Plasma-Modified Carbon Materials for Radionuclide Absorption
Abstract
1. Introduction
2. Carbon Materials for Radioactive Nuclide Removal
2.1. Graphene and GO
2.2. Biochar
2.3. CNTs
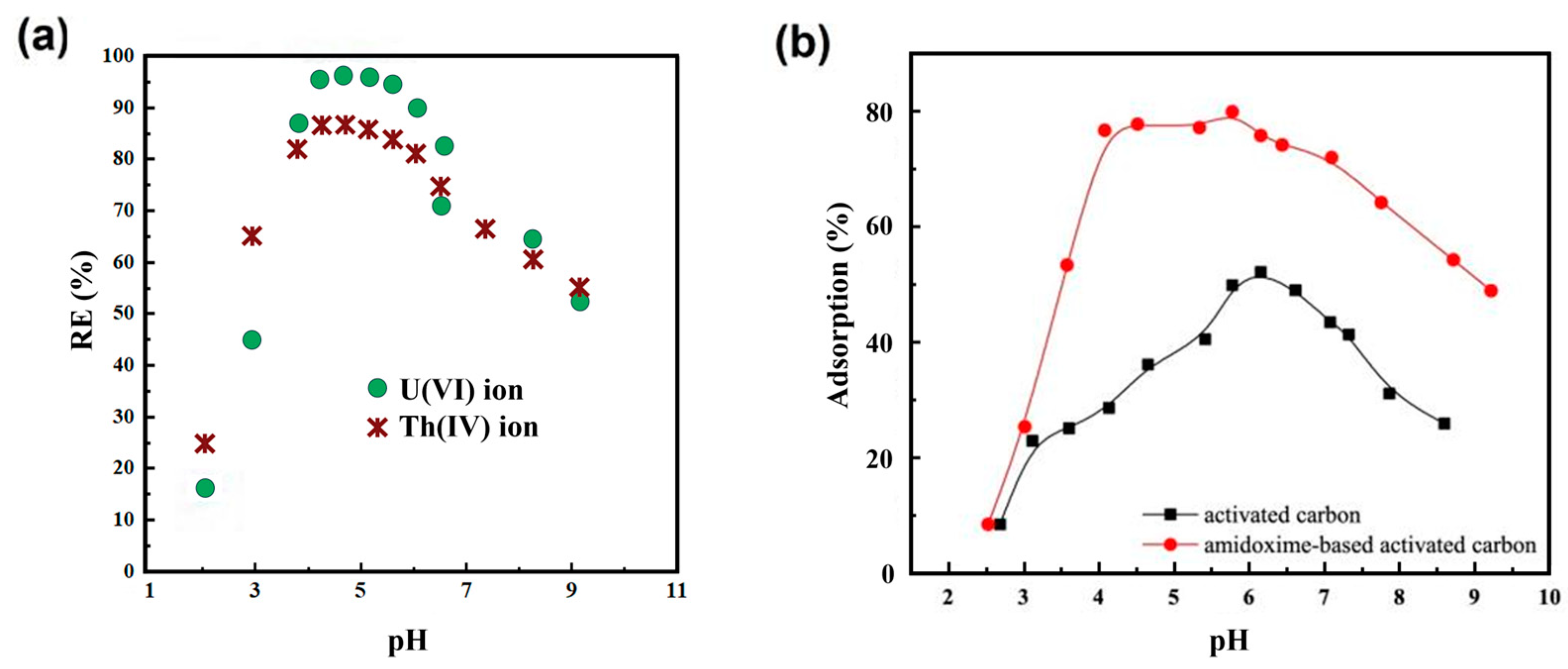
2.4. AC
2.5. Fullerenes
2.6. Carbon Based Composite Materials
3. Plasma-Modified Carbon Materials for Nuclides Absorption
3.1. Plasma-Modified Carbon Materials for U Absorption
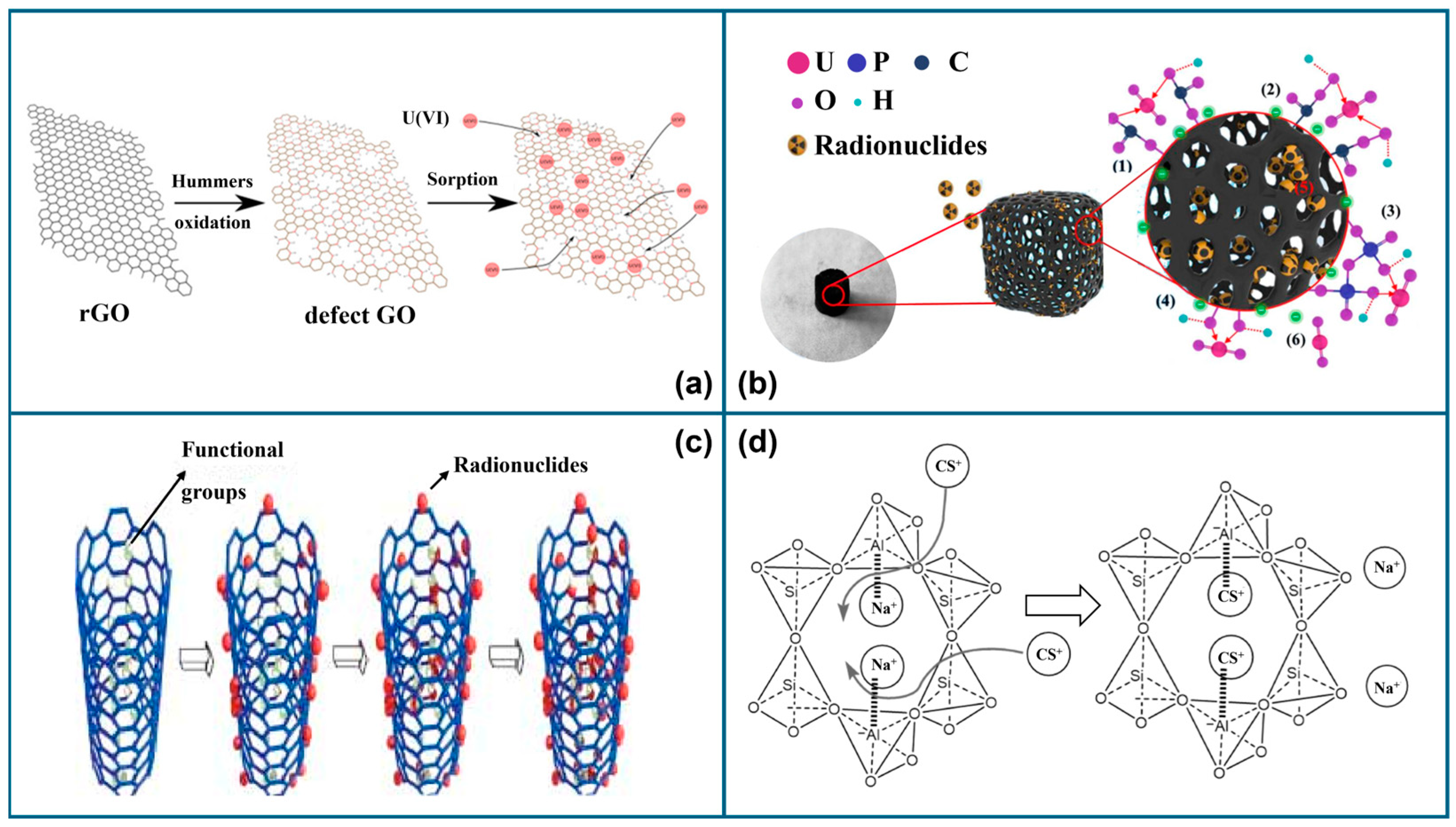
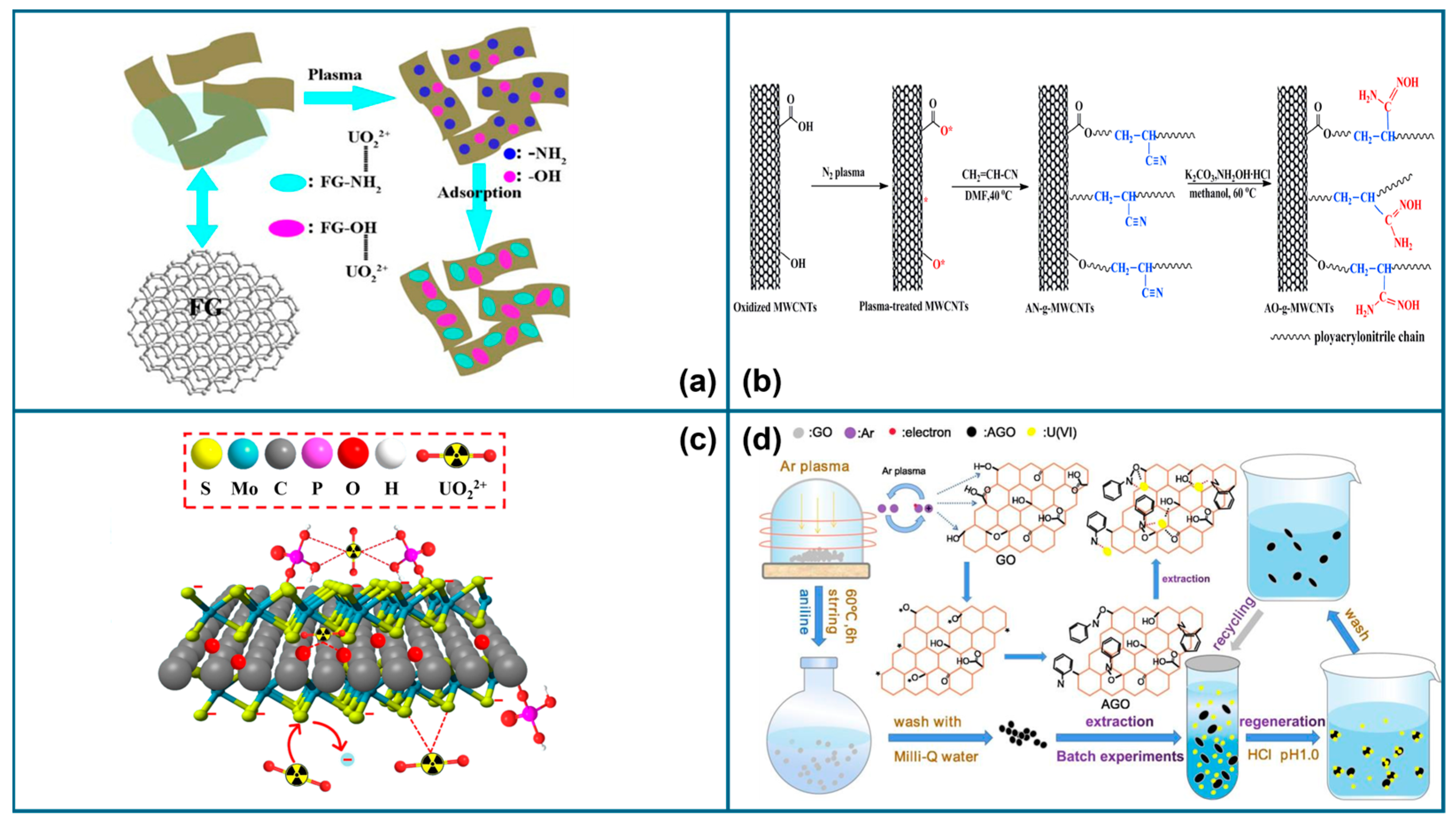
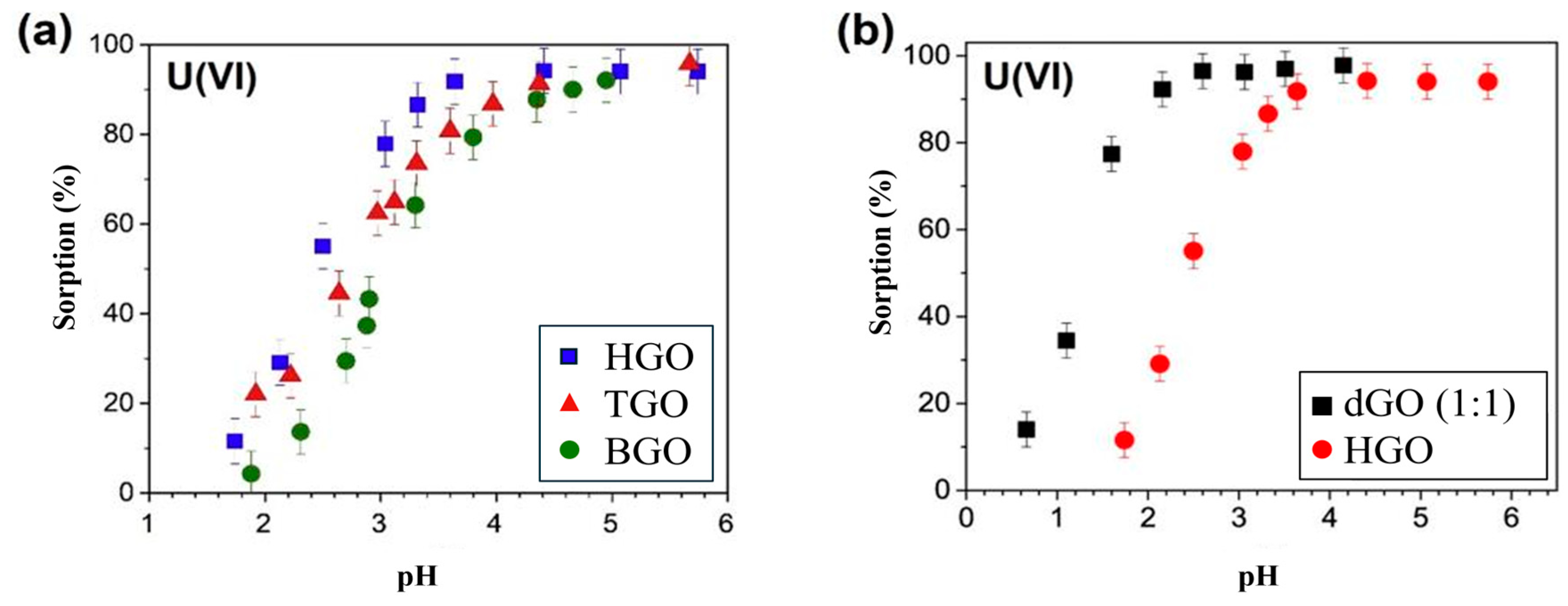
3.1.1. Plasma-Modified GO
3.1.2. Plasma-Modified Biochar
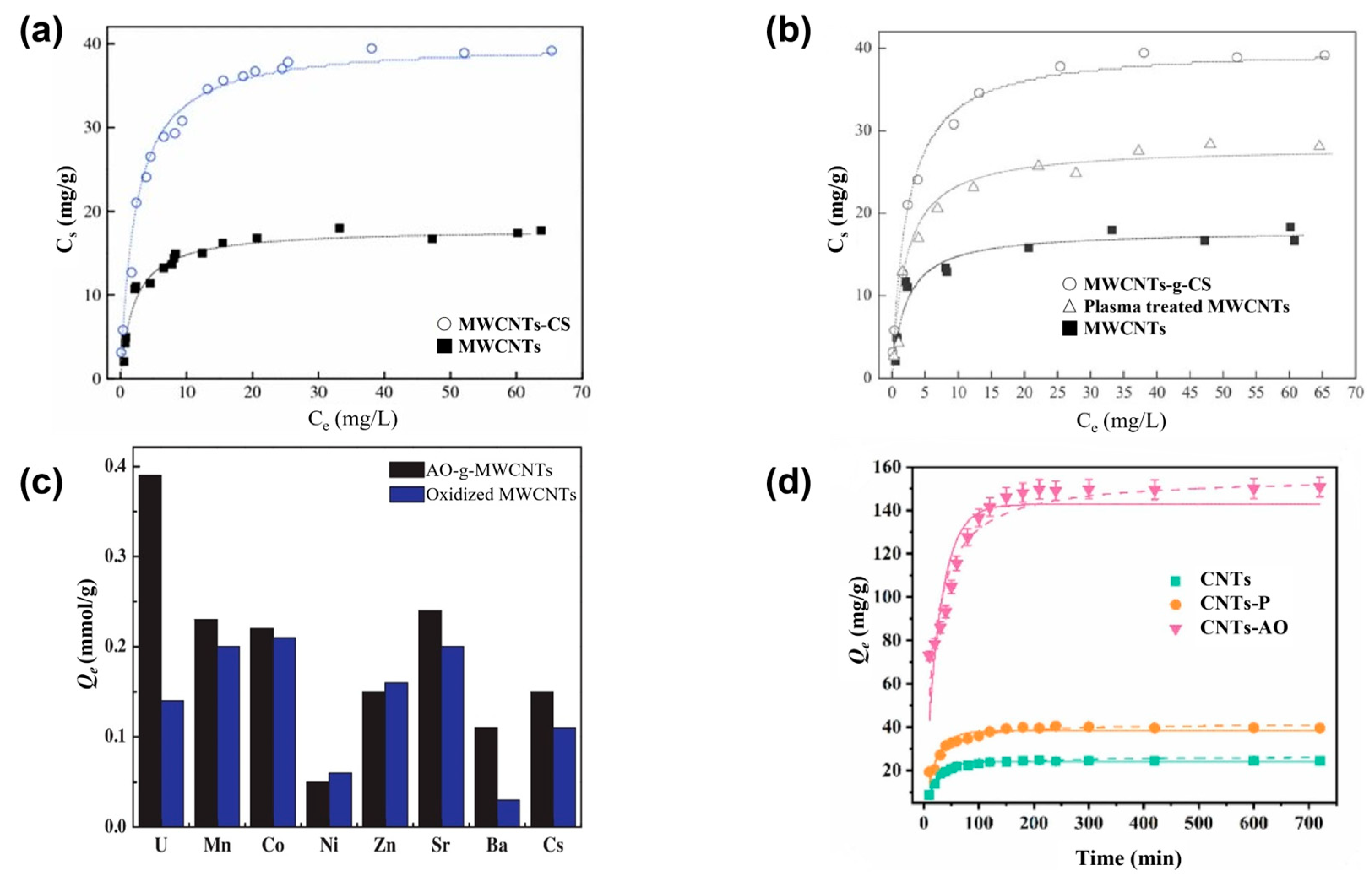
3.1.3. Plasma-Modified CNTs
3.1.4. Plasma-Modified Graphite
3.1.5. Plasma-Modified Carbon Composites
3.1.6. Other Plasma-Modified Carbon Materials (Carbon Dots and Carbon Black)
3.2. Plasma-Modified Carbon Materials for Absorption of Other Nuclides
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nathaniel, S.P.; Alam, M.S.; Murshed, M.; Mahmood, H.; Ahmad, P. The roles of nuclear energy, renewable energy, and economic growth in the abatement of carbon dioxide emissions in the G7 countries. Environ. Sci. Pollut. Res. 2021, 28, 47957–47972. [Google Scholar] [CrossRef] [PubMed]
- Saidi, K.; Omri, A. Reducing CO2 emissions in OECD countries: Do renewable and nuclear energy matter? Prog. Nucl. Energy 2020, 126, 103425. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Dai, L.; Liu, M.; Xiao, Y.; Cong, T.; Gu, H. The role of nuclear energy in the carbon neutrality goal. Prog. Nucl. Energy 2023, 162, 104772. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, X.; Rong, Q.; Liu, X.; Zhou, Y.; Yang, H.; Wang, G.; Chen, Z.; Wang, X. Enrichment and separation of radionuclides by organic polymer materials: A review. ACS ES&T Eng. 2024, 4, 250–268. [Google Scholar]
- Kiang, J.G.; Olabisi, A.O. Radiation: A poly-traumatic hit leading to multi-organ injury. Cell Biosci. 2019, 9, 25. [Google Scholar] [CrossRef]
- Kiang, J.G.; Blakely, W.F. Combined radiation injury and its impacts on radiation countermeasures and biodosimetry. Int. J. Radiat. Biol. 2023, 99, 1055–1065. [Google Scholar] [CrossRef]
- Ohba, T.; Tanigawa, K.; Liutsko, L. Evacuation after a nuclear accident: Critical reviews of past nuclear accidents and proposal for future planning. Environ. Int. 2021, 148, 106379. [Google Scholar] [CrossRef] [PubMed]
- Patra, K.; Sengupta, A. Recent advances in functionalized porous adsorbents for radioactive waste water decontamination: Current status, research gap and future outlook. Mater. Today Sustain. 2024, 25, 100703. [Google Scholar] [CrossRef]
- Fahad, S.A.; Nawab, M.S.; Shaida, M.A.; Verma, S.; Khan, M.U.; Siddiqui, V.; Naushad, M.; Saleemd, L.; Saleem, L.; Farooqi, I.H. Carbon based adsorbents for the removal of U(VI) from aqueous medium: A state of the art review. J. Water Process. Eng. 2023, 52, 103458. [Google Scholar] [CrossRef]
- Hao, M.; Liu, Y.; Wu, W.; Wang, S.; Yang, X.; Chen, Z.; Tang, Z.; Huang, Q.; Wang, S.; Yang, H.; et al. Advanced porous adsorbents for radionuclides elimination. EnergyChem 2023, 5, 100101. [Google Scholar] [CrossRef]
- Xu, M.; Cai, Y.; Chen, G.; Li, B.; Chen, Z.; Hu, B.; Wang, X. Efficient selective removal of radionuclides by sorption and catalytic reduction using nanomaterials. Nanomaterials 2022, 12, 1443. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Luo, Y.; Liu, Z.; Xie, X.; Xue, M.; Gao, B. Engineering carbon materials for organic pollutant removal via adsorption and photodegradation: A review. Sep. Purif. Technol. 2024, 359, 130872. [Google Scholar] [CrossRef]
- Kwak, C.H.; Lim, C.; Kim, S.; Lee, Y.S. Surface modification of carbon materials and its application as adsorbents. J. Ind. Eng. Chem. 2022, 116, 21–31. [Google Scholar] [CrossRef]
- Wu, M.; Jia, L.; Lu, S.; Qin, Z.; Wei, S.; Yan, R. Interfacial performance of high-performance fiber-reinforced composites improved by cold plasma treatment: A review. Surf. Interfaces 2021, 24, 101077. [Google Scholar]
- Duan, S.; Wang, Y.; Liu, X.; Shao, D.; Hayat, T.; Alsaedi, A.; Li, J. Removal of U(VI) from aqueous solution by amino functionalized flake graphite prepared by plasma treatment. ACS Sustain. Chem. Eng. 2017, 5, 4073–4085. [Google Scholar] [CrossRef]
- Boulanger, N.; Kuzenkova, A.S.; Iakunkov, A.; Romanchuk, A.Y.; Trigub, A.L.; Egorov, A.V.; Talyzin, A.V. Enhanced sorption of radionuclides by defect-rich graphene oxide. ACS Appl. Mater. Interfaces 2020, 12, 45122–45135. [Google Scholar] [CrossRef]
- Liao, J.; Ding, L.; Zhang, Y.; Zhu, W. Efficient removal of uranium from wastewater using pig manure biochar: Understanding adsorption and binding mechanisms. J. Hazard. Mater. 2022, 423, 127190. [Google Scholar] [CrossRef]
- Tan, X.L.; Xu, D.; Chen, C.L.; Wang, X.K.; Hu, W.P. Adsorption and kinetic desorption study of 152+ 154Eu (III) on multiwall carbon nanotubes from aqueous solution by using chelating resin and XPS methods. Radiochim. Acta 2008, 96, 23–29. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.; Almazán-Sánchez, P.T.; Solache-Ríos, M. Radioactive waste treatments by using zeolites. A short review. J. Environ. Radioact. 2021, 233, 106610. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Yang, J.; Liao, J.; Yang, Y.; Liu, N.; Tang, J. Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium (VI). Appl. Surf. Sci. 2014, 320, 10–20. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, N.; Ge, Y.; Ye, T.; Yang, Z.; Zou, L.; Ma, W.; Lu, L. Adsorption behavior and mechanism of U (VI) onto phytic Acid-modified Biochar/MoS2 heterojunction materials. Sep. Purif. Technol. 2022, 294, 121158. [Google Scholar] [CrossRef]
- Liao, S.; Li, Y.; Cheng, J.; Yu, J.; Ren, W.; Yang, S. Active and selective removal of U(VI) from contaminated water by plasma-initiated polymerization of aniline/GO. J. Mol. Liq. 2021, 344, 117687. [Google Scholar] [CrossRef]
- Lv, C.; Li, S.; Liu, L.; Zhu, X.; Yang, X. Enhanced electrochemical characteristics of the glucose oxidase bioelectrode constructed by carboxyl-functionalized mesoporous carbon. Sensors 2020, 20, 3365. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.; Chen, C. Adsorption of radionuclides on carbon-based nanomaterials. In Interface Science and Technology, 1st ed.; Chen, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 29, pp. 141–215. [Google Scholar]
- Pan, X.; Ji, J.; Zhang, N.; Xing, M. Research progress of graphene-based nanomaterials for the environmental remediation. Chin. Chem. Lett. 2020, 31, 1462–1473. [Google Scholar] [CrossRef]
- Li, M.; Tang, X.; Tan, J.; Cheng, G.; Wu, F.; Zhou, N. Properties and mechanism of uranium adsorption on single-sided fluorinated graphene: A first-principles study. Surf. Interfaces 2024, 50, 104504. [Google Scholar] [CrossRef]
- Zhou, C.; Li, B.; Li, Y.; Zhao, J.; Mei, Q.; Wu, Y.; Li, M.; Chen, Y.; Fan, Y. A review of graphene oxide-based adsorbents for removing lead ions in water. J. Environ. Chem. Eng. 2024, 12, 111839. [Google Scholar] [CrossRef]
- Kuzenkova, A.S.; Romanchuk, A.Y.; Trigub, A.L.; Maslakov, K.I.; Egorov, A.V.; Amidani, L.; Kittrell, C.; Kvashnina, K.N.; Tour, J.M.; Talyzin, A.V.; et al. New insights into the mechanism of graphene oxide and radionuclide interaction. Carbon 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A. Carbon based materials: A review of adsorbents for inorganic and organic compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar composites: Emerging trends, field successes and sustainability implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Cleaner Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Wang, H.; Mašek, O.; Hou, R.; O’Connor, D.; Hou, D. New trends in biochar pyrolysis and modification strategies: Feedstock, pyrolysis conditions, sustainability concerns and implications for soil amendment. Soil Use Manag. 2020, 36, 358–386. [Google Scholar] [CrossRef]
- Guo, L.; Peng, L.; Li, J.; Zhang, W.; Shi, B. Graphitic N-doped biochar for superefficient uranium recycling from nuclear wastewater. Sci. Total Environ. 2023, 882, 163462. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, X.; Jiang, J. Phosphate-modified hydrothermal biochar: Green and efficient uranium adsorption. Mater. Lett. 2024, 377, 137363. [Google Scholar] [CrossRef]
- Sumalatha, B.; Narayana, A.V.; Khan, A.A.; Venkateswarulu, T.C.; Reddy, G.S.; Reddy, P.R.; Babu, D.J. A Sustainable Green Approach for Efficient Capture of Strontium from Simulated Radioactive Wastewater Using Modified Biochar. Int. J. Environ. Res. 2022, 16, 75. [Google Scholar] [CrossRef]
- Apul, O.G.; Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res. 2015, 68, 34–55. [Google Scholar] [CrossRef]
- Yu, J.-G.; Zhao, X.-H.; Yang, H.; Chen, X.-H.; Yang, Q.; Yu, L.-Y.; Jiang, J.-H.; Chen, X.-Q. Aqueous adsorption and removal of organic contaminants by carbon nanotubes. Sci. Total Environ. 2014, 482, 241–251. [Google Scholar] [CrossRef]
- Hassan, S.S.; Abdel Rahman, E.M.; El-Subruiti, G.M.; Kamel, A.H.; Diab, H.M. Removal of uranium-238, thorium-232, and potassium-40 from wastewater via adsorption on multiwalled carbon nanotubes. ACS Omega 2022, 7, 12342–12353. [Google Scholar] [CrossRef]
- Yılmaz, C.E.; Aslani, M.A.; Aslani, C.K. Removal of thorium by modified multi-walled carbon nanotubes: Optimization, thermodynamic, kinetic, and molecular dynamic viewpoint. Prog. Nucl. Energy 2020, 127, 103445. [Google Scholar] [CrossRef]
- Mozhiarasi, V.; Natarajan, T.S. Bael fruit shell–derived activated carbon adsorbent: Effect of surface charge of activated carbon and type of pollutants for improved adsorption capacity. Biomass Convers. Biorefin. 2024, 14, 8761–8774. [Google Scholar] [CrossRef]
- He, S.; Chen, G.; Xiao, H.; Shi, G.; Ruan, C.; Ma, Y.; Dai, H.; Yuan, B.; Chen, X.; Yang, X. Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture. J. Colloid Interface Sci. 2021, 582, 90–101. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Xu, X.; Meng, X.; Qu, J.; Wang, Z.; Liu, C.; Qu, B. Preparation, characterization and application of activated carbon from corn cob by KOH activation for removal of Hg(II) from aqueous solution. Bioresour. Technol. 2020, 306, 123154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jena, H.M. Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. J. Cleaner Prod. 2016, 137, 1246–1259. [Google Scholar] [CrossRef]
- Raut, E.R.; Bedmohata, M.A.; Chaudhari, A.R. Comparative study of preparation and characterization of activated carbon obtained from sugarcane bagasse and rice husk by using H3PO4 and ZnCl2. Mater. Today Proc. 2022, 66, 1875–1884. [Google Scholar] [CrossRef]
- Wang, S.; Nam, H.; Nam, H. Preparation of activated carbon from peanut shell with KOH activation and its application for H2S adsorption in confined space. J. Environ. Chem. Eng. 2020, 8, 103683. [Google Scholar] [CrossRef]
- Abd El-Magied, M.O.; Hassan, A.M.; El-Aassy, I.K.; Gad, H.M.; Youssef, M.A.; Mohammaden, T.F. Development of functionalized activated carbon for uranium removal from groundwater. Int. J. Environ. Res. 2021, 15, 543–558. [Google Scholar] [CrossRef]
- Alahabadi, A.; Singh, P.; Raizada, P.; Anastopoulos, I.; Sivamani, S.; Dotto, G.L.; Landarani, M.; Ivanets, A.; Kyzas, G.Z.; Hosseini-Bandegharaei, A. Activated carbon from wood wastes for the removal of uranium and thorium ions through modification with mineral acid. Colloids Surf. A 2020, 607, 125516. [Google Scholar] [CrossRef]
- Liu, P.; Yu, Q.; Xue, Y.; Chen, J.; Ma, F. Adsorption performance of U(VI) by amidoxime-based activated carbon. J. Radioanal. Nucl. Chem. 2020, 324, 813–822. [Google Scholar] [CrossRef]
- Du, J.; Jiang, G. Adsorption of actinide ion complexes on C60O: An all-electron ZORA-DFT-D3 study. Spectrochim. Acta Part A 2019, 223, 117375. [Google Scholar] [CrossRef]
- Jena, N.K.; Sundararajan, M.; Ghosh, S.K. On the interaction of uranyl with functionalized fullerenes: A DFT investigation. RSC Adv. 2012, 2, 2994–2999. [Google Scholar] [CrossRef]
- Du, J.; Jiang, G. The strong interaction of actinyl ions with fullerenol driven by multiple hydrogen bonds. Dalton Trans. 2022, 51, 5118–5126. [Google Scholar] [CrossRef]
- Bonifazi, D.; Enger, O.; Diederich, F. Supramolecular [60]fullerene chemistry on surfaces. Chem. Soc. Rev. 2007, 36, 390–414. [Google Scholar] [CrossRef]
- Le-Minh, N.; Sivret, E.C.; Shammay, A.; Stuetz, R.M. Factors affecting the adsorption of gaseous environmental odors by activated carbon: A critical review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 341–375. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Liberati, B.; Novak, M.; Cooper, M.; Kruse, N.; Young, D.; Trembly, J. Radium-226 removal from simulated produced water using natural zeolite and ion-exchange resin. Ind. Eng. Chem. Res. 2016, 55, 12502–12505. [Google Scholar] [CrossRef]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating hierarchical pores in zeolite catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, Z.; Wang, X.; Ying, D.; Niu, F.; Cao, X.; Wang, Y.; Hua, R.; Liu, Y.; Wang, X. Ordered mesoporous polymer–carbon composites containing amidoxime groups for uranium removal from aqueous solutions. Chem. Eng. J. 2018, 341, 208–217. [Google Scholar] [CrossRef]
- Chen, L.; Feng, S.; Zhao, D.; Chen, S.; Li, F.; Chen, C. Efficient sorption and reduction of U(VI) on zero-valent iron-polyaniline-graphene aerogel ternary composite. J. Colloid Interface Sci. 2017, 490, 197–206. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, C.; Liu, Z.; Zhang, L.; Chen, L.; Wang, J.; Wang, X.; Yang, S.; Wang, S. Fabrication of a phosphorylated graphene oxide–chitosan composite for highly effective and selective capture of U(VI). Environ. Sci. Nano 2017, 4, 1876–1886. [Google Scholar] [CrossRef]
- Wu, J.; Tian, K.; Wang, J. Adsorption of uranium (VI) by amidoxime modified multiwalled carbon nanotubes. Prog. Nucl. Energy 2018, 106, 79–86. [Google Scholar] [CrossRef]
- Song, W.; Liu, M.; Hu, R.; Tan, X.; Li, J. Water-soluble polyacrylamide coated-Fe3O4 magnetic composites for high-efficient enrichment of U(VI) from radioactive wastewater. Chem. Eng. J. 2014, 246, 268–276. [Google Scholar] [CrossRef]
- Das, S.; Pandey, A.K.; Athawale, A.A.; Manchanda, V.K. Exchanges of uranium (VI) species in amidoxime-functionalized sorbents. J. Phys. Chem. B 2009, 113, 6328–6335. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Hydrophobic plasma polymer coated silica particles for petroleum hydrocarbon removal. ACS Appl. Mater. Interfaces 2013, 5, 8563–8571. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Plasma polymer-functionalized silica particles for heavy metals removal. ACS Appl. Mater. Interfaces 2015, 7, 4265–4274. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, J.; Luo, W.; Liao, X.; Shi, B. In situ chemical oxidation-grafted amidoxime-based collagen fibers for rapid uranium extraction from radioactive wastewater. Sep. Purif. Technol. 2023, 307, 122826. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic surface functionalization of electrospun nanofibrous scaffolds in tissue engineering. Polymers 2020, 12, 2636. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Goh, P.S.; Ismail, A.F.; Hilal, N. Surface design of liquid separation membrane through graft polymerization: A state of the art review. Membranes 2021, 11, 832. [Google Scholar] [CrossRef]
- Ran, C.; Zhou, X.; Dong, P.; Liu, K.; Ostrikov, K. Ultralong-lasting plasma-activated water inhibits pathogens and improves plant disease resistance in soft rot-infected hydroponic lettuce. Plasma Process. Polym. 2024, 21, e2400039. [Google Scholar] [CrossRef]
- Zhou, X.F.; Xiang, H.F.; Yang, M.H.; Geng, W.Q.; Liu, K. Temporal evolution characteristics of the excited species in a pulsed needle-water discharge: Effect of voltage and frequency. J. Phys. D Appl. Phys. 2023, 56, 455202. [Google Scholar] [CrossRef]
- Yan, A.; Kong, X.; Xue, S.; Guo, P.; Chen, Z.; Li, D.; Liu, Z.; Zhang, H.; Ning, W.; Wang, R. Atmospheric pressure plasma jet interacting with a droplet on dielectric surface. Plasma Sources Sci. Technol. 2024, 33, 105011. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhang, R.; Wang, X. Reductive immobilization of Re(VII) by graphene modified nanoscale zero-valent iron particles using a plasma technique. Sci. China Chem. 2016, 59, 150–158. [Google Scholar] [CrossRef]
- Zhu, H.; Duan, S.; Chen, L.; Alsaedi, A.; Hayat, T.; Li, J. Plasma-induced grafting of acrylic acid on bentonite for the removal of U(VI) from aqueous solution. Plasma Sci. Technol. 2017, 19, 115501. [Google Scholar] [CrossRef]
- Kadela, K.; Grzybek, G.; Kotarba, A.; Stelmachowski, P. Enhancing graphene nanoplatelet reactivity through low-temperature plasma modification. ACS Appl. Mater. Interfaces 2024, 16, 19771–19779. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Xue, S.; Guo, P.; He, J.; Wang, G.; Zeng, Y.; Qie, L.; Wang, R. Research progress of low-temperature-plasma polishing technology in chip material processing. Clean Energy Sci. Technol. 2024, 2, 263. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Cui, J.; Zhao, B.; Yang, W.; Li, H.; Wang, R. Preparation of highly efficient antibacterial non-woven by facile plasma-induced graft polymerizing of DADMAC. Plasma Sci. Technol. 2023, 25, 114001. [Google Scholar] [CrossRef]
- Liang, H.; Ming, F.; Alshareef, H.N. Applications of plasma in energy conversion and storage materials. Adv. Energy Mater. 2018, 8, 1801804. [Google Scholar] [CrossRef]
- Gupta, B.; Plummer, C.; Bisson, I.; Frey, P.; Hilborn, J. Plasma-induced graft polymerization of acrylic acid onto poly (ethylene terephthalate) films: Characterization and human smooth muscle cell growth on grafted films. Biomaterials 2002, 23, 863–871. [Google Scholar] [CrossRef]
- Song, W.; Wang, X.; Wang, Q.; Shao, D.; Wang, X. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys. Chem. Chem. Phys. 2015, 17, 398–406. [Google Scholar] [CrossRef]
- Hu, B.; Guo, X.; Zheng, C.; Song, G.; Chen, D.; Zhu, Y.; Song, X.; Sun, Y. Plasma-enhanced amidoxime/magnetic graphene oxide for efficient enrichment of U(VI) investigated by EXAFS and modeling techniques. Chem. Eng. J. 2019, 357, 66–74. [Google Scholar] [CrossRef]
- Ulusoy, U.; Şimşek, S.; Ceyhan, Ö. Investigations for modification of polyacrylamide-bentonite by phytic acid and its usability in Fe3+, Zn2+ and UO22+ adsorption. Adsorption 2003, 9, 165–175. [Google Scholar] [CrossRef]
- Kelley, S.P.; Barber, P.S.; Mullins, P.H.; Rogers, R.D. Structural clues to UO22+/VO2+ competition in seawater extraction using amidoxime-based extractants. Chem. Commun. 2014, 50, 12504–12507. [Google Scholar] [CrossRef]
- Yi, J.; Huo, Z.; Tan, X.; Chen, C.; Asiri, A.M.; Alamry, K.A.; Li, J. Plasma-facilitated modification of pumpkin vine-based biochar and its application for efficient elimination of uranyl from aqueous solution. Plasma Sci. Technol. 2019, 21, 095502. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Li, Q.; Yang, H.; Liu, Y.; Wu, L.; Liu, Z.; Hu, B.; Wang, X. Low-temperature plasma induced phosphate groups onto coffee residue-derived porous carbon for efficient U(VI) extraction. J. Environ. Sci. 2022, 122, 1–13. [Google Scholar] [CrossRef]
- Wang, R.; Xia, Z.; Kong, X.; Liang, L.; Ostrikov, K.K. Etching and annealing treatment to improve the plasma-deposited SiOx film adhesion force. Surf. Coat. Technol. 2021, 427, 127840. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, J.; Zhang, Y.; Zhang, J.; Liang, J.; Zhang, J.; Li, J. The electrosorption of uranium (VI) onto the modified porous biocarbon with ammonia low-temperature plasma: Kinetics and mechanism. Chem. Eng. J. 2023, 463, 142413. [Google Scholar] [CrossRef]
- Shao, D.; Jiang, Z.; Wang, X.; Li, J.; Meng, Y. Plasma induced grafting carboxymethyl cellulose on multiwalled carbon nanotubes for the removal of UO22+ from aqueous solution. J. Phys. Chem. B 2009, 113, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Hu, J.; Wang, X. Plasma induced grafting multiwalled carbon nanotube with chitosan and its application for removal of UO22+, Cu2+, and Pb2+ from aqueous solutions. Plasma Process. Polym. 2010, 7, 977–985. [Google Scholar] [CrossRef]
- Lazaridis, N.K.; Kyzas, G.Z.; Vassiliou, A.A.; Bikiaris, D.N. Chitosan derivatives as biosorbents for basic dyes. Langmuir 2007, 23, 7634–7643. [Google Scholar] [CrossRef]
- Chen, J.; Lu, D.; Chen, B.; OuYang, P. Removal of U(VI) from aqueous solutions by using MWCNTs and chitosan modified MWCNTs. J. Radioanal. Nucl. Chem. 2013, 295, 2233–2241. [Google Scholar] [CrossRef]
- Geng, J.; Ma, L.; Wang, H.; Liu, J.; Bai, C.; Song, Q.; Li, J.; Hou, M.; Li, S. Amidoxime-grafted hydrothermal carbon microspheres for highly selective separation of uranium. J. Nanosci. Nanotechnol. 2012, 12, 7354–7363. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, S.; Shaban, M.; Ren, X.; Chen, S.; Li, Z.; Li, H.; Chen, C. Plasma-assisted preparation of amidoxime-carbon nanotubes hybrids for effective uranium extraction. J. Environ. Chem. Eng. 2024, 12, 114495. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Tsumura, S.; Nagatsu, M. High-efficiency plasma surface modification of graphite-encapsulated magnetic nanoparticles using a pulsed particle explosion technique. Jpn. J. Appl. Phys. 2013, 53, 010205. [Google Scholar] [CrossRef]
- Xiao, J.; Song, W.; Hu, R.; Chen, L.; Tian, X. One-step arc-produced amino-functionalized graphite-encapsulated magnetic nanoparticles for the efficient removal of radionuclides. ACS Appl. Nano Mater. 2018, 2, 385–394. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, P. Adsorption of methylene blue from aqueous solutions by modified expanded graphite powder. Desalination 2009, 249, 331–336. [Google Scholar] [CrossRef]
- Li, C.; Bai, H.; Shi, G. Conducting polymer nanomaterials: Electrosynthesis and applications. Chem. Soc. Rev. 2009, 38, 2397–2409. [Google Scholar] [CrossRef]
- Choi, M.; Jang, J. Heavy metal ion adsorption onto polypyrrole-impregnated porous carbon. J. Colloid Interface Sci. 2008, 325, 287–289. [Google Scholar] [CrossRef]
- Bhaumik, M.; Maity, A.; Srinivasu, V.V.; Onyango, M.S. Enhanced removal of Cr(VI) from aqueous solution using polypyrrole/Fe3O4 magnetic nanocomposite. J. Hazard. Mater. 2011, 190, 381–390. [Google Scholar] [CrossRef]
- Hu, R.; Shao, D.; Wang, X. Graphene oxide/polypyrrole composites for highly selective enrichment of U(VI) from aqueous solutions. Polym. Chem. 2014, 5, 6207–6215. [Google Scholar] [CrossRef]
- Zong, P.; Cao, D.; Cheng, Y.; Wang, S.; Zhang, J.; Guo, Z.; Hayat, T.; Alharbi, N.S.; He, C. Carboxymethyl cellulose supported magnetic graphene oxide composites by plasma induced technique and their highly efficient removal of uranium ions. Cellulose 2019, 26, 4039–4060. [Google Scholar] [CrossRef]
- Kim, B.C.; Lee, J.; Um, W.; Kim, J.; Joo, J.; Lee, J.H.; Kwak, J.H.; Kim, J.H.; Lee, C.; Lee, H.; et al. Magnetic mesoporous materials for removal of environmental wastes. J. Hazard. Mater. 2011, 192, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Qu, G.; Yan, L.; Wang, R.; Guo, P.; Yang, Y.; Li, X. Efficient Removal of Representative Chemical Agents by Rapid and Sufficient Adsorption via Magnetic Graphene Oxide Composites. Appl. Sci. 2023, 13, 10731. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, X.; He, B.; Pan, D.; Liang, J.; Wang, F.; Fan, Q. Arsenazo-functionalized magnetic carbon composite for uranium (VI) removal from aqueous solution. J. Mol. Liq. 2018, 269, 441–449. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L.; Zhang, K.; Lu, Y.; Chen, J.; Wang, S.; Hu, B.; Wang, X. Application of carbon dots and their composite materials for the detection and removal of radioactive ions: A review. Chemosphere 2022, 287, 132313. [Google Scholar] [CrossRef]
- Wang, Z.; Lu, Y.; Yuan, H.; Ren, Z.; Xu, C.; Chen, J. Microplasma-assisted rapid synthesis of luminescent nitrogen-doped carbon dots and their application in pH sensing and uranium detection. Nanoscale 2015, 7, 20743–20748. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Zhu, A.; Wang, R. Multiple chemical warfare agent simulant decontamination by self-driven microplasma. Plasma Sci. Technol. 2023, 25, 114002. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Lu, Y.; Wu, F.; Ye, G.; Wei, G.; Sun, T.; Chen, J. Visualization of adsorption: Luminescent mesoporous silica-carbon dots composite for rapid and selective removal of U(VI) and in situ monitoring the adsorption behavior. ACS Appl. Mater. Interfaces 2017, 9, 7392–7398. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Man, H.; Sun, L.; Zang, S. Carbon black: A good adsorbent for triclosan removal from water. Water 2022, 14, 576. [Google Scholar] [CrossRef]
- Sha, H.; Su, X.; Chen, X.; Wang, L.; Wang, S.; Ke, Y.; Zhou, P. Effect of dissolution for black carbon on the adsorption capacity for methylene blue: Equilibrium, kinetics and thermodynamics. J. Dispers. Sci. Technol. 2024, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, C.; Li, X.; Ren, J.; Zhang, G.; Xie, H.; Liu, B.; Zhou, G. Preparation of carbon black-based porous carbon adsorbents and study of toluene adsorption properties. J. Chem. Technol. Biotechnol. 2023, 98, 117–128. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, P.; Li, C.; Fan, X.; Wen, Q.; Zhan, Q.; Shu, X.; Xu, T.; Zeng, G. Adsorption mechanism of sodium dodecyl benzene sulfonate on carbon blacks by adsorption isotherm and zeta potential determinations. Environ. Technol. 2013, 34, 201–207. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yu, S.; Ren, X.; Chen, S.; Li, Z.; Li, H.; Chen, C. Amidoxime functionality of carbon black by plasma technology for efficient uranium extraction from aqueous solution and simulated seawater. Sep. Purif. Technol. 2025, 356, 129931. [Google Scholar] [CrossRef]
- Park, S.; Ham, D.; Lee, W. Effect of Underwater Plasma Discharge on the Chemical Regeneration of Spent Granular Activated Carbon. J. Korean Soc. Environ. Eng. 2018, 40, 458–464. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; Bondouk, I.I.; Omar, K.M.; Hamdy, A.; Abbas, M.I.; El-Khatib, M.; Hammoury, S.I.; Gouda, M.M. Impact of (nano ZnO/multi-wall CNTs) prepared by arc discharge method on the removal efficiency of stable iodine 127I and radioactive iodine 131I from water. Sci. Rep. 2024, 14, 4242. [Google Scholar] [CrossRef]
- Zong, P.; Cao, D.; Cheng, Y.; Wang, S.; Hayat, T.; Alharbi, N.S.; Guo, Z.; Zhao, Y.; He, C. Enhanced performance for Eu(III) ion remediation using magnetic multiwalled carbon nanotubes functionalized with carboxymethyl cellulose nanoparticles synthesized by plasma technology. Inorg. Chem. Front. 2018, 5, 3184–3196. [Google Scholar] [CrossRef]
- Ali, S.; Shah, I.A.; Huang, H. Selectivity of Ar/O2 plasma-treated carbon nanotube membranes for Sr(II) and Cs(I) in water and wastewater: Fit-for-purpose water treatment. Sep. Purif. Technol. 2020, 237, 116352. [Google Scholar] [CrossRef]
- Song, M.; Wang, Q.; Meng, Y. Removal of UO22+ from aqueous solution by plasma functionalized MWCNTs. J. Radioanal. Nucl. Chem. 2012, 293, 899–906. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, S.; Wang, X.; Xu, C.; Li, J.; Chen, C.; Chen, J.; Hayat, T.; Alsaedi, A.; Alharbi, N.S.; et al. Plasma-facilitated synthesis of amidoxime/carbon nanofiber hybrids for effective enrichment of 238U(VI) and 241Am(III). Environ. Sci. Technol. 2017, 51, 12274–12282. [Google Scholar] [CrossRef]


| Adsorbent | Advantages | Disadvantages |
|---|---|---|
| AC | Widely applicable, strong adsorption capacity, low cost, and wide array of sources [40,41,42]. | Low selectivity, and adsorption capacity is influenced by environmental factors [53]. |
| Biochar | High permeability, good porosity, large surface area, and environmentally friendly [30]. | Poor environmental stability [42], and adsorption efficiency depends on raw materials [55]. |
| Graphene | Excellent thermal/electrical conductivity, large specific surface area, and multiple oxygen-containing functional groups [25]. | Limited oxygen-containing functional groups, high cost, and complex preparation process [54]. |
| CNTs | High elastic modulus and tensile strength, and excellent electrical and thermal conductivity [36]. | High cost, challenges in large-scale production, and insolubility issues [37]. |
| Zeolites | High ion-exchange capacity, excellent selectivity, low cost, and compatibility with natural environments [56]. | Small pore size and long diffusion paths reduce transport efficiency [57]. |
| No. | Adsorption Target | Carbon Material | Structure of Plasma Source and Its Discharge Modes | Excitation Source | Working Gas and Gas Pressure | Adsorption Mechanism | Functional Groups and Modification Methods | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | U(VI) Eu(III) Co(II) | GO | Dielectric barrier discharge (DBD) plasma | Power: 240 W; voltage: 120 V; time: 30 min; room temperature | Atmospheric pressure | Complexation between nitrogen- and oxygen-containing functional groups and radionuclides | A large number of nitrogenous and oxygen-containing functional groups; grafting | At pH = 5.0 ± 0.1 and T = 295 K, the adsorption capacity of PAM/GO for U(VI), Eu(III), and Co(II) was 0.698, 1.245, and 1.621 mmol/g, respectively | Song et al., 2015 [80] |
| 2 | U(VI) | Magnetic GO | - | Power: 120 W; voltage: 600 V; current: 20 mA | N2, 10 Pa | Inner-sphere surface complexation | Oxygen- and nitrogen-containing functional groups; grafting | At pH = 4 and T = 293 K, the adsorption capacity of AO/mGO was 435 mg/g and 2.85 mg/g in the South China Sea | Hu et al., 2018 [81] |
| 3 | Uranyl | Biochar | - | A high-voltage pulsed DC voltage device; power: 100 W | - | Surface complexation and electrostatic interactions | C-O, C=O, and -COO; grafting | At pH = 5 and T = 298 K, the adsorption capacity was 207.02 mg/g | Yi et al., 2019 [84] |
| 4 | U(VI) | Biochar | - | Power: 100 W; time: 2 hr | N2, 1.8 Pa | 1. Electron transfer reaction 2. Complexation of -NH2, P-OH/P=O and C-OH groups | -NH2, phosphate group, -OH group; grafting | At pH = 6, T = 298 K, and time = 1 h, the adsorption capacity was 648.54 mg/g | Chen et al., 2022 [85] |
| 5 | U(VI) | Biochar | Radio frequency (RF) plasma | Power: 200 W | NH3, 4.0 Pa | The Faraday side reaction was mainly introduced | Nitrogen-containing and oxygen-containing groups; etching | At pH = 4 and T = 298 K, the adsorption capacity was 466.72 mg/g and the electroadsorption efficiency of biocarbon for U(VI) was 94.45%; the electroadsorption capacity in seawater was 78.34 mg/g | Wang et al., 2023 [87] |
| 6 | UO22+ | MWCNTs | Customized grafting reactors | Power: 70 W; voltage: 650 V; current: 60 mA | N2, 10 Pa | Strong complexation ability of CMC with metal ions | -NH2 and CMC; grafting | At pH = 5, T = 298 K, and m/v = 0.4 g/L, the ionic strength was 0.01 mol NaClO4 and the adsorption capacity was 111.86 mg/g | Shao et al., 2009 [88] |
| 7 | UO22+ Cu2+ Pb2+ | MWCNTs | Customized grafting reactors | Power: 70 W; voltage: 650 V; current: 60 mA | N2, 10 Pa | The functional groups of the material formed strong complexes with metal ions | UO22+: -OH and other functional groups; grafting | At pH = 5.0 ± 0.1, T = 20 ± 1 °C, time = 24 h, m/v = 0.4 g/L, and C[NaClO4] = 0.01 mol/L, the adsorption capacity of UO22+ was 39.2 mg/g | Shao et al., 2010 [89] |
| 8 | U(VI) | MWCNTs | Graft reactor | Power: 70 W; voltage: 650 V; current: 60 mA | N2, 10 Pa | Inner-sphere surface complexation dominated | The functional groups of CS; grafting | At pH = 5.0 ± 0.1, T = 20 ± 1 °C, time = 48 h, m/v = 0.4 g/L, and C[NaClO4] = 0.01 mol/L, the adsorption capacity was 41 mg/g | Chen et al., 2012 [91] |
| 9 | U(VI) | MWCNTs | In a custom-made grafting reactor | Power: 100 W; voltage: 800 V; current: 15 mA | N2, 10 Pa | Surface complexation | AO; grafting | At pH = 4.5, the adsorption capacity for U(VI) was 145 mg/g (0.61 mmol/g) | Wang et al., 2014 [20] |
| 10 | U(VI) | CNTs | RF plasma | Power: 100 W; time: 20 min | O2, vacuum environment | The synergistic effect of abundant oxygen- and nitrogen-containing functional groups within AO groups on CNTs facilitated the process, and when U(VI) reached the surface of CNTs-AO, complex formation or ion exchange reactions took place | Oxygen-containing functional groups and nitrogen-containing functional groups; grafting | At pH = 6 and T = 303 K, the adsorption capacity was 275.98 mg/g | He et al., 2024 [93] |
| 11 | UO22+ | MWCNTs | RF plasma | Power: 80 W | O2, 20 Pa | Ion exchange and outer-sphere surface complexation | -COOH, carbonyl (C=O), and -OH groups; modification | At pH = 5.6 ± 0.1, T = 343.15 K, m/v = 0.3 g/L, C(NaClO4) = 0.01 M, the adsorption capacity was 4.06 × 10−4 mol/g | Song et al., 2012 [118] |
| 12 | U(VI) Th(IV) Eu(III) | Graphite-embedded magnetic nanoparticles | One-step arc discharge | Voltage: 650 V; current: 120 A; the discharge was produced by gradually decreasing the distance between the two rods | Gas mixture of He/CH4/NH (NH3: 0–5.0%; He/CH4 = 2:1), 80 Torr | Inner-sphere surface complexation | Quaternary, pyrrolic, amino, and pyridinic N | At pH = 4.0 ± 0.1 and T = 298.15 K, the adsorption capacities for U(VI), Th(IV), and Eu(III) were 47.28 mg/g, 45.48 mg/g, and 32.21 mg/g, respectively | Xiao et al., 2018 [95] |
| 13 | U(VI) | FG | - | The HV pulsed DC voltage; power: 100 W | 3.9 Pa | Complexation of U(VI) with -NH2 and phenolic hydroxyl groups on the surface of modified FG | -NH2 and -OH; grafting | At pH 6.0 ± 0.1 and T = 333.15 K, the adsorbent concentration = 0.25 g/L, the adsorption capacity was 140.68 mg/g | Duan et al., 2017 [15] |
| 14 | U(VI) | GO/PPy | DBD plasma | Power: 200 W; voltage: 100–110 V; time: 30 min; room temperature | N2 | Mainly attributed to surface complexation due to the coordination of U(VI) ions with oxygen- and nitrogen-containing functional groups | Nitrogen- and oxygen-containing functional groups; grafting | At pH = 5.0 ± 0.1 and T = 298 ± 2 K, the adsorption capacity was 147.1 mg/g | Hu et al., 2014 [100] |
| 15 | U(VI) | AGO | RF plasma | Power: 100 W | Ar, 10 Pa | Coordination of -NH2 functional groups | Graphite’s original functional group and -NH2 group | At pH = 5 and T = 298 K, the adsorption capacity was 341.5 mg/g | Liao et al., 2021 [22] |
| 16 | U(VI) | CMC/MGOs | Customized reactors | Power: 120 W; voltage: 950 V | N2, 10 Pa | Inner-sphere surface complexation | Hydroxyl group, carboxymethyl group, epoxy group, etc. | At pH = 5.5 ± 0.1, T = 301 K, and m/v = 0.25 g/L, the adsorption capacity was 7.94 × 10−4 mol/g | Zong et al., 2019 [101] |
| 17 | U(VI) | Biochar/MoS2 composites | RF plasma | Power: 180 W | H2, 30 Pa | The S vacancies, S, C-O and P-O of the BDC/MoS2-PO4 were bonded to [O = U = O]2+ in the solution | Modification | At pH = 6, the adsorption capacity was 204.08 mg/g | Sun et al., 2022 [21] |
| 18 | 238U (VI)241 Am(III) | AO/carbon nanofiber hybrids | Customized grafting reactors | Power: 70 W; voltage: 650 V; current: 60 mA | N2, 10 Pa | At pH = 5.0–7.0: inner-sphere surface complexation/surface precipitated; at pH = 3.5: inner-sphere surface complexation was formed on AO/CNF | AO; grafting | At pH = 3.5 and T = 293 K, the adsorption capacities for 238U(VI) and 241Am(III) were 588.24 mg/g and 40.79 mg/g, respectively | Sun et al., 2017 [119] |
| 19 | U(VI) | CDs | Atmospheric-pressure microplasma | Current: 10 mA | 60 sccm Ar | - | −COOH, -OH, etc. | At pH = 5, T = 298.15 K, and m/v = 0.5 mg/mL, the adsorption capacity was 173.60 mg/g | Wang et al., 2017 [108] |
| 20 | U(VI) | CB | RF plasma | Power: 60 W; time: 30 min | Carrier gas O2/Ar (5:25 ratio), <30 Pa | Adsorption was closely related to the single-site or double-site chelation of U(VI) with -NH2 and -C=N-OH, respectively | AO, oxygen-containing functional groups (mainly -COO); grafting | At pH = 6, T = 303 K, dosage = 0.4 g/L, and time = 24 h, the adsorption capacity was 220.95 mg/g in aqueous solution; at pH = 8.3, T = 293 K, dosage = 0.1 mg/L, and C[U (VI)] = 4.0 μg/L, the adsorption capacity was 3.2 mg/g in dynamic simulated seawater | He et al., 2024 [113] |
| 21 | U(VI) | C core–shell | RF plasma | Voltage: 5000 V; current: 1.0 mA | Ar, 200 sccm | The uranyl and -AsO2(OH) groups produced a strong affinity through chelation | Arsenazo III; grafting | At pH = 4, T = 298 K, C[U(VI)] = 2 × 10−5 mol/L, m/v = 0.6 g/L, and ionic strength = 0.01 mol/L NaCl, the adsorption capacity was 46.2 mg/g | Li et al., 2018 [104] |
| 22 | I | AC | Underwater plasma discharge | Power: 600 W; voltage: 2100 V | - | - | -OH, etc. | At 20% NaOH and 50% ethanol and time = 48 h, the adsorption capacity was 849 mg/g in water and the recovery of I adsorption capacity was 89% | Park et al., 2018 [114] |
| 23 | 127I− 131I− | ZnO/ MWCNTs nanocomposite | Arc discharge | Constant voltage: 2100 V alternating current: 15 A; each discharge time: 2 min; total discharge time: 30 min | - | Multi-layer physical adsorption | - | At pH = 5, T = 25 °C, and time = 60 min, the adsorption capacity was 15.25 mg/g | El- Khatib et al., 2024 [115] |
| 24 | Eu(III) | CMC/iron oxides/ MWCNTs | Customized grafting reactors | Power: 70 W; voltage: 650 V; current: 60 mA | N2, 10 Pa | At low pH, the main interaction mechanism was outer-sphere surface complexation, and at high pH, it was inner-sphere surface complexation | Multiple hydroxyl and carboxyl functional groups; grafting | At pH = 6.0 ± 0.1, T = 298 K, m/v = 0.6 g/L, and ionic strength = 0.01 mol/L NaNO3, the adsorption capacity was 3.36 × 10−4 mol/g | Zong et al., 2018 [116] |
| 25 | Sr(II) Cs(I) | CNT membrane | RF plasma | Power: 80 W | 70 Sccm3 min−1 for Ar and 40 Sccm3 min−1 for O2, 2 Pa | The removal mechanisms of divalent cations by adsorbents usually involved inner-sphere complexation reactions between the metal ions and the electron-pair donor atoms available on the surface of the adsorbents and the monovalent cations was primarily induced by electrostatic or Coulombic attraction between negatively charged CNTs | Functionalization | The partition coefficient was 4.14 for Sr and 0.81 for Cs | Ali et al., 2020 [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Y.; Guo, Z.; Lian, B.; Kang, J.; Fang, Z.; Qie, L.; Liu, L.; Zhao, L.; Wang, R. Plasma-Modified Carbon Materials for Radionuclide Absorption. C 2025, 11, 28. https://doi.org/10.3390/c11020028
Fang Y, Guo Z, Lian B, Kang J, Fang Z, Qie L, Liu L, Zhao L, Wang R. Plasma-Modified Carbon Materials for Radionuclide Absorption. C. 2025; 11(2):28. https://doi.org/10.3390/c11020028
Chicago/Turabian StyleFang, Yifan, Zixuan Guo, Bing Lian, Jing Kang, Zhou Fang, Longfei Qie, Lili Liu, Luxiang Zhao, and Ruixue Wang. 2025. "Plasma-Modified Carbon Materials for Radionuclide Absorption" C 11, no. 2: 28. https://doi.org/10.3390/c11020028
APA StyleFang, Y., Guo, Z., Lian, B., Kang, J., Fang, Z., Qie, L., Liu, L., Zhao, L., & Wang, R. (2025). Plasma-Modified Carbon Materials for Radionuclide Absorption. C, 11(2), 28. https://doi.org/10.3390/c11020028










