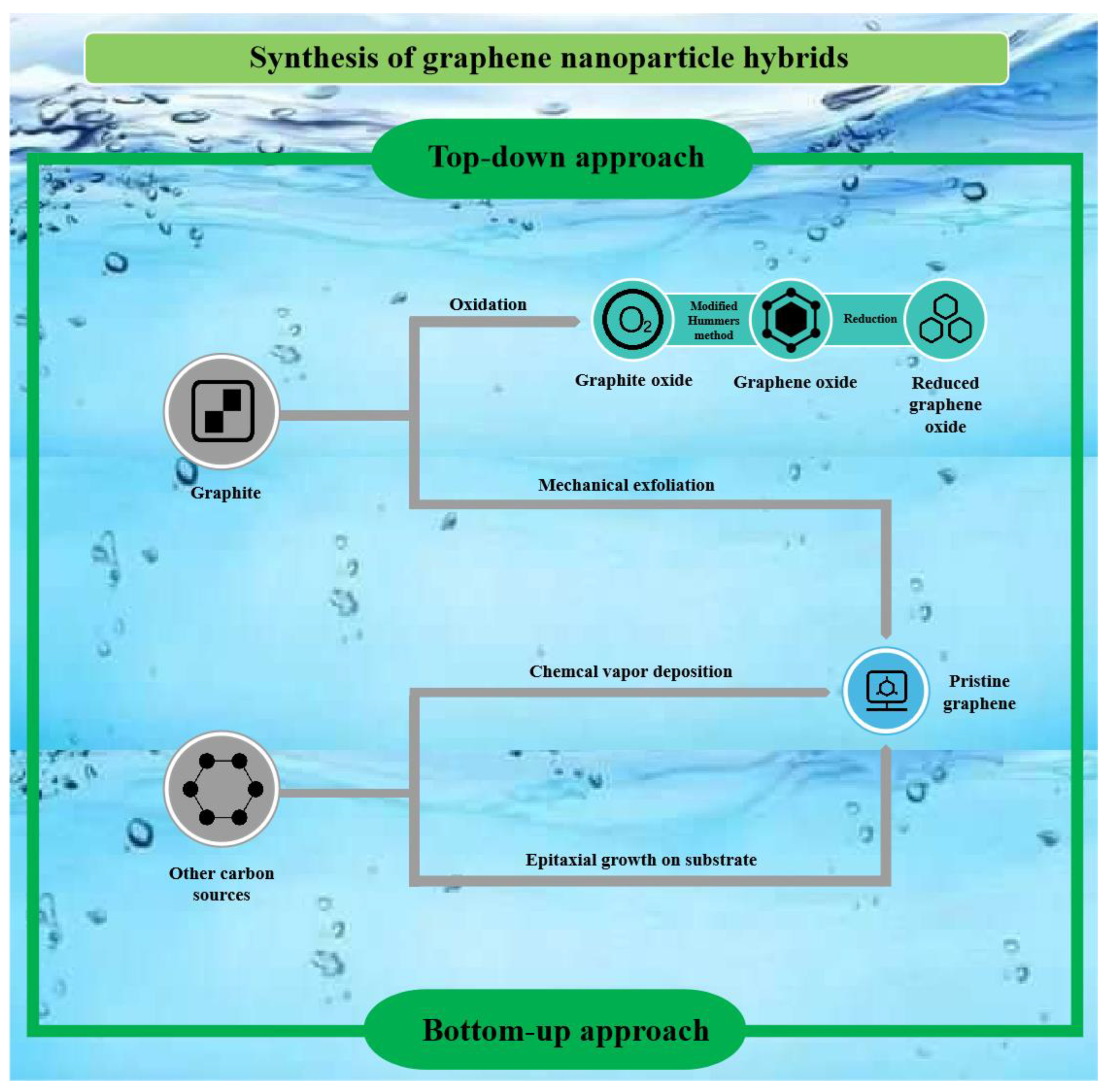
2. Preparation of Graphene and Its Derivatives
2.1. Preparation of Graphene and GO
2.1.1. Graphene
2.1.2. Graphene Oxide
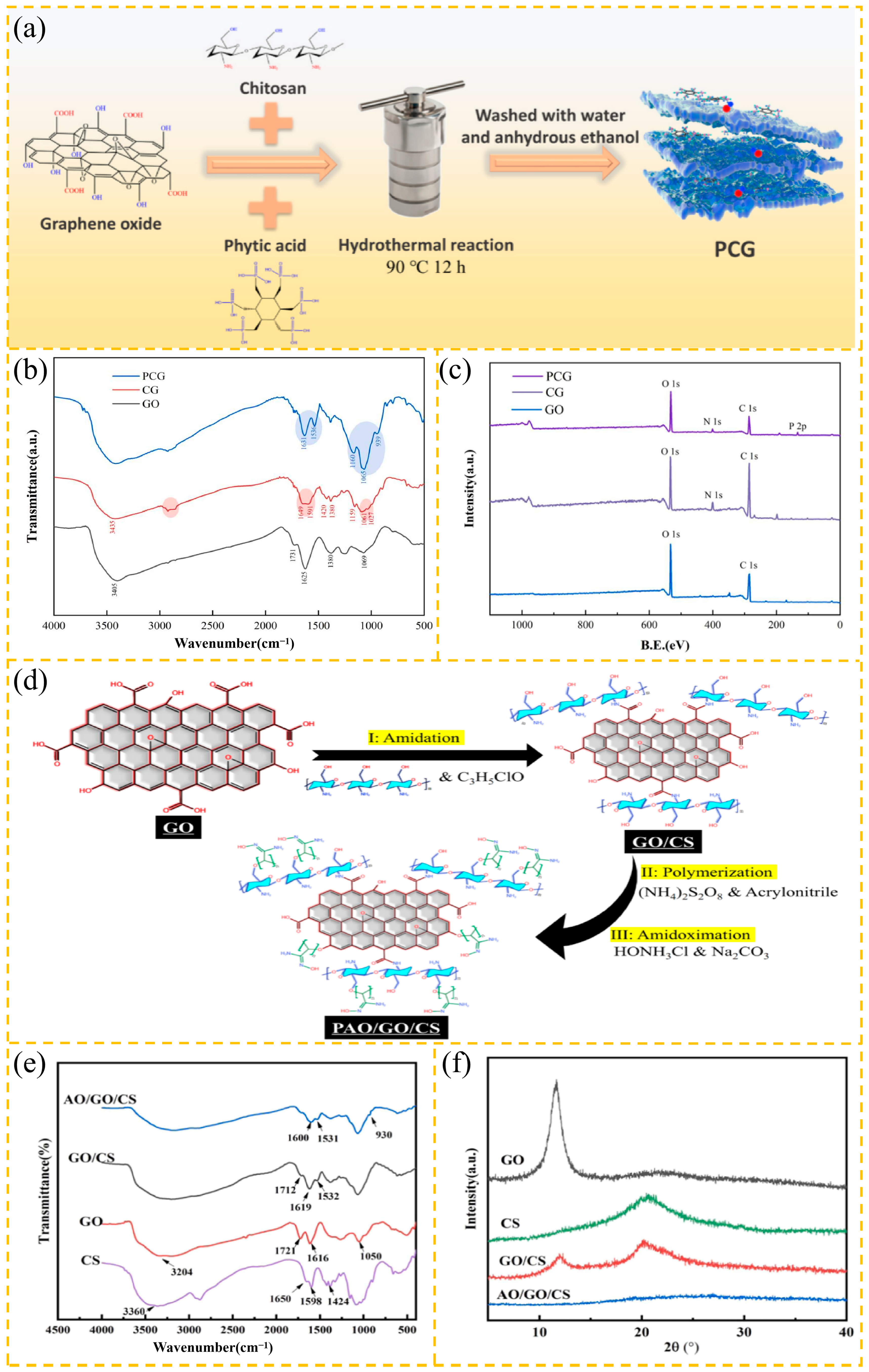
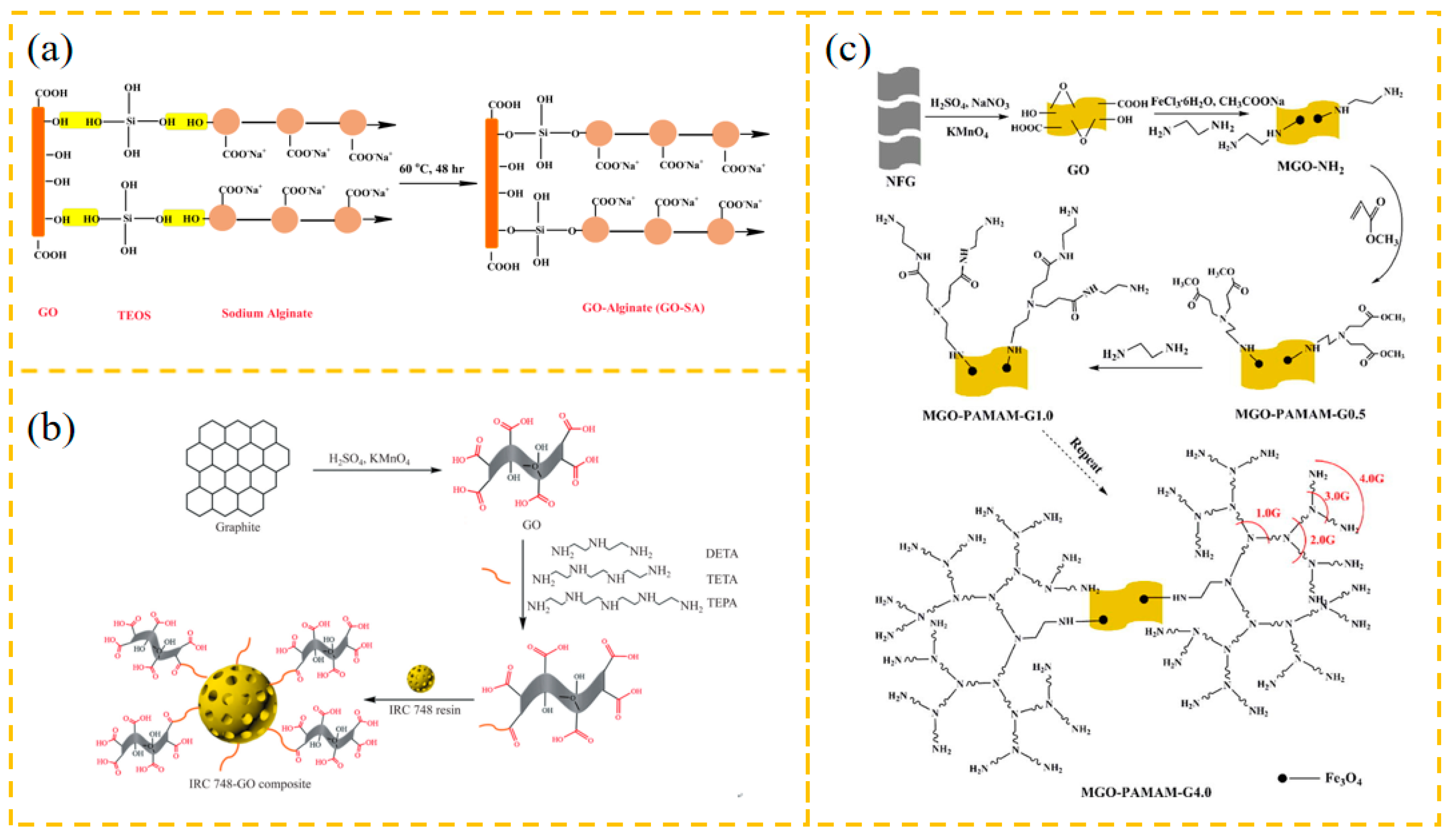
2.2. Preparation of GO Derivatives
2.2.1. GO Inorganic Composites
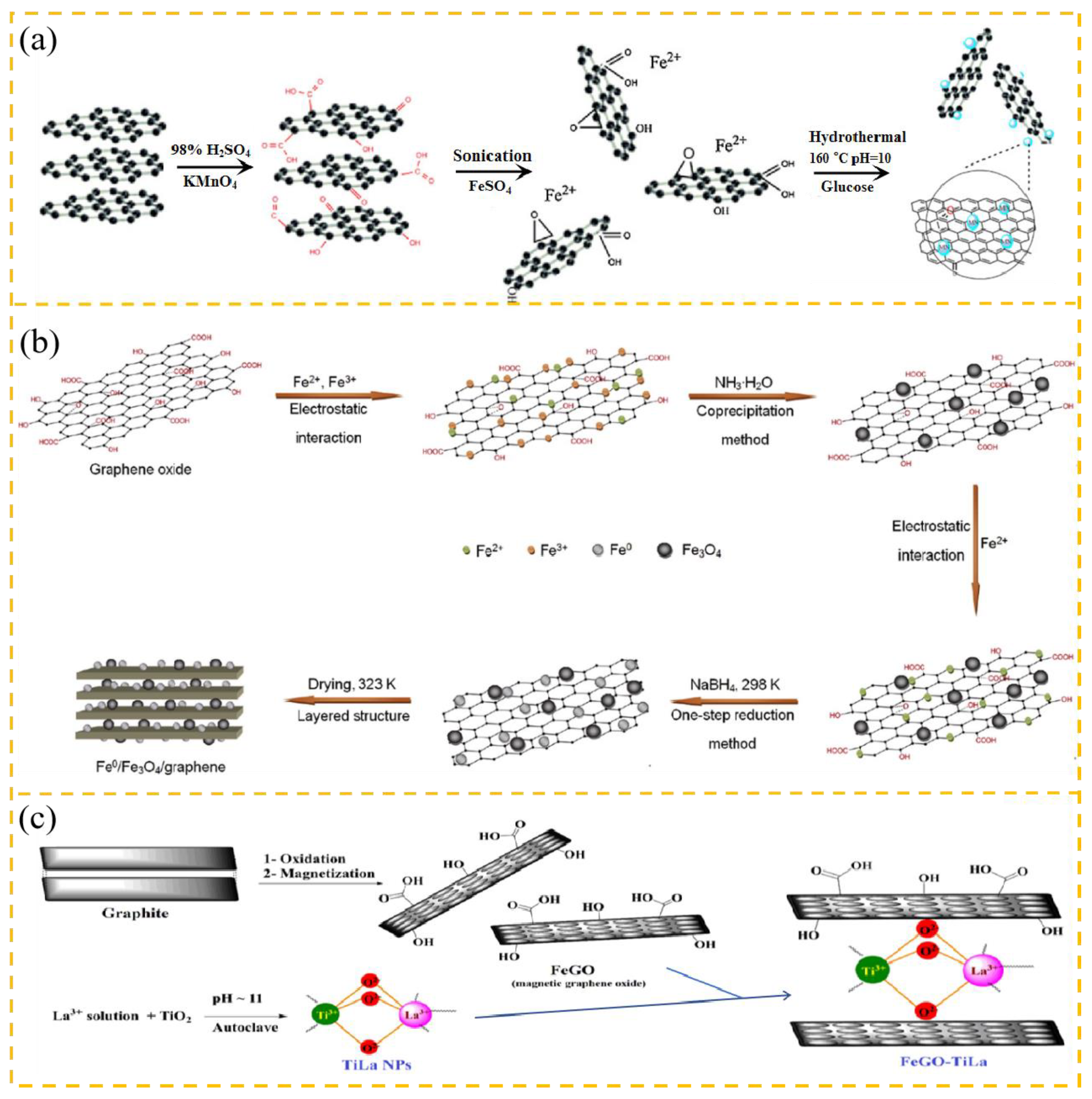
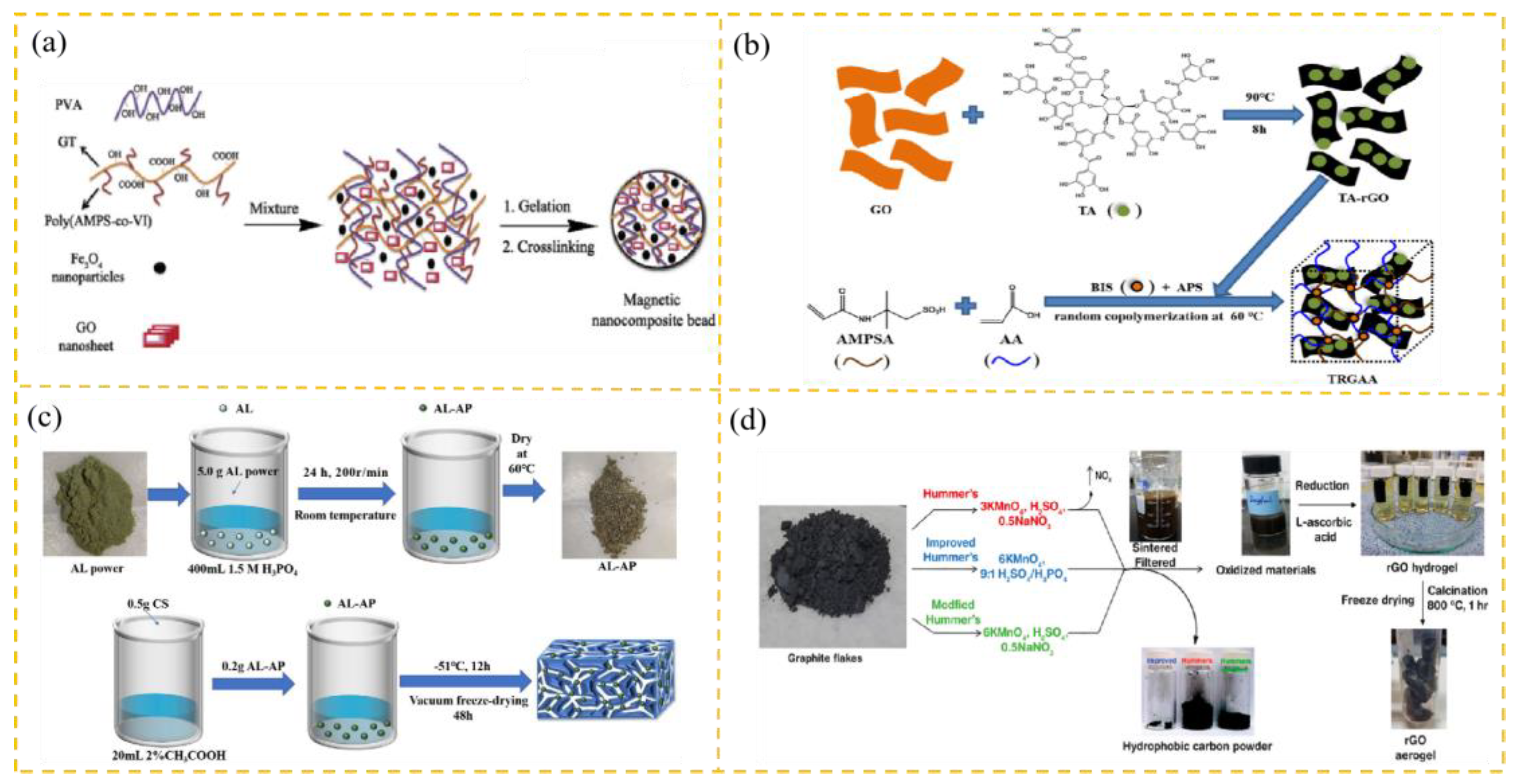
2.2.2. GO Polymer Composites
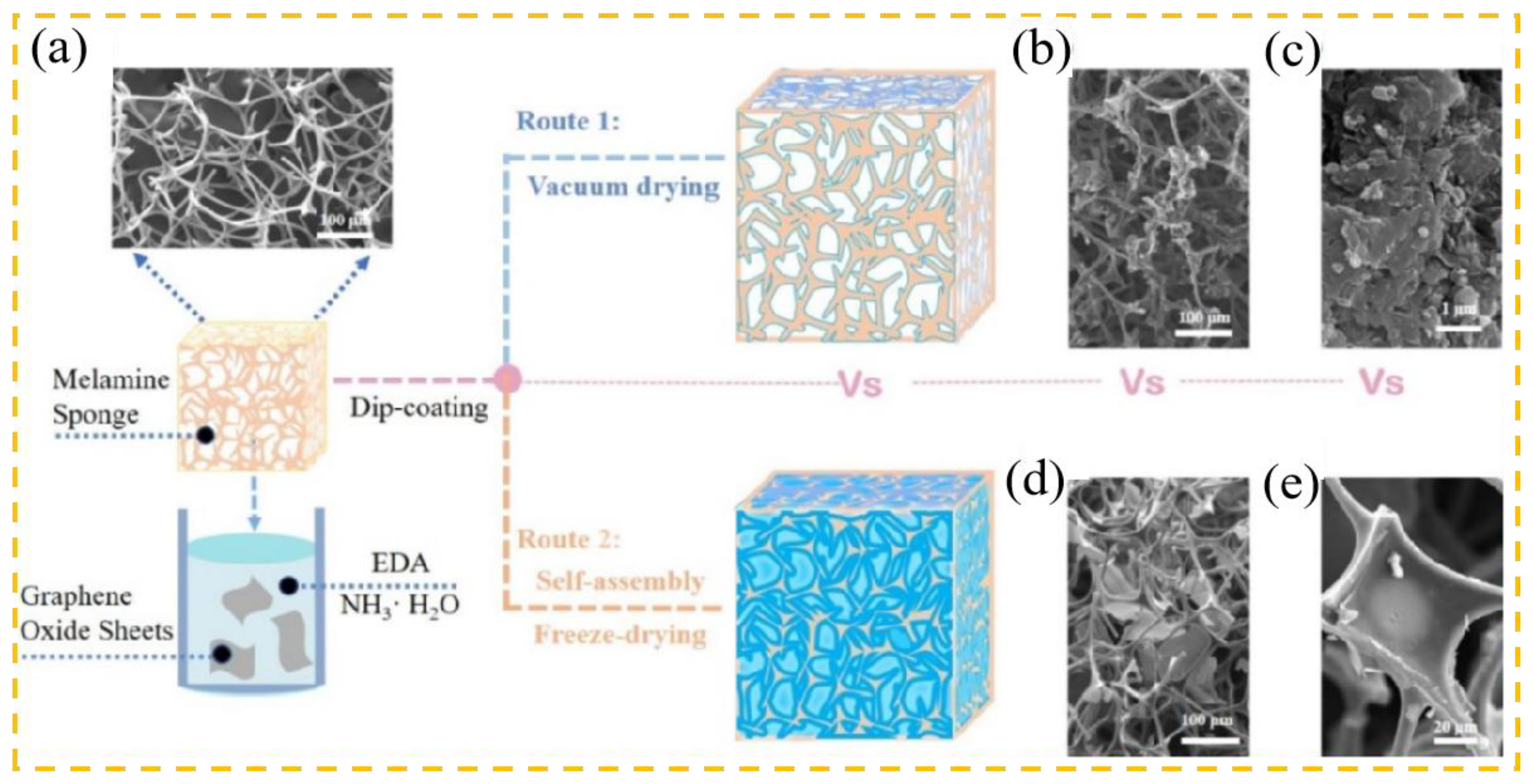
2.3. Preparation of 3D Graphene-Based Adsorbents
2.3.1. Graphene Foam
2.3.2. Graphene Sponge
2.3.3. Graphene Gel
3. Adsorption of Inorganic Pollutants with Graphene-Based Adsorbents
3.1. Heavy Metals
3.1.1. Arsenic
3.1.2. Chromium
3.1.3. Lead
3.1.4. Copper and Cobalt
3.1.5. Selenium
3.1.6. Summary of Adsorption of Heavy Metal Ions
3.2. Rare Metals
3.2.1. Radioactive Metals
3.2.2. Rare Earth Metals
3.3. Other Inorganic Pollutants
3.3.1. Fluorides
3.3.2. Phosphates
4. Adsorption of Organic Pollutants with Graphene-Based Adsorbents
4.1. Organic Dyes
4.1.1. Organic Cationic Dyes
4.1.2. Organic Anionic Dyes
4.1.3. Summary of Adsorption of Organic Dyes
4.2. Pharmaceuticals
4.2.1. Antibiotics
4.2.2. Other Organic Pharmaceuticals
4.3. Aromatic Pollutants
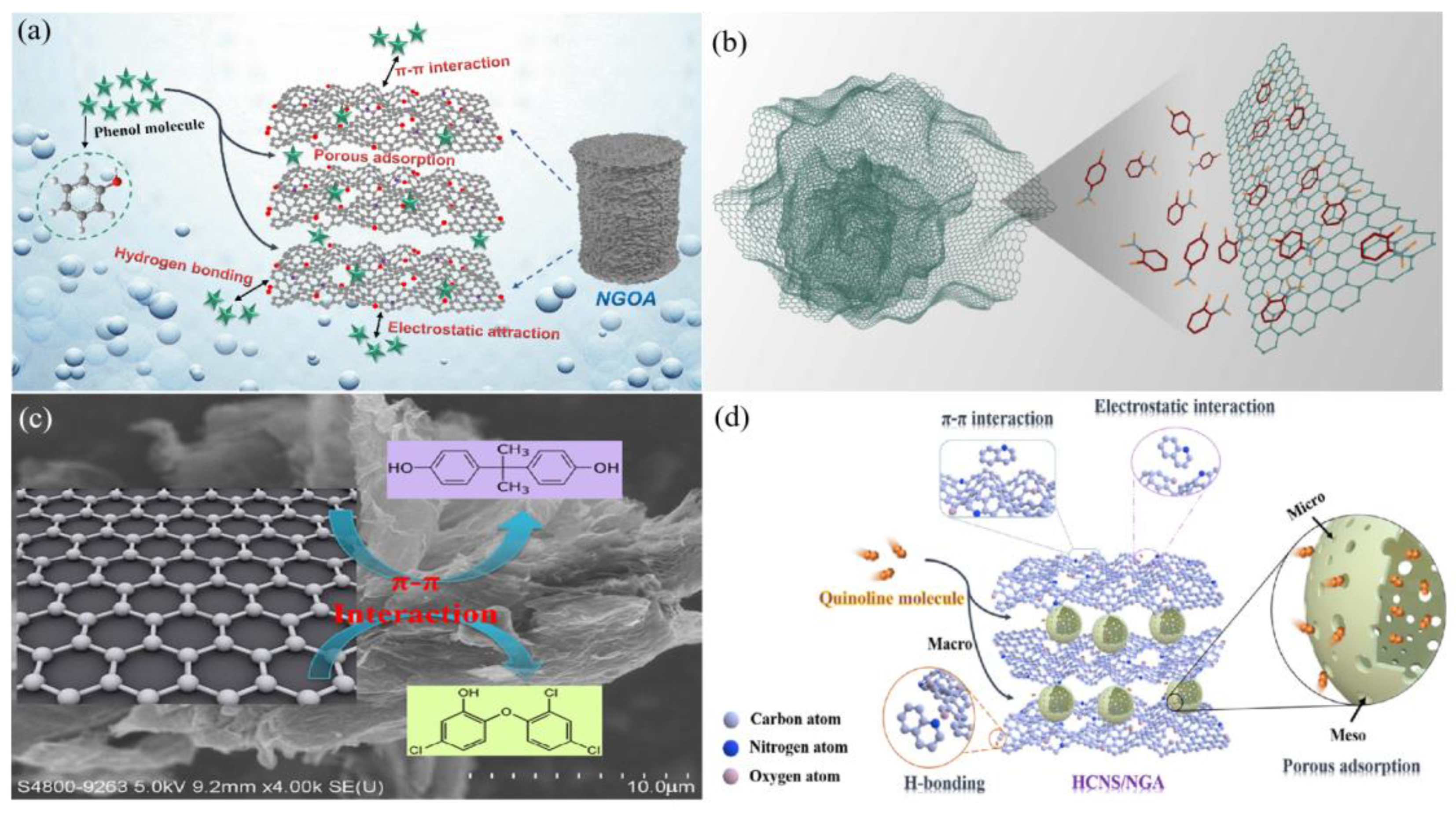
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarath, N.G.; Puthur, J.T. Heavy metal pollution assessment in a mangrove ecosystem scheduled as a community reserve. Wetl. Ecol. Manag. 2021, 29, 719–730. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, L.; Wang, M.; Wang, X.; Cui, H.; Wei, J.; Li, X. The role of metal-organic frameworks in removing emerging contaminants in wastewater. J. Clean. Prod. 2023, 429, 139526. [Google Scholar] [CrossRef]
- Wójcik, G. Sorption behaviors of light lanthanides(III) (La(III), Ce(III), Pr(III), Nd(III)) and Cr(III) using nitrolite. Materials 2020, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Araya, M.; Oeltze, H.; Radeva, J.; Roth, A.G.; Göbbert, C.; Niestroj-Pahl, R.; Dähne, L.; Wiese, J. Operation of hybrid membranes for the removal of pharmaceuticals and pollutants from water and wastewater. Membranes 2022, 12, 502. [Google Scholar] [CrossRef]
- Duan, G.; Cao, Z.; Zhong, H.; Ma, X.; Wang, S. Highly efficient poly(6-acryloylamino-N-hydroxyhexanamide) resin for adsorption of heavy metal ions. J. Environ. Manag. 2022, 308, 114631. [Google Scholar] [CrossRef]
- Zhou, S.; Xie, Y.; Zhu, F.; Gao, Y.; Liu, Y.; Tang, Z.; Duan, Y. Amidoxime modified chitosan/graphene oxide composite for efficient adsorption of U(VI) from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 106363. [Google Scholar] [CrossRef]
- Tang, X.; Xue, H.; Li, J.; Wang, S.; Yu, J.; Zeng, T. Degradation of bisphenol A by nitrogen-rich ZIF-8-derived carbon materials-activated peroxymonosulfate. Toxics 2024, 12, 359. [Google Scholar] [CrossRef]
- Cui, Y.; Li, S.; Yu, N.; Yu, X.; Ji, X.; Wang, L. Highly biocompatible hemoglobin-stabilized gold nanoparticles for an enhanced catalytic reduction of 4-nitrophenol. Inorganics 2024, 12, 136. [Google Scholar] [CrossRef]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of heavy metals from industrial wastewater by chemical precipitation: Mechanisms and sludge characterization. Arab. J. Sci. Eng. 2022, 47, 5587–5599. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Li, H.; Yu, D.; Wang, Y.; Wang, W.; Wu, M. Preparation and selective adsorption of surface-imprinted microspheres based on hyperbranched polyamide-functionalized sodium alginate for the removal of Sb(III). Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124106. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Narita, H.; Tanaka, M. Solvent extraction equilibrium modeling for the separation of ammonia, nickel(II), and copper(II) from the loaded LIX84-I. Miner. Eng. 2021, 172, 107132. [Google Scholar] [CrossRef]
- Pei, J.; Huang, L.; Jiang, H.; Liu, H.; Liu, X.; Hu, X. Inhibitory effect of hydrogen ion on the copper ions separation from acid solution across graphene oxide membranes. Sep. Purif. Technol. 2019, 210, 651–658. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Wang, S.; Zhong, H.; Wu, Z.-k.; Cao, Z.-f. Reactivation of nano-Fe3O4/diethanolamine/rGO catalyst by using electric field in Fenton reaction. J. Taiwan Inst. Chem. Eng. 2019, 99, 113–122. [Google Scholar] [CrossRef]
- Jiang, C.-L.; Wang, R.; Chen, X.; Zheng, L.-G.; Cheng, H. Preparation of chitosan modified fly ash under acid condition and its adsorption mechanism for Cr(VI) in water. J. Cent. South Univ. 2021, 28, 1652–1664. [Google Scholar] [CrossRef]
- Shi, T.-S.; Jiang, F.; Wang, P.; Yue, T.; Sun, W. Deep purification of As(V) in drinking water by silica gel loaded with FeOOH and MnO2. J. Cent. South Univ. 2021, 28, 1692–1706. [Google Scholar] [CrossRef]
- Qiu, S.; Yan, L.; Jing, C. Simultaneous removal of arsenic and antimony from mining wastewater using granular TiO2: Batch and field column studies. J. Environ. Sci. 2019, 75, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuozor, K.O.; Igwegbe, C.A.; Adeniyi, A.G. Verification of pore size effect on aqueous-phase adsorption kinetics: A case study of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127119. [Google Scholar] [CrossRef]
- Alshabib, M.; Oluwadamilare, M.A.; Tanimu, A.; Abdulazeez, I.; Alhooshani, K.; Ganiyu, S.A. Experimental and DFT investigation of ceria-nanocomposite decorated AC derived from groundnut shell for efficient removal of methylene-blue from wastewater effluent. Appl. Surf. Sci. 2021, 536, 147749. [Google Scholar] [CrossRef]
- Yu, W.; Xu, J.; Li, J.; Zhu, S.; Xie, J.; Zhou, Z.; Wang, B.; Li, J.; Chen, K. Hollow structured kapok fiber-based hierarchical porous biocarbons for ultrahigh adsorption of organic dyes. Ind. Eng. Chem. Res. 2022, 61, 4114–4124. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Q.; Yang, Y.; Wang, Q.; He, Y.; Dong, N. Synergetic effect on methylene blue adsorption to biochar with gentian violet in dyeing and printing wastewater under competitive adsorption mechanism. Case Stud. Therm. Eng. 2021, 26, 101099. [Google Scholar] [CrossRef]
- Park, C.; Engel, E.S.; Crowe, A.; Gilbert, T.R.; Rodriguez, N.M. Use of carbon nanofibers in the removal of organic solvents from water. Langmuir 2000, 16, 8050–8056. [Google Scholar] [CrossRef]
- Mishakov, I.V.; Bauman, Y.I.; Brzhezinskaya, M.; Netskina, O.V.; Shubin, Y.V.; Kibis, L.S.; Stoyanovskii, V.O.; Larionov, K.B.; Serkova, A.N.; Vedyagin, A.A. Water purification from chlorobenzenes using heteroatom-functionalized carbon nanofibers produced on self-organizing Ni-Pd catalyst. J. Environ. Chem. Eng. 2022, 10, 107873. [Google Scholar] [CrossRef]
- Dehghani, Z.; Ostovari, F.; Sharifi, S. A comparison of the crystal structure and optical properties of reduced graphene oxide and aminated graphene nanosheets for optoelectronic device applications. Optik 2023, 274, 170551. [Google Scholar] [CrossRef]
- Kim, J.B.; Koo, S.H.; Kim, I.H.; Kim, J.T.; Kim, J.G.; Jayaraman, B.; Lim, J.; Kim, S.O. Characteristic dual-domain composite structure of reduced graphene oxide and its application to higher specific capacitance. Chem. Eng. J. 2022, 446, 137390. [Google Scholar] [CrossRef]
- Bryan, M.Y.K.; Chai, P.V.; Law, J.Y.; Mahmoudi, E. Graphene oxide-chitosan composite material as adsorbent in removing methylene blue dye from synthetic wastewater. Mater. Today Proc. 2022, 64, 1587–1596. [Google Scholar] [CrossRef]
- Li, D.; Hua, T.; Yuan, J.; Xu, F. Methylene blue adsorption from an aqueous solution by a magnetic graphene oxide/humic acid composite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127171. [Google Scholar] [CrossRef]
- Hua, Y.; Li, F.; Hu, N.; Fu, S.-Y. Frictional characteristics of graphene oxide-modified continuous glass fiber reinforced epoxy composite. Compos. Sci. Technol. 2022, 223, 109446. [Google Scholar] [CrossRef]
- Jun, S.-Y.; Park, S.H.; Sohn, M.K.; Kim, S.; Lee, J.M.; Kong, D.S.; Lee, T.-Y.; Jung, J.H.; Kim, M.-S.; Yoo, S.; et al. Reduction time effect on the dielectric characteristics of reduced-graphene-oxide--encapsulated barium titanate powder fillers. Carbon 2022, 199, 23–32. [Google Scholar] [CrossRef]
- Anandaraj, C.; Venkatapathy, R.; Bharath Sabarish, V.C.; Kalaivani, P.; Durairajan, A.; Graça, M.P.; Valente, M.A.; Gajendiran, J.; Gokul Raj, S.; Ramesh Kumar, G. Structural characteristics, optical, electrical and electrochemical sensing properties of graphene and multi walled carbon nanotube admixtured bismuth iron oxide composite ceramics. Ceram. Int. 2021, 47, 28042–28049. [Google Scholar] [CrossRef]
- Hu, H.; Wen, W.; Ou, J.Z. Construction of adsorbents with graphene and its derivatives for wastewater treatment: A review. Environ. Sci. Nano 2022, 9, 3226–3276. [Google Scholar] [CrossRef]
- Wang, Y.; Gai, Z.; Guo, F.; Zhang, M.; Zhang, L.; Xia, G.; Chai, X.; Ren, Y.; Zhang, X.; Jiang, X. A diamond/graphene/diamond eectrode for waste water treatment. Nanomaterials 2023, 13, 3043. [Google Scholar] [CrossRef] [PubMed]
- Faysal Hossain, M.D.; Akther, N.; Zhou, Y. Recent advancements in graphene adsorbents for wastewater treatment: Current status and challenges. Chin. Chem. Lett. 2020, 31, 2525–2538. [Google Scholar] [CrossRef]
- Asif, F.C.; Saha, G.C. Graphene-like carbon structure synthesis from biomass pyrolysis: A critical review on feedstock–process–properties relationship. C 2023, 9, 31. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Laraba, S.R.; Luo, W.; Rezzoug, A.; Zahra, Q.u.a.; Zhang, S.; Wu, B.; Chen, W.; Xiao, L.; Yang, Y.; Wei, J.; et al. Graphene-based composites for biomedical applications. Green Chem. Lett. Rev. 2022, 15, 724–748. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Irzhak, A.; Irzhak, D.; Kang, T.W.; Kononenko, O.; Matveev, V.; Panin, G.; Roshchupkin, D. Direct growth of graphene film on piezoelectric La3Ga5.5Ta0.5O14 crystal. Phys. Status Solidi (RRL)—Rapid Res. Lett. 2016, 10, 639–644. [Google Scholar] [CrossRef]
- Kononenko, O.; Brzhezinskaya, M.; Zotov, A.; Korepanov, V.; Levashov, V.; Matveev, V.; Roshchupkin, D. Influence of numerous Moiré superlattices on transport properties of twisted multilayer graphene. Carbon 2022, 194, 52–61. [Google Scholar] [CrossRef]
- Santhiran, A.; Iyngaran, P.; Abiman, P.; Kuganathan, N. Graphene synthesis and its recent advances in applications—A review. C 2021, 7, 76. [Google Scholar] [CrossRef]
- Jana, A.; Scheer, E.; Polarz, S. Synthesis of graphene–transition metal oxide hybrid nanoparticles and their application in various fields. Beilstein J. Nanotechnol. 2017, 8, 688–714. [Google Scholar] [CrossRef]
- Pedrazzetti, L.; Gibertini, E.; Bizzoni, F.; Russo, V.; Lucotti, A.; Nobili, L.; Magagnin, L. Graphene growth on electroformed copper substrates by atmospheric pressure CVD. Materials 2022, 15, 1572. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, B.; Wang, W.; Li, Y.; Zeng, X.; Liu, H.; Liang, Y.; Zhao, Z.; Cai, A.; Zhang, R.; et al. Toward epitaxial growth of misorientation-free graphene on Cu(III) foils. ACS Nano 2022, 16, 285–294. [Google Scholar] [CrossRef]
- Olorunkosebi, A.A.; Eleruja, M.A.; Adedeji, A.V.; Olofinjana, B.; Fasakin, O.; Omotoso, E.; Oyedotun, K.O.; Ajayi, E.O.B.; Manyala, N. Optimization of graphene oxide through various Hummers’ methods and comparative reduction using green approach. Diam. Relat. Mater. 2021, 117, 108456. [Google Scholar] [CrossRef]
- Vacacela Gomez, C.; Guevara, M.; Tene, T.; Villamagua, L.; Usca, G.T.; Maldonado, F.; Tapia, C.; Cataldo, A.; Bellucci, S.; Caputi, L.S. The liquid exfoliation of graphene in polar solvents. Appl. Surf. Sci. 2021, 546, 149046. [Google Scholar] [CrossRef]
- Tan, H.; Navik, R.; Liu, Z.; Xiang, Q.; Zhao, Y. Scalable massive production of defect-free few-layer graphene by ball-milling in series with shearing exfoliation in supercritical CO2. J. Supercrit. Fluids 2022, 181, 105496. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Karthik, N.; Chandrasekaran, P.; Perumal, S.; Arunachalam, P.; Raja, P.B.; Sethuraman, M.G.; Lee, Y.R. Electrochemically exfoliated graphene sheets as electrode material for aqueous symmetric supercapacitors. Surf. Coat. Technol. 2021, 416, 127150. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.V.; Glukhova, O.E.; Brzhezinskaya, M.; Stolyarova, D.Y.; Varezhnikov, A.S.; Solomatin, M.A.; Barkov, P.V.; Kirilenko, D.A.; Pavlov, S.I.; et al. Guiding graphene derivatization for the on-chip multisensor arrays: From the synthesis to the theoretical background. Adv. Mater. Technol. 2022, 7, 2101250. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Wei, Y.; Sun, H.; Li, Z.; Zhao, X.; Gao, C. An iron-based green approach to 1-h production of single-layer graphene oxide. Nat. Commun. 2015, 6, 5716. [Google Scholar] [CrossRef]
- Ye, C.; Wang, G.; Yuan, H.; Li, J.; Ni, K.; Pan, F.; Guo, M.; Wu, Y.; Ji, H.; Zhang, F.; et al. Microfluidic oxidation of graphite in two minutes with capability of real-time monitoring. Adv. Mater. 2022, 34, 2107083. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, G.; Pan, Y.; Liu, L.; Yang, B.; Zhang, S.; Lai, D.; Che, C. An improved Hummers method to synthesize graphene oxide using much less concentrated sulfuric acid. Chin. Chem. Lett. 2022, 33, 4541–4544. [Google Scholar] [CrossRef]
- Zhou, A.; Yu, T.; Liang, X.; Yin, S. H2O2-free strategy derived from Hummers method for preparing graphene oxide with high oxidation degree. FlatChem 2023, 38, 100487. [Google Scholar] [CrossRef]
- Sahila Grace, A.; Littis Malar, G.S.P. Synthesis and characterization of graphene oxide from coconut husk ash. Orient. J. Chem. 2020, 36, 348–352. [Google Scholar] [CrossRef]
- Amir Faiz, M.S.; Che Azurahanim, C.A.; Yazid, Y.; Suriani, A.B.; Siti Nurul Ain, M.J. Preparation and characterization of graphene oxide from tea waste and it’s photocatalytic application of TiO2/graphene nanocomposite. Mater. Res. Express 2020, 7, 015613. [Google Scholar] [CrossRef]
- Sujiono, E.H.; Zurnansyah; Zabrian, D.; Dahlan, M.Y.; Amin, B.D.; Samnur; Agus, J. Graphene oxide based coconut shell waste: Synthesis by modified Hummers method and characterization. Heliyon 2020, 6, e04568. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Development of magnetite/graphene oxide hydrogels from agricultural wastes for water treatment. J. Alloys Compd. 2022, 10, 1889–1909. [Google Scholar] [CrossRef]
- Harres, A.; Garcia, W.J.S.; Salles, T.R.; Bruckmann, F.S.; Sulzenco, J.B.; Schneider, A.D.; Rhoden, C.R.B. Magnetic properties of graphene oxide decorated with magnetite nanoparticles. Diam. Relat. Mater. 2023, 138, 110238. [Google Scholar] [CrossRef]
- Shen, J.; Shi, M.; Ma, H.; Yan, B.; Li, N.; Ye, M. Hydrothermal synthesis of magnetic reduced graphene oxide sheets. Mater. Res. Bull. 2011, 46, 2077–2083. [Google Scholar] [CrossRef]
- Wang, X.; Yu, J. Application of Fe3O4/graphene oxide composite for the separation of Cs(I) and Sr(II) from aqueous solution. J. Radioanal. Nucl. Chem. 2015, 303, 807–813. [Google Scholar] [CrossRef]
- Chong, S.; Zhang, G.; Tian, H.; Zhao, H. Rapid degradation of dyes in water by magnetic Fe0/Fe3O4/graphene composites. J. Environ. Sci. 2016, 44, 148–157. [Google Scholar] [CrossRef]
- Das, T.K.; Sakthivel, T.S.; Jeyaranjan, A.; Seal, S.; Bezbaruah, A.N. Ultra-high arsenic adsorption by graphene oxide iron nanohybrid: Removal mechanisms and potential applications. Chemosphere 2020, 253, 126702. [Google Scholar] [CrossRef]
- Mosleh, N.; Joolaei Ahranjani, P.; Parandi, E.; Rashidi Nodeh, H.; Nawrot, N.; Rezania, S.; Sathishkumar, P. Titanium lanthanum three oxides decorated magnetic graphene oxide for adsorption of lead ions from aqueous media. Environ. Res. 2022, 214, 113831. [Google Scholar] [CrossRef]
- Pan, N.; Li, L.; Ding, J.; Li, S.; Wang, R.; Jin, Y.; Wang, X.; Xia, C. Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). J. Hazard. Mater. 2016, 309, 107–115. [Google Scholar] [CrossRef]
- Bagbi, Y.; Solanki, P.R. Fabrication of mesoporous silica nanoparticle-decorated graphene oxide sheets for the effective removal of lead (Pb2+) from water. ACS Omega 2023, 9, 304–316. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Kumar Naik, T.S.S.; Subramanian, S.; Kumar, V.; Singh, J.; et al. Sustainable removal of Cr(VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2022, 203, 111891. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Li, Q.; Ma, J.; Zhu, M. Preparation, characterization, and application of mesoporous silica-grafted graphene oxide for highly selective lead adsorption. Chem. Eng. J. 2015, 273, 630–637. [Google Scholar] [CrossRef]
- Li, L.; Zhao, L.; Ma, J.; Tian, Y. Preparation of graphene oxide/chitosan complex and its adsorption properties for heavy metal ions. Green Process. Synth. 2020, 9, 294–303. [Google Scholar] [CrossRef]
- Sherlala, A.I.A.; Raman, A.A.A.; Bello, M.M.; Buthiyappan, A. Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J. Environ. Manag. 2019, 246, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Ren, Q.; Lv, J.; Wang, Y.; Feng, Z.; Li, Y.; Wang, C.; Liu, Y.; Wang, Y. Hydrothermal fabrication of phytic acid decorated chitosan-graphene oxide composites for efficient and selective adsorption of uranium (VI). J. Environ. Chem. Eng. 2023, 11, 110760. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, H.; Liu, P.; Fang, W.; Geng, J. A novel modified graphene oxide/chitosan composite used as an adsorbent for Cr(VI) in aqueous solutions. Int. J. Biol. Macromol. 2016, 87, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhamid, A.I.; Elgoud, E.M.A.; Aly, H.F. Alginate modified graphene oxide for rapid and effective sorption of some heavy metal ions from an aqueous solution. Cellulose 2022, 29, 6231–6245. [Google Scholar] [CrossRef]
- Ma, X.; Duan, G.-y.; Huang, J.-q.; Yang, J.; Cao, Z.-f.; Wang, S. Preparation of graphene oxide/polyiminodiacetic acid resin as a high-performance adsorbent for Cu(II). J. Cent. South Univ. 2023, 30, 3881–3896. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Abu Haija, M. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- Peer, F.E.; Bahramifar, N.; Younesi, H. Removal of Cd (II), Pb (II) and Cu (II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J. Taiwan Inst. Chem. Eng. 2018, 87, 225–240. [Google Scholar] [CrossRef]
- Ma, Y.-X.; Xing, D.; Shao, W.-J.; Du, X.-Y.; La, P.-Q. Preparation of polyamidoamine dendrimers functionalized magnetic graphene oxide for the adsorption of Hg(II) in aqueous solution. J. Colloid Interface Sci. 2017, 505, 352–363. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Ren, R.-P.; Li, W.; Lv, Y.-K. A robust, superhydrophobic graphene aerogel as a recyclable sorbent for oils and organic solvents at various temperatures. J. Colloid Interface Sci. 2017, 500, 63–68. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, W.; Liu, H.; Gu, H. Hyperelastic magnetic reduced graphene oxide three-dimensional framework with superb oil and organic solvent adsorption capability. Adv. Compos. Hybrid Mater. 2020, 3, 473–484. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Mahesh, K.; Le, N.H.; Kemp, K.C.; Timilsina, R.; Tiwari, R.N.; Kim, K.S. Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon 2013, 56, 173–182. [Google Scholar] [CrossRef]
- Sahraei, R.; Sekhavat Pour, Z.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean. Prod. 2017, 142, 2973–2984. [Google Scholar] [CrossRef]
- Yu, B.; Bai, Y.; Ming, Z.; Yang, H.; Chen, L.; Hu, X.; Feng, S.; Yang, S.-T. Adsorption behaviors of tetracycline on magnetic graphene oxide sponge. Mater. Chem. Phys. 2017, 198, 283–290. [Google Scholar] [CrossRef]
- Jayanthi, S.; KrishnaRao Eswar, N.; Singh, S.A.; Chatterjee, K.; Madras, G.; Sood, A.K. Macroporous three-dimensional graphene oxide foams for dye adsorption and antibacterial applications. RSC Adv. 2016, 6, 1231–1242. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, F.; Luo, Y.; Zhang, L. Synthesis of three-dimensional graphene oxide foam for the removal of heavy metal ions. Chem. Phys. Lett. 2014, 593, 122–127. [Google Scholar] [CrossRef]
- Wu, R.; Yu, B.; Liu, X.; Li, H.; Wang, W.; Chen, L.; Bai, Y.; Ming, Z.; Yang, S.-T. One-pot hydrothermal preparation of graphene sponge for the removal of oils and organic solvents. Appl. Surf. Sci. 2016, 362, 56–62. [Google Scholar] [CrossRef]
- Bagoole, O.; Rahman, M.M.; Shah, S.; Hong, H.; Chen, H.; Al Ghaferi, A.; Younes, H. Functionalized three-dimensional graphene sponges for highly efficient crude and diesel oil adsorption. Environ. Sci. Pollut. Res. 2018, 25, 23091–23105. [Google Scholar] [CrossRef]
- Maimaiti, T.; Hu, R.; Yuan, H.; Liang, C.; Liu, F.; Li, Q.; Lan, S.; Yu, B.; Yang, S.-T. Magnetic Fe3O4/TiO2/graphene sponge for the adsorption of methylene blue in aqueous solution. Diam. Relat. Mater. 2022, 123, 108811. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, F.; Li, L.; Ma, J. Adsorption-reduction synergistic effect for rapid removal of Cr (VI) ions on superelastic NH2-graphene sponge. Chem. Eng. J. 2021, 421, 129933. [Google Scholar] [CrossRef]
- Myung, Y.; Jung, S.; Tung, T.T.; Tripathi, K.M.; Kim, T. Graphene-based aerogels derived from biomass for energy storage and environmental remediation. ACS Sustain. Chem. Eng. 2019, 7, 3772–3782. [Google Scholar] [CrossRef]
- Kaushik, J.; Gunture; Tripathi, K.M.; Singh, R.; Sonkar, S.K. Thiourea-functionalized graphene aerogel for the aqueous phase sensing of toxic Pb(II) metal ions and H2O2. Chemosphere 2022, 287, 132105. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, S.; Lee, M.; Jeong, D.H.; Baek, Y.; Yoon, J.; Kim, Y.H. Carbon nanotube-bonded graphene hybrid aerogels and their application to water purification. Nanoscale 2015, 7, 6782–6789. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, J.; Yao, G.; Liu, H. Self-adhesive, self-healable, and triple-responsive hydrogel doped with polydopamine as an adsorbent toward methylene blue. Ind. Eng. Chem. Res. 2019, 58, 17075–17087. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient removal of dye from aqueous solution using natural locust bean gum based hydrogels as adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef]
- Gutekunst, S.B.; Siemsen, K.; Huth, S.; Moehring, A.; Hesseler, B.; Timmermann, M.; Paulowicz, I.; Mishra, Y.K.; Siebert, L.; Adelung, R.; et al. 3D hydrogels containing interconnected microchannels of subcellular size for capturing human pathogenic acanthamoeba castellanii. ACS Biomater. Sci. Eng. 2019, 5, 1784–1792. [Google Scholar] [CrossRef]
- Taye, A.; Yifru, A.; Getachew, N.; Mehretie, S.; Admassie, S. Adsorption of hexavalent chromium using water hyacinth leaf protein concentrate/graphene oxide hydrogel. Environ. Monit. Assess. 2023, 195, 1342. [Google Scholar] [CrossRef]
- Yao, G.; Liu, X.; Zhang, G.; Han, Z.; Liu, H. Green synthesis of tannic acid functionalized graphene hydrogel to efficiently adsorb methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126972. [Google Scholar] [CrossRef]
- Phiri, J.; Johansson, L.-S.; Gane, P.; Maloney, T. A comparative study of mechanical, thermal and electrical properties of graphene-, graphene oxide- and reduced graphene oxide-doped microfibrillated cellulose nanocomposites. Compos. Part B Eng. 2018, 147, 104–113. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, J.; Chen, S.; Varley, R.J.; Pan, K. 1D/2D nanomaterials synergistic, compressible, and response rapidly 3D graphene aerogel for piezoresistive sensor. Adv. Funct. Mater. 2020, 30, 2003618. [Google Scholar] [CrossRef]
- Deng, M.; Zhao, L.; Wang, Z.; Yang, P.; Sun, Y. Preparation of phosphoric-modified aloe vera/chitosan aerogels and their efficient adsorption of U(VI). Environ. Sci. Pollut. Res. 2023, 30, 33229–33242. [Google Scholar] [CrossRef] [PubMed]
- Nundy, S.; Ghosh, A.; Nath, R.; Paul, A.; Tahir, A.A.; Mallick, T.K. Reduced graphene oxide (rGO) aerogel: Efficient adsorbent for the elimination of antimony (III) and (V) from wastewater. J. Hazard. Mater. 2021, 420, 126554. [Google Scholar] [CrossRef] [PubMed]
- Sreedevi, P.R.; Suresh, K.; Jiang, G. Bacterial bioremediation of heavy metals in wastewater: A review of processes and applications. J. Water Process Eng. 2022, 48, 102884. [Google Scholar] [CrossRef]
- Bodrud-Doza, M.; Islam, S.M.D.-U.; Hasan, M.T.; Alam, F.; Haque, M.M.; Rakib, M.A.; Asad, M.A.; Rahman, M.A. Groundwater pollution by trace metals and human health risk assessment in central west part of Bangladesh. Groundw. Sustain. Dev. 2019, 9, 100219. [Google Scholar] [CrossRef]
- Manzoor, Q.; Shahab, M.R.; Sajid, A.; Yaseen, H.M.; Alqahtani, F.O.; Malik, Q.M.; Nazir, A.; Arif, K.; Iqbal, M. Eco-benign preparation of biosorbent using momordica charantia for the efficient removal of Cr(VI) ions from wastewater. Z. Für Phys. Chem. 2022, 236, 1461–1491. [Google Scholar] [CrossRef]
- Ghosal, P.S.; Kattil, K.V.; Yadav, M.K.; Gupta, A.K. Adsorptive removal of arsenic by novel iron/olivine composite: Insights into preparation and adsorption process by response surface methodology and artificial neural network. J. Environ. Manag. 2018, 209, 176–187. [Google Scholar] [CrossRef]
- da Silva, E.B.; Mussoline, W.A.; Wilkie, A.C.; Ma, L.Q. Arsenic removal and biomass reduction of As-hyperaccumulator Pteris vittata: Coupling ethanol extraction with anaerobic digestion. Sci. Total Environ. 2019, 666, 205–211. [Google Scholar] [CrossRef]
- Criscuoli, A.; Figoli, A. Pressure-driven and thermally-driven membrane operations for the treatment of arsenic-contaminated waters: A comparison. J. Hazard. Mater. 2019, 370, 147–155. [Google Scholar] [CrossRef]
- Nieto-Delgado, C.; Gutiérrez-Martínez, J.; Rangel-Méndez, J.R. Modified activated carbon with interconnected fibrils of iron-oxyhydroxides using Mn2+ as morphology regulator, for a superior arsenic removal from water. J. Environ. Sci. 2019, 76, 403–414. [Google Scholar] [CrossRef]
- Su, H.; Ye, Z.; Hmidi, N. High-performance iron oxide-graphene oxide nanocomposite adsorbents for arsenic removal. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 161–172. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Lo, I.M.C. Magnetic nanoparticles: Essential factors for sustainable environmental applications. Water Res. 2013, 47, 2613–2632. [Google Scholar] [CrossRef]
- Qu, G.; Li, R.; Zhou, Y.; Wu, B.; Cai, Y.; Ning, P. Preparation of ferric nitrate-graphene nanocomposite and its adsorption of arsenic(V) from simulated arsenic-containing wastewater. Appl. Organomet. Chem. 2019, 33, e5221. [Google Scholar] [CrossRef]
- Ren, L.; Xu, J.; Zhang, Y.; Zhou, J.; Chen, D.; Chang, Z. Preparation and characterization of porous chitosan microspheres and adsorption performance for hexavalent chromium. Int. J. Biol. Macromol. 2019, 135, 898–906. [Google Scholar] [CrossRef]
- Pavesi, T.; Moreira, J.C. Mechanisms and individuality in chromium toxicity in humans. J. Appl. Toxicol. 2020, 40, 1183–1197. [Google Scholar] [CrossRef]
- Ben Khalifa, E.; Rzig, B.; Chakroun, R.; Nouagui, H.; Hamrouni, B. Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent. Chemom. Intell. Lab. Syst. 2019, 189, 18–26. [Google Scholar] [CrossRef]
- Panda, H.; Tiadi, N.; Mohanty, M.; Mohanty, C.R. Studies on adsorption behavior of an industrial waste for removal of chromium from aqueous solution. S. Afr. J. Chem. Eng. 2017, 23, 132–138. [Google Scholar] [CrossRef]
- Govindaraju, K.; Vinu, R.; Gautam, R.; Vasantharaja, R.; Niranjan, M.; Sundar, I. Microwave-assisted torrefaction of biomass kappaphycus alvarezii based biochar and magnetic biochar for removal of hexavalent chromium Cr(VI) from aqueous solution. Biomass Convers. Biorefinery 2024, 14, 3643–3653. [Google Scholar] [CrossRef]
- Mondal, N.K.; Chakraborty, S. Adsorption of Cr(VI) from aqueous solution on graphene oxide prepared from graphite: Equilibrium, kinetic and thermodynamic studies. Appl. Water Sci. 2020, 10, 61. [Google Scholar] [CrossRef]
- Heidari, A.; Younesi, H.; Mehraban, Z.; Heikkinen, H. Selective adsorption of Pb(II), Cd(II), and Ni(II) ions from aqueous solution using chitosan–MAA nanoparticles. Int. J. Biol. Macromol. 2013, 61, 251–263. [Google Scholar] [CrossRef]
- Kołodyńska, D. Chitosan as an effective low-cost sorbent of heavy metal complexes with the polyaspartic acid. Chem. Eng. J. 2011, 173, 520–529. [Google Scholar] [CrossRef]
- Jia, B.; Li, G.; Cao, E.; Luo, J.; Zhao, X.; Huang, H. Recent progress of antibacterial hydrogels in wound dressings. Mater. Today Bio 2023, 19, 100582. [Google Scholar] [CrossRef]
- Shang, M.-R.; Liu, Y.-G.; Liu, S.-B.; Zeng, G.-M.; Tan, X.-F.; Jiang, L.-H.; Huang, X.-X.; Ding, Y.; Guo, Y.-M.; Wang, S.-F. A novel graphene oxide coated biochar composite: Synthesis, characterization and application for Cr(VI) removal. RSC Adv. 2016, 6, 85202–85212. [Google Scholar] [CrossRef]
- Kumar, P.; Chauhan, M.S. Adsorption of chromium (VI) from the synthetic aqueous solution using chemically modified dried water hyacinth roots. J. Environ. Chem. Eng. 2019, 7, 103218. [Google Scholar] [CrossRef]
- Worku, Z.; Tibebu, S.; Nure, J.F.; Tibebu, S.; Moyo, W.; Ambaye, A.D.; Nkambule, T.T.I. Adsorption of chromium from electroplating wastewater using activated carbon developed from water hyacinth. BMC Chem. 2023, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Sun, T.; Saleem, A.; Wang, C. Enhanced removal of Cr(III)-EDTA chelates from high-salinity water by ternary complex formation on DETA functionalized magnetic carbon-based adsorbents. Ecotoxicol. Environ. Saf. 2021, 209, 111858. [Google Scholar] [CrossRef] [PubMed]
- Kinuthia, G.K.; Ngure, V.; Beti, D.; Lugalia, R.; Wangila, A.; Kamau, L. Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: Community health implication. Sci. Rep. 2020, 10, 8434. [Google Scholar] [CrossRef]
- Narayana, P.L.; Lingamdinne, L.P.; Karri, R.R.; Devanesan, S.; AlSalhi, M.S.; Reddy, N.S.; Chang, Y.-Y.; Koduru, J.R. Predictive capability evaluation and optimization of Pb(II) removal by reduced graphene oxide-based inverse spinel nickel ferrite nanocomposite. Environ. Res. 2022, 204, 112029. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, L.-Y.; Yang, H.; Liu, Q.; Chen, X.-H.; Jiang, X.-Y.; Chen, X.-Q.; Jiao, F.-P. Graphene nanosheets as novel adsorbents in adsorption, preconcentration and removal of gases, organic compounds and metal ions. Sci. Total Environ. 2015, 502, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Azam, M.G.; Kabir, M.H.; Shaikh, M.A.A.; Ahmed, S.; Mahmud, M.; Yasmin, S. A rapid and efficient adsorptive removal of lead from water using graphene oxide prepared from waste dry cell battery. J. Water Process Eng. 2022, 46, 102597. [Google Scholar] [CrossRef]
- Lingamdinne, L.P.; Godlaveeti, S.K.; Angaru, G.K.R.; Chang, Y.-Y.; Nagireddy, R.R.; Somala, A.R.; Koduru, J.R. Highly efficient surface sequestration of Pb2+ and Cr3+ from water using a Mn3O4 anchored reduced graphene oxide: Selective removal of Pb2+ from real water. Chemosphere 2022, 299, 134457. [Google Scholar] [CrossRef]
- Mu, R.; Liu, B.; Chen, X.; Wang, N.; Yang, J. Adsorption of Cu (II) and Co (II) from aqueous solution using lignosulfonate/chitosan adsorbent. Int. J. Bio. Macromol. 2020, 163, 120–127. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Manivel, P.; Rajendrakumar, R.T.; Uyar, T. Multifunctional ZnO nanorod-reduced graphene oxide hybrids nanocomposites for effective water remediation: Effective sunlight driven degradation of organic dyes and rapid heavy metal adsorption. Chem. Eng. J. 2017, 325, 588–600. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total Environ. 2015, 521, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Awual, M.R.; Yaita, T.; Suzuki, S.; Shiwaku, H. Ultimate selenium(IV) monitoring and removal from water using a new class of organic ligand based composite adsorbent. J. Hazard. Mater. 2015, 291, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, S.S.; Memon, S.A.; Rafi ul, Z.; Ram, N.; Saeed, S.; Mubarak, N.M.; Karri, R.R. Rapid adsorption of selenium removal using iron manganese-based micro adsorbent. Sci. Rep. 2022, 12, 17207. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, R.; Joshi, T.P.; Peng, J.; Qu, J. Highly efficient removal of selenite by electrolysis-assisted nano-zerovalent iron (nZVI): Implication for corrosion and reduction. Chem. Eng. J. 2021, 405, 126564. [Google Scholar] [CrossRef]
- Okonji, S.O.; Dominic, J.A.; Pernitsky, D.; Achari, G. Removal and recovery of selenium species from wastewater: Adsorption kinetics and co-precipitation mechanisms. J. Water Process Eng. 2020, 38, 101666. [Google Scholar] [CrossRef]
- Latva, S.; Peräniemi, S.; Ahlgrén, M. Study of metal-loaded activated charcoals for the separation and determination of selenium species by energy dispersive X-ray fluorescence analysis. Anal. Chim. Acta 2003, 478, 229–235. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Otto, M. Preparation and evaluation of Fe-loaded activated carbon for enrichment of selenium for analytical and environmental purposes. Chemosphere 2013, 90, 683–690. [Google Scholar] [CrossRef]
- Jankovský, O.; Šimek, P.; Klímová, K.; Sedmidubský, D.; Pumera, M.; Sofer, Z. Highly selective removal of Ga3+ ions from Al3+/Ga3+ mixtures using graphite oxide. Carbon 2015, 89, 121–129. [Google Scholar] [CrossRef]
- Xiao, W.; Yan, B.; Zeng, H.; Liu, Q. Dendrimer functionalized graphene oxide for selenium removal. Carbon 2016, 105, 655–664. [Google Scholar] [CrossRef]
- Lu, Z.; Yu, J.; Zeng, H.; Liu, Q. Polyamine-modified magnetic graphene oxide nanocomposite for enhanced selenium removal. Sep. Purif. Technol. 2017, 183, 249–257. [Google Scholar] [CrossRef]
- Sun, F.; Zhu, Y.; Liu, X.; Chi, Z. Highly efficient removal of Se(IV) using reduced graphene oxide-supported nanoscale zero-valent iron (nZVI/rGO): Selenium removal mechanism. Environ. Sci. Pollut. Res. 2023, 30, 27560–27569. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Park, W.K.; Hwang, T.-M.; Yoon, D.H.; Yang, W.S.; Kang, J.-W. Comparative evaluation of magnetite–graphene oxide and magnetite-reduced graphene oxide composite for As(III) and As(V) removal. J. Hazard. Mater. 2016, 304, 196–204. [Google Scholar] [CrossRef]
- Ye, Y.; Yin, D.; Wang, B.; Zhang, Q. Synthesis of three-dimensional Fe3O4 graphene aerogels for the removal of Arsenic Ions from Water. J. Nanomater. 2015, 2015, 864864. [Google Scholar] [CrossRef]
- Singh, N.; Naseh, M.F.; Ansari, J.R.; Sarkar, T.; Datta, A. Removal of aqueous arsenic (III) by graphene-based systems at micro-trace level. Carbon Lett. 2023, 33, 233–243. [Google Scholar] [CrossRef]
- Chen, H.; Liu, F.; Cai, C.; Wu, H.; Yang, L. Removal of Hg2+ from desulfurization wastewater by tannin-immobilized graphene oxide. Environ. Sci. Pollut. Res. 2022, 29, 17964–17976. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, C.; Li, X.; Duan, H.; Wang, X. Preparation of magnetic ionic liquid/chitosan/graphene oxide composite and application for water treatment. Int. J. Biol. Macromol. 2014, 66, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Lingamdinne, L.P.; Koduru, J.R.; Choi, Y.-L.; Chang, Y.-Y.; Yang, J.-K. Studies on removal of Pb(II) and Cr(III) using graphene oxide based inverse spinel nickel ferrite nano-composite as sorbent. Hydrometallurgy 2016, 165, 64–72. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, W.; Chen, Y.; Sun, J.; Liang, M.; Guo, Y.; Liu, X.; Liu, M.; Wei, Y.; Wei, J.; et al. Effective removal of hexavalent chromium from aqueous solutions using quaternary ammonium-functionalized magnetic graphene oxide composites. Separations 2023, 10, 463. [Google Scholar] [CrossRef]
- Fan, L.; Luo, C.; Sun, M.; Li, X.; Qiu, H. Highly selective adsorption of lead ions by water-dispersible magnetic chitosan/graphene oxide composites. Colloids Surf. B Biointerfaces 2013, 103, 523–529. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solution. J. Alloys Compd. 2020, 381, 120914. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, X.; Wu, C.; Xie, R.; Feng, S.; Chen, C. Facile preparation of amino functionalized graphene oxide decorated with Fe3O4 nanoparticles for the adsorption of Cr(VI). Appl. Surf. Sci. 2016, 384, 1–9. [Google Scholar] [CrossRef]
- Wu, W.P.; Ge, H.C. Preparation of amino-silane and thiosemicarbazide modified graphene oxide composite for high uptake adsorption of Hg(II) and Pb(II). J. Alloys Compd. 2024, 2312839. [Google Scholar] [CrossRef]
- Joya-Cárdenas, D.R.; Rodríguez-Caicedo, J.P.; Corona-Rivera, M.A.; Saldaña-Robles, N.; Damián-Ascencio, C.E.; Saldaña-Robles, A. Removal of As(V) in the presence of Cr(VI) in contaminated water from the Bajio region of Mexico using ferrihydrite-functionalized graphene oxide (GOFH): A case study. Emerg. Contam. 2024, 10, 100312. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, W.; Zhou, W.; Luo, J. Novel magnetic polysaccharide/graphene oxide @Fe3O4 gel beads for adsorbing heavy metal ions. Carbohydr. Polym. 2019, 216, 119–128. [Google Scholar] [CrossRef]
- Verma, M.; Lee, I.; Oh, J.; Kumar, V.; Kim, H. Synthesis of EDTA-functionalized graphene oxide-chitosan nanocomposite for simultaneous removal of inorganic and organic pollutants from complex wastewater. Chemosphere 2022, 287, 132385. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Han, J.; Niu, W.; Wang, B.; Wu, Z.; Wei, Z.; Zhu, Y.; Guo, Q.; Wang, X. Acid-resistant chitosan/graphene oxide adsorbent for Cu2+ removal: The role of mixed cross-linking and amino-functionalized. Int. J. Biol. Macromol. 2024, 273, 133096. [Google Scholar] [CrossRef] [PubMed]
- Azfar Shaida, M.; Daniyal; Ali, S.; Saalim Badar, M.; Salim Mahtab, M.; Umar Khan, M.; Ullah Khan, S.; Ahmad, I.; Atika; Haq Farooqi, I.; et al. Synthesis, characterization, and breakthrough modeling of low-cost graphene oxide-sand supported adsorbent filter for the separation of lead and chromium ions. J. Mol. Liq. 2024, 403, 124611. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Tewari, S.; Kumari, N.; Bhardwaj, A.; Misra, N.; Shukla, S.; Dwivedi, J.; Sharma, S. Adsorption of Pb(II) and Cd(II) by functionalized graphene oxide (GO-MBT): Mechanisms, antibacterial activity, and DFT studies. Ind. Eng. Chem. Res. 2024, 164, 112464. [Google Scholar] [CrossRef]
- Elumalai, N.S.; Jaisankar, S.M.; Kumaran, C. Utilization of graphene oxide-chitosan nanocomposite for the removal of heavy metals: Kinetics, isotherm, and error analysis. Water Conserv. Sci. Eng. 2024, 9, 9. [Google Scholar] [CrossRef]
- Zhang, C.-Z.; Chen, B.; Bai, Y.; Xie, J. A new functionalized reduced graphene oxide adsorbent for removing heavy metal ions in water via coordination and ion exchange. Sep. Sci. Technol. 2018, 53, 2896–2905. [Google Scholar] [CrossRef]
- Attia, N.F.; Diab, M.A.; Attia, A.S.; El-Shahat, M.F. Greener approach for fabrication of antibacterial graphene-polypyrrole nanoparticle adsorbent for removal of Mn2+ from aqueous solution. Synth. Met. 2021, 282, 107132. [Google Scholar] [CrossRef]
- Hadadian, M.; Goharshadi, E.K.; Fard, M.M.; Ahmadzadeh, H. Synergistic effect of graphene nanosheets and zinc oxide nanoparticles for effective adsorption of Ni(II) ions from aqueous solutions. ACS Biomater. Sci. Eng. 2018, 124, 239. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Z.; Yang, Q.; Huang, R. Efficient removal of lead, copper and cadmium ions from water by a porous calcium alginate/graphene oxide composite aerogel. Nanomaterials 2018, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-f.; Wen, X.; Wang, J.; Yang, F.; Zhong, H.; Wang, S.; Wu, Z.-k. In situ nano-Fe3O4/triisopropanolamine functionalized graphene oxide composites to enhance Pb2+ ions removal. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 209–217. [Google Scholar] [CrossRef]
- Ibrahim, A.I.; Onaizi, S.A.; Vohra, M.S. Novel CTAB functionalized graphene oxide for selenium removal: Adsorption results and ANN & RSM modeling. Emergent Mater. 2024, 7, 547–564. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, Y.; Yang, Y.; Zou, K.; Wu, R.; Xia, K.; Xie, S. Synthesis of polyethylenimine/graphene oxide for the adsorption of U(VI) from aqueous solution. Appl. Surf. Sci. 2019, 471, 88–95. [Google Scholar] [CrossRef]
- Nan, Y.; Wang, J.; Chang, X.; Shao, K.; Lin, Y.; Qian, L.; Li, Z.; Hu, P. Functionalized graphene oxide/sodium alginate beads with ion responsiveness for uranium trapping. Carbohydr. Polym. 2023, 300, 120259. [Google Scholar] [CrossRef]
- Wu, F.; Huang, H.; Sun, X.; Xie, S.; Yuan, H.; Liu, Y.; Guo, Y. Persimmon tannin-modified graphene oxide/chitosan microsphere for removing U(VI) in rare earth wastewater. J. Radioanal. Nucl. Chem. 2023, 332, 3617–3633. [Google Scholar] [CrossRef]
- Kim, Y.; Eom, H.H.; Kim, Y.K.; Harbottle, D.; Lee, J.W. Effective removal of cesium from wastewater via adsorptive filtration with potassium copper hexacyanoferrate-immobilized and polyethyleneimine-grafted graphene oxide. Chemosphere 2020, 250, 126262. [Google Scholar] [CrossRef]
- Kadam, A.A.; Jang, J.; Lee, D.S. Facile synthesis of pectin-stabilized magnetic graphene oxide Prussian blue nanocomposites for selective cesium removal from aqueous solution. Bioresour. Technol. 2016, 216, 391–398. [Google Scholar] [CrossRef]
- Huang, T.; Qu, J.; Tan, L.; Yao, R.; Jiao, W.; Wang, Y.; Lin, T.; Hao, Y.; Yang, H.; Yang, H.; et al. Efficient adsorption of Ce (III) using cellulose graft poly (2-acrylamido-2-methyl-1-propanesulfonic acid)/graphene oxide composite. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132981. [Google Scholar] [CrossRef]
- Abu Elgoud, E.M.; Abd-Elhamid, A.I.; Aly, H.F. Modification of graphene oxide with imidazolium-based ionic liquid for significant sorption of La(III) and Pr(III) from aqueous solutions. Appl. Water Sci. 2023, 13, 152. [Google Scholar] [CrossRef]
- Gbadebo, A.M. Groundwater fluoride and dental fluorosis in southwestern Nigeria. Environ. Geochem. Health 2012, 34, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zang, Z.; Zhang, S.; Ouyang, G.; Han, R. Enhanced fluoride adsorption from aqueous solution by zirconium (IV)-impregnated magnetic chitosan graphene oxide. Int. J. Biol. Macromol. 2021, 182, 1759–1768. [Google Scholar] [CrossRef]
- Rashid, U.S.; Das, T.K.; Sakthivel, T.S.; Seal, S.; Bezbaruah, A.N. GO-CeO2 nanohybrid for ultra-rapid fluoride removal from drinking water. Sci. Total Environ. 2021, 793, 148547. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J. The adsorption of phosphate by graphene from aqueous solution. RSC Adv. 2012, 2, 5234–5242. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Ibrahim, K.; El-Monaem, E.M.A.; El-Subruiti, G.M.; Omer, A.M. Phosphate removal by Lanthanum-doped aminated graphene oxide@aminated chitosan microspheres: Insights into the adsorption mechanism. J. Clean. Prod. 2023, 385, 135640. [Google Scholar] [CrossRef]
- Wang, P.; Li, L.; Tian, Y.; Sun, L.; Zhan, W.; Chen, S.; Zhang, J.; Zuo, W. Three-dimensional graphene/La(OH)3-nanorod aerogel adsorbent by self-assembly process for enhanced removal and recovery of phosphate in wastewater. Sci. Total Environ. 2022, 809, 152124. [Google Scholar] [CrossRef]
- He, J.; Du, Y.-e.; Bai, Y.; An, J.; Cai, X.; Chen, Y.; Wang, P.; Yang, X.; Feng, Q. Facile formation of anatase/rutile TiO2 nanocomposites with enhanced photocatalytic activity. Molecules 2019, 24, 2996. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M.; Bhila, B.; Moloto, H.; Naidoo, E.B. Lead ions and methylene blue dye removal from aqueous solution by mucuna beans (velvet beans) adsorbents. J. Environ. Chem. Eng. 2020, 8, 103557. [Google Scholar] [CrossRef]
- Cao, Z.-f.; Wen, X.; Chen, P.; Yang, F.; Ou, X.-l.; Wang, S.; Zhong, H. Synthesis of a novel heterogeneous fenton catalyst and promote the degradation of methylene blue by fast regeneration of Fe2+. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 94–104. [Google Scholar] [CrossRef]
- Raees, A.; Jamal, M.; Ahmed, I.; Silanpaa, M.; Saad Algarni, T. Synthesis and characterization of CeO2/CuO nanocomposites for photocatalytic degradation of methylene blue in visible light. Coatings 2021, 11, 305. [Google Scholar] [CrossRef]
- Tabish, T.A.; Memon, F.A.; Gomez, D.E.; Horsell, D.W.; Zhang, S. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep. 2018, 8, 1817. [Google Scholar] [CrossRef]
- Joshi, B.; Khalil, A.M.E.; Zhang, S.; Memon, F.A. Investigating the potential of greener-porous graphene for the treatment of organic pollutants in wastewater. C 2023, 9, 97. [Google Scholar] [CrossRef]
- Naeem, H.; Tofil, H.M.; Soliman, M.; Hai, A.; Zaidi, S.H.H.; Kizilbash, N.; Alruwaili, D.; Ajmal, M.; Siddiq, M. Reduced graphene oxide-zinc sulfide nanocomposite decorated with silver nanoparticles for wastewater treatment by adsorption, photocatalysis and antimicrobial action. Molecules 2023, 28, 926. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Eldridge, D.S.; Malherbe, F. Sonochemical synthesis of improved graphene oxide for enhanced adsorption of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129587. [Google Scholar] [CrossRef]
- Dang, A.; Yuan, Z.; Liu, X.; Ma, S.; Yang, Y.; Zada, A.; Gao, Y.; Liu, Y.; Li, T.; Han, Y. Graphene oxide mediated carbon foam/CNTs composites for highly efficient adsorption of methylene blue and mechanism insight. Ceram. Int. 2023, 49, 36970–36978. [Google Scholar] [CrossRef]
- Rashad, M.; Helali, S.; Shaalan, N.M.; Albalawi, A.E.; Alatawi, N.S.; Al-Faqiri, B.; Al-Belwi, M.M.; Alsharari, A.M. Dual studies of photo degradation and adsorptions of Congo red in wastewater on graphene–copper oxide heterostructures. Materials 2023, 16, 3721. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, X.; Luo, H.; Ling, H.; Mo, W.; Fang, H.; Shen, C.; Lei, J.; Sun, M.; Li, J. Efficient removal of Congo red with graphene aerogel derived from recycled anode of lithium-ion battery. Int. J. Environ. Sci. Technol. 2021, 18, 3995–4006. [Google Scholar] [CrossRef]
- Yang, Z.; He, C.; Liao, W.; Zhang, X.; Liu, W.; Zou, B. Adsorption of orange G in liquid solution by the amino functionalized GO. Separations 2022, 9, 391. [Google Scholar] [CrossRef]
- Labiadh, L.; Kamali, A.R. 3D graphene nanoedges as efficient dye adsorbents with ultra-high thermal regeneration performance. Appl. Surf. Sci. 2019, 490, 383–394. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Liu, X.; Wu, J.; Zhu, X.; Bai, Z.; Yu, Z. Preparation of β-cyclodextrin/graphene oxide and its adsorption properties for methylene blue. Colloids Surf. B Biointerfaces 2021, 200, 111605. [Google Scholar] [CrossRef]
- Wu, Z.; Zhong, H.; Yuan, X.; Wang, H.; Wang, L.; Chen, X.; Zeng, G.; Wu, Y. Adsorptive removal of methylene blue by rhamnolipid-functionalized graphene oxide from wastewater. Water Res. 2014, 67, 330–344. [Google Scholar] [CrossRef]
- Bu, J.; Yuan, L.; Zhang, N.; Liu, D.; Meng, Y.; Peng, X. High-efficiency adsorption of methylene blue dye from wastewater by a thiosemicarbazide functionalized graphene oxide composite. Diam. Relat. Mater. 2020, 101, 107604. [Google Scholar] [CrossRef]
- Banaei, A.; Saadat, A.; Javadi, R.; Pargolghasemi, P. Preparation magnetic graphene oxide/diethylenetriamine composite for removal of methylene blue from aqueous solutions. Sci. Rep. 2024, 14, 15457. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jing, K.; Peng, S.; Shi, Q.; Liu, H. Facile preparation of graphene oxide-based composite aerogel to efficiently adsorb methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2024, 681, 132754. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, X.; Chen, B. Stable graphene oxide/poly(ethyleneimine) 3D aerogel with tunable surface charge for high performance selective removal of ionic dyes from water. Chem. Eng. J. 2018, 334, 1119–1127. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Wang, H.; Du, Q.; Li, M.; Sun, Y.; Cui, M.; Li, L. Study on the adsorption performance of casein/graphene oxide aerogel for methylene blue. ACS Omega 2021, 6, 29243–29253. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Z.; Tian, T.; Li, G.; Gu, J.; Zhou, J.; Dong, S. Preparation of carboxymethyl cellulose/graphene oxide/ZIF-8 aerogels for efficient methylene blue adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2024, 696, 134338. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, J.; Ma, Q.; Baihetiyaer, B.; Sun, J.; Zhang, Y.; Jiang, Y.; Wang, J.; Yin, X. Montmorillonite-reduced graphene oxide composite aerogel (M−rGO): A green adsorbent for the dynamic removal of cadmium and methylene blue from wastewater. Sep. Purif. Technol. 2022, 296, 121416. [Google Scholar] [CrossRef]
- E, T.; Ma, D.; Yang, S.; Hao, X. Graphene oxide-montmorillonite/sodium alginate aerogel beads for selective adsorption of methylene blue in wastewater. J. Alloys Compd. 2020, 832, 154833. [Google Scholar] [CrossRef]
- Li, Y.; Ni, M.; He, Q.; Li, X.; Zhang, W.; Wang, H. Adsorption of rhodamine B and Cr3+ ion onto graphene/chitosan composite. J. Comput. Methods Sci. Eng. 2021, 21, 927–938. [Google Scholar] [CrossRef]
- Natasha; Khan, A.; Rahman, U.U.; Sadaf; Yaseen, M.; Abumousa, R.A.; Khattak, R.; Rehman, N.; Bououdina, M.; Humayun, M. Effective removal of nile blue dye from wastewater using silver-decorated reduced graphene oxide. ACS Omega 2024, 9, 19461–19480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; AlGarni, T.S.; Kumar, P.S.; Bhogal, S.; Kumar, A.; Sharma, S.; Naushad, M.; Alothman, Z.A.; Stadler, F.J. Utilization of Ag2O-Al2O3-ZrO2 decorated onto rGO as adsorbent for the removal of Congo red from aqueous solution. Environ. Res. 2021, 197, 111179. [Google Scholar] [CrossRef]
- Dissanayake, N.S.L.; Pathirana, M.A.; Wanasekara, N.D.; Mahltig, B.; Nandasiri, G.K. Removal of methylene blue and congo red using a chitosan–graphene oxide-electrosprayed functionalized polymeric nanofiber membrane. Nanomaterials 2023, 13, 1350. [Google Scholar] [CrossRef]
- Su, J.; He, S.; Zhao, Z.; Liu, X.; Li, H. Efficient preparation of cetyltrimethylammonium bromide-graphene oxide composite and its adsorption of Congo red from aqueous solutions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 554, 227–236. [Google Scholar] [CrossRef]
- Mahmood, T.; Noreen, U.; Ali, R.; Ullah, A.; Naeem, A.; Aslam, M. Adsorptive removal of Congo red from aqueous phase using graphene–tin oxide composite as a novel adsorbent. Int. J. Environ. Sci. Technol. 2022, 19, 10275–10290. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Nie, R.; Li, Y.; Li, Q.; Lei, Y.; Guo, M.; Zhang, Y. Highly efficient adsorption of anionic dyes on a porous graphene oxide nanosheets/chitosan composite aerogel. Ind. Crops Prod. 2024, 220, 119146. [Google Scholar] [CrossRef]
- Dong, J.; Chen, Q.; Zhang, J.; Wang, Z.; Cai, J.; Yan, H.; Chen, C. Effects of rainfall events on behavior of tetracycline antibiotics in a receiving river: Seasonal differences in dominant processes and mechanisms. Sci. Total Environ. 2019, 692, 511–518. [Google Scholar] [CrossRef]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, W.-J.; Jiang, H.; Chen, J.-J.; Li, W.-W.; Yu, H.-Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Moussavi, G.; Hossaini, Z.; Pourakbar, M. High-rate adsorption of acetaminophen from the contaminated water onto double-oxidized graphene oxide. Chem. Eng. J. 2016, 287, 665–673. [Google Scholar] [CrossRef]
- Peng, B.; Chen, L.; Que, C.; Yang, K.; Deng, F.; Deng, X.; Shi, G.; Xu, G.; Wu, M. Adsorption of antibiotics on graphene and biochar in aqueous solutions induced by π-π interactions. Sci. Rep. 2016, 6, 31920. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, R.; Behnejad, H. A comprehensive adsorption study and modeling of antibiotics as a pharmaceutical waste by graphene oxide nanosheets. Ecotoxicol. Environ. Saf. 2018, 147, 117–123. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, H.; Wang, X.; Wang, C.; Wei, X. Effect of pH on adsorption of tetracycline antibiotics on graphene oxide. Int. J. Environ. Res. Public Health 2023, 20, 2448. [Google Scholar] [CrossRef] [PubMed]
- Shaha, C.K.; Mahmud, M.A.A.; Saha, S.; Karmaker, S.; Saha, T.K. Efficient removal of sparfloxacin antibiotic from water using sulfonated graphene oxide: Kinetics, thermodynamics, and environmental implications. Heliyon 2024, 10, e33644. [Google Scholar] [CrossRef]
- Song, X.; Shui, B.; Wang, Y.; Zhou, J.; Wang, S.; Wu, N. Adsorption performance of GO-doped activated ATP composites towards tetracycline. RSC Adv. 2022, 12, 19917–19928. [Google Scholar] [CrossRef]
- Parashar, D.; Harafan, A.; Achari, G.; Kumar, M. Ciprofloxacin and metronidazole adsorption on chitosan-modified graphene oxide as single-compound and binary mixtures: Kinetics, isotherm, and sorption mechanism. J. Hazard. Toxic Radioact. Waste 2023, 27, 04022042. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, K.; Xu, X.; Liu, X.; Zhao, B.; Ding, H.; Yu, Z.; Deng, C. Preparation of β-cyclodextrin/dopamine hydrochloride-graphene oxide and its adsorption properties for sulfonamide antibiotics. Environ. Sci. Pollut. Res. 2022, 29, 70192–70201. [Google Scholar] [CrossRef] [PubMed]
- Fatholahi, P.; Salehzadeh, H.; Hosseini, K.; Wantala, K.; Shivaraju, H.P.; Jenkins, D.; Shahmoradi, B. Removal of erythromycin antibiotic from the aqueous media using magnetic graphene oxide nanoparticles. Desalination Water Treat. 2023, 303, 142–150. [Google Scholar] [CrossRef]
- Bahar, P.; Hassani, A.H.; Panahi, H.A.; Moniri, E. Application of modified graphene oxide with thermosensitive polymers for adsorption of antibiotics from synthetic contaminated water. Dalton Trans. 2021, 210, 281–295. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Q.; Sheng, C.; Jin, C.; Sun, Q. One-step preparation of graphene oxide/cellulose nanofibril hybrid aerogel for adsorptive removal of four kinds of antibiotics. J. Nanomater. 2017, 2017, 5150613. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Ma, J.; Chen, J. Enhanced adsorption removal of antibiotics from aqueous solutions by modified alginate/graphene double network porous hydrogel. J. Colloid Interface Sci. 2017, 507, 250–259. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B-Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Tang, L.; Ma, X.Y.; Wang, Y.; Zhang, S.; Zheng, K.; Wang, X.C.; Lin, Y. Removal of trace organic pollutants (pharmaceuticals and pesticides) and reduction of biological effects from secondary effluent by typical granular activated carbon. Sci. Total Environ. 2020, 749, 141611. [Google Scholar] [CrossRef]
- Gouveia, T.I.A.; Mota, I.H.; Silva, A.M.T.; Alves, A.; Santos, M.S.F. Are cytostatic drugs in surface waters a potential threat? Sci. Total Environ. 2022, 853, 158559. [Google Scholar] [CrossRef]
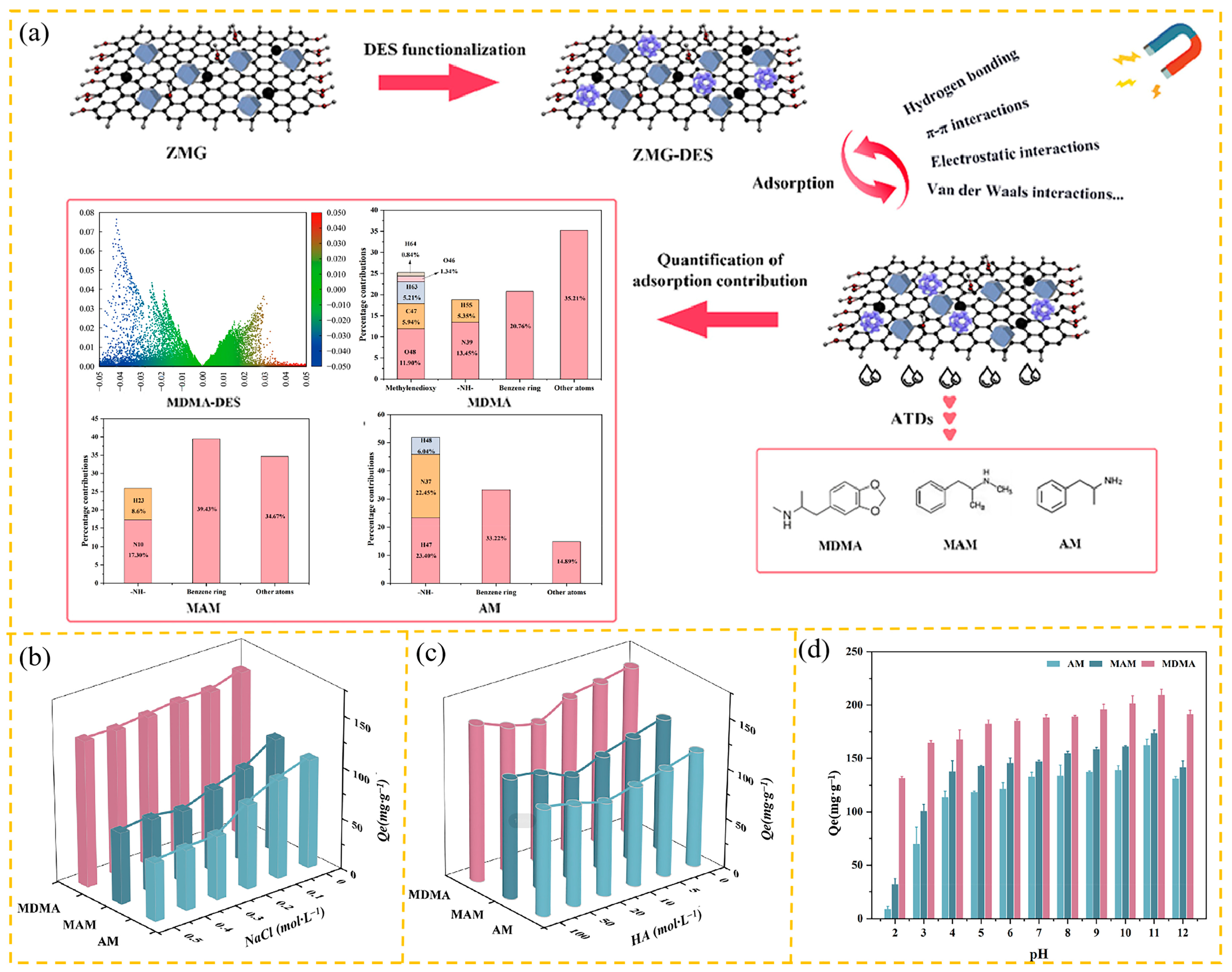
- Cao, S.; Liu, Y.; Ming, D.; Tian, J.; You, J.; Chen, Z. Evaluation of the difference in adsorption of amphetamine-type drugs on deep eutectic solvent-functionalized graphene oxide/ZIF-67 composite: Experiment and theoretical calculations. Environ. Res. 2024, 249, 118356. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Sayma, J.; Munira, N.; Mohamed, D.; Da’na, D.A.; Qiblawey, H.; Alkhouzaam, A. Effective removal of phenol from wastewater using a hybrid process of graphene oxide adsorption and UV-irradiation. Environ. Technol. Innov. 2022, 27, 102525. [Google Scholar] [CrossRef]
- Cui, Y.; Kang, W.; Hu, J. Effectiveness and mechanisms of the adsorption of phenol from wastewater onto N-doped graphene oxide aerogel. J. Water Process Eng. 2023, 53, 103665. [Google Scholar] [CrossRef]
- Severo, L.S.; Thue, P.S.; Lima, D.R.; Didó, C.A.; Vasconcellos, M.A.Z.; Armas, L.E.G.; Lima, E.C.; Benvenutti, E.V.; de Menezes, E.W. 3D graphene sponge biomass-derived with high surface area applied as adsorbent for nitrophenols. J. Environ. Chem. Eng. 2023, 11, 109924. [Google Scholar] [CrossRef]
- Wang, F.; Lu, X.; Peng, W.; Deng, Y.; Zhang, T.; Hu, Y.; Li, X.-y. Sorption behavior of bisphenol A and triclosan by graphene: Comparison with activated carbon. ACS Omega 2017, 2, 5378–5384. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Tian, W.; Qiao, K.; Zhao, J.; Chu, M.; Du, Z.; Wang, L.; Xie, W. Adsorption behaviors of polycyclic aromatic hydrocarbons and oxygen derivatives in wastewater on n-doped reduced graphene oxide. Sep. Purif. Technol. 2021, 254, 117565. [Google Scholar] [CrossRef]
- Kang, W.; Cui, Y.; Yang, Y.; Guo, M.; Zhao, Z.; Wang, X.; Liu, X. Preparation of nitrogen-doped hollow carbon nanosphere/graphene composite aerogel for efficient removal of quinoline from wastewater. J. Hazard. Mater. 2021, 417, 126160. [Google Scholar] [CrossRef]
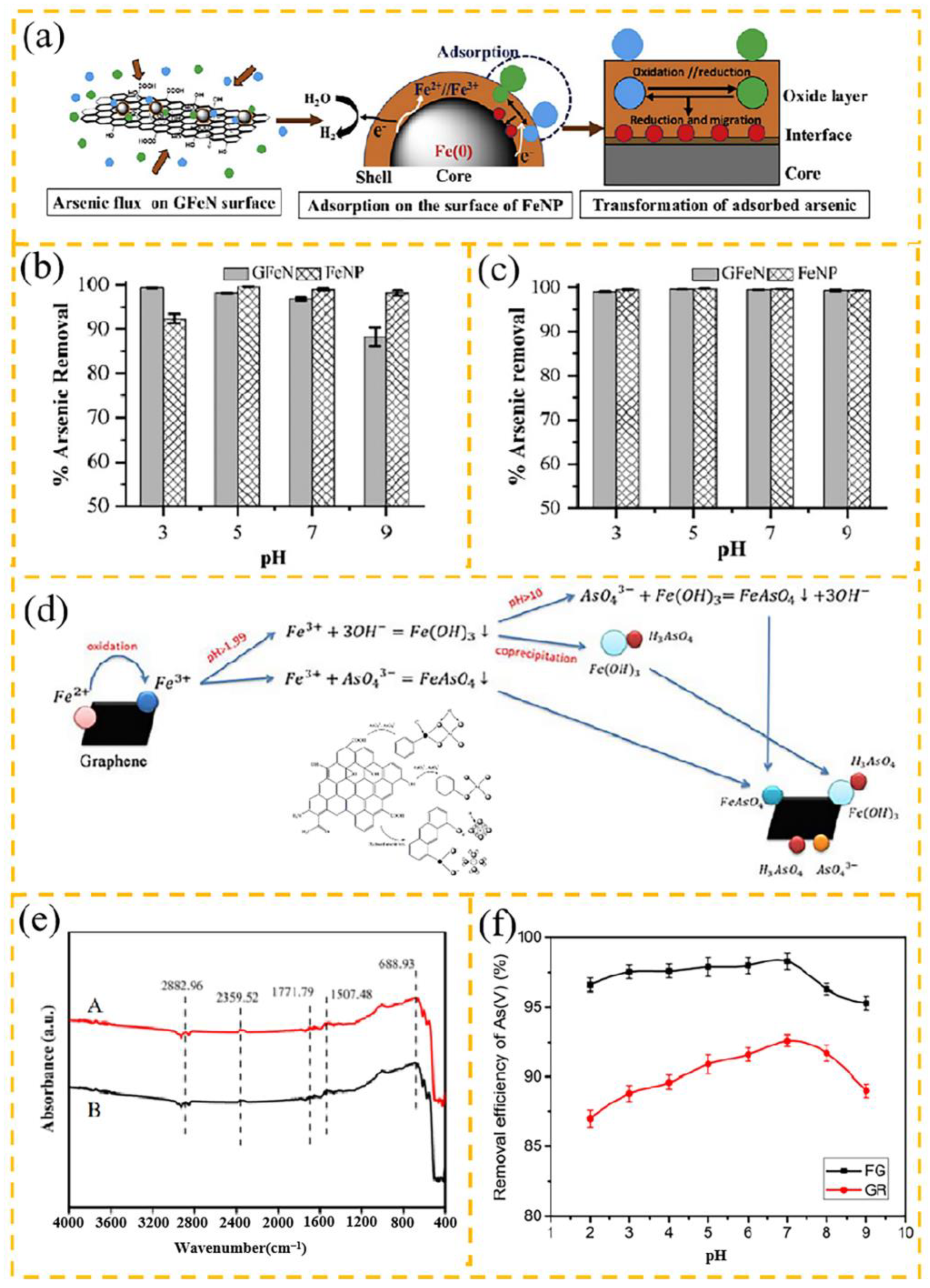


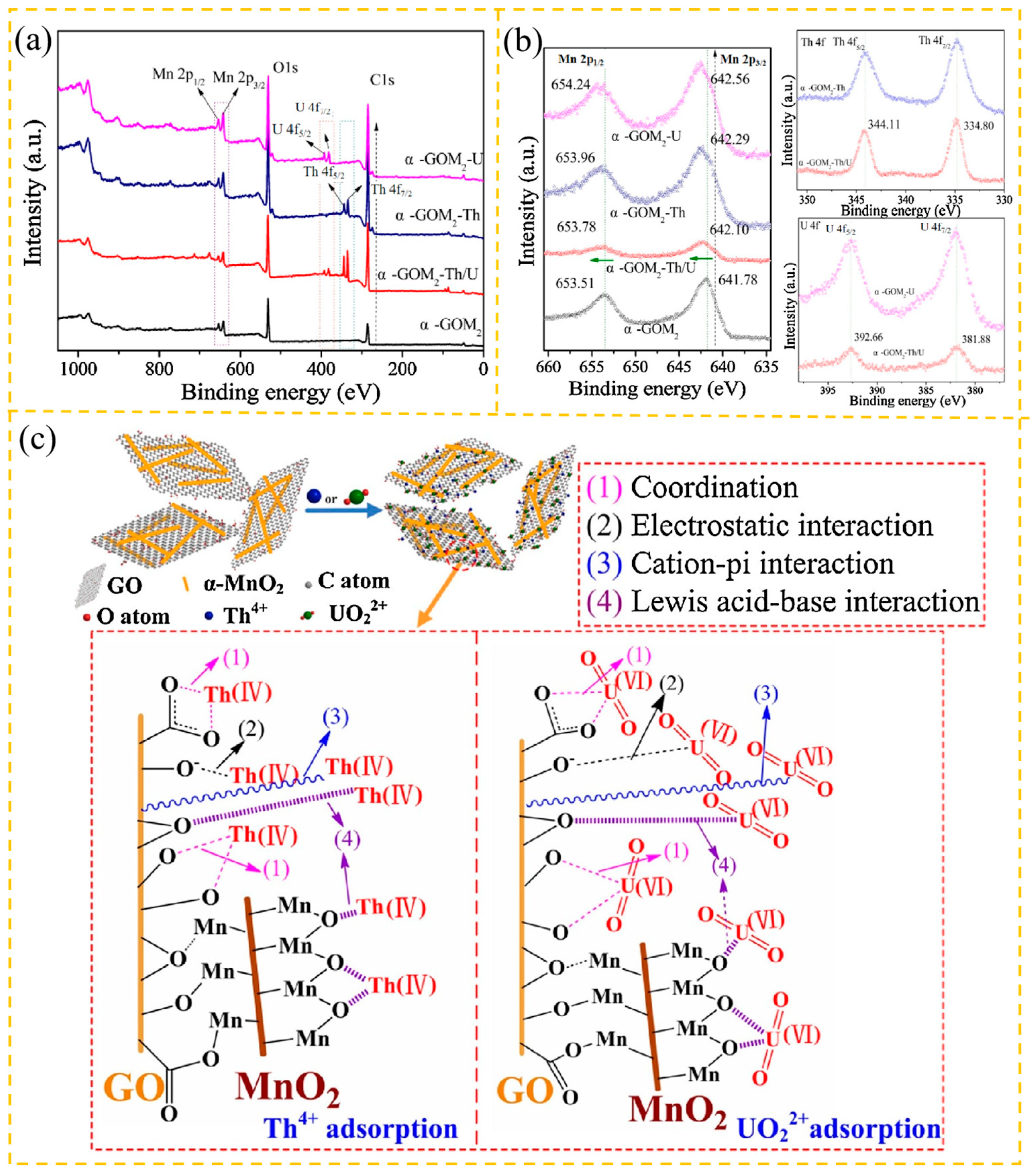
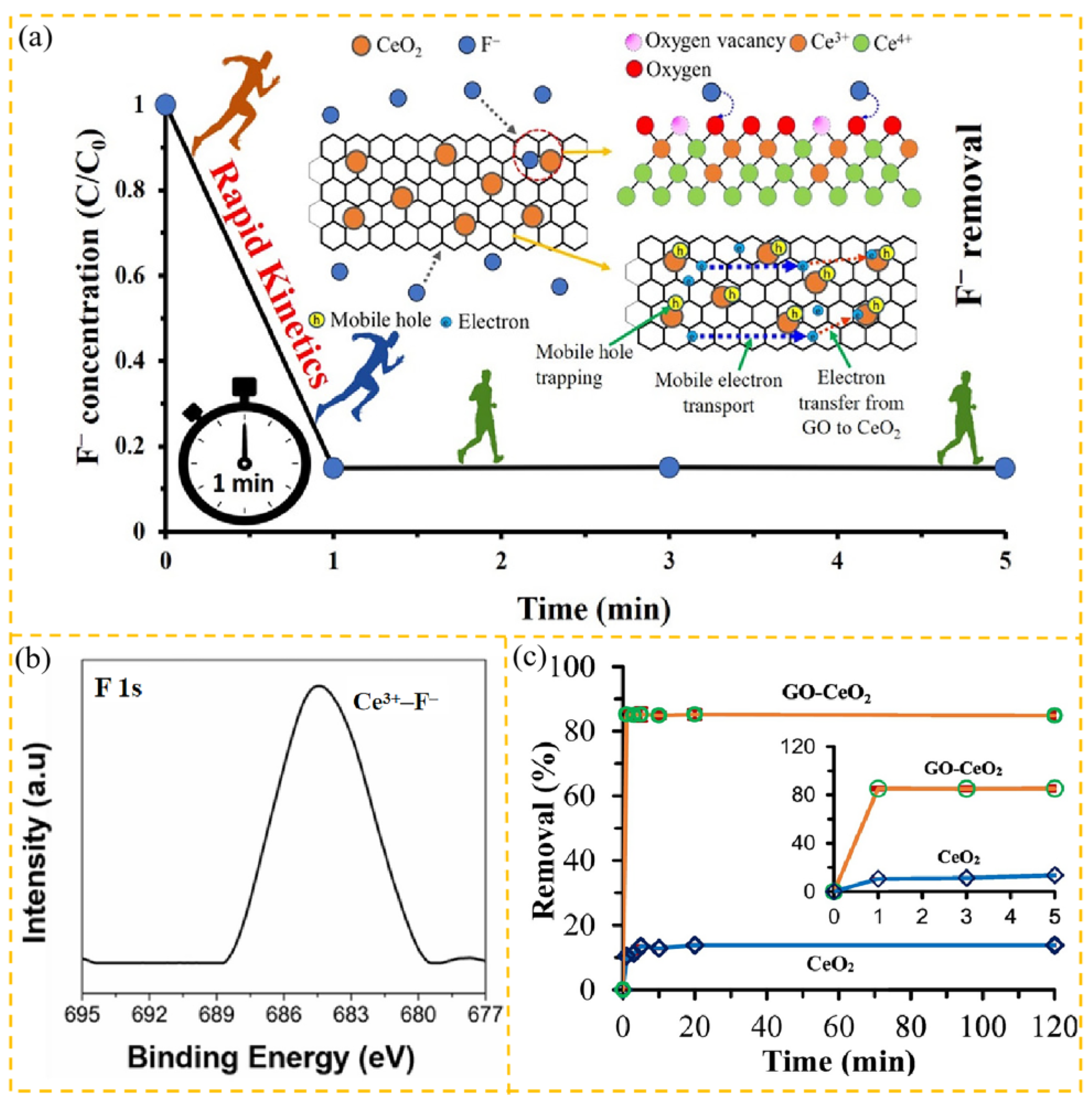
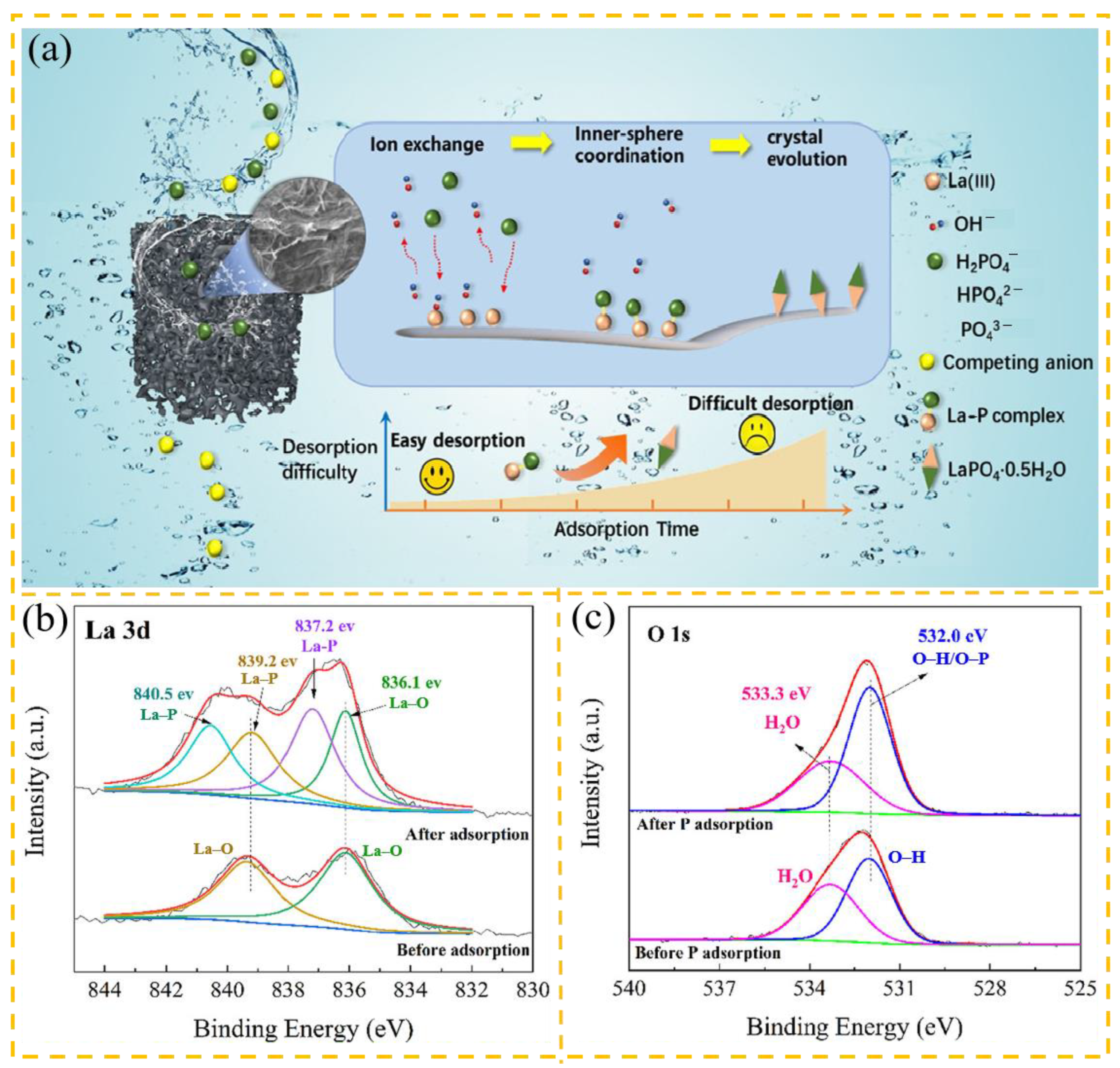

| Adsorbent | Heavy Metal Ion | Adsorption Conditions | Adsorption Capacity/ Removal Efficiency | Reference | |
|---|---|---|---|---|---|
| Material | Form | ||||
| CMGO | Powder | As(III) | pH = 7.3, 25 °C, 4 h | 45 mg·g−1 | [68] |
| Fe3O4–GO | Powder | As(III) | pH = 7, 25 °C, 12 h | 85 mg·g−1 | [142] |
| As(V) | pH = 7, 25 °C, 12 h | 38 mg·g−1 | |||
| Fe3O4–rGO | Powder | As(III) | pH = 7, 25 °C, 12 h | 57 mg·g−1 | [142] |
| As(V) | pH = 7, 25 °C, 12 h | 12 mg·g−1 | |||
| Fe3O4–GO | Aerogel | As(V) | Neutral pH, 25 °C, 12 h | 40.048 mg·g−1 | [143] |
| Fe3O4–rGO | Powder | As(III) | pH = 7, 30 °C, 2 h | 0.81 mg·g−1 | [144] |
| GO | Powder | As(III) | pH = 7, 30 °C, 2 h | 0.70 mg·g−1 | [144] |
| rGO | Powder | As(III) | pH = 7, 30 °C, 2 h | 0.68 mg·g−1 | [144] |
| Co–rGO | Powder | As(III) | pH = 7, 30 °C, 2 h | 0.75 mg·g−1 | [144] |
| GFeN | Powder | As(III) | pH = 7, 22 ± 2 °C | 306.10 ± 9.92 mg·g−1 | [61] |
| As(V) | pH = 7, 22 ± 2 °C | 431.41 ± 25.95 mg·g−1 | |||
| FG | Powder | As(V) | pH = 7, 35 °C | 112.4 mg·g−1 | [109] |
| TAIGO | Powder | Hg(II) | Natural pH, 15 min | 23.81 mg·g−1 | [145] |
| GO | Powder | Cr(VI) | pH = 4 | 1.222 mg·g−1 | [115] |
| ZnO–GO | Powder | Cr(VI) | Neutral pH, 303 K | 96% | [65] |
| GEC | Powder | Cr(VI) | pH = 2, 25 °C | 86.17 mg·g−1 | [70] |
| MCGO–IL | Powder | Cr(VI) | pH = 3, 30 °C | 145.35 mg·g−1 | [146] |
| GONF | Powder | Cr(III) | pH = 4, 298 ± 2 K | 45.5 mg·g−1 | [147] |
| Pb(II) | pH = 5–6, 298 ± 2 K | 25.0 mg·g−1 | |||
| WHLPC/GO | Hydrogel | Cr(VI) | pH = 1, 7 h | 322.00 mg·g−1 | [94] |
| AFGS | Sponge | Cr(VI) | pH > 4, 25 °C | 166.46 mg·g−1 | [87] |
| M–PAS–GO | Powder | Cr(VI) | pH = 3.2, 25 °C | 46.48 mg·g−1 | [148] |
| rGNF | Powder | Pb(II) | pH = 5, 30 °C, 100 min | 99.8% | [124] |
| GO | Powder | Pb(II) | pH = 4, 25 °C, 20 min | 98.87% | [127] |
| rGO–Mn3O4 | Powder | Pb(II) | pH = 4, 25 ± 2 °C, 60 min | 130.28 ± 0.52 mg·g−1 | [128] |
| MCGO | Powder | Pb(II) | pH = 5, 1 h | 50.23 mg·g−1 | [149] |
| FeGO–TiLa | Powder | Pb(II) | pH = 5, 2 h | 112 mg·g−1 | [62] |
| MSiO2–GO | Powder | Pb(II) | pH = 6, 25 °C | 90.48% | [64] |
| Magnetic GO | Powder | Cd(II) | pH = 7, 45 °C | 128.2 mg·g−1 | [150] |
| Pb(II) | pH = 7, 45 °C | 385.1 mg·g−1 | |||
| ZnO NR–rGO | Powder | Co(II) | pH = 6 | 36.354 mg·g−1 | [130] |
| Cu(II) | pH = 6 | 67.399 mg·g−1 | |||
| AMGO | Powder | Cr(VI) | pH = 2 | 123.4 mg·g−1 | [151] |
| TSCGO | Powder | Hg(II) | pH = 6, 298 K | 933.2 mg·g−1 | [152] |
| Pb(II) | pH = 5, 298 K | 890.4 mg·g−1 | |||
| GOFH | Powder | As(V) | pH = 4, 298 K | 160 mg·g−1 | [153] |
| Cr(VI) | pH = 4, 298 K | 66 mg·g−1 | |||
| IRC748–GO | Powder | Cu(II) | pH = 5.81, 30 °C | 127.22 mg·g−1 | [72] |
| CMC/SA/GO@Fe3O4 | Powder | Cu(II) | pH = 5, 30 °C | 55.96 mg·g−1 | [154] |
| Cd(II) | pH = 6, 30 °C | 86.28 mg·g−1 | |||
| Pb(II) | pH = 5, 30 °C | 189.04 mg·g−1 | |||
| GO–EDTA–CS | Powder | Cu(II) | pH = 5.1, room temperature | 130 ± 2.80 mg·g−1 | [155] |
| Hg(II) | pH = 5.1, room temperature | 324 ± 3.30 mg·g−1 | |||
| GO/CS | Powder | Cu(II) | pH = 5, 313.15 K, 210 min | 269 mg·g−1 | [156] |
| GO/CS | Powder | Cu(II) | pH = 6, 20 °C | 60.7 mg·g−1 | [67] |
| Cr(VI) | pH = 6, 20 °C | 48.7 mg·g−1 | |||
| Pb(II) | pH = 6, 20 °C | 32.3 mg·g−1 | |||
| mGO–PAMAM | Powder | Cu(II) | pH = 7, 25 °C | 353.59 mg·g−1 | [74] |
| Cd(II) | pH = 6, 25 °C | 435.85 mg·g−1 | |||
| Pb(II) | pH = 7, 25 °C | 326.729 mg·g−1 | |||
| GO–SA | Powder | Pb(II) | pH = 4, 25 °C | 887.21 mg·g−1 | [71] |
| Zn(II) | pH = 5, 25 °C | 161.25 mg·g−1 | |||
| Cd(II) | pH = 7, 25 °C | 139.62 mg·g−1 | |||
| GO–sand | Powder | Pb(II) | pH = 6, 298 K | 289.68 mg·g−1 | [157] |
| Cr(III) | pH = 6, 298 K | 258.87 mg·g−1 | |||
| GO–MBT | Powder | Pb(II) | pH = 6 | 116.959 mg·g−1 | [158] |
| Cd(II) | pH = 7 | 112.99 mg·g−1 | |||
| GO–CNC | Powder | Cr(VI) | pH = 5, 105 min | 90.74% | [159] |
| Ni(II) | pH = 8, 90 min | 58.56% | |||
| RGOS | Powder | Pb(II) | pH = 5.0, <10 min | 689 mg·g−1 | [160] |
| Cu(II) | pH = 5.0, <10 min | 59 mg·g−1 | |||
| Ni(II) | pH = 5.5, <10 min | 66 mg·g−1 | |||
| Cd((II) | pH = 5.0, <10 min | 267 mg·g−1 | |||
| Cr(III) | pH = 5.5, <10 min | 191 mg·g−1 | |||
| GRP–PPY–NP | Powder | Mn(II) | pH = 5.5, 90 min | 89% | [161] |
| ZnO–Gr | Powder | Ni(II) | pH = 8.2, 25 °C | 66.66 mg·g−1 | [162] |
| Graphene | Powder | Ni(II) | pH = 8.2, 25 °C | 3.78 mg·g−1 | [162] |
| mp–CA/GO | Aerogel | Pb(II) | 25 °C, 40 min | 368.2 mg·g−1 | [163] |
| Cu(II) | 25 °C, 40 min | 98.1 mg·g−1 | |||
| Cd(II) | 25 °C, 40 min | 183.6 mg·g−1 | |||
| GOF | Foam | Zn(II) | pH = 10 | 326.4 mg·g−1 | [83] |
| Cd(II) | pH = 10 | 252.5 mg·g−1 | |||
| Pb(II) | pH = 10 | 381.3 mg·g−1 | |||
| Fe(III) | pH = 10 | 587.6 mg·g−1 | |||
| rGO | Aerogel | Sb(III) | pH = 6 ± 0.5, 298 K | 168.59 mg·g−1 | [99] |
| Sb(V) | pH = 6 ± 0.5, 298 K | 206.72 mg·g−1 | |||
| TI/GO@ Fe3O4 | Powder | Pb(II) | pH = 5, 293 K | 461.00 mg·g−1 | [164] |
| PAMAM–GO | Powder | Se(IV) | pH = 6 | 60.9 mg·g−1 | [139] |
| Se(VI) | pH = 6 | 77.9 mg·g−1 | |||
| PAA–MGO | Powder | Se(IV) | pH = 5.8 | 120.1 mg·g−1 | [140] |
| Se(VI) | pH = 5.8 | 83.7 mg·g−1 | |||
| NZVI/rGO | Powder | Se(IV) | pH = 3, 25 °C | 173.53 mg·g−1 | [141] |
| CTAB@GO | Powder | Se(IV) | pH = 2–3 | 139.95 mg·g−1 | [165] |
| Se(VI) | pH = 2–3 | 468.01 mg·g−1 | |||
| Adsorbent | Organic Dye | Adsorption Conditions | Adsorption Capacity (mg·g−1) | Reference | |
|---|---|---|---|---|---|
| Material | Form | ||||
| GO | Powder | MB | pH = 12 | 2301 | [186] |
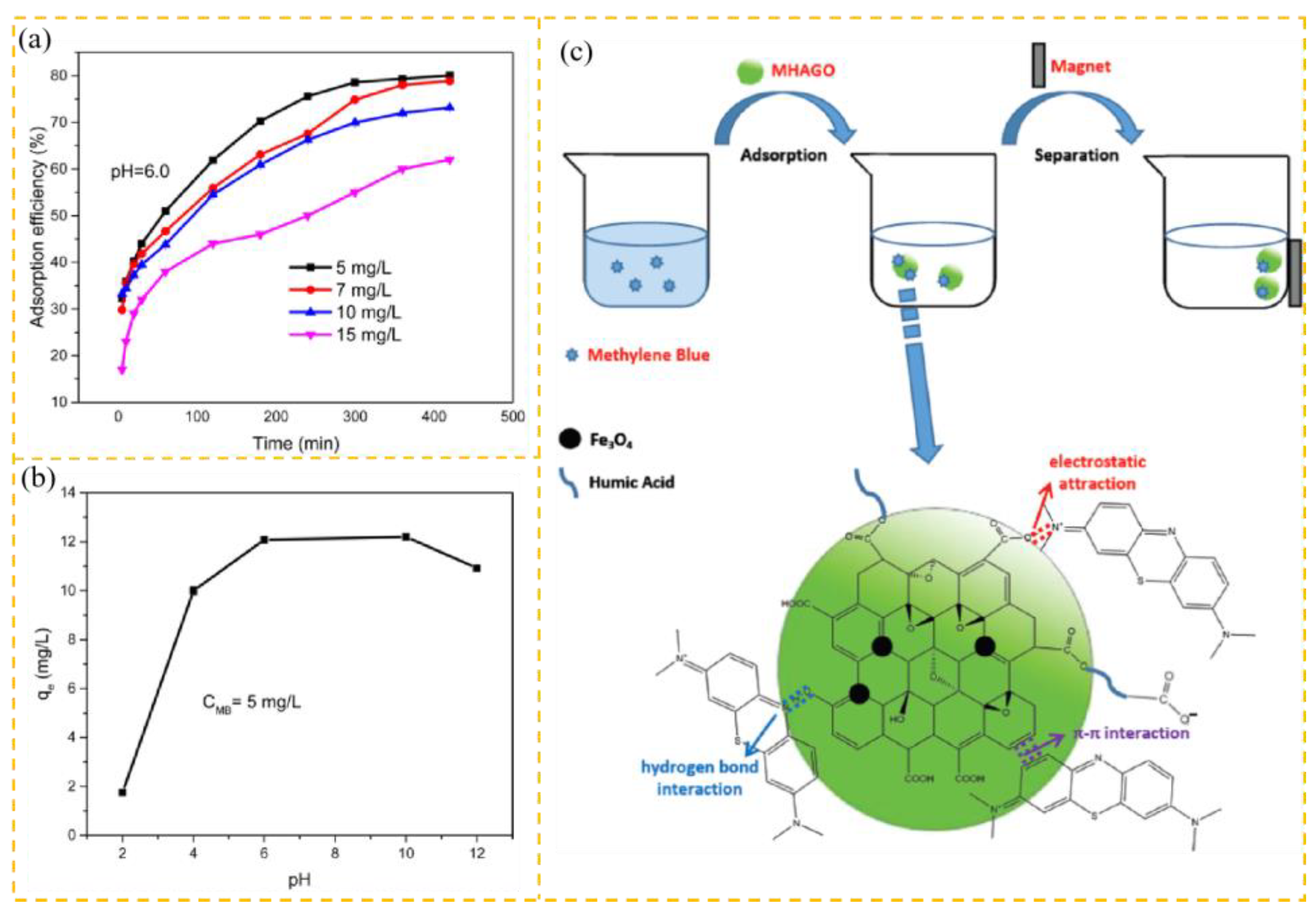
| MHAGO | Powder | MB | pH = 6.0, 45 °C | 59.00 | [27] |
| β-CD/GO | Powder | MB | 30 °C, 1 h | 76.4 | [192] |
| RL–GO | Powder | MB | pH = 11, 318 K | 581.40 | [193] |
| GO–TSC–GO | Powder | MB | pH > 7, 25 °C, 120 min | 596.642 | [194] |
| GO | Powder | MB | pH > 7, 25 °C, 120 min | 196.8 | [194] |
| GO/DETA/MnFe2O4@SiO2 | Powder | MB | pH = 7, 25 °C | 243.91 | [195] |
| MFTGS | Sponge | MB | 308 K, 10 min | 224 | [86] |
| CF/CNTs/GO | Foam | MB | C0 = 100 mg·L−1 | 146 | [187] |
| APAM/DTPA–CS/GO | Aerogel | MB | 303 K, 95 min | 652.99 | [196] |
| GO/PEI | Aerogel | MB | pH = 10.5, 25 ± 1 °C | 249.6 | [197] |
| MO | pH = 2.0, 25 ± 1 °C | 331.0 | |||
| CS/GO | Aerogel | MB | pH = 8, 293 K | 437.29 | [198] |
| CGZ-3 | Aerogel | MB | pH = 9, 298 K | 427.35 | [199] |
| M−rGO | Aerogel | MB | 298 K | 450.90 | [200] |
| GO-MMT/SA | Aerogel | MB | 60 min | 150.66 | [201] |
| TA–rGO | Hydrogel | MB | 25 °C | 1000 | [95] |
| GO–EDTA–CS | Powder | MB | pH = 8.3, room temperature | 141 ± 6.60 mg·g−1 | [155] |
| CV | pH = 8.3, room temperature | 121 ± 3.50 mg·g−1 | |||
| CS/GO | Powder | RhB | pH = 4, 25 °C | 858.0 | [202] |
| rGO–ZnS | Powder | MG | 25 °C | 27.54 | [185] |
| EV | 25 °C | 20.04 | |||
| Ag–rGO | Powder | NB | pH = 6, 30 °C | 36.8 | [203] |
| rGO | Powder | NB | pH = 6, 30 °C | 20 | [203] |
| GO | Powder | NB | pH = 6, 30 °C | 8.8 | [203] |
| Ag2O–Al2O3–ZrO2–rGO | Powder | CR | 30 °C, 90 min | 333.32 | [204] |
| CS/GO | Powder | CR | pH = 7, 300 K | 182.5 | [205] |
| MB | pH = 7, 300 K | 219.3 | |||
| Graphene | Aerogel | CR | pH = 2, 60 °C | 53.91 | [189] |
| CTAB–GO | Powder | CR | pH = 3, 298 K | 2767 | [206] |
| G/SnO2 | Powder | CR | pH = 2–4, 318 K | 359.71 | [207] |
| AS–GO | Powder | OG | pH = 3, 313 K | 576.6 | [190] |
| 3D graphene | Powder | MO | pH = 2–11, 20 °C | 27.932 | [191] |
| GONs | Powder | MO | pH = 6.7, 298 K | 179.21 | [208] |
| BB | pH = 6.7, 298 K | 236.42 | |||
| Adsorbent | Antibiotic | Adsorption Conditions | Adsorption Capacity/Removal Efficiency | Reference | |
|---|---|---|---|---|---|
| Material | Form | ||||
| Graphene | Powder | Sulfamethoxazole | pH = 7, 298 K | 181.3 μg·g−1 | [215] |
| Sulfamethazine | pH = 7, 298 K | 196.3 μg·g−1 | |||
| Sulfadiazine | pH = 7, 298 K | 184.9 μg·g−1 | |||
| Cefalexin | pH = 7, 298 K | 207.2 μg·g−1 | |||
| Olfloxain | pH = 7, 298 K | 199.2 μg·g−1 | |||
| Amoxicillin | pH = 7, 298 K | 198.2 μg·g−1 | |||
| Tetracycline | pH = 7, 298 K | 200.2 μg·g−1 | |||
| GO | Powder | Sulfamethazine | pH = 5 | 240 mg·g−1 | [216] |
| Ciprofloxacin | pH = 9 | 379 mg·g−1 | |||
| GO | Powder | Doxycycline | pH = 6, 25 °C | 116.5099 mg·g−1 | [217] |
| Ciprofloxacin | pH = 6, 25 °C | 156.7634 mg·g−1 | |||
| Tetracycline | pH = 6, 25 °C | 80.980 mg·g−1 | |||
| GO | Powder | Chlortetracycline | pH = 5, 298 K | 218.60 mg·g−1 | [218] |
| Tetracycline | pH = 5, 308 K | 182.91 mg·g−1 | |||
| Oxytetracycline | pH = 5, 308 K | 176.62 mg·g−1 | |||
| SGO | Powder | Sparfloxacin | pH = 5.5, 25 °C | 1428.57 μmol·g−1 | [219] |
| GO/α–ATP | Powder | Tetracycline | pH = 5, 55 °C | 38.73 mg·g−1 | [220] |
| CS–GO | Powder | Metronidazole | Neutral pH, 298 K | 29.76 mg·g−1 | [221] |
| Ciprofloxacin | Neutral pH, 298 K | 102.04 mg·g−1 | |||
| CD–DGO | Powder | Sulfamethoxazole | pH = 2, 35 °C | 144.2804 mg·g−1 | [222] |
| Sulfadiazine | pH = 2, 35 °C | 152.2888 mg·g−1 | |||
| MGO | Powder | Erythromycin | pH = 3, 298 K | 285.71 mg·g−1 | [223] |
| TS–GO | Powder | Ciprofloxacin | pH = 5, 25 °C | 769.23 mg·g−1 | [224] |
| Amoxicillin | pH = 3, 25 °C | 625 mg·g−1 | |||
| GO–CNF | Aerogel | Doxycycline | 25 °C | 469.7 mg·g−1 | [225] |
| Chlortetracycline | 25 °C | 396.5 mg·g−1 | |||
| Oxytetracycline | 25 °C | 386.5 mg·g−1 | |||
| Tetracycline | 25 °C | 343.8 mg·g−1 | |||
| Alginate/rGO | Hydrogel | Tetracycline | pH = 8, 298 K | 290.70 mg·g−1 | [226] |
| Ciprofloxacin | pH = 8, 298 K | 344.83 mg·g−1 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Du, R.; Cao, Z.; Li, C.; Xue, J.; Ma, X.; Wang, S. Research Progress in Graphene-Based Adsorbents for Wastewater Treatment: Preparation, Adsorption Properties and Mechanisms for Inorganic and Organic Pollutants. C 2024, 10, 78. https://doi.org/10.3390/c10030078
Li G, Du R, Cao Z, Li C, Xue J, Ma X, Wang S. Research Progress in Graphene-Based Adsorbents for Wastewater Treatment: Preparation, Adsorption Properties and Mechanisms for Inorganic and Organic Pollutants. C. 2024; 10(3):78. https://doi.org/10.3390/c10030078
Chicago/Turabian StyleLi, Guangqian, Ruiling Du, Zhanfang Cao, Changxin Li, Jianrong Xue, Xin Ma, and Shuai Wang. 2024. "Research Progress in Graphene-Based Adsorbents for Wastewater Treatment: Preparation, Adsorption Properties and Mechanisms for Inorganic and Organic Pollutants" C 10, no. 3: 78. https://doi.org/10.3390/c10030078
APA StyleLi, G., Du, R., Cao, Z., Li, C., Xue, J., Ma, X., & Wang, S. (2024). Research Progress in Graphene-Based Adsorbents for Wastewater Treatment: Preparation, Adsorption Properties and Mechanisms for Inorganic and Organic Pollutants. C, 10(3), 78. https://doi.org/10.3390/c10030078










