Biosensors Based on Lipid Modified Graphene Microelectrodes
Abstract
:1. Introduction
1.1. Protein and Peptide-Based Biosensors Based on Nanostructured Materials
1.2. Graphene Nanomaterials Used in Electrochemical (Bio)Sensors Fabrication
2. Experimental
2.1. Materials and Solutions
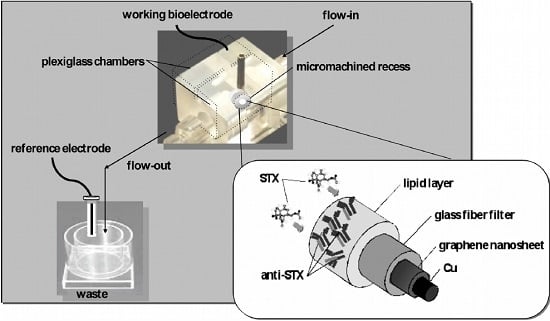
2.2. Fabrication of the Lipid-Based Sensor Electrode with Incorporated Receptor on a Graphene Electrode
2.3. Electrochemical Measurements
3. Mechanism of Signal Generation
4. Examples of Biosensors Based on Lipid Modified Graphene Microelectrodes
5. Summary and Conclusions
- The highly suitable microenvironment for the attachment of “receptor” molecules due to the same chemistry of lipid membrane and “receptor” molecules which coexists in living cell membranes.
- The stabilized lipid membrane has the ability to increase the life time of biosensor compared to previous reported biosensors without lipid membrane on the transducer surface.
- Due to the stabilized membrane, the sensitivity of presented cholesterol biosensor is improved.
- The wide range of analyte concentrations that is detected by the proposed biosensor.
- The biosensor is successfully applied in the real time sample analysis which strongly supported that the present biosensor could be used for the monitoring of an analyte in real samples, however without the stabilized lipid membrane, it is quite difficult to apply the biosensor for the detection of analyte with precise and accurate measurements.
- The negligible response to the common interfererents demonstrates the good selectivity of the presented biosensor.
- The detection limit of the present sensors is lower than that previously-described by three fold of a decade in most of the cases.
Conflicts of Interest
References
- Fullerenes and Carbon Nanotubes. Available online: http://www.docbrown.info/page03/nanochem04.htm#Diagrams (accessed on 16 March 2017).
- Polat, E.O.; Balci, O.; Kakenov, N.; Uzlu, H.B.; Kocabas, C.; Dahiya, R. Synthesis of large area graphene for high performance in flexible optoelectronic devices. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nikoleli, G.-P.; Tzamtzis, N.; Nikolelis, D.P.; Psaroudakis, N.; Danielsson, B.; Israr, M.Q.; Willander, M. Potentiometric Cholesterol Biosensor Based on ZnO Nanowalls and Stabilized Polymerized Lipid Film. Electroanalysis 2013, 25, 367–372. [Google Scholar]
- Campuzano, S.; Wang, J. Nanobioelectroanalysis Based on Carbon/Inorganic Hybrid Nanoarchitectures. Electroanalysis 2011, 23, 1289–1300. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–20134. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Sasaki, T.; Pumera, M. Platelet Graphite Nanofibers for Electrochemical Sensing and Biosensing: The Influence of Graphene Sheet Orientation. Chem. Asian J. 2010, 5, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new nanocarbon. Mater. J. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Artiles, M.S.; Rout, C.S.; Fisher, T.S. Graphene-based hybrid materials and devices for biosensing. Adv. Drug Deliv. Rev. 2011, 63, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Pan, Y.; Li, L. Recent advances in graphene-based nanomaterials for biomedical applications. Nano Life 2012, 2, 1230001. [Google Scholar] [CrossRef]
- Zheng, D.; Vashist, S.K.; Dykas, M.M.; Saha, S.; Al-Rubeaan, K.; Lam, E.; Luong, J.H.T.; Sheu, F.-S. Graphene versus Multi-Walled Carbon Nanotubes for Electrochemical Glucose Biosensing. Materials 2013, 6, 1011–1027. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Pan, C. Influence of graphene microstructures on electrochemical performance for supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 379–385. [Google Scholar] [CrossRef]
- Israr, M.Q.; ul Hasan, K.; Sadaf, J.R.; Engquist, I.; Nur, O.; Willander, M.; Danielsson, B. Structural Characterization and Biocompatible Applications of Graphene Nanosheets for Miniaturization of Potentiometric Cholesterol Biosensor. J. Biosens. Bioelectron. 2011, 2, 109–114. [Google Scholar] [CrossRef]
- Pumera, P. Graphene in biosensing. Mater. Today 2011, 14, 308–315. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemically Modified Graphenes as Detectors in Lab-on-Chip Device. Electroanalysis 2013, 25, 945–950. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Raftopoulou, G.; Nikoleli, G.-P.; Simantiraki, M. Stabilized Lipid Membrane Based Biosensors with Incorporated Enzyme for Repetitive Uses. Electroanalysis 2006, 18, 2467–2474. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Ntanos, N.; Nikoleli, G.-P.; Tampouris, K. Development of an Electrochemical Biosensor for the Rapid Detection of Naphthalene Acetic Acid in Fruits by Using Air Stable Lipid Films with Incorporated Auxin-Binding Protein 1 Receptor. Protein Pept. Lett. 2008, 15, 789–794. [Google Scholar] [CrossRef]
- Nikolelis, D.P.; Chaloulakos, T.-I.; Nikoleli, G.-P.; Psaroudakis, N. A portable sensor for the rapid detection of naphthalene acetic acid in fruits and vegetables using stabilized in air lipid films with incorporated auxin-binding protein 1 receptor. Talanta 2008, 77, 786–792. [Google Scholar] [CrossRef]
- Bratakou, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Karapetis, S.; Tzamtzis, N. Development of an Electrochemical Biosensor for the Rapid Detection of Saxitoxin Based on Air Stable Lipid Films with Incorporated Anti-STX Using Graphene Electrodes. Electroanalysis 2017, in press. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Ibupoto, Z.H.; Nikolelis, D.P.; Likodimos, V.; Psaroudakis, N.; Tzamtzis, N.; Willander, M.; Hianik, T. Potentiometric cholesterol biosensing application of graphene electrode with stabilized polymeric lipid membrane. Cent. Eur. J. Chem. 2013, 11, 1554–1561. [Google Scholar] [CrossRef]
- Charpentier, L.; Murr, I.E. Amperometric determination of cholesterol in serum with use of a renewable surface peroxidase electrode. Anal. Chim. Acta 1995, 318, 89–93. [Google Scholar] [CrossRef]
- Huang, Y.; Palkar, P.V.; Li, L.-J.; Zhang, H.; Chen, P. Integrating carbon nanotubes and lipid bilayer for biosensing. Biosens. Bioelectron. 2010, 25, 1834–1837. [Google Scholar] [CrossRef] [PubMed]
- Ang, P.K.; Jaiswal, M.; Haley, C.; Lim, Y.X.; Wang, Y.; Sankaran, J.; Li, A.; Lim, C.T.; Wohland, T.; Barbaros, Ö.; et al. A Bioelectronic Platform Using a Graphene-Lipid Bilayer Interface. ACS Nano 2010, 4, 7387–7394. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.K.; Jaffa, A.A. Recent Advances in Application of Biosensors in Tissue Engineering. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Jackowska, K.; KrysinskI, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, F.; Isin, D.; Polat, D. Graphene Oxide Modified Chemically Activated Graphite Electrodes for Detection of microRNA. Electroanalysis 2017. [Google Scholar] [CrossRef]
- Liu, J.; Guo, S.; Han, L.; Wang, T.; Hong, W.; Liu, Y.; Wang, E. Synthesis of phospholipid monolayer membrane functionalized graphene for drug delivery. J. Mater. Chem. 2012, 22, 20634–20640. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Israr, M.Q.; Tzamtzis, N.; Nikolelis, D.P.; Willander, M.; Psaroudakis, N. Structural Characterization of Graphene Nanosheets for Miniaturization of Potentiometric Urea Lipid Film Based Biosensors. Electroanalysis 2012, 24, 1285–1295. [Google Scholar] [CrossRef]
- Karapetis, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Tzamtzis, N.; Psaroudakis, N. Development of an Electrochemical Biosensor for the Rapid Detection of Cholera Toxin Based on Air Stable Lipid Films with Incorporated Ganglioside GM1 Using Graphene Electrodes. Electroanalysis 2016, 28, 1584–1590. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Nikolelis, D.P.; Tzamtzis, N.; Psaroudakis, N. A Selective Immunosensor for D-dimer Based on Antibody Immobilized on a Graphene Electrode with Incorporated Lipid Films. Electroanalysis 2014, 26, 1522–1527. [Google Scholar] [CrossRef]
- Bratakou, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Psaroudakis, N. Development of a Potentiometric Chemical Sensor for the Rapid Detection of Carbofuran Based on Air Stable Lipid Films with Incorporated Calix[4]arene Phosphoryl Receptor Using Graphene Electrodes. Electroanalysis 2015, 27, 2608–2613. [Google Scholar] [CrossRef]
- Bratakou, S.; Nikoleli, G.-P.; Siontorou, C.G.; Karapetis, S.; Nikolelis, D.P.; Tzamtzis, N. Electrochemical Biosensor for Naphthalene Acetic Acid in Fruits and Vegetables Based on Lipid Films with Incorporated Auxin-binding Protein Receptor Using Graphene Electrodes. Electroanalysis 2016, 28, 2171–2177. [Google Scholar] [CrossRef]




| Analyte | “Receptor” | Reference |
|---|---|---|
| Urea | Urease | [29] |
| Cholesterol | Cholesterol oxidase | [21] |
| Cholera toxin | Ganglioside GM1 | [30] |
| D-dimer | Mouse anti human D-dimer antibody | [31] |
| Carbofuran | Artificial receptor for carbofuran | [32] |
| NAA | Auxin receptor | [33] |
| Saxitoxin | Anti-STX | [20] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Bratakou, S.; Karapetis, S.; Tzamtzis, N. Biosensors Based on Lipid Modified Graphene Microelectrodes. C 2017, 3, 9. https://doi.org/10.3390/c3010009
Nikoleli G-P, Siontorou CG, Nikolelis DP, Bratakou S, Karapetis S, Tzamtzis N. Biosensors Based on Lipid Modified Graphene Microelectrodes. C. 2017; 3(1):9. https://doi.org/10.3390/c3010009
Chicago/Turabian StyleNikoleli, Georgia-Paraskevi, Christina G. Siontorou, Dimitrios P. Nikolelis, Spyridoula Bratakou, Stephanos Karapetis, and Nikolaos Tzamtzis. 2017. "Biosensors Based on Lipid Modified Graphene Microelectrodes" C 3, no. 1: 9. https://doi.org/10.3390/c3010009
APA StyleNikoleli, G.-P., Siontorou, C. G., Nikolelis, D. P., Bratakou, S., Karapetis, S., & Tzamtzis, N. (2017). Biosensors Based on Lipid Modified Graphene Microelectrodes. C, 3(1), 9. https://doi.org/10.3390/c3010009