Probing the Catalytic Activity of Tin-Platinum Decorated Graphene; Liquid Phase Oxidation of Cyclohexane
Abstract
:1. Introduction
2. Results and Discussion
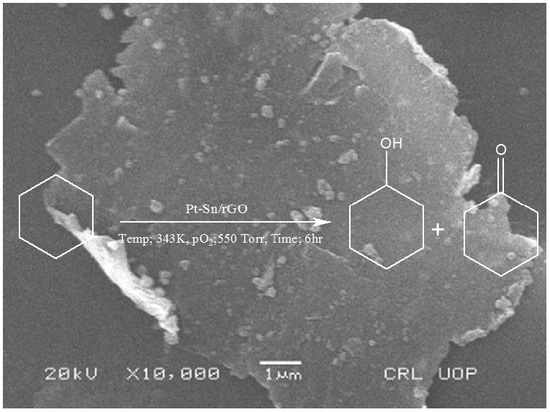
2.1. Characterization of the Catalyst
2.2. Oxidation of Cyclohexane: Effect of Reaction Conditions
2.3. Kinetic Analysis of the Data
3. Experimental Section
3.1. General
3.2. Synthesis of Pt-Sn Supported on Graphene Oxide (Pt-Sn/rGO)
3.3. Characterization of the Catalyst
3.4. Catalytic Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ebadi, A.; Safari, N.; Peyrovi, M.H. Aerobic oxidation of cyclohexane with γ-alumina supported metallophthalocyanines in the gas phase. Appl. Catal. A Gen. 2007, 321, 135–139. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, T.; Chen, L.; Ye, Y.; Qi, Z.; Freund, H.; Sundmacher, K. Selective oxidation of cyclohexanol to cyclohexanone in the ionic liquid 1-octyl-3-methylimidazolium chloride. Chem. Commun. 2011, 47, 9354–9356. [Google Scholar] [CrossRef] [PubMed]
- Salavati-Niasari, M.; Zamani, E.; Ganjali, M.R.; Norouzi, P. Synthesis, characterization and liquid phase oxidation of cyclohexanol using tert-butylhydroperoxide over host (zeolite-Y)/guest (copper (II) complexes of 12-and 13-membered diaza dioxa Schiff-base macrocyclic ligand) nanocomposite materials (HGNM). J. Mol. Catal. A Chem. 2007, 261, 196–201. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, J.; Huang, W.; Liu, X. Electrocatalytic oxidation of cyclohexanol on a nickel oxyhydroxide modified nickel electrode in alkaline solutions. Catal. Commun. 2007, 8, 1017–1022. [Google Scholar] [CrossRef]
- Sakthivel, A.; Selvam, P. Mesoporous (Cr) MCM-41: A mild and efficient heterogeneous catalyst for selective oxidation of cyclohexane. J. Catal. 2002, 211, 134–143. [Google Scholar] [CrossRef]
- Conte, M.; Liu, X.; Murphy, D.M.; Whiston, K.; Hutchings, G.J. Cyclohexane oxidation using Au/MgO: An investigation of the reaction mechanism. Phys. Chem. Chem. Phys. 2012, 14, 16279–16285. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, F.; Tan, J.; Hu, X.; Wang, H.; Yang, J.; Zheng, W. Selective catalysis of the aerobic oxidation of cyclohexane in the liquid phase by carbon nanotubes. Angew. Chem. Int. Ed. Engl. 2011, 50, 3978–3982. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.V.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Cyclohexane selective oxidation over metal-organic frameworks of MIL-101 family: Superior catalytic activity and selectivity. Chem. Commun. 2012, 48, 6812–6814. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, U.; Pereira, R.; Krähembühl, C.E.; Rufo, M.; Buffon, R. Synergistic effect of iron and copper oxides supported on silica in the room temperature oxidation of cyclohexane. Appl. Catal. A Gen. 1995, 131, 135–141. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, J.; Miao, H.; Wang, F.; Li, X. Catalytic oxidation of cyclohexane to cyclohexanol and cyclohexanone over Co3O4 nanocrystals with molecular oxygen. Appl. Catal. A Gen. 2005, 292, 223–228. [Google Scholar] [CrossRef]
- Da Cruz, R.S.; Silva, J.M.D.S.; Arnold, U.; Schuchardt, U. Catalytic activity and stability of a chromium containing silicate in liquid phase cyclohexane oxidation. J. Mol. Catal. A Chem. 2001, 171, 251–257. [Google Scholar] [CrossRef]
- Huang, H.; Sun, D.; Wang, X. Pt-Co alloy nanoparticles supported on graphene nanosheets with high performance for methanol oxidation. Chin. Sci. Bull. 2012, 57, 3071–3079. [Google Scholar] [CrossRef]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Marinkas, A.; Arena, F.; Mitzel, J.; Prinz, G.M.; Heinzel, A.; Peinecke, V.; Natter, H. Graphene as catalyst support: The influences of carbon additives and catalyst preparation methods on the performance of PEM fuel cells. Carbon 2013, 58, 139–150. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef]
- Metin, Ö.; Kayhan, E.; Özkar, S.; Schneider, J.J. Palladium nanoparticles supported on chemically derived graphene: An efficient and reusable catalyst for the dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2012, 37, 8161–8169. [Google Scholar] [CrossRef]
- Li, Y.; Gao, W.; Ci, L.; Wang, C.; Ajayan, P.M. Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 2010, 48, 1124–1130. [Google Scholar] [CrossRef]
- Hsu, K.C.; Chen, D.H. Green synthesis and synergistic catalytic effect of Ag/reduced graphene oxide nanocomposite. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Huo, Y.; Yang, J.; Chang, S.; Ma, Y.; Huang, W. Reduced graphene oxide supported Au nanoparticles as an efficient catalyst for aerobic oxidation of benzyl alcohol. Appl. Surf. Sci. 2013, 280, 450–455. [Google Scholar] [CrossRef]
- Metin, Ö.; Ho, S.F.; Alp, C.; Can, H.; Mankin, M.N.; Gültekin, M.S.; Sun, S. Ni/Pd core/shell nanoparticles supported on graphene as a highly active and reusable catalyst for Suzuki-Miyaura cross-coupling reaction. Nano Res. 2013, 6, 10–18. [Google Scholar] [CrossRef]
- Sadiq, M.; Aman, R.; Saeed, K.; Ahmad, M.S.; Zia, M.A. Green and sustainable heterogeneous organo-catalyst for asymmetric aldol reactions. Mod. Res. Catal. 2015, 4, 43. [Google Scholar] [CrossRef]
- Su, B.J.; Wang, K.W.; Tseng, C.J.; Wang, C.H.; Hsueh, Y.J. Synthesis and catalytic property of PtSn/C toward the ethanol oxidation reaction. Int. J. Electrochem. Sci. 2012, 7, 5246–5255. [Google Scholar]
- Zhang, W.; Li, Y.; Zeng, X.; Peng, S. Synergetic effect of metal nickel and graphene as a cocatalyst for enhanced photocatalytic hydrogen evolution via dye sensitization. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiq, M.; Sadiq, S.; Abid Zia, M.; Ali, M.; Saeed, K.; Sohail Ahmad, M.; Ali, R. Probing the Catalytic Activity of Tin-Platinum Decorated Graphene; Liquid Phase Oxidation of Cyclohexane. C 2016, 2, 8. https://doi.org/10.3390/c2010008
Sadiq M, Sadiq S, Abid Zia M, Ali M, Saeed K, Sohail Ahmad M, Ali R. Probing the Catalytic Activity of Tin-Platinum Decorated Graphene; Liquid Phase Oxidation of Cyclohexane. C. 2016; 2(1):8. https://doi.org/10.3390/c2010008
Chicago/Turabian StyleSadiq, Mohammad, Saima Sadiq, Muhammad Abid Zia, Muhammad Ali, Khalid Saeed, Muhammad Sohail Ahmad, and Rahmat Ali. 2016. "Probing the Catalytic Activity of Tin-Platinum Decorated Graphene; Liquid Phase Oxidation of Cyclohexane" C 2, no. 1: 8. https://doi.org/10.3390/c2010008
APA StyleSadiq, M., Sadiq, S., Abid Zia, M., Ali, M., Saeed, K., Sohail Ahmad, M., & Ali, R. (2016). Probing the Catalytic Activity of Tin-Platinum Decorated Graphene; Liquid Phase Oxidation of Cyclohexane. C, 2(1), 8. https://doi.org/10.3390/c2010008