Environmental Impacts and Adsorption Isotherms of Coconut Shell Activated Carbon: Effect of Acid Activation, Water, and Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AC
- The quantification of the inputs and outputs was based on lab-scale experiments.
- All the materials for the recycling process were sourced locally.
- The recycling process’s energy was derived from electricity, which was sourced from natural gas. In Qatar, where natural gas is the dominant energy source, these assumptions were accurate. However, the results may vary in regions with different energy mixes.
- The water was reused in successive processes.
- Deionized-desalinated water was used as a base case. Deionizing water, which is more energy-intensive than underground water, can be produced via distillation, ion exchange, or reverse osmosis. In this dataset, ion exchange was modeled, with cations and anions exchanged with protons and hydroxide ions. The ion exchanger was regenerated with hydrochloric acid and caustic soda.
- Energy consumption and emissions from the equipment were not considered. The functional unit was defined as 1 kg of AC produced from CSs, with comparisons to commercial AC based on equal weights. The functional unit was the basis for comparing the environmental impacts of the different production scenarios.
- The EI data for commercial AC was sourced from the LCA for Experts (GaBi) database.
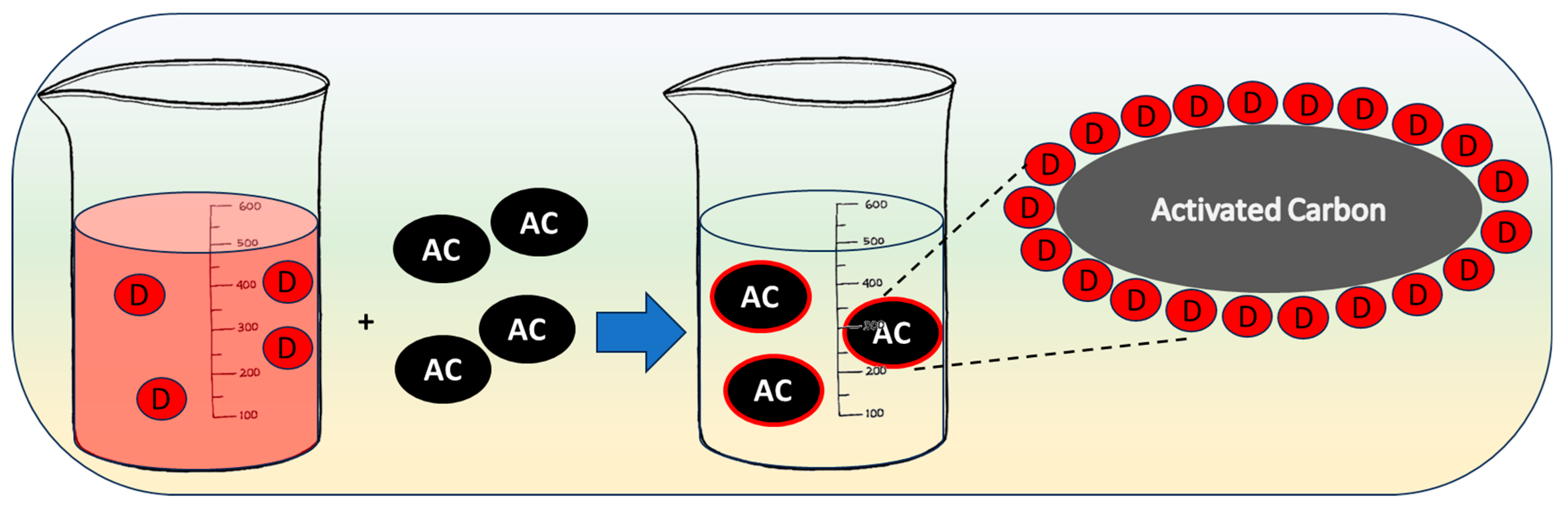
2.2. Dye Adsorption
3. Results and Discussion
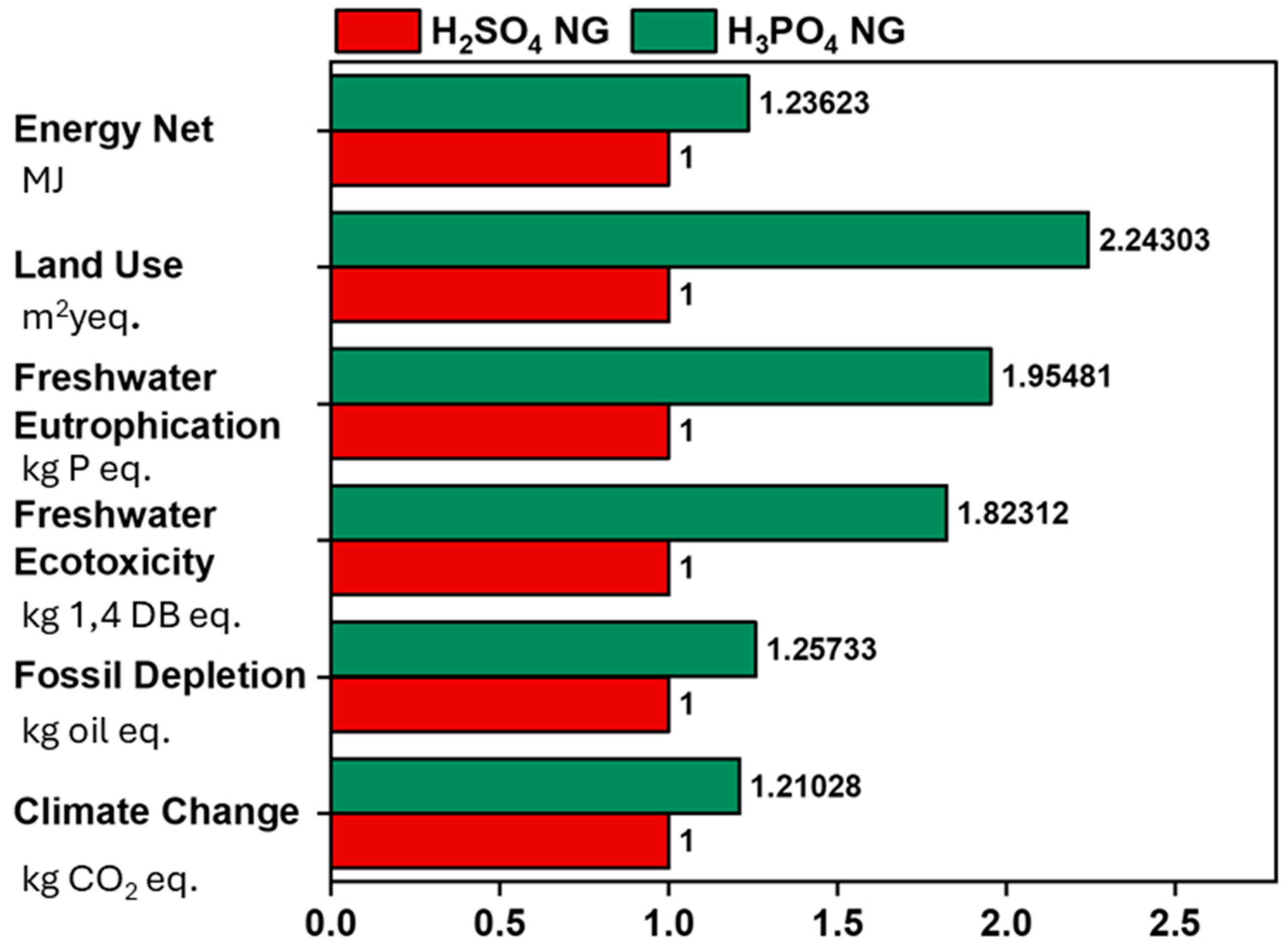
3.1. EI Assessment
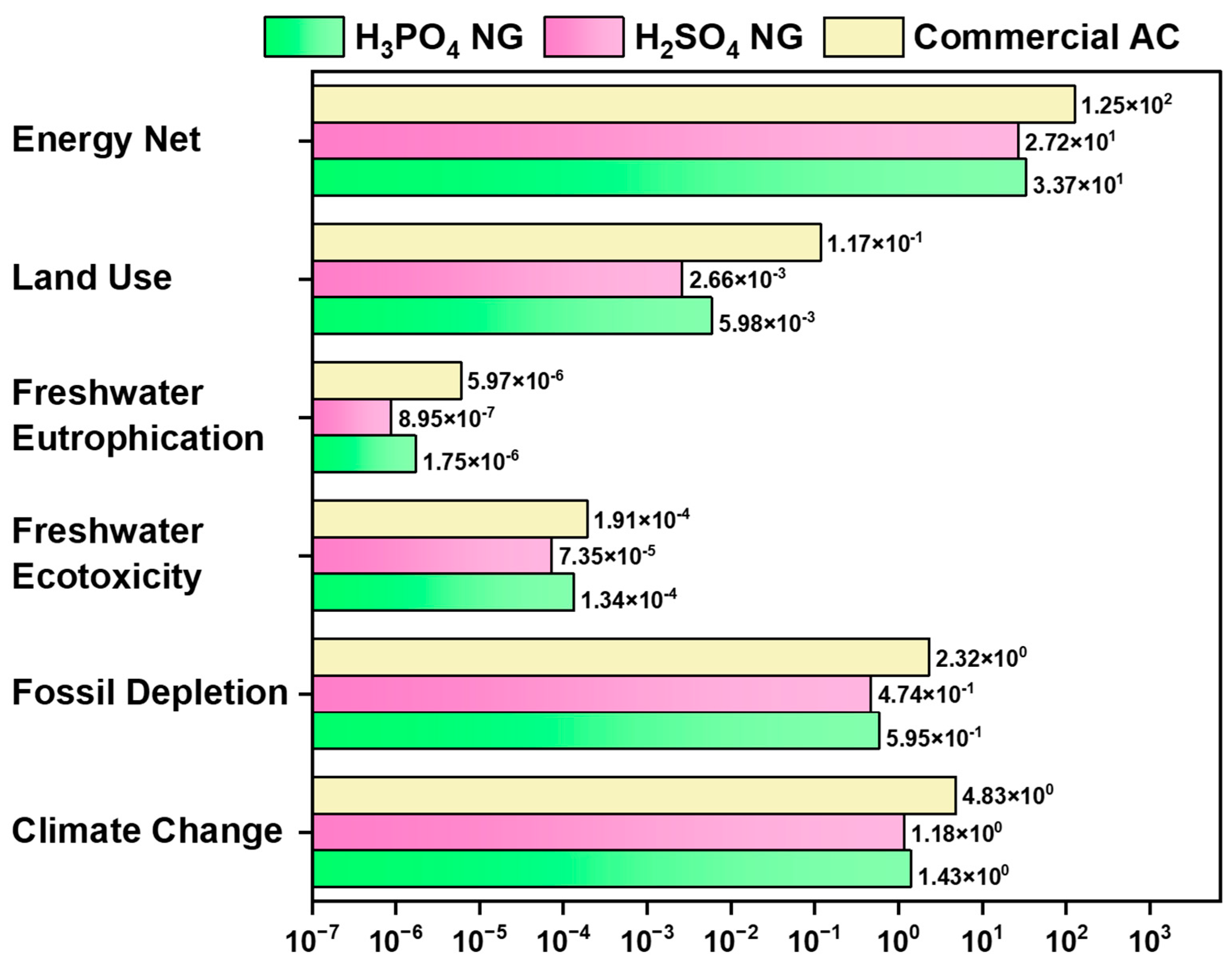
3.2. Comparison with Commercial AC
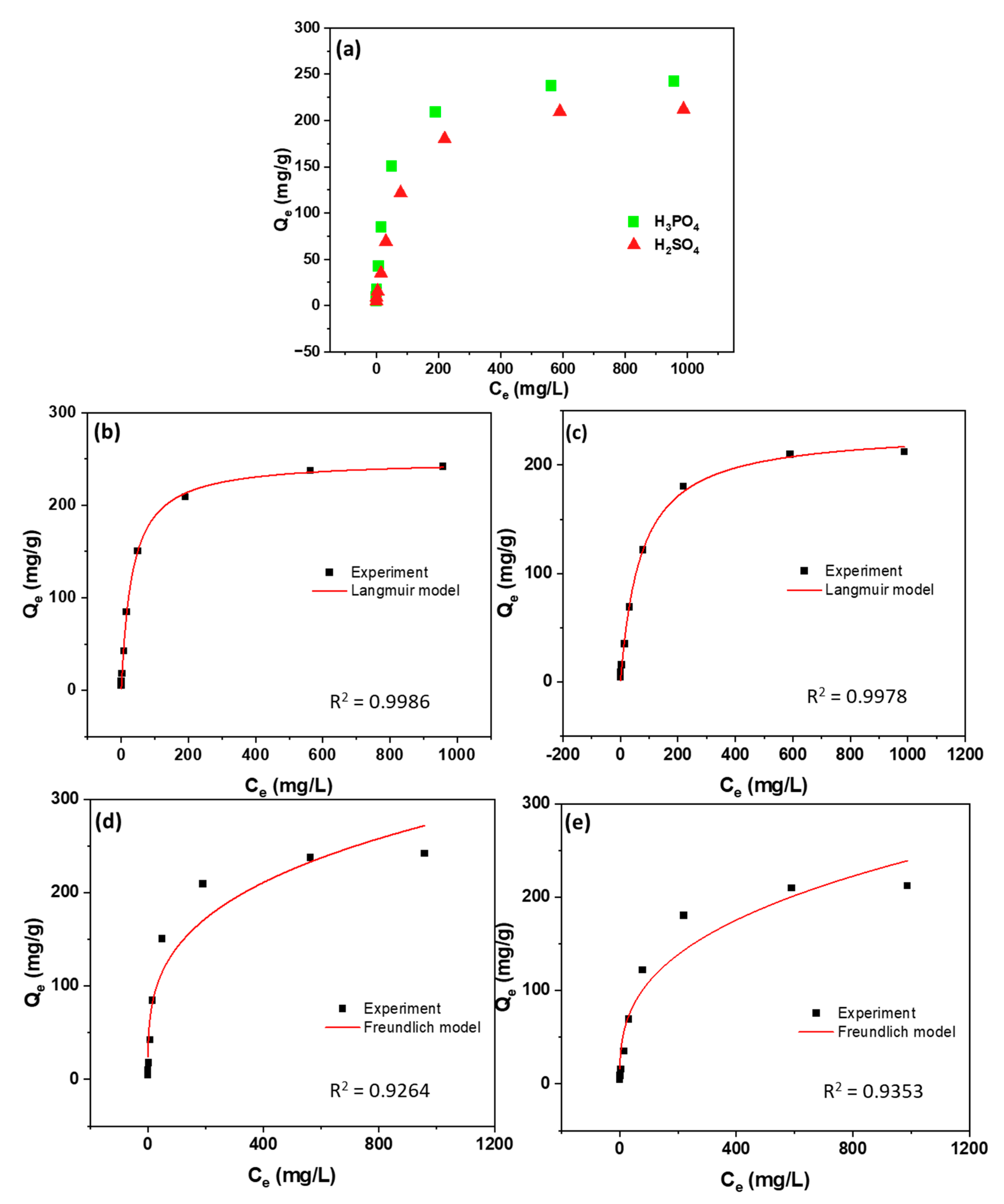
3.3. Adsorption Capacity
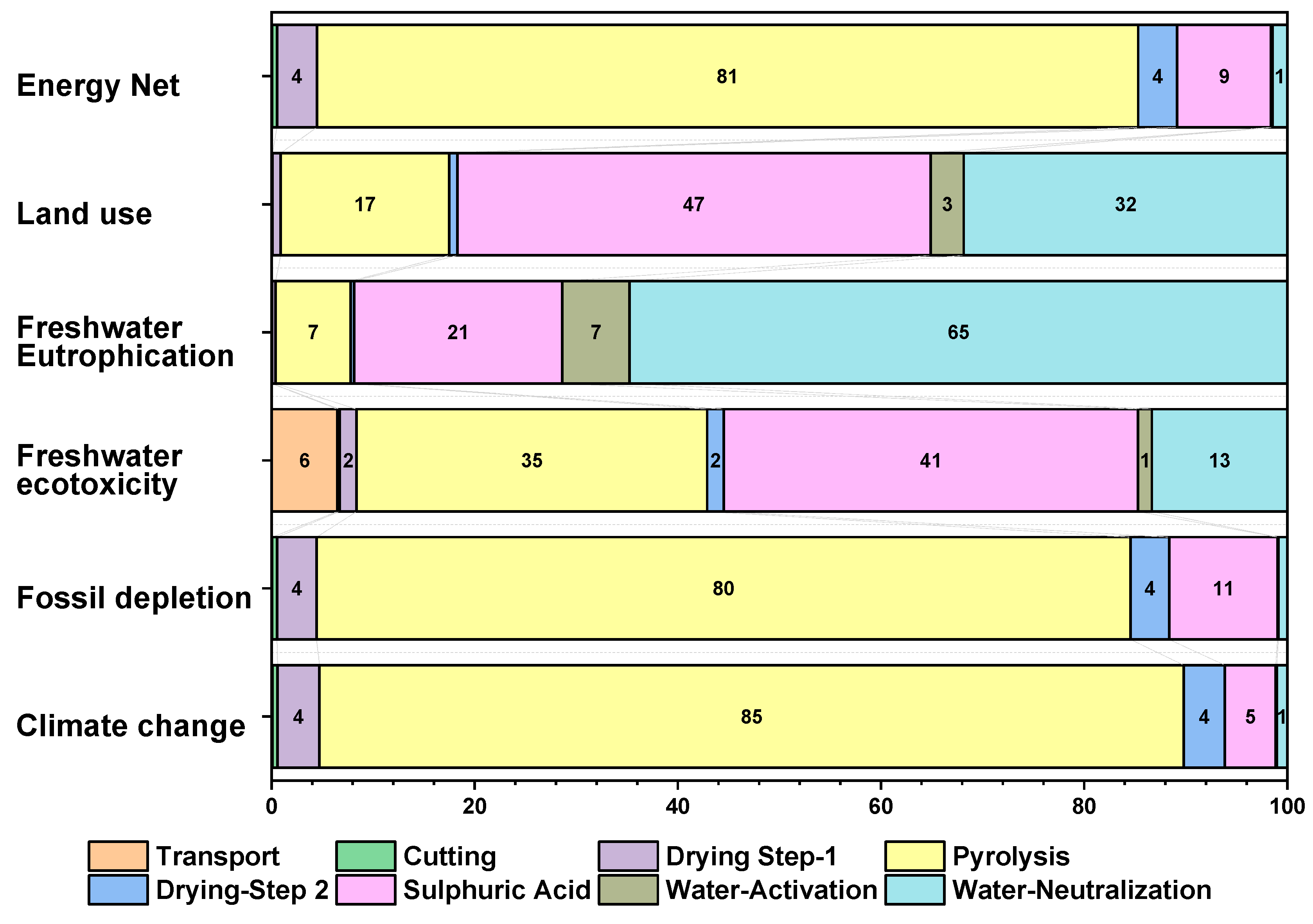
3.4. Contribution Analysis
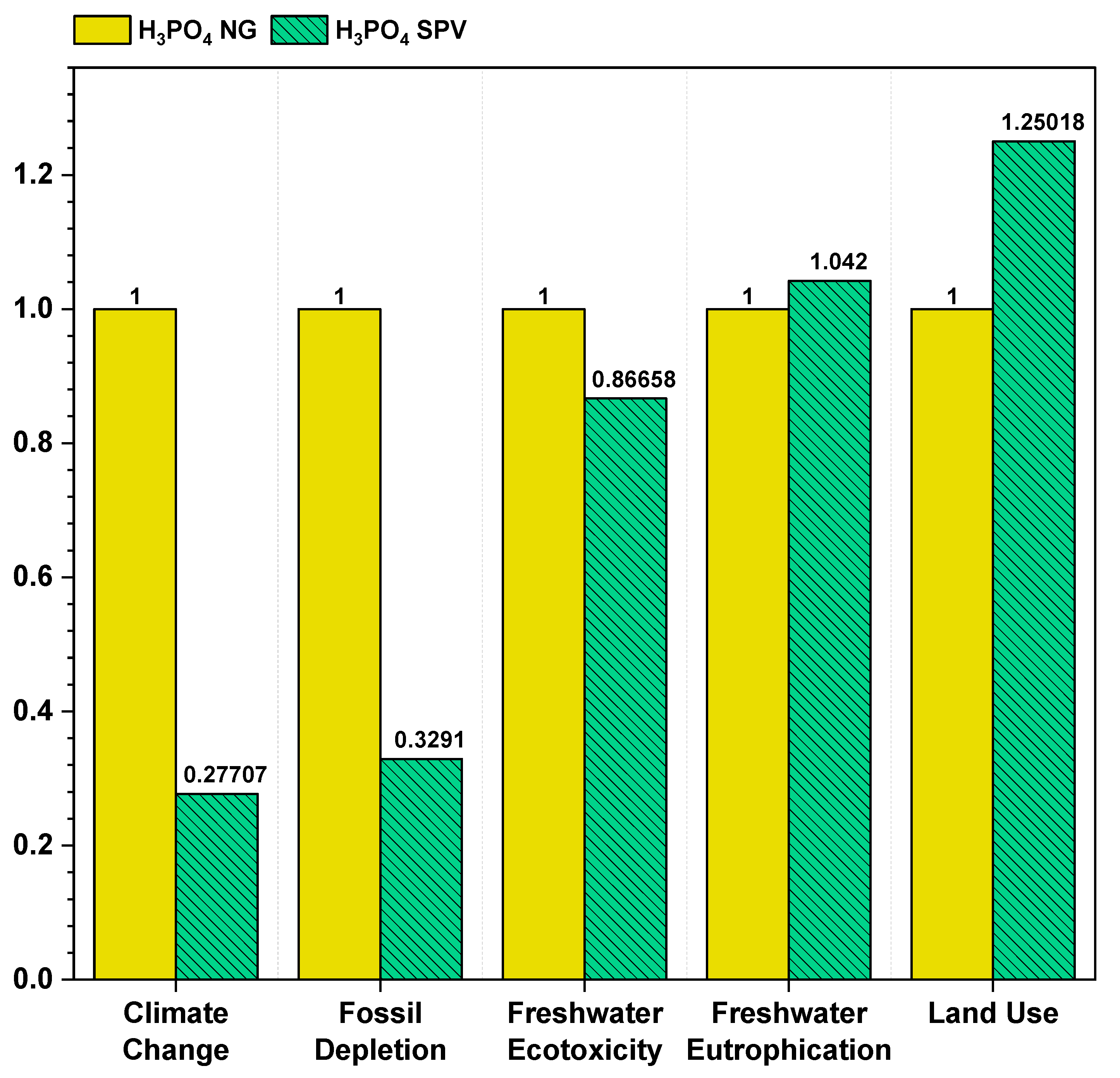
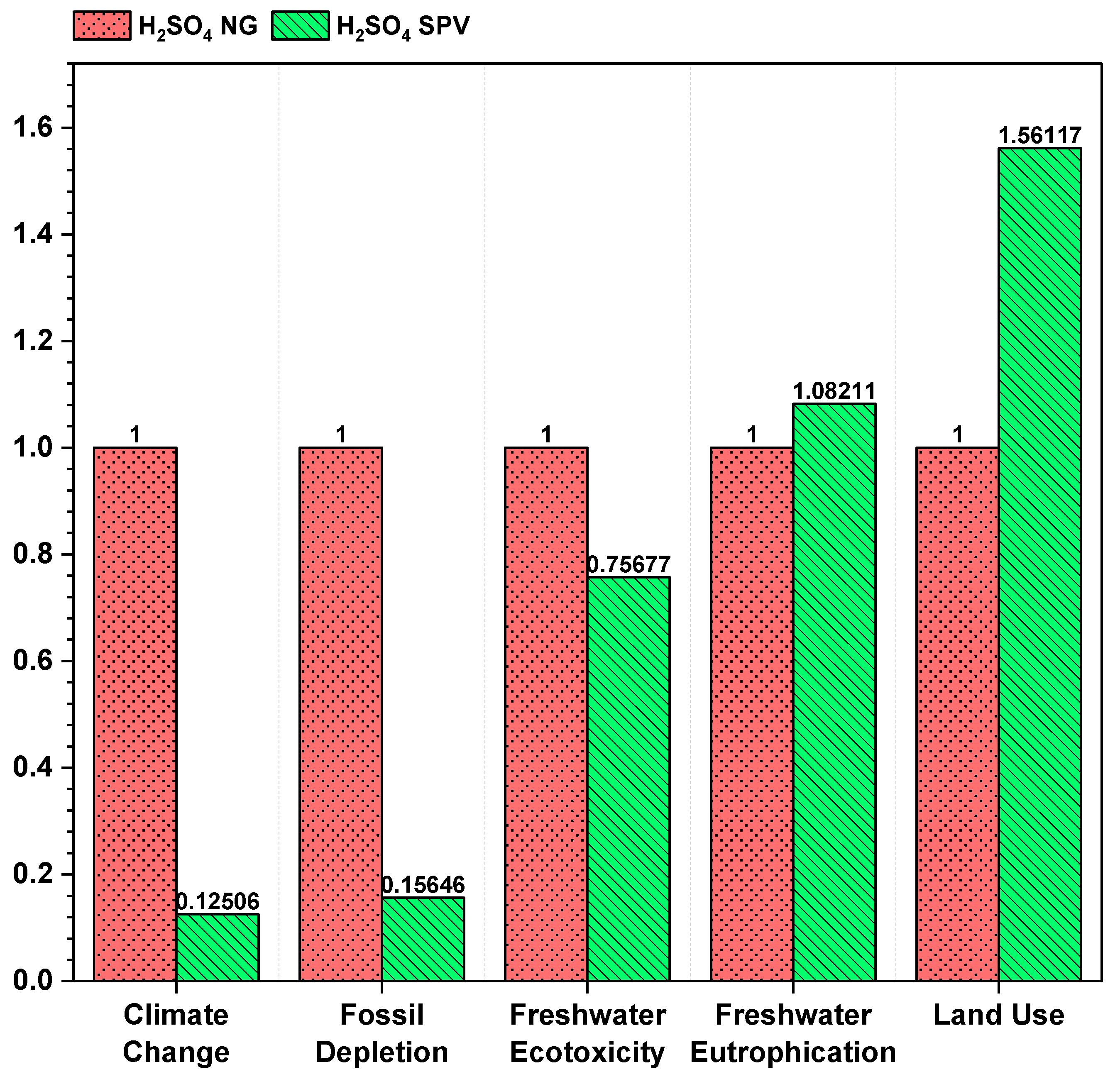
3.5. Source of Fuel
3.6. Source of Water
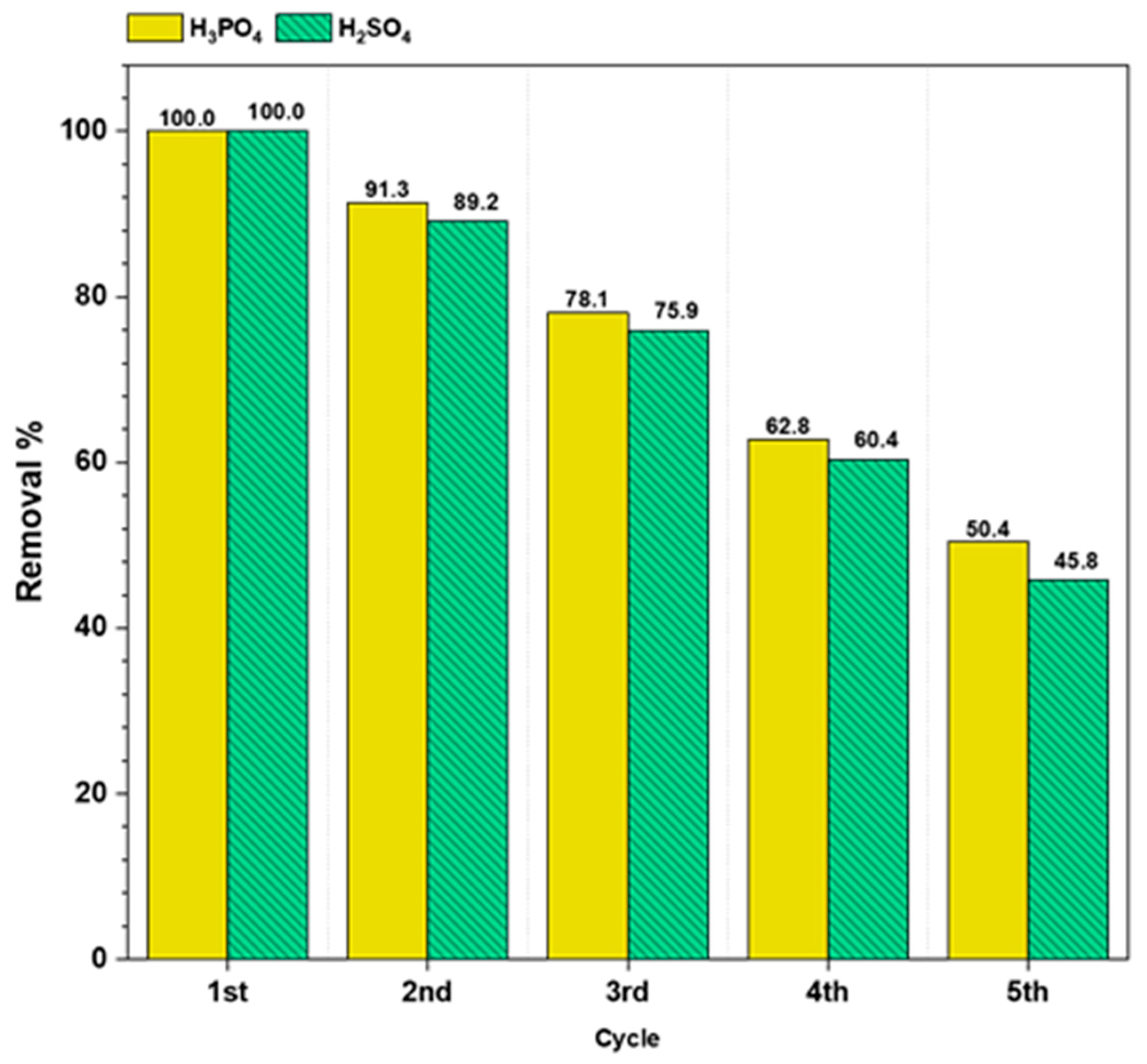
3.7. Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AC | Activated Carbon |
| AR | Alizarin Red |
| CC | Climate Change |
| CO2 | Carbon Dioxide |
| CS | Coconut Shell |
| EI | Environmental Impact |
| EN | Energy Net |
| FD | Fossil Depletion |
| FE | Freshwater Ecotoxicity |
| FU | Freshwater Eutrophication |
| H3PO4 | Phosphoric Acid |
| H2SO4 | Sulfuric Acid |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| LU | Land Use |
| NG | Natural Gas |
| SPV | Solar Photovoltaic |
References
- Limousy, L.; Ghouma, I.; Ouederni, A.; Jeguirim, M. Amoxicillin removal from aqueous solution using activated carbon prepared by chemical activation of olive stone. Environ. Sci. Pollut. Res. 2017, 24, 9993–10004. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Das, P. High-grade activated carbon from pyrolytic biochar of Jatropha and Karanja oil seed cakes—Indian biodiesel industry wastes. Biomass Convers. Biorefinery 2018, 8, 545–561. [Google Scholar] [CrossRef]
- Mckay, G. The adsorption of dyestuffs from aqueous solutions using activated carbon. iii. intraparticle diffusion processes. J. Chem. Technol. Biotechnol. Chem. Technol. 2007, 33, 196–204. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sajjad, S.; Saleem, J.; Alherbawi, M.; Mckay, G. A review of the removal of dyestuffs from effluents onto biochar. Separations 2022, 9, 139. [Google Scholar] [CrossRef]
- Munonde, T.S.; Madima, N.; Ratshiedana, R.; Nosizo Nomngongo, P.; Mofokeng, L.E.; Dima, R.S. Synergistic adsorption-photocatalytic remediation of methylene blue dye from textile industry wastewater over NiFe LDH supported on tyre-ash derived activated carbon. Appl. Surf. Sci. 2025, 679, 161205. [Google Scholar] [CrossRef]
- Saleem, J.; Tahir, F.; Zubair Khalid Baig, M.; McKay, G. Environmental impacts of activated carbon flakes from Palm fiber and polyolefin wastes using life cycle assessment. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Hu, Z.; Srinivasan, M.P.; Ni, Y. Preparation of Mesoporous High-Surface-Area Activated Carbon. Adv. Mater. 2000, 12, 62–65. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Dimbo, D.; Abewaa, M.; Adino, E.; Mengistu, A.; Takele, T.; Oro, A.; Rangaraju, M. Methylene blue adsorption from aqueous solution using activated carbon of spathodea campanulata. Results Eng. 2024, 21, 101910. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lim, M.S.W.; Hong, Z.-Y.; Chong, S.; Tiong, T.J.; Pan, G.-T.; Huang, C.-M. Coconut shell-derived activated carbon for high-performance solid-state supercapacitors. Energies 2021, 14, 4546. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Taer, E.; Taslim, R.; Putri, A.W.; Apriwandi, A.; Agustino, A. Activated Carbon Electrode Made From Coconut Husk Waste For Supercapacitor Application. Int. J. Electrochem. Sci. 2018, 13, 12072–12084. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Song, C.; Wu, S.; Cheng, M.; Tao, P.; Shao, M.; Gao, G. Adsorption Studies of Coconut Shell Carbons Prepared by KOH Activation for Removal of Lead(II) From Aqueous Solutions. Sustainability 2013, 6, 86–98. [Google Scholar] [CrossRef]
- Saleh, A.; Amhadin, F.A.; Novianty, I. Synthesis of Reduced Graphene Oxide and Zinc Oxide Composite From Candlenut Shell Charcoal (Aleuritas moluccana). Elkawnie 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Jiang, T.-J. Mesoporous activated carbon produced from coconut shell using a single-step physical activation process. Biomass Convers. Biorefinery 2018, 8, 711–718. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated coconut shell-derived hydrochar prepared via hydrothermal carbonization-NaOH activation for methylene blue adsorption. J. Environ. Manag. 2017, 203, 237–244. [Google Scholar] [CrossRef]
- Saleem, J.; Tahir, F.; Baig, M.Z.K.; Al-Ansari, T.; McKay, G. Assessing the environmental footprint of recycled plastic pellets: A life-cycle assessment perspective. Environ. Technol. Innov. 2023, 32, 103289. [Google Scholar] [CrossRef]
- Chan, A.A.; Buthiyappan, A.; Raman, A.A.A.; Ibrahim, S. Recent advances on the coconut shell derived carbonaceous material for the removal of recalcitrant pollutants: A review. Korean J. Chem. Eng. 2022, 39, 2571–2593. [Google Scholar] [CrossRef]
- Muralikrishnan, R.; Jodhi, C. Biodecolorization of Reactive Dyes Using Biochar Derived from Coconut Shell: Batch, Isotherm, Kinetic and Desorption Studies. ChemistrySelect 2020, 5, 7734–7742. [Google Scholar] [CrossRef]
- Pundlik, R.C.; Chowdhury, S.D.; Dash, R.R.; Bhunia, P. Life-Cycle Assessment of Agricultural Waste-Based and Biomass-Based Adsorbents. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 669–695. [Google Scholar]
- Vilén, A.; Laurell, P.; Vahala, R. Comparative life cycle assessment of activated carbon production from various raw materials. J. Environ. Manag. 2022, 324, 116356. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, C.C.; Muraleedharan, C.; Arun, P. Life cycle assessment of producer gas derived from coconut shell and its comparison with coal gas: An Indian perspective. Int. J. Energy Environ. Eng. 2013, 4, 8. [Google Scholar] [CrossRef]
- Arena, N.; Lee, J.; Clift, R. Life Cycle Assessment of activated carbon production from coconut shells. J. Clean. Prod. 2016, 125, 68–77. [Google Scholar] [CrossRef]
- Glogic, E.; Kamali, A.K.; Keppetipola, N.M.; Alonge, B.; Kumara, G.R.A.; Sonnemann, G.; Toupance, T.; Cojocaru, L. Life Cycle Assessment of Supercapacitor Electrodes Based on Activated Carbon from Coconut Shells. ACS Sustain. Chem. Eng. 2022, 10, 15025–15034. [Google Scholar] [CrossRef]
- Noijuntira, I.; Kittisupakorn, P. Life Cycle Assessment for the Activated Carbon Production by Coconut Shells and Palm-Oil Shells. 2010. Available online: https://repository.rmutp.ac.th/handle/123456789/708 (accessed on 1 June 2024).
- Kabir Ahmad, R.; Anwar Sulaiman, S.; Yusup, S.; Sham Dol, S.; Inayat, M.; Aminu Umar, H. Exploring the potential of coconut shell biomass for charcoal production. Ain Shams Eng. J. 2022, 13, 101499. [Google Scholar] [CrossRef]
- Saleem, J.; Moghal, Z.K.B.; Pradhan, S.; McKay, G. High-performance activated carbon from coconut shells for dye removal: Study of isotherm and thermodynamics. RSC Adv. 2024, 14, 33797–33808. [Google Scholar] [CrossRef]
- ISO 14044; Environmental Management—Life Cycle Assessment: Principles and Framework. ISO: Geneva, Switzerland, 1997.
- ISO 14040; Environmental Management—Life Cycle Assessment: Requirements and Guidelines. ISO: Geneva, Switzerland, 2006.
- Saleem, J.; Shahid, U.B.; McKay, G. Environmental Nanotechnology. In Handbook of Environmental Materials Management; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–32. [Google Scholar]
- Thinkstep GaBi [Computer Software], Thinkstep, Sphera Solutions; Sphera: Leinfelden-Echterdingen, Germany, 2020.
- Guendouzi, M.E.; Rifai, A.; Skafi, M. Properties of fluoride in wet phosphoric acid processes: Fluorosilicic acid in an aqueous solution of H2SiF6–H20 at temperatures ranging from 298.15K to 353.15K. Fluid Phase Equilib. 2015, 396, 43–49. [Google Scholar] [CrossRef]
- Altintaş, N.; Ayok, T.; Gözmen, T. Fluorine pollution in the marmara region, Turkey. Production methods for fluorine removal and recovery. Water Res. 1987, 21, 781–788. [Google Scholar] [CrossRef]
- Ghaedi, M.; Najibi, A.; Hossainian, H.; Shokrollahi, A.; Soylak, M. Kinetic and equilibrium study of Alizarin Red S removal by activated carbon. Toxicol. Environ. Chem. 2012, 94, 40–48. [Google Scholar] [CrossRef]
- Fayazi, M.; Ghanei-Motlagh, M.; Taher, M.A. The adsorption of basic dye (Alizarin red S) from aqueous solution onto activated carbon/γ-Fe2O3 nano-composite: Kinetic and equilibrium studies. Mater. Sci. Semicond. Process. 2015, 40, 35–43. [Google Scholar] [CrossRef]
- Tahir, F.; Baloch, A.A.B.; Ali, H. Resilience of Desalination Plants for Sustainable Water Supply in Middle East. In Sustainability Perspectives: Science, Policy and Practice: A Global View of Theories, Policies and Practice in Sustainable Development; Springer: Cham, Switzerland, 2020; pp. 303–329. [Google Scholar]
- Darwish, M. Qatar water problem and solar desalination. Desalin. Water Treat. 2014, 52, 1250–1262. [Google Scholar] [CrossRef]
- Dawoud, M.A. Environmental Impacts of Seawater Desalination: Arabian Gulf Case Study. Int. J. Environ. Sustain. 2012, 1, 22–37. [Google Scholar] [CrossRef]



| Process | H2SO4 | H3PO4 | Remarks |
|---|---|---|---|
| Transportation | 2 km | 2 km | Transportation of waste—diesel truck |
| Shredding | 42.408 kJ | 42.408 kJ | - |
| Drying | 339.12 kJ | 339.12 kJ | Drying under oven |
| Activation | 188.379 g H2SO4 0.946 L water | 205.71 g H3PO4 0.939 L water | - |
| Neutralization | 9.236 L water | 9.236 L water | - |
| Drying | 332.28 kJ | 332.28 kJ | Drying under oven |
| Pyrolysis | 6984 kJ | 6984 kJ |
| Impact Category | Unit | Scenarios | ||
|---|---|---|---|---|
| H3PO4 | H2SO4 | Commercial AC | ||
| Climate change | kg CO2 eq. | 1.43103 | 1.182398 | 4.831549 |
| Fossil depletion | kg oil eq. | 0.595454 | 0.473587 | 2.318179 |
| Freshwater ecotoxicity | kg 1,4 DB eq. | 0.000134 | 7.35 × 10−5 | 0.000191 |
| Freshwater eutrophication | kg P eq. | 1.75 × 10−6 | 8.95 × 10−7 | 5.97 × 10−6 |
| Land use | m2y eq. | 0.005976 | 0.002664 | 0.117129 |
| Energy net | MJ | 33.65999 | 27.22802 | 125.3223 |
| Impact Category | H2SO4 | Commercial AC | % Improvement |
|---|---|---|---|
| Climate change | 1.182398 | 4.831549 | 75.5 |
| Fossil depletion | 0.473587 | 2.318179 | 79.5 |
| Freshwater ecotoxicity | 7.35 × 10−5 | 0.000191 | 61.6 |
| Freshwater eutrophication | 8.95 × 10−7 | 5.97 × 10−6 | 85.1 |
| Land use | 0.002664 | 0.117129 | 97.7 |
| Energy net | 27.22802 | 125.3223 | 78.3 |
| EI | Natural Gas (NG) | Solar Photovoltaic (SPV) | ||
|---|---|---|---|---|
| H3PO4 | H2SO4 | H3PO4 | H2SO4 | |
| Climate Change | 1.43103 | 1.182398 | 0.396499 | 0.147868 |
| Fossil Depletion | 0.595454 | 0.473587 | 0.195963 | 0.074097 |
| Freshwater Ecotoxicity | 0.000134 | 7.35 × 10−5 | 0.000116 | 5.56 × 10−5 |
| Freshwater Eutrophication | 1.75 × 10−6 | 8.95 × 10−7 | 1.82 × 10−6 | 9.68 × 10−7 |
| Land Use | 0.005976 | 0.002664 | 0.007472 | 0.00416 |
| Impact Category | Deionized-Desalinated Water | Scenario 1 Groundwater | Scenario 2 Surface Water |
|---|---|---|---|
| Climate Change | 1.182 | 1.170 | 1.171 |
| Fossil Depletion | 0.474 | 0.469 | 0.470 |
| Freshwater Ecotoxicity | 7.35 × 10−5 | 6.47 × 10−5 | 6.48 × 10−5 |
| Freshwater Eutrophication | 8.95 × 10−7 | 3.74 × 10−7 | 3.76 × 10−7 |
| Land Use | 0.00266 | 0.00176 | 0.00179 |
| Energy Net | 27.228 | 26.805 | 26.825 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, J.; Moghal, Z.K.B.; Tahir, F.; Al-Ansari, T.; McKay, G. Environmental Impacts and Adsorption Isotherms of Coconut Shell Activated Carbon: Effect of Acid Activation, Water, and Fuel. C 2025, 11, 22. https://doi.org/10.3390/c11010022
Saleem J, Moghal ZKB, Tahir F, Al-Ansari T, McKay G. Environmental Impacts and Adsorption Isotherms of Coconut Shell Activated Carbon: Effect of Acid Activation, Water, and Fuel. C. 2025; 11(1):22. https://doi.org/10.3390/c11010022
Chicago/Turabian StyleSaleem, Junaid, Zubair Khalid Baig Moghal, Furqan Tahir, Tareq Al-Ansari, and Gordon McKay. 2025. "Environmental Impacts and Adsorption Isotherms of Coconut Shell Activated Carbon: Effect of Acid Activation, Water, and Fuel" C 11, no. 1: 22. https://doi.org/10.3390/c11010022
APA StyleSaleem, J., Moghal, Z. K. B., Tahir, F., Al-Ansari, T., & McKay, G. (2025). Environmental Impacts and Adsorption Isotherms of Coconut Shell Activated Carbon: Effect of Acid Activation, Water, and Fuel. C, 11(1), 22. https://doi.org/10.3390/c11010022






