Functionalized Graphene–Polyoxometalate Nanodots Assembly as “Organic–Inorganic” Hybrid Supercapacitors and Insights into Electrode/Electrolyte Interfacial Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microscopic Structural Characterization
2.2. Optical and Vibrational Spectroscopy
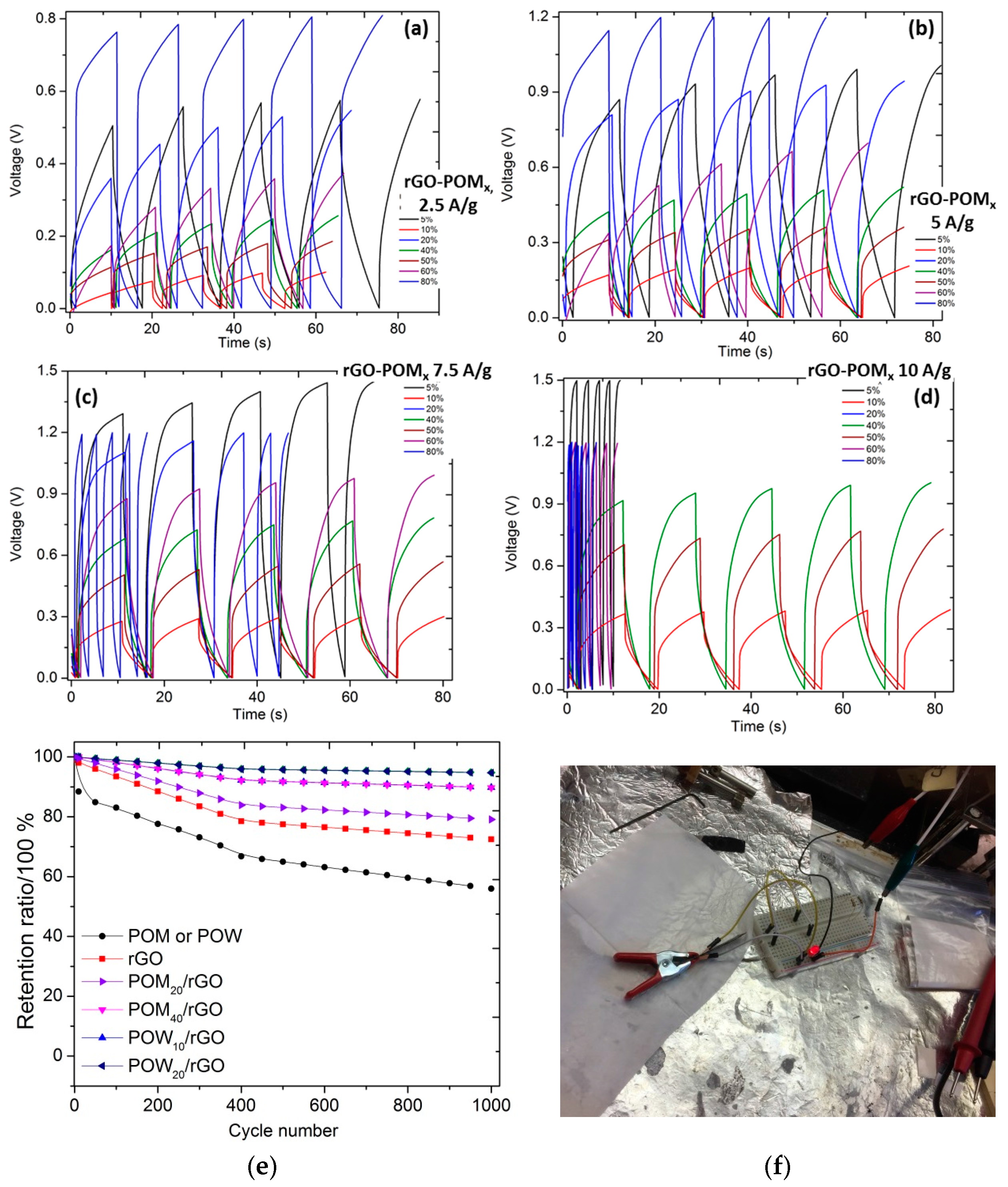
2.3. Electrochemical Properties
2.4. Scanning Electrochemical Microscopy
3. Materials and Methods
3.1. Hydrothermal Synthesis and Electrodes Preparation
3.2. Sample Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Conway, B.E.; Bliss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Crabtree, G.; Lewis, N. Renewable energy: Progress and prospects. Phys. Today 2007, 60, 37–42. [Google Scholar] [CrossRef]
- Romero, P.G.; Ayyad, O.; Guevara, J.S.; Rojas, D.M. Hybrid organic-inorganic materials: From Child’s play to energy applications. J. Sol. Stat. Electrochem. 2010, 14, 1939–1945. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, P.G.; Scrosat, B.; Raeascon, J.-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Carrizosa, S.B.; McDonald, B.; Jasinski, J.; Dimakis, N. Graphene-family nanomaterials assembled with cobalt oxides and cobalt nanoparticles as hybrid supercapacitive electrodes and enzymeless glucose detection platforms. J. Mater. Res. 2017, 32, 301–322. [Google Scholar] [CrossRef]
- Laforgue, A.; Simon, P.; Fauvarque, J.F.; Conte, M.; Sarrau, J.F.; Lailler, P. Hybrid supercapacitors—From laboratory to industrial development. Work 2002, 6, 8. [Google Scholar]
- Gupta, S.; McDonald, B.; Carrizosa, S.B.; Price, C. Microstructure, residual stress, and intermolecular force distribution maps of graphene/polymer hybrid composites: Nanoscale morphology-promoted synergistic effects. Compos. Part B Eng. 2016, 92, 175–192. [Google Scholar] [CrossRef]
- Gupta, S.; Price, C. Investigating graphene/conducting polymer hybrid layered composites as pseudocapacitors: Interplay of heterogeneous electron transfer, electric double layers and mechanical stability. Compos. Part B Eng. 2016, 105, 46–59. [Google Scholar] [CrossRef]
- Geim, A.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.; Eda, G.; Chowalla, M. Graphene oxide as a chemically tunable platform for optical applications. Nat. Chem. 2010, 2, 1015. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Chowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.P.; Bao, Q.L.; Ang, P.K.; Yang, J.X. The chemistry of grapheme. J. Mater. Chem. 2010, 20, 2277. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene transistors. Nat. Nanotechnol. 2010, 5, 487. [Google Scholar] [CrossRef] [PubMed]
- Blake, P.; Brimicombe, P.D.; Nair, R.R.; Booth, T.J.; Jiang, D.; Schedin, F.; Ponomarenko, L.A.; Morozov, S.V.; Gleeson, H.F.; Hill, E.W.; et al. Graphene-based liquid crystal device. Nano Lett. 2008, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Maehashi, K.; Yamashiro, Y.; Matsumoto, K. Electrolyte-gated graphene field-effect transistors for detecting pH and protein adsorption. Nano Lett. 2009, 9, 3318. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, I.V.; Patila, M.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Graphene-based nanobiocatalytic systems: Recent advances and future prospects. Trends Biotechnol. 2014, 32, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, J.; Zhao, Q.; Liu, L.; Zhang, Z. Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 2010, 6, 537. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Picraux, T. Enhanced lithium ion battery cycling of silicon nanowire anodes by template growth to eliminate silicon underlayer islands. Nano Lett. 2013, 13, 5740–5747. [Google Scholar] [CrossRef] [PubMed]
- Vaillant, J.; Cantu, M.L.; Gallegos, K.C.; Pastor, N.C.; Romero, P.G. Chemical synthesis of hybrid materials based on PAni and PEDOT with polyoxometalates for electrochemical superapacitors. Prog. Sol. Stat. Chem. 2006, 34, 147–159. [Google Scholar] [CrossRef]
- Cericola, D.; Kötz, R. Hybridization of electrochemical capacitors and rechargeable batteries: Principles and limits. Electroch. Acta 2012, 72, 1–17. [Google Scholar] [CrossRef]
- Brousse, T.; Maarchand, R.; Taberna, P.-L.; Simon, P. TiO2-activated carbon non-aqueous hybrid system for energy storage. J. Power Sources 2006, 158, 571–577. [Google Scholar] [CrossRef]
- Naoi, K.; Ishimoto, S.; Miyamoto, J.-I.; Naoi, W. Second generation “nanohybrid supercapacitor”: Evolution of capacitive energy storage devices. Energy Environ. Sci. 2012, 5, 9363–9373. [Google Scholar] [CrossRef]
- Gupta, S.; Carrizosa, S.B. Graphene–inorganic hybrids with cobalt oxide polymorphs for electrochemical energy systems and electrocatalysis: Synthesis, processing and properties. J. Electron. Mater. 2015, 44, 4492–4509. [Google Scholar] [CrossRef]
- Gupta, S.; van Meveren, M.; Jasinski, J. Investigating electrochemical properties and interfacial processes of manganese oxides/graphene hybrids as high-performance supercapacitor electrodes. Int. J. Electrochem. Sci. 2015, 10, 10272–10291. [Google Scholar]
- Gupta, S.; Aberg, B.; Carrizosa, S.B.; Dimakis, N. Vanadium pentoxide nanobelt-reduced graphene oxide nanosheet composites as high-performance pseudocapacitive electrodes: AC impedance spectroscopy data modeling and theoretical calculations. Materials 2016, 9, 615. [Google Scholar] [CrossRef]
- Wang, H.; Dai, H. Strongly coupled inorganic–nano-carbon hybrid materials for energy storage. Chem. Soc. Rev. 2013, 42, 3088–3113. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef] [PubMed]
- Avansi, W., Jr.; Ribeiro, C.; Leite, E.R.; Mastelaro, V.R. Vanadium pentoxide nanostructures: An effective control of morphology and crystal structure in hydrothermal conditions. Cryst. Growth Des. 2009, 9, 3626–3631. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Cheng, T.; Wang, Y.; Lai, W.-Y.; Pang, H.; Huang, W. A Simple approach to boost capacitance: Flexible supercapacitors based on manganese Oxides@MOFs via chemically induced in situ self-transformation. Adv. Mater. 2016, 28, 5242–5249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Lai, W.-Y.; Pang, H.; Huang, W. Flexible supercapacitors based on paper substrates: A new paradigm for low-cost energy storage. Chem. Soc. Rev. 2015, 44, 5181. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.-Z.; Zhang, J.-D.; Lai, W.-Y.; Huang, W. High-performance free-standing PEDOT:PSS electrodes for flexible and transparent all-solid-state supercapacitors. J. Mater. Chem. A 2016, 4, 10493–10499. [Google Scholar] [CrossRef]
- Romero, P.G. Polyoxometalates as photoelectrochemical models for quantum-sized semiconducting oxides. Solid State Ionics 1997, 101, 243–248. [Google Scholar] [CrossRef]
- Symes, M.D.; Cronin, L. Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nat. Chem. 2013, 5, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Krickemeyer, E.; Meyer, J.; Bogge, H.; Peters, F.; Plass, W.; Diemann, E.; Dillinger, S.; Nonnenbrunch, F.; Randernath, M.; et al. [Mo154(NO)14O420(OH)28(H2O)70](25 ± 5)−: A water-soluble big wheel with more than 700 atoms and a relative molecular mass of about 24000. Angew. Chem. Int. Ed. 1995, 34, 2122–2124. [Google Scholar] [CrossRef]
- Muller, A.; Beckemann, E.; Bogge, H.; Schmidtmann, M.; Dress, A. Inorganic chemistry goes protein size: A Mo368 nano-hedgehog initiating nanochemistry by symmetry breaking. Angew. Chem. Int. Ed. 2002, 41, 1163–1167. [Google Scholar] [CrossRef]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Guevara, J.; Ruiz, V.; Romero, P.G. Hybrid energy storage: High voltage aqueous supercapacitors based on activated carbon–phosphotungstate hybrid materials. J. Mater. Chem. A 2014, 2, 1014–1021. [Google Scholar] [CrossRef]
- Dubal, D.P.; Guervara, J.S.; Tonti, D.; Encisco, E.; Romero, P.G. A high voltage solid state symmetric supercapacitor based on graphene–polyoxometalate hybrid electrodes with hydroquinone doped hybrid gel-electrolyte. J. Mater. Chem. A 2015, 3, 23483–23492. [Google Scholar] [CrossRef]
- Kim, Y.; Shanmugam, S. Polyoxometalate-reduced graphene oxide hybrid catalyst: Synthesis, structure, and electrochemical properties. ACS Appl. Mater. Interfaces 2013, 5, 12197–12204. [Google Scholar] [CrossRef] [PubMed]
- Tessonnier, J.-P.; Renaudin, S.G.; Alia, S.; Yan, Y.; Barteau, M.A. Structure, stability and electronic interactions of polyoxometalates on functionalized graphene sheets. Lnagmuir 2013, 29, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Byrappa, K.; Yoshimura, M. Handbook of Hydrothermal Technology; Noyes Publications: Park Ridge, NJ, USA, 2001. [Google Scholar]
- Gupta, S.; Price, C. Scanning electrochemical microscopy of graphene/polymer hybrid thin films as supercapacitors: Physical–chemical interfacial processes. AIP Adv. 2015, 5, 107113-1–107113-8. [Google Scholar] [CrossRef]
- Gupta, S.; Carrizosa, S.B. Insights into electrode/electrolyte interfacial processes and the effect of nanostructured cobalt oxides loading on graphene-based hybrids by scanning electrochemical microscopy. Appl. Phys. Lett. 2016, 109, 243903–243907. [Google Scholar] [CrossRef]
- Bard, A.J.; Mirkin, M.V. (Eds.) Scanning Electrochemical Microscopy; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Song, I.K.; Kaba, M.S.; Barteau, M.A.; Lee, W.Y. Investigation of redox potential and negative differential resistance behavior of heteropolyacids by scanning tunneling microscopy. Catal. Today 1998, 44, 285–291. [Google Scholar] [CrossRef]
- Wen, S.; Guan, W.; Wang, J.; Lang, Z.; Yan, L.; Su, Z. Theoretical investigation of structural and electronic properties of [PW12O40]3− on graphene layer. Dalton Trans. 2012, 41, 4602–4607. [Google Scholar] [CrossRef] [PubMed]
- Schwegler, M.A.; Vinke, P.; van der Eijk, M.; van Bekkum, H. Activated carbon as a support for heteropolyanion catalysts. Appl. Catal. A 2012, 80, 41–57. [Google Scholar] [CrossRef]
- Jorio, A.; Dresselhaus, M.S.; Saito, R.; Dresselhaus, G. Raman Spectroscopy in Graphene Related Systems; Wiley-VCH Verlag: New York, NY, USA, 2011. [Google Scholar]
- Ratajczak, H.; Barnes, A.J.; Bielanski, A.; Lutz, H.D.; Müller, A. Polyoxometalate Chemistry from Topology via Self-Assembly to Applications; Muller, A., Pope, M.T., Eds.; Springer: Amesterdam, The Netherlands, 2011; p. 101. [Google Scholar]
- Lee, C.-Y.; Bond, A.M. Evaluation of levels of defect sites present in highly ordered pyrolytic graphite electrodes using capacitive and faradaic current components derived simultaneously from large-amplitude fourier transformed ac voltammetric experiments. Anal. Chem. 2009, 81, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.S. Effect of local structure on the UV-visible absorption edges of molybdenum oxide clusters and supported molybdenum oxides. J. Catal. 1995, 151, 470–474. [Google Scholar] [CrossRef]
- Cox, P.A. Transition Metal Oxides: An Introduction to Their Electronic Structure and Properties; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Gupta, S.; Saxena, A. Nanocarbon materials: Probing the curvature and topology effects using phonon spectra. J. Raman Spectrosc. 2009, 40, 1127–1137. [Google Scholar] [CrossRef]
- Vidoeski, B.A.; Jovanic, S.P.; Antunovic, I.D.H.; Bogdanovic, D.V.B.; Budimir, M.D.; Markovic, Z.M.; Markovic, B.M.T. Raman study of the interactions between highly ordered pyrolytic graphite (HOPG) and polyopxometalates: The effects of acid concentration. J. Serb. Chem. Soc. 2016, 81, 777–787. [Google Scholar] [CrossRef]
- Nomiya, K.; Sugie, Y.; Amimoto, K.; Miwa, M. Charge-transfer absorption spectra of some tungsten(VI) and molybdenum(VI) polyoxoanions. Polyhedron 1987, 6, 519–524. [Google Scholar] [CrossRef]
- Sasca, V.; Popa, A. Band-gap energy of heteropoly compounds containing Keggin polyanion-[PVxMo12−xO40]−(3+x) relates to counter-cations and temperature studied by UV-VIS diffuse reflectance spectroscopy. J. Appl. Phys. 2013, 114, 133503. [Google Scholar] [CrossRef]
- Gupta, S.; Heintzman, E.; Jasinski, J. Multiphonon Raman spectroscopy properties and Raman mapping of 2D van der Waals solids: Graphene and beyond. J. Raman Spectrosc. 2015, 45, 217–230. [Google Scholar] [CrossRef]
- Deltcheff, C.R.; Fournier, M.; Franck, R.; Thouvenot, R. Vibrational investigations of polyoxometalates. 2. Evidence for anion-anion interactions in molybdenum (VI) and tungsten (VI) compounds related to the Keggin structure. Inorg. Chem. 1983, 22, 207. [Google Scholar] [CrossRef]
- Grinenval, E.; Rozanska, X.; Baudouin, A.; Berrier, E.; Delbecq, F.; Sautet, P.; Basset, J.-M.; Lefebvre, F. Controlled interactions between anhydrous Keggin-type heteropolyacids and silica support: Preparation and characterization of well-defined silica-supported polyoxometalates species. J. Phys. Chem. C 2010, 114, 19024–19034. [Google Scholar] [CrossRef]
- Aparicio-Angles, X.; Closet, A.; Bo, C.; Poblet, J.M. Towards the computational modeling of polyoxoanions on metal surfaces: IR spectrum characterization of [SiW12O40]4− on Ag (111). Phys. Chem. Chem. Phys. 2011, 13, 15143–15147. [Google Scholar] [CrossRef] [PubMed]
- Sadakane, M.; Steckhan, E. Electrochemical properties of polyoxometalates as electrocatalysts. Chem. Rev. 1998, 98, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483. [Google Scholar] [CrossRef]
- Celzard, A.; Collas, F.; Marêché, J.F.; Furdin, G.; Rey, I. Porous electrodes-based double-layer supercapacitors: Pore structure versus series resistance. J. Power Sources 2002, 108, 153. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Constant-phase-element behavior caused by resistivity distributions in films I. Theory. J. Electrochim. Soc. 2010, 157, C452. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jorcin, J.-B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473. [Google Scholar] [CrossRef] [Green Version]
- Grover, F.W. Inductance Calculations; Dover Publications: Schenectady, NY, USA, 2009. [Google Scholar]
- Tüken, T.; Yazici, B.; Erbil, M. A new multilayer coating for mild steel protection. Progr. Org. Coat. 2004, 50, 115. [Google Scholar] [CrossRef]
- Taberna, P.L.; Simon, P.; Fauvarque, J.F. Electrochemical characteristics and impedance spectroscopy studies of carbon–carbon supercapacitors. J. Electrochem. Soc. 2003, 150, A292. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True performance metrics in electrochemical energy storage. Sci. Mag. 2011, 334, 917–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafabadi, A.I.; Yasud, S.; Kobashi, K.; Yamada, T.; Futaba, D.N.; Hatori, H.; Yumura, M.; Iijima, S.; Hata, K. Extracting the full potential of single-walled carbon nanotubes as durable supercapacitor electrodes operable at 4 V with high power and energy density. Adv. Energy Mater. 2010, 22, E235–E241. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; López, J.R.; Parks, J.J.; Ritzert, N.L.; Ralph, D.C.; Abruňa, H.D. Reactivity of monolayer chemical vapor deposited graphene imperfections studied using scanning electrochemical microscopy. ACS Nano 2012, 6, 3070. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Tegou, E.; Pseiropoulos, G.; Filippidou, M.K.; Chatzandroulis, S. Low-temperature thermal reduction of graphene oxide films in ambient atmosphere: Infra-red spectroscopic studies and gas sensing applications, Hydrazine-reduction of graphite- and graphene oxide. Microelectron. Eng. 2016, 159, 146–150. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Damyanova, S.; Gomez, L.M.; Bañares, M.A.; Fierro, J.L.G. Thermal behavior of 12-molybdophosphoric acid supported on zirconium-loaded silica. Chem. Mater. 2000, 12, 501. [Google Scholar] [CrossRef]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, S.; Aberg, B.; Carrizosa, S.B. Functionalized Graphene–Polyoxometalate Nanodots Assembly as “Organic–Inorganic” Hybrid Supercapacitors and Insights into Electrode/Electrolyte Interfacial Processes. C 2017, 3, 24. https://doi.org/10.3390/c3030024
Gupta S, Aberg B, Carrizosa SB. Functionalized Graphene–Polyoxometalate Nanodots Assembly as “Organic–Inorganic” Hybrid Supercapacitors and Insights into Electrode/Electrolyte Interfacial Processes. C. 2017; 3(3):24. https://doi.org/10.3390/c3030024
Chicago/Turabian StyleGupta, Sanju, Bryce Aberg, and Sara B. Carrizosa. 2017. "Functionalized Graphene–Polyoxometalate Nanodots Assembly as “Organic–Inorganic” Hybrid Supercapacitors and Insights into Electrode/Electrolyte Interfacial Processes" C 3, no. 3: 24. https://doi.org/10.3390/c3030024
APA StyleGupta, S., Aberg, B., & Carrizosa, S. B. (2017). Functionalized Graphene–Polyoxometalate Nanodots Assembly as “Organic–Inorganic” Hybrid Supercapacitors and Insights into Electrode/Electrolyte Interfacial Processes. C, 3(3), 24. https://doi.org/10.3390/c3030024




