Recent Advances in Carbon-Based Interfacial Photothermal Converters for Seawater Desalination: A Review
Abstract
1. Introduction
2. Development of Solar-Driven Water Evaporation Technologies
2.1. Evaporation System Based on Bottom Heating
2.2. Evaporation System Based on Bulk Heating
2.3. Evaporation System Based on Interfacial Heating
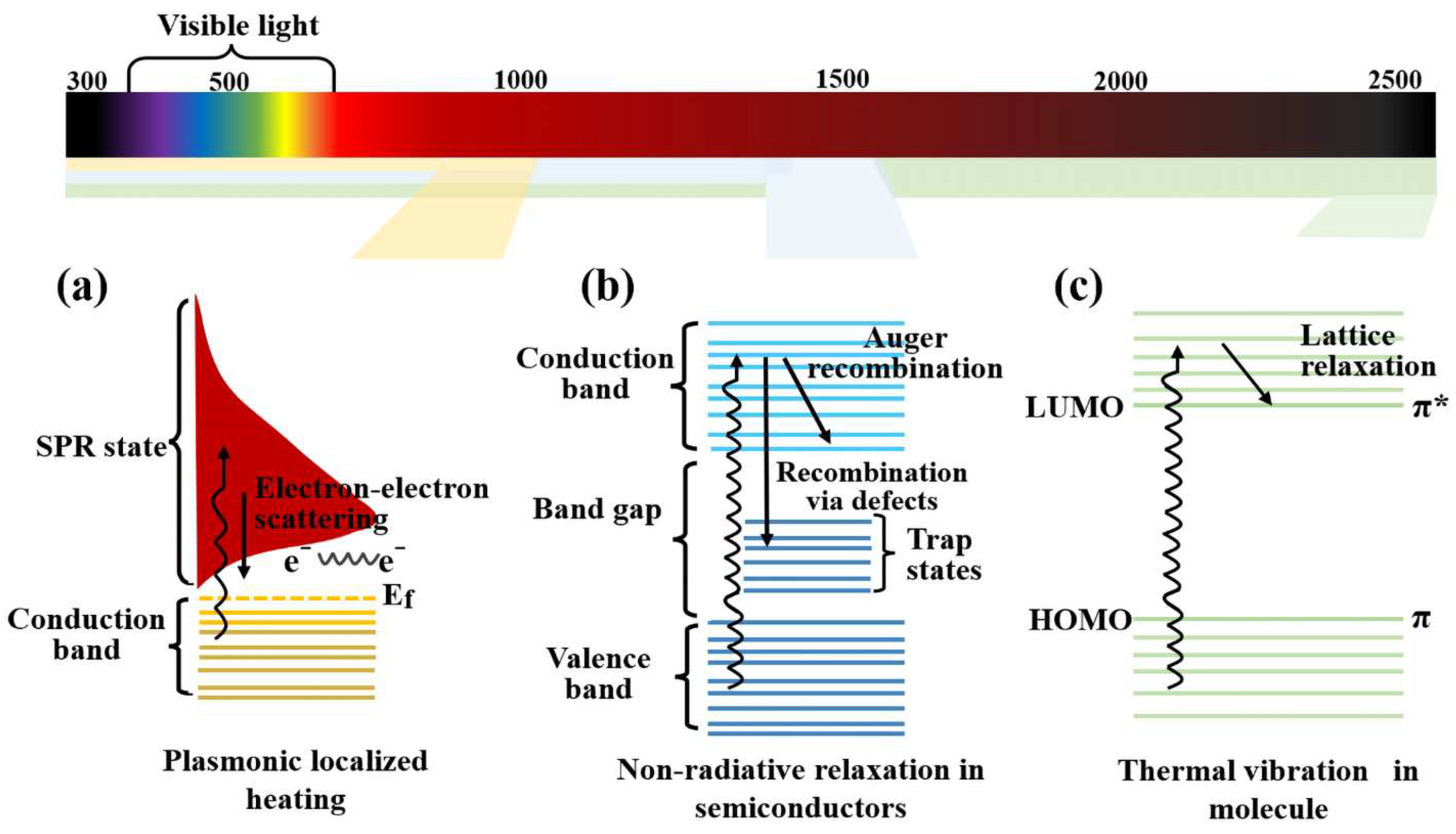
3. Photothermal Materials and Their Conversion Mechanisms
3.1. Metal-Based Materials
3.2. Semiconductor Materials
3.3. Organic Polymer Materials
3.4. Carbon-Based Materials
4. Preparation Methods of Light Absorber
4.1. Preparation of a 2D Absorber
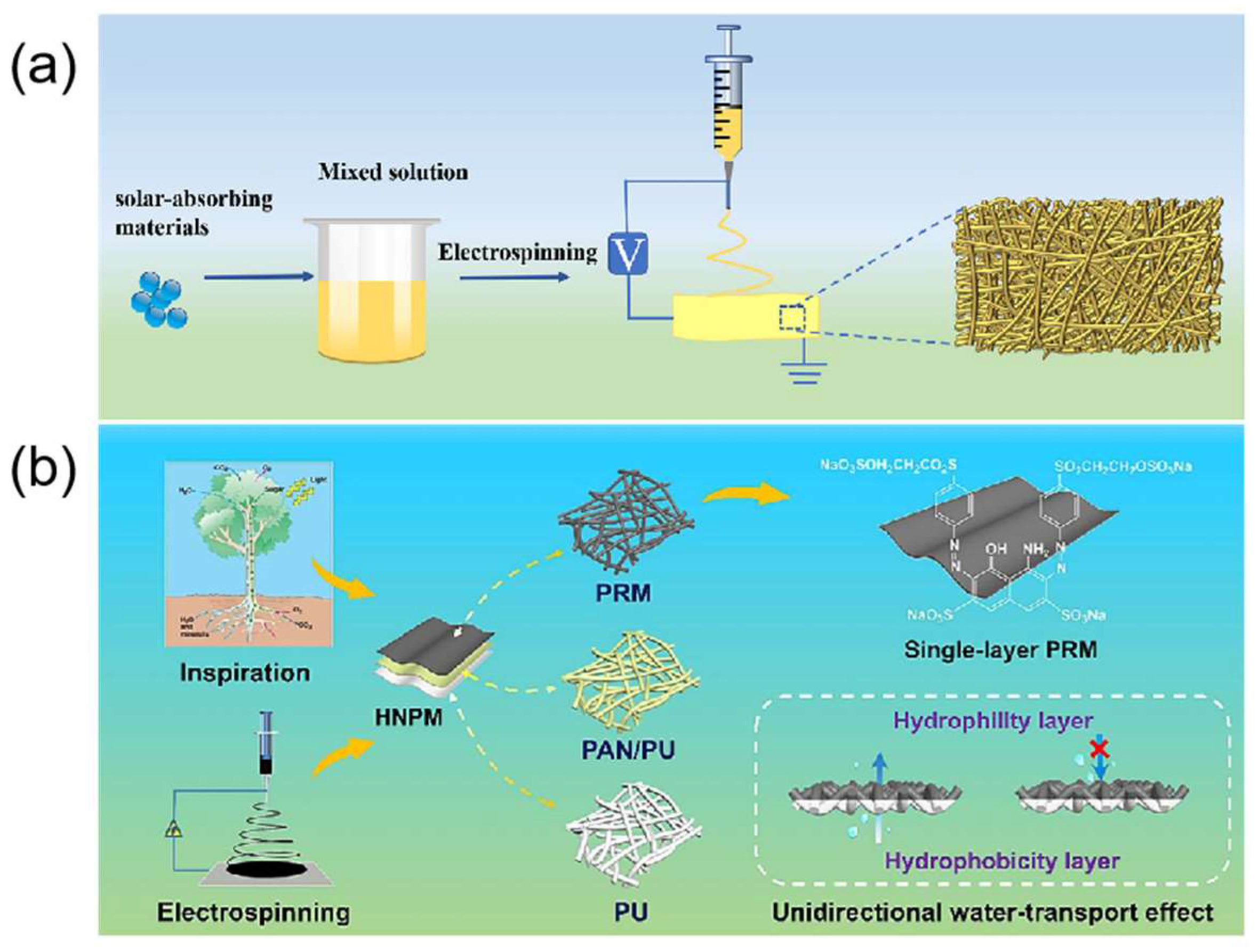
4.1.1. Electrospinning
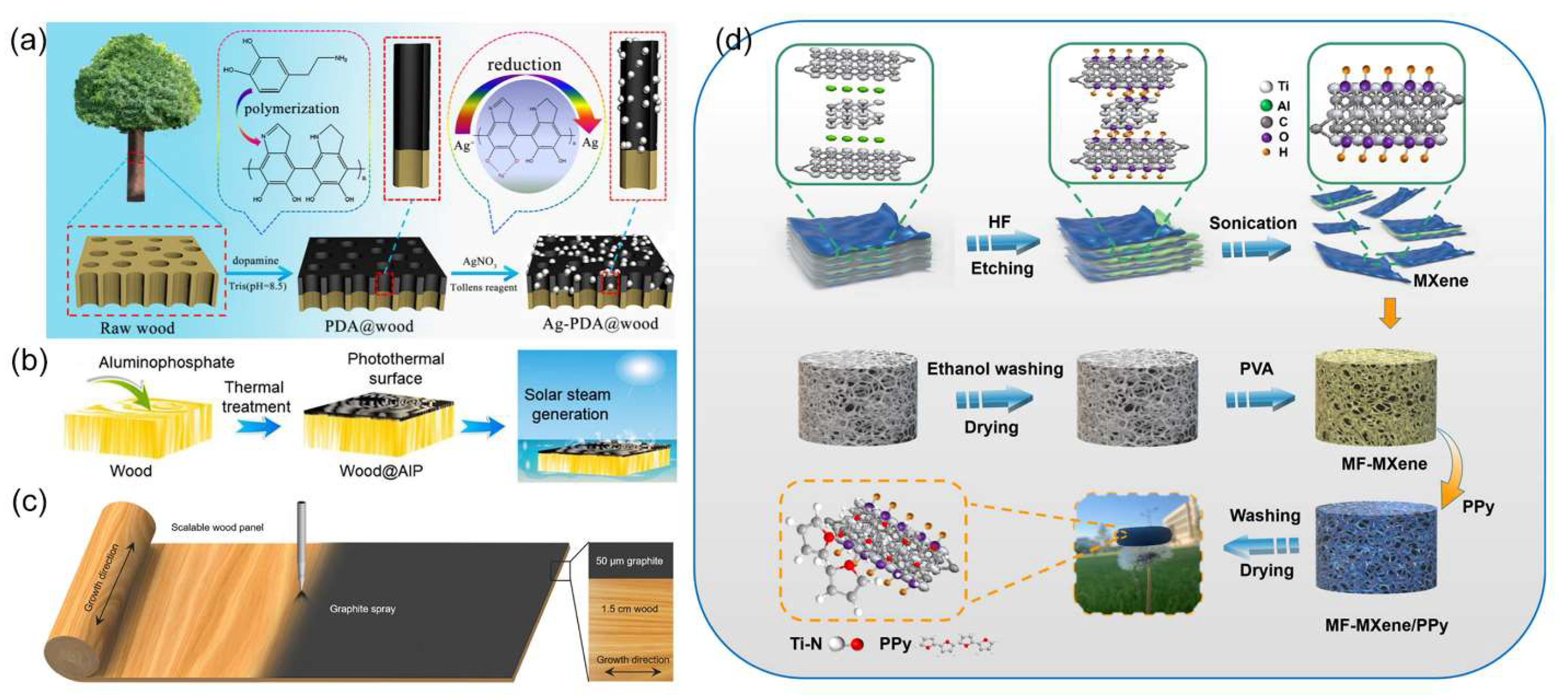
4.1.2. Surface Treatment
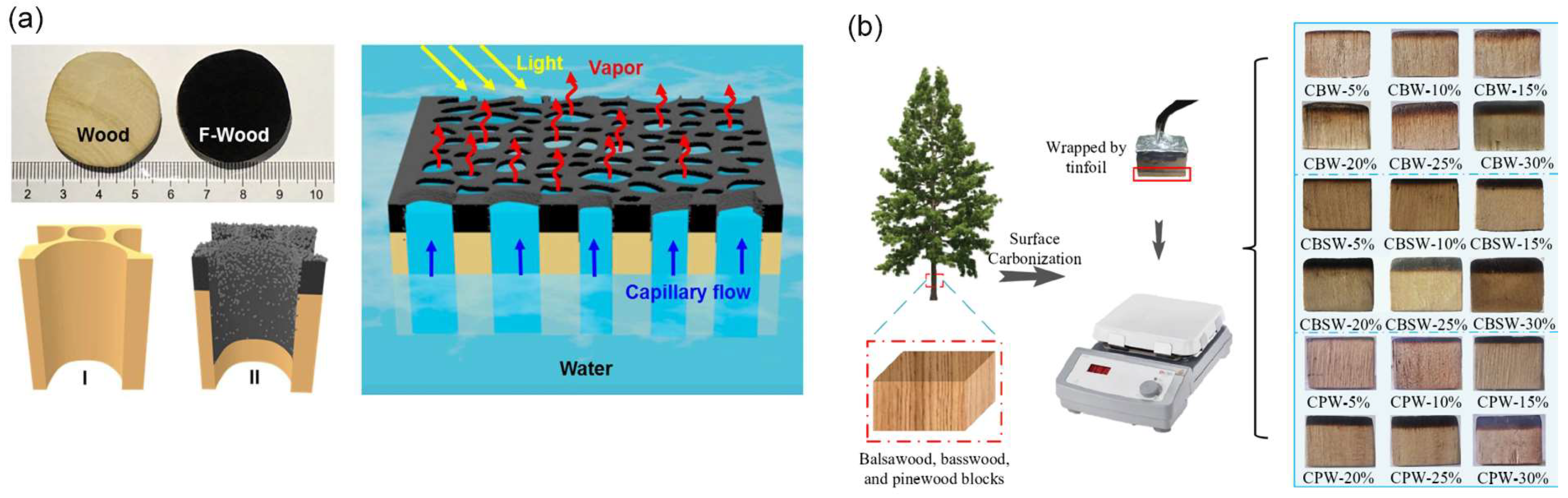
4.1.3. Carbonizing Methods
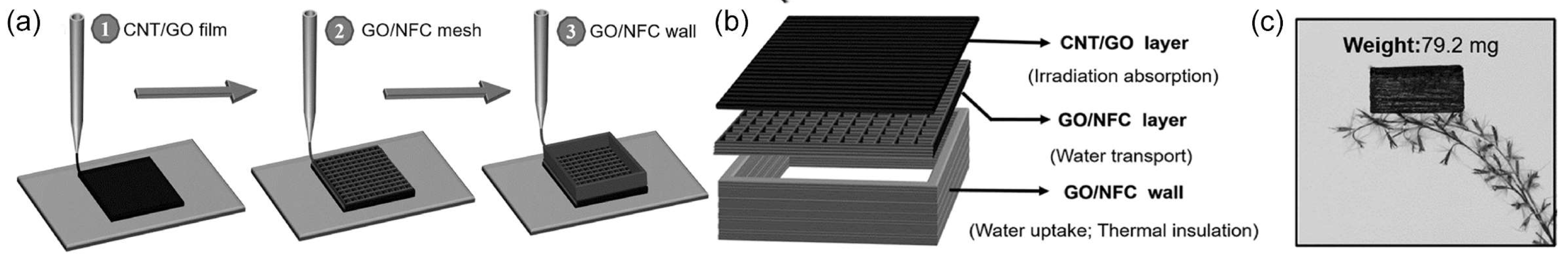
4.1.4. Combining Multiple Methods
4.2. Fabrication of 3D Absorbers
4.2.1. Carbonization Methods
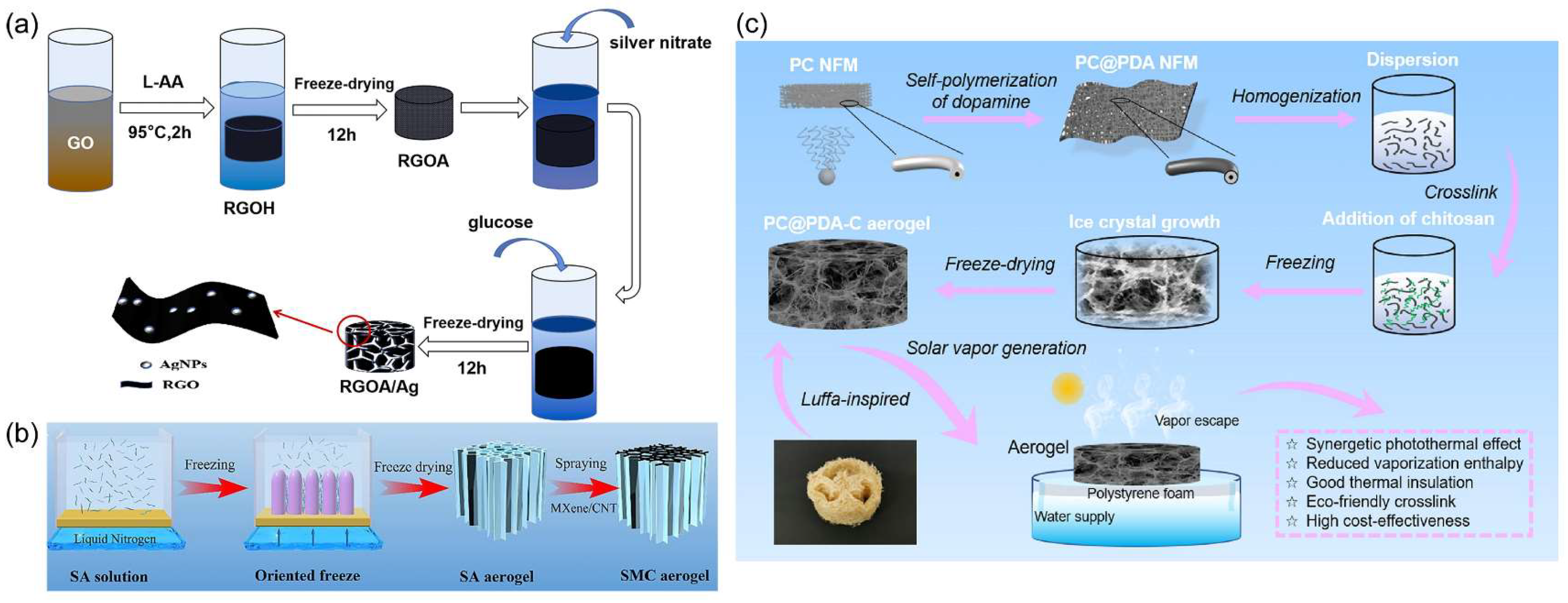
4.2.2. Surface Modification Methods
4.2.3. Aerogel Method
4.2.4. Hydrogel Methods
4.2.5. 3D Printing
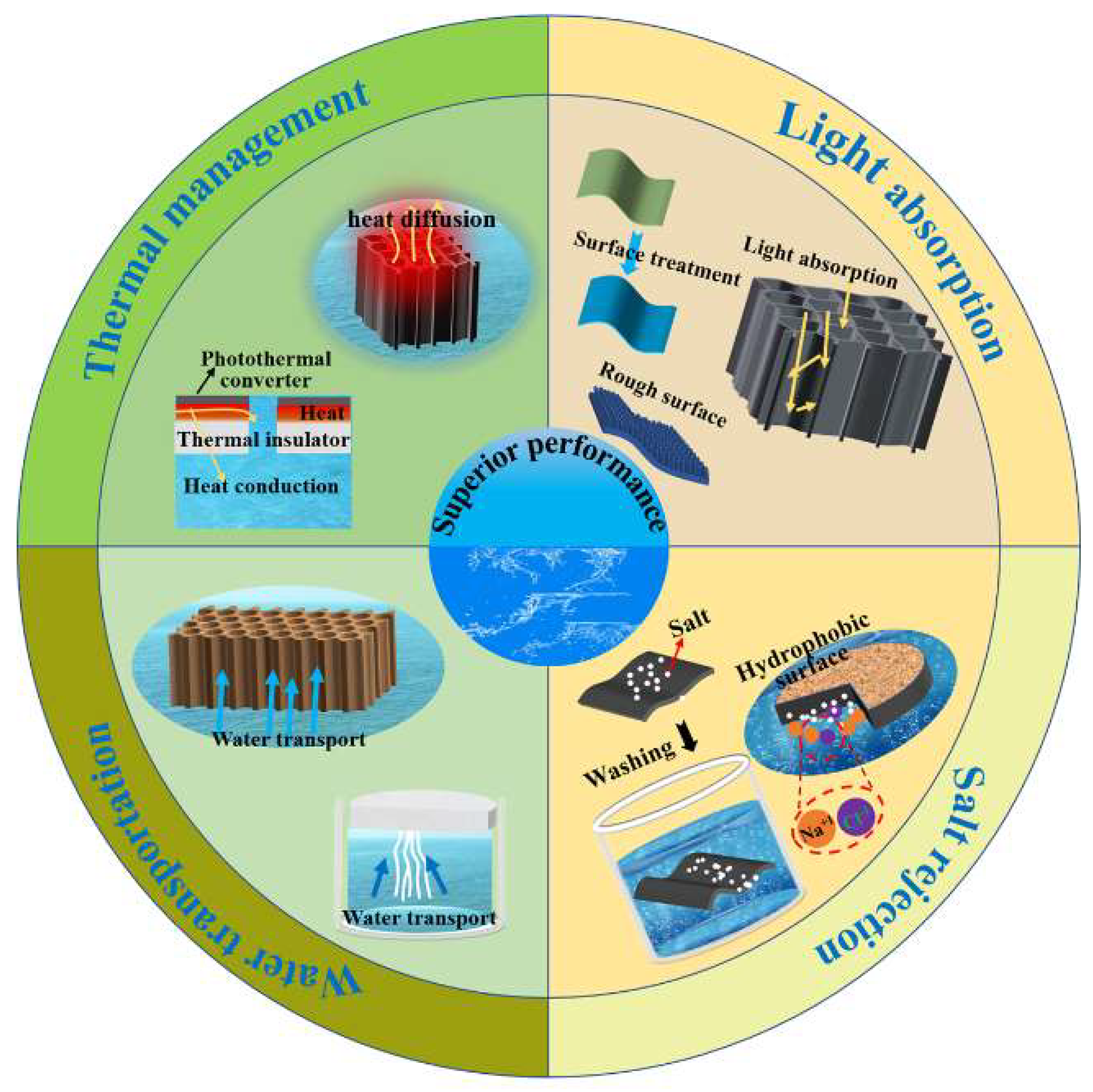
5. Methods to Improve the Efficiency of Interfacial Photothermal Conversion
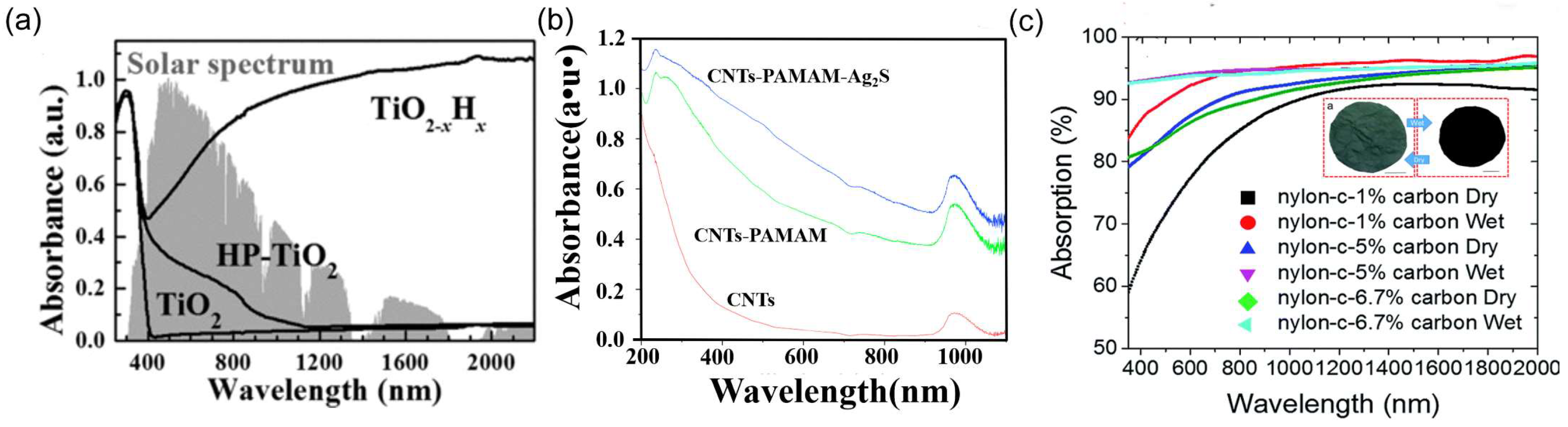
5.1. Increase Light Absorption
5.1.1. Material Selection
5.1.2. Solar Absorber Structure Design
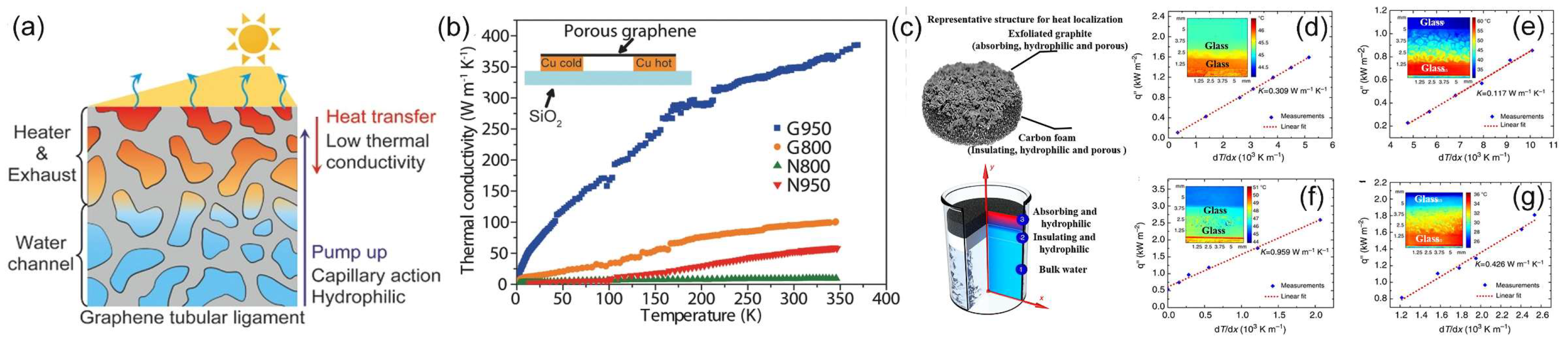
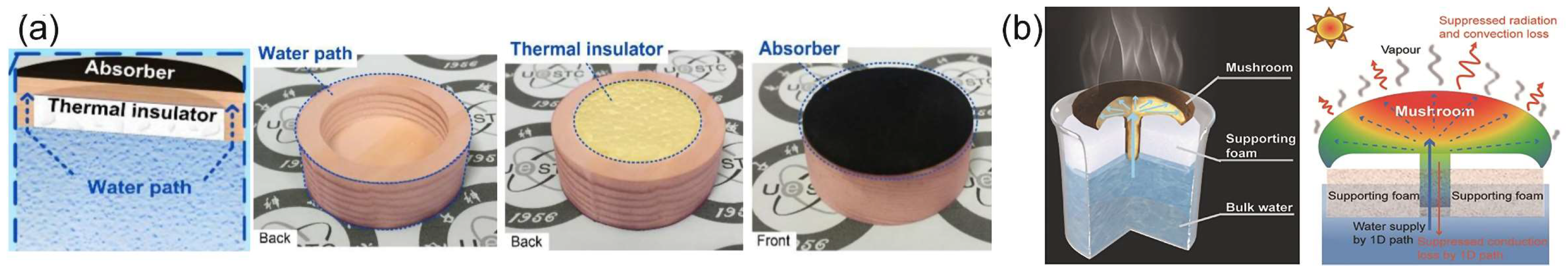
5.2. Thermal Management
5.3. Efficient Water Transportation
5.4. Improve Salt Rejection
6. Summary and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- He, F.; Wu, X.; Gao, J.; Wang, Z. Solar-driven interfacial evaporation toward clean water production: Burgeoning materials, concepts and technologies. J. Mater. Chem. A 2021, 9, 27121–27139. [Google Scholar] [CrossRef]
- Li, Z.; Wei, S.; Ge, Y.; Zhang, Z.; Li, Z. Biomass-based materials for solar-powered seawater evaporation. Sci. Total Environ. 2023, 858, 160003. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Sheng, X.; Lin, P.; Tang, J.; Pan, L.; Kaneti, Y.V.; Yang, T.; Yamauchi, Y. Solar-powered sustainable water production: State-of-the-art technologies for sunlight–energy–water nexus. ACS Nano 2021, 15, 12535–12566. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Gu, X.; Hang, G.; Yin, L.; Wang, S.; Dai, Y.; Feng, C. Photothermal conversion materials and their applications in the field of desalination. Prog. Chem. 2019, 31, 580–596. [Google Scholar]
- Ghafurian, M.M.; Niazmand, H.; Ebrahimnia-Bajestan, E.; Elhami Nik, H. Localized solar heating via graphene oxide nanofluid for direct steam generation. J. Therm. Anal. Calorim. 2018, 135, 1443–1449. [Google Scholar] [CrossRef]
- Yu, F.; Chen, Y.; Liang, X.; Xu, J.; Lee, C.; Liang, Q.; Tao, P.; Deng, T. Dispersion stability of thermal nanofluids. Prog. Nat. Sci. Mater. Int. 2017, 27, 531–542. [Google Scholar] [CrossRef]
- Zhang, Q.; Yi, G.; Fu, Z.; Yu, H.; Chen, S.; Quan, X. Vertically Aligned Janus MXene-Based Aerogels for Solar Desalination with High Efficiency and Salt Resistance. ACS Nano 2019, 13, 13196–13207. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Y.; Lu, X.; Wang, S.; Chen, M.; Huang, W.; Wu, Z.; Lu, G.; Nie, L. Ag-Hybridized plasmonic Au-triangular nanoplates: Highly sensitive photoacoustic/Raman evaluation and improved antibacterial/photothermal combination therapy. J. Mater. Chem. B 2018, 6, 2813–2820. [Google Scholar] [CrossRef]
- Chang, Y.C.; Zeng, C.J.; Chen, C.Y.; Tsay, C.Y.; Lee, G.J.; Wu, J.J. NiS/Pt loaded on electrospun TiO2 nanofiber with enhanced visible-light-driven photocatalytic hydrogen production. Mater. Res. Bull. 2023, 157, 112041. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Xiang, B.; Zeng, X.; Luo, Y.; Meng, X.; Tang, S. Scalable carbon black enhanced nanofiber network films for high-efficiency solar steam generation. Adv. Mater. Interfaces 2021, 8, 2101160. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.; Wang, F.; Su, Y.; Zhou, X.; Zhu, Z.; Sun, H.; Liang, W.; Li, A. Potentially scalable fabrication of salt-rejection evaporator based on electrogenerated polypyrrole-coated nickel foam for efficient solar steam generation. Desalination 2021, 505, 114982. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Dao, V.D.; Vu, N.H.; Yun, S. Recent advances and challenges for solar-driven water evaporation system toward applications. Nano Energy 2020, 68, 104324. [Google Scholar] [CrossRef]
- Liu, X.; Mishra, D.D.; Wang, X.; Peng, H.; Hu, C. Towards highly efficient solar-driven interfacial evaporation for desalination. J. Mater. Chem. A 2020, 8, 17907–17937. [Google Scholar] [CrossRef]
- Sun, W.; Zhong, G.; Kübel, C.; Ali, F.M.; Qian, C.; Wang, L.; Ebrahimi, M.; Reyes, L.M.; Helmy, A.S.; Ozin, G.A. Size-tunable photothermal germanium nanocrystals. Angew. Chem. Int. Ed. 2017, 56, 6329–6334. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, Z.; Xiang, W.; Shen, C.; Wang, Z.; Jia, X.; Sun, J.; Liu, C.J. Plasmonic wooden flower for highly efficient solar vapor generation. Nano Energy 2020, 76, 104998. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Wang, J.; Xu, W.; Yuan, Y.; Cai, W.; Zhu, S.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photonics 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Bae, K.; Kang, G.; Cho, S.K.; Park, W.; Kim, K.; Padilla, W.J. Flexible thin-film black gold membranes with ultrabroadband plasmonic nanofocusing for efficient solar vapour generation. Nat. Commun. 2015, 6, 10103. [Google Scholar] [CrossRef]
- Xu, J.; Xu, F.; Qian, M.; Li, Z.; Sun, P.; Hong, Z.; Huang, F. Copper nanodot-embedded graphene urchins of nearly full-spectrum solar absorption and extraordinary solar desalination. Nano Energy 2018, 53, 425–431. [Google Scholar] [CrossRef]
- Raza, A.; Lu, J.Y.; Alzaim, S.; Li, H.; Zhang, T.J. Novel receiver-enhanced solar vapor generation: Review and perspectives. Energies 2018, 11, 253. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Song, L.; Xiong, Y. Coupling solar energy into reactions: Materials design for surface plasmon-mediated catalysis. Small 2015, 11, 3873–3889. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.; Bardhan, R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale 2014, 6, 2502–2530. [Google Scholar] [CrossRef]
- Aberasturi, D.J.; Serrano-Montes, A.B.; Liz-Marzán, L.M. Modern applications of plasmonic nanoparticles: From energy to health. Adv. Opt. Mater. 2015, 3, 602–617. [Google Scholar] [CrossRef]
- Ibrahim, I.; Seo, D.H.; Park, M.J.; Angeloski, A.; McDonagh, A.; Bendavid, A.; Shon, H.K.; Tijing, L. Highly stable gold nanolayer membrane for efficient solar water evaporation under a harsh environment. Chemosphere 2022, 299, 134394. [Google Scholar] [CrossRef] [PubMed]
- Goharshadi, K.; Sajjadi, S.A.; Goharshadi, E.K.; Mehrkhah, R. Highly efficient plasmonic wood/Ag/Pd photoabsorber in interfacial solar steam generation. Mater. Res. Bull. 2022, 154, 111916. [Google Scholar] [CrossRef]
- Xiao, J.K.; Gong, J.Z.; Dai, M.; Zhang, Y.F.; Wang, S.G.; Lin, Z.D.; Du, F.P.; Fu, P. Reduced graphene oxide/Ag nanoparticle aerogel for efficient solar water evaporation. J. Alloys Compd. 2023, 930, 167404. [Google Scholar] [CrossRef]
- Xiao, C.; Liang, W.; Hasi, Q.M.; Chen, L.; He, J.; Liu, F.; Wang, C.; Sun, H.; Zhu, Z.; Li, A. Ag/polypyrrole co-modified poly (ionic liquid) s hydrogels as efficient solar generators for desalination. Mater. Today Energy 2020, 16, 100417. [Google Scholar] [CrossRef]
- Ibrahim, I.; Seo, D.H.; McDonagh, A.M.; Shon, H.K.; Tijing, L. Semiconductor photothermal materials enabling efficient solar steam generation toward desalination and wastewater treatment. Desalination 2021, 500, 114853. [Google Scholar] [CrossRef]
- Sha, C.; Chen, Y.; Wang, X.; Wang, W. Research Progress of Carbon—Based Solar Absorbers for Solar Driven Water Evaporation Equipment. J. Petrochem. Univ. 2019, 32, 1. [Google Scholar]
- Chen, L.; Wang, H.; Kuravi, S.; Kota, K.; Park, Y.H.; Xu, P. Low-cost and reusable carbon black based solar evaporator for effective water desalination. Desalination 2020, 483, 114412. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Cheng, G.; Shi, L.; Liu, X.; Zhu, J. Direct vapor generation through localized solar heating via carbon-nanotube nanofluid. Energy Convers. Manag. 2016, 130, 176–183. [Google Scholar] [CrossRef]
- Hong, Z.; Pei, J.; Wang, Y.; Cao, B.; Mao, M.; Liu, H.; Jiang, H.; An, Q.; Liu, X.; Hu, X. Characteristics of the direct absorption solar collectors based on reduced graphene oxide nanofluids in solar steam evaporation. Energy Convers. Manag. 2019, 199, 112019. [Google Scholar] [CrossRef]
- Dao, V.D.; Choi, H.S. Carbon-based sunlight absorbers in solar-driven steam generation devices. Glob. Chall. 2018, 2, 1700094. [Google Scholar] [CrossRef]
- Jin, Y.; Chang, J.; Shi, Y.; Shi, L.; Hong, S.; Wang, P. A highly flexible and washable nonwoven photothermal cloth for efficient and practical solar steam generation. J. Mater. Chem. A 2018, 6, 7942–7949. [Google Scholar] [CrossRef]
- Ou, K.; Li, J.; Hou, Y.; Qi, K.; Dai, Y.; Wang, M.; Wang, B. Hierarchical nanofibrous and recyclable membrane with unidirectional water-transport effect for efficient solar-driven interfacial evaporation. J. Colloid Interface Sci. 2024, 656, 474–484. [Google Scholar] [CrossRef]
- Xue, G.; Liu, K.; Chen, Q.; Yang, P.; Li, J.; Ding, T.; Duan, J.; Qi, B.; Zhou, J. Robust and low-cost flame-treated wood for high-performance solar steam generation. ACS Appl. Mater. Interfaces 2017, 9, 15052–15057. [Google Scholar] [CrossRef]
- Liu, K.K.; Jiang, Q.; Tadepalli, S.; Raliya, R.; Biswas, P.; Naik, R.R.; Singamaneni, S. Wood–graphene oxide composite for highly efficient solar steam generation and desalination. ACS Appl. Mater. Interfaces 2017, 9, 7675–7681. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Tang, M.; Zhou, L.; Zhu, B.; Zhu, S.; Zhu, J. Graphene oxide-based efficient and scalable solar desalination under one sun with a confined 2D water path. Proc. Natl. Acad. Sci. USA 2016, 113, 13953–13958. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Tang, B.; Wang, P. Solar-thermal conversion and thermal energy storage of graphene foam-based composites. Nanoscale 2016, 8, 14600–14607. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Yang, Z.; Chen, C.; Kuang, Y.; Song, J.; Jia, C.; Hitz, E.M.; Yang, B.; Hu, L. Graphene oxide-based evaporator with one-dimensional water transport enabling high-efficiency solar desalination. Nano Energy 2017, 41, 201–209. [Google Scholar] [CrossRef]
- Wang, X.; Lei, Z.; Ma, X.; He, G.; Xu, T.; Tan, J.; Wang, L.; Zhang, X.; Qu, L.; Zhang, X. A lightweight MXene-coated nonwoven fabric with excellent flame retardancy, EMI shielding, and electrothermal/photothermal conversion for wearable heater. Chem. Eng. J. 2022, 430, 132605. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Wu, S.; Xu, B.; Xu, H. Multilayer polypyrrole nanosheets with self-organized surface structures for flexible and efficient solar–thermal energy conversion. Adv. Mater. 2019, 31, 1807716. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Cao, M.; Wang, C.; Guo, P. Highly efficient solar steam generation of polyamide 6 membrane modified with graphene oxide and Au nanoparticles. J. Mater. Res. 2022, 37, 1475–1485. [Google Scholar] [CrossRef]
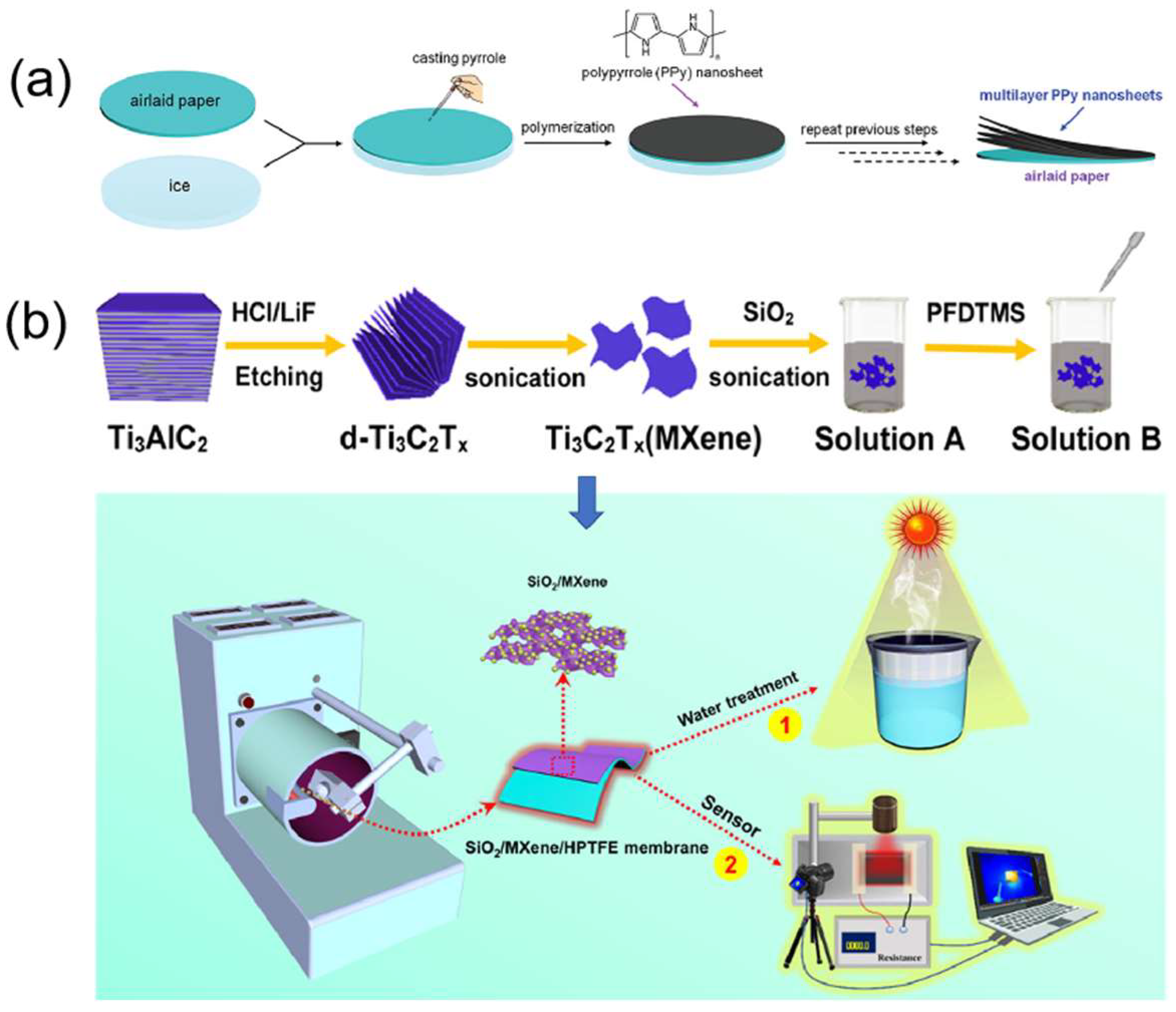
- Li, H.; Li, L.; Xiong, L.; Wang, B.; Wang, G.; Ma, S.; Han, X. SiO2/MXene/Poly (tetrafluoroethylene)-Based Janus membranes as solar absorbers for solar steam generation. ACS Appl. Nano Mater. 2021, 4, 14274–14284. [Google Scholar] [CrossRef]
- Wan, P.; Gu, X.; Ouyang, X.; Shi, S.; Deng, B.; Liu, J.; Chu, P.K.; Yu, X.F. A versatile solar-powered vapor generating membrane for multi-media purification. Sep. Purif. Technol. 2021, 260, 117952. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, L.; Dou, T.; Zou, J.; Zhang, Y.; Sun, L. Willow catkins-derived porous carbon membrane with hydrophilic property for efficient solar steam generation. ACS Omega 2020, 5, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
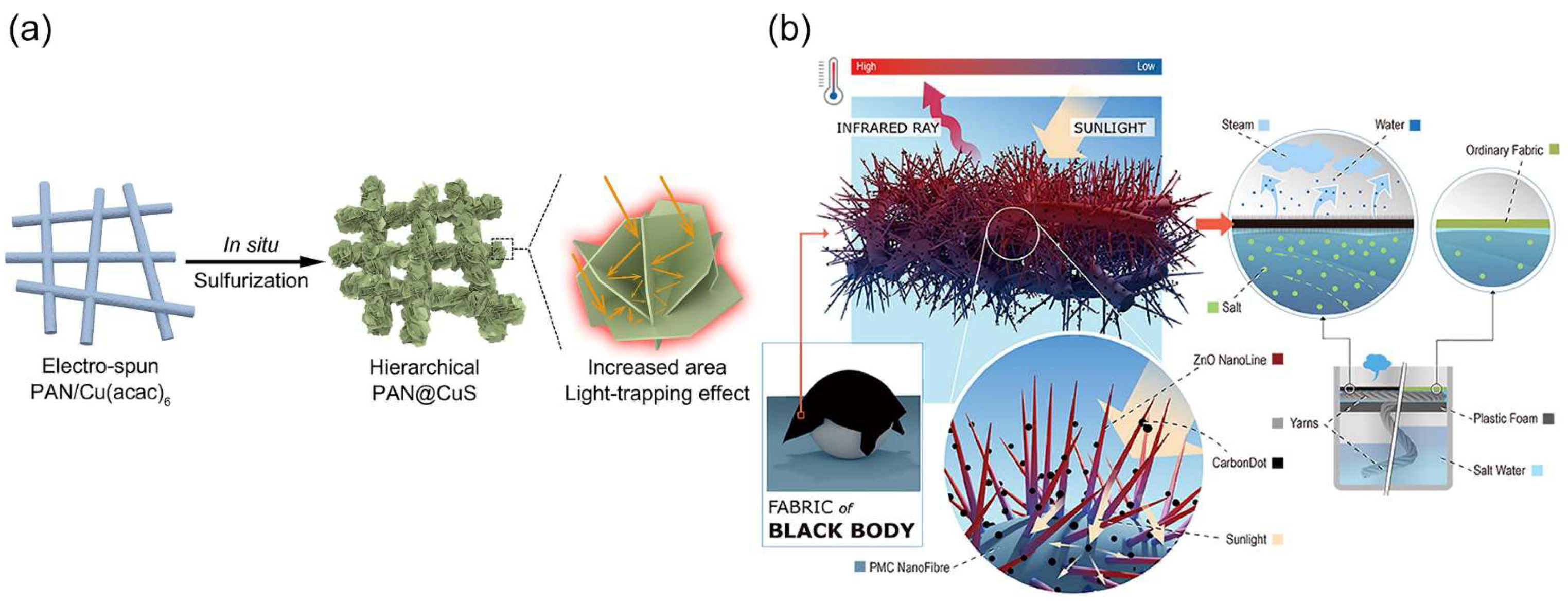
- Liu, Z.; Zhou, Z.; Wu, N.; Zhang, R.; Zhu, B.; Jin, H.; Zhang, Y.; Zhu, M.; Chen, Z. Hierarchical photothermal fabrics with low evaporation enthalpy as heliotropic evaporators for efficient, continuous, salt-free desalination. ACS Nano 2021, 15, 13007–13018. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, J.; Wang, R.; Li, Y.; Liu, C.; Liu, Y. Multi-angle wide-spectrum light-trapping nanofiber membrane for highly efficient solar desalination. Appl. Energy 2022, 328, 120203. [Google Scholar] [CrossRef]
- Guo, D.; Yang, X. Highly efficient solar steam generation of low cost TiN/bio-carbon foam. Sci. China Mater. 2018, 62, 711–718. [Google Scholar] [CrossRef]
- Zhu, M.; Li, Y.; Chen, G.; Jiang, F.; Yang, Z.; Luo, X.; Wang, Y.; Lacey, S.D.; Dai, J.; Wang, C.; et al. Tree-inspired design for high-efficiency water extraction. Adv. Mater. 2017, 29, 1704107. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hong, K.; Sun, X.; Natan, A.; Luan, P.; Yang, Y.; Zhu, H. An ‘antifouling’ porous loofah sponge with internal microchannels as solar absorbers and water pumpers for thermal desalination. J. Mater. Chem. A 2020, 8, 12323–12333. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.; Xu, W.; Li, X.; Zhou, L.; Zhu, S.; Zhu, J. Mushrooms as efficient solar steam-generation devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qu, J.; Yin, G.; Zhang, T.; Zhao, H.Y.; Jiao, F.Z.; Liu, J.; Li, X.; Yu, Z.Z. Omnidirectionally irradiated three-dimensional molybdenum disulfide decorated hydrothermal pinecone evaporator for solar-thermal evaporation and photocatalytic degradation of wastewaters. J. Colloid Interface Sci. 2023, 637, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, W.; Zada, I.; Zhang, Y.; Gu, J.; Liu, Q.; Su, H.; Pantelic, D.; Jelenkovic, B.; Zhang, D. 3D-structured carbonized sunflower heads for improved energy efficiency in solar steam generation. ACS Appl. Mater. Interfaces 2019, 12, 2171–2179. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Qiu, Y.; Feng, H. High-efficiency wood-based evaporators for solar-driven interfacial evaporation. Sol. Energy 2022, 244, 322–330. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Jia, X.; Li, Y.; Wang, S.; Song, H. Wood-based solar interface evaporation device with self-desalting and high antibacterial activity for efficient solar steam generation. ACS Appl. Mater. Interfaces 2020, 12, 47029–47037. [Google Scholar] [CrossRef]
- Chen, T.; Wu, Z.; Liu, Z.; Aladejana, J.T.; Wang, X.; Niu, M.; Wei, Q.; Xie, Y. Hierarchical porous aluminophosphate-treated wood for high-efficiency solar steam generation. ACS Appl. Mater. Interfaces 2020, 12, 19511–19518. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Zhao, X.; Chen, G.; Dai, J.; Pastel, G.; Jia, C.; Chen, C.; Hitz, E.; Siddhartha, D.; et al. Scalable and highly efficient mesoporous wood-based solar steam generation device: Localized heat, rapid water transport. Adv. Funct. Mater. 2018, 28, 1707134. [Google Scholar] [CrossRef]
- Mu, X.; Chen, L.; Qu, N.; Yu, J.; Jiang, X.; Xiao, C.; Luo, X.; Hasi, Q. MXene/polypyrrole coated melamine-foam for efficient interfacial evaporation and photodegradation. J. Colloid Interface Sci. 2023, 636, 291–304. [Google Scholar] [CrossRef]
- Sun, A.; Hou, X.; Hu, X. Super-performance photothermal conversion of 3D macrostructure graphene-CuFeSe2 aerogel contributes to durable and fast clean-up of highly viscous crude oil in seawater. Nano Energy 2020, 70, 104511. [Google Scholar] [CrossRef]
- Hu, X.; Xu, W.; Zhou, L.; Tan, Y.; Wang, Y.; Zhu, S.; Zhu, J. Tailoring Graphene Oxide-Based Aerogels for Efficient Solar Steam Generation under One Sun. Adv. Mater. 2016, 29, 1604031. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Derami, H.G.; Ghim, D.; Cao, S.; Jun, Y.S.; Singamaneni, S. Polydopamine-filled bacterial nanocellulose as a biodegradable interfacial photothermal evaporator for highly efficient solar steam generation. J. Mater. Chem. A 2017, 5, 18397–18402. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Xiong, J.; Li, A.; Wang, R.; Wang, L.; Qin, X.; Yu, J. Bioinspired design of electrospun nanofiber based aerogel for efficient and cost-effective solar vapor generation. Chem. Eng. J. 2022, 427, 131539. [Google Scholar] [CrossRef]
- Ma, X.; Tian, N.; Wang, G.; Wang, W.; Miao, J.; Fan, T. Biomimetic vertically aligned aerogel with synergistic photothermal effect enables efficient solar-driven desalination. Desalination 2023, 550, 116397. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhu, Y.J.; Chen, Y.Q.; Yu, H.P.; Xiong, Z.C. Bioinspired Aerogel with Vertically Ordered Channels and Low Water Evaporation Enthalpy for High-Efficiency Salt-Rejecting Solar Seawater Desalination and Wastewater Purification. Small 2023, 19, 2206917. [Google Scholar] [CrossRef]
- Liu, Z.; Qing, R.K.; Xie, A.Q.; Liu, H.; Zhu, L.; Chen, S. Self-contained Janus aerogel with antifouling and salt-rejecting properties for stable solar evaporation. ACS Appl. Mater. Interfaces 2021, 13, 18829–18837. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, F.; Liu, X.Y.; Yu, J.; Liu, Y.T.; Ding, B. Multiscale synergetic bandgap/structure engineering in semiconductor nanofibrous aerogels for enhanced solar evaporation. Nano Lett. 2023, 23, 11907–11915. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Khan, S.; Chen, L.; Suzuki, N.; Terashima, C.; Liu, K.; Fujishima, A.; Liu, M. Hierarchical structures hydrogel evaporator and superhydrophilic water collect device for efficient solar steam evaporation. Nano Res. 2021, 14, 1135–1140. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, X.; Huang, Q.; Zhang, H.; Liu, C.; Liu, Y. Simple preparation of external-shape and internal-channel size adjustable porous hydrogels by fermentation for efficient solar interfacial evaporation. Sol. Energy 2020, 208, 778–786. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Gao, J.; Yao, J.; Zhang, Q. Recent progress in metal-organic frameworks-based hydrogels and aerogels and their applications. Coord. Chem. Rev. 2019, 398, 213016. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Y.; Guo, Q.; Cai, X.; Xiao, J.; Ding, Z.; Yang, J. Macroporous double-network hydrogel for high-efficiency solar steam generation under 1 sun illumination. ACS Appl. Mater. Interfaces 2018, 10, 10998–11007. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cai, X.; Jing, G.; Huang, R.; Song, G.; Wang, D.; Chen, W. Facile synthesis of hierarchical SnSe nanosheets–hydrogel evaporators for sustainable solar-powered desalination. J. Mater. Chem. A 2022, 10, 10672–10681. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Wei, P.; Hou, K.; Zhu, M. Scalable carbon black deposited fabric/hydrogel composites for affordable solar-driven water purification. J. Mater. Sci. Technol. 2022, 106, 10–18. [Google Scholar] [CrossRef]
- Li, C.; Zhu, B.; Liu, Z.; Zhao, J.; Meng, R.; Zhang, L.; Chen, Z. Polyelectrolyte-based photothermal hydrogel with low evaporation enthalpy for solar-driven salt-tolerant desalination. Chem. Eng. J. 2022, 431, 134224. [Google Scholar] [CrossRef]
- Alsharari, M.; Chen, B.; Shu, W. 3D Printing of Highly Stretchable and Sensitive Strain Sensors Using Graphene Based Composites. Proceedings 2018, 2, 792. [Google Scholar] [CrossRef]
- Li, Y.; Gao, T.; Yang, Z.; Chen, C.; Luo, W.; Song, J.; Hitz, E.; Jia, C.; Zhou, Y.; Liu, B.; et al. 3D-printed, all-in-one evaporator for high-efficiency solar steam generation under 1 sun illumination. Adv. Mater. 2017, 29, 1700981. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Nilghaz, A.; Wu, Z.; Wang, T.; Zhu, B.; Tang, G.; Su, B.; Tian, J. Large-scale production of spent coffee ground-based photothermal materials for high-efficiency solar-driven interfacial evaporation. Chem. Eng. J. 2023, 455, 140361. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Zhang, L.; Wang, P. Rational design of a bi-layered reduced graphene oxide film on polystyrene foam for solar-driven interfacial water evaporation. J. Mater. Chem. A 2017, 5, 16212–16219. [Google Scholar] [CrossRef]
- Deng, Z.; Miao, L.; Liu, P.F.; Zhou, J.; Wang, P.; Gu, Y.; Wang, X.; Cai, H.; Sun, L.; Tanemura, S. Extremely high water-production created by a nanoink-stained PVA evaporator with embossment structure. Nano Energy 2019, 55, 368–376. [Google Scholar] [CrossRef]
- Guo, C.L.; Miao, E.D.; Zhao, J.X.; Liang, L.; Liu, Q. Paper based integrated evaporation device for efficient solar steam generation through localized heating. Solar Energy 2019, 188, 1283–1291. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Okolie, M.C.; Williams, F.K.; Oki, A. Ag2S nanocrystallites deposited over polyamidoamine grafted carbon nanotubes: An efficient NIR active photothermal agent. Mater. Chem. Phys. 2019, 234, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jia, X.; Ni, Y.; Pan, B.; Long, Y.; Miao, D.; Yan, X. A 3D solar-driven evaporator based on electrospun recycled PET film for efficient seawater desalination. J. Clean. Prod. 2023, 408, 137113. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, H.; Zhao, F.; Zhou, X.; Shi, W.; Yu, G. Biomass-derived hybrid hydrogel evaporators for cost-effective solar water purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef]
- Hong, S.; Shi, Y.; Li, R.; Zhang, C.Y.; Wang, P. Nature-inspired, 3D origami solar steam generator toward near full utilization of solar energy. ACS Appl. Mater. Interfaces 2018, 10, 28517–28524. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, H.; Yin, Z.; Zhao, J.; Yin, X.; Li, N.; Que, W. A general salt-resistant hydrophilic/hydrophobic nanoporous double layer design for efficient and stable solar water evaporation distillation. Mater. Horiz. 2018, 5, 1143–1150. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, X.; Zhao, F.; Bae, J.; Rosenberger, B.; Yu, G. Synergistic energy nanoconfinement and water activation in hydrogels for efficient solar water desalination. ACS Nano 2019, 13, 7913–7919. [Google Scholar] [CrossRef]
- Ren, H.; Tang, M.; Guan, B.; Wang, K.; Yang, J.; Wang, F.; Liu, Z. Hierarchical graphene foam for efficient omnidirectional solar–thermal energy conversion. Adv. Mater. 2017, 29, 1702590. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Feng, R.; Bernard, A.; Liu, Y.; Zhang, Y.; Duan, H.; Shang, W.; Tao, P.; Song, C.; et al. A bioinspired, reusable, paper-based system for high-performance large-scale evaporation. Adv. Mater. 2015, 27, 2768–2774. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, W.; Liang, F.; Wang, C.; Song, X.; Huang, M.; Jiang, H. Wettable photothermal hollow fibers arrays for efficient solar-driven desalination under omnidirectional illumination without salt precipitation. Mater. Today Energy 2020, 16, 100391. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, B.; Hu, N.; Wang, H. Efficient 3D-interfacial solar steam generation enabled by photothermal nanodiamonds paint-coat with optimized heat management. Appl. Therm. Eng. 2020, 171, 115059. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, B.; Hu, N.; Wang, H. Low-cost and facile fabrication of a candle soot/adsorbent cotton 3D-interfacial solar steam generation for effective water evaporation. Sol. Energy Mater. Sol. Cells 2021, 221, 110876. [Google Scholar] [CrossRef]
- Wang, J.T.; Hong, J.L. Effect of folding on 3D photothermal cones with efficient solar-driven water evaporation. Appl. Therm. Eng. 2020, 178, 115636. [Google Scholar] [CrossRef]
- Ni, F.; Xiao, P.; Zhang, C.; Liang, Y.; Gu, J.; Zhang, L.; Chen, T. Micro-/macroscopically synergetic control of switchable 2D/3D photothermal water purification enabled by robust, portable, and cost-effective cellulose papers. ACS Appl. Mater. Interfaces 2019, 11, 15498–15506. [Google Scholar] [CrossRef]
- Lu, X.; Mu, C.; Liu, Y.; Wu, L.; Tong, Z.; Huang, K. Recent advances in solar-driven interfacial evaporation coupling systems: Energy conversion, water purification, and seawater resource extraction. Nano Energy 2023, 120, 109180. [Google Scholar] [CrossRef]
- Ito, Y.; Tanabe, Y.; Han, J.; Fujita, T.; Tanigaki, K.; Chen, M. Multifunctional Porous Graphene for High-Efficiency Steam Generation by Heat Localization. Adv. Mater. 2015, 27, 4302–4307. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; He, S.; Xia, G.; Wang, X.; Xiang, Q.; Wen, T.; Zhong, Z.; Liao, Y. Thermal insulation design for efficient and scalable solar water interfacial evaporation and purification. J. Mater. Sci. Technol. 2021, 66, 157–162. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, H.; Li, Y.; Kuang, Y.; Xu, X.; Chen, C.; Huang, H.; Jia, C.; Zhao, X.; Hitz, E.; et al. Lightweight, mesoporous, and highly absorptive all-nanofiber aerogel for efficient solar steam generation. ACS Appl. Mater. Interfaces 2018, 10, 1104–1112. [Google Scholar] [CrossRef]
- Qi, Q.; Wang, W.; Wang, Y.; Yu, D. Robust light-driven interfacial water evaporator by electrospinning SiO2/MWCNTs-COOH/PAN photothermal fiber membrane. Sep. Purif. Technol. 2020, 239, 116595. [Google Scholar] [CrossRef]
- Kou, H.; Liu, Z.; Zhu, B.; Macharia, D.K.; Ahmed, S.; Wu, B.; Zhu, M.; Liu, X.; Chen, Z. Recyclable CNT-coupled cotton fabrics for low-cost and efficient desalination of seawater under sunlight. Desalination 2019, 462, 29–38. [Google Scholar] [CrossRef]
- Shi, P.; Song, Y.; Zhu, F. Research progress on photothermal concentration technology of anti-salt and anti-scaling interface. J. Nanjing Univ. Nat. Sci. Ed. 2022, 58, 971–988. [Google Scholar]
- Zhang, Y.; Xiong, T.; Suresh, L.; Qu, H.; Zhang, X.; Zhang, Q.; Yang, J.; Tan, S.C. Guaranteeing complete salt rejection by channeling saline water through fluidic photothermal structure toward synergistic zero energy clean water production and in situ energy generation. ACS Energy Lett. 2020, 5, 3397–3404. [Google Scholar] [CrossRef]
- Zhang, B.; Gu, Q.; Wang, C.; Gao, Q.; Guo, J.; Wong, P.W.; Liu, C.T.; An, A.K. Self-Assembled Hydrophobic/Hydrophilic Porphyrin-Ti3C2Tx MXene Janus Membrane for Dual-Functional Enabled Photothermal Desalination. ACS Appl. Mater. Interfaces 2021, 13, 3762–3770. [Google Scholar] [CrossRef]
- Lei, Z.; Sun, X.; Zhu, S.; Dong, K.; Liu, X.; Wang, L.; Zhang, X.; Qu, L.; Zhang, X. Nature inspired MXene-decorated 3D honeycomb-fabric architectures toward efficient water desalination and salt harvesting. Nano-Micro Lett. 2022, 14, 10. [Google Scholar] [CrossRef]






| Ref | Materials | Efficiency (%) | Cost ($/m2) | Q (m2/$) | Characteristic |
|---|---|---|---|---|---|
| [67] | Carbonized chitosan aerogel | 91 | 0.79 | ≈5.71 | Low cost and mechanical properties, high efficiency |
| [51] | Carbonized wood | 57.3 | <1 | <4.048 | Low cost and efficiency |
| [84] | Carbon black, rPET | 93.91 | 1.19 | 3.81 | Low cost and high efficiency |
| [59] | Graphite wood block | 80 | <3 | 1.4607 | Low cost and efficiency |
| [85] | Fe-MOF | 90 | 14.9 | 0.302 | Complicated synthesis process, excellent photothermal performance |
| [86] | Graphene oxide, carbon nanotube, porous cellulose filter paper | ≈100 | 36.97 | 0.125 | High cost, excellent photothermal performance |
| [87] | Cu2SnSe3, Cu2ZnSnSe4 and hydrophilic filter membrane | 86.6 | 41.5 | 0.1075 | Good anti-salt deposition effect, high cost |
| [88] | Ti2O3, PVA | 90 | 293.21 | 0.0153 | High cost, excellent photothermal performance |
| [89] | Graphene, commercial Ni foams | 91.4 | 124.07 | 0.0364 | High cost, excellent photothermal performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Niu, Y.; Zhu, S.; He, H.; Yan, X. Recent Advances in Carbon-Based Interfacial Photothermal Converters for Seawater Desalination: A Review. C 2024, 10, 86. https://doi.org/10.3390/c10030086
Jia X, Niu Y, Zhu S, He H, Yan X. Recent Advances in Carbon-Based Interfacial Photothermal Converters for Seawater Desalination: A Review. C. 2024; 10(3):86. https://doi.org/10.3390/c10030086
Chicago/Turabian StyleJia, Xiaoyu, Yuke Niu, Shufang Zhu, Hongwei He, and Xu Yan. 2024. "Recent Advances in Carbon-Based Interfacial Photothermal Converters for Seawater Desalination: A Review" C 10, no. 3: 86. https://doi.org/10.3390/c10030086
APA StyleJia, X., Niu, Y., Zhu, S., He, H., & Yan, X. (2024). Recent Advances in Carbon-Based Interfacial Photothermal Converters for Seawater Desalination: A Review. C, 10(3), 86. https://doi.org/10.3390/c10030086






