Catalytic Growth of Carbon Nanotubes by Direct Liquid Injection CVD Using the Nanocluster [HxPMo12O40?H4Mo72Fe30(O2CMe)15O254(H2O)98-y(EtOH)y]
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Characterization
2.2. FeMoC Synthesis
2.3. CNT Growth
3. Results and Discussion
3.1. Optimization of FeMoC Synthesis
3.2. Direct Liquid Injection Chemical Vapor Deposition (DLICVD)
3.3. Variation of Aspiration Temperature
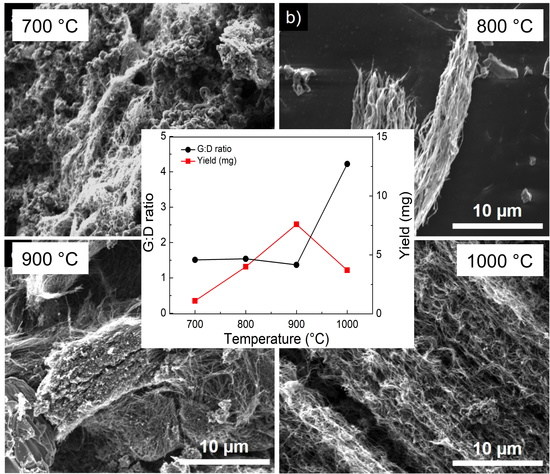
3.4. Variation of Growth Temperature
3.5. Variation of Injection Rate
3.6. Variation of Catalyst Concentration
3.7. Variation in Carbon Source
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Ameli, A.; Arjmand, M.; Sundararaj, U. Effects of synthesis catalyst and temperature on broadband dielectric properties of nitrogen-doped carbon nanotube/polyvinylidene fluoride nanocomposites. Carbon 2016, 106, 260–278. [Google Scholar] [CrossRef]
- Arjmand, M.; Ameli, A.; Sundararaj, U. Employing nitrogen doping as innovative technique to improve broadband dielectric properties of carbon nanotube/polymer nanocomposites. Macromol. Mater. Eng. 2016, 301, 555–565. [Google Scholar] [CrossRef]
- Pawar, S.P.; Arjmand, M.; Gandi, M.; Bose, S.; Sundararaj, U. Critical insights in understanding effects of synthesis temperature and nitrogen doping towards charge storage capability and microwave shielding in nitrogen-doped carbon nanotube/polymer nanocomposites. RSC Adv. 2016, 6, 63224–63234. [Google Scholar] [CrossRef]
- McEuen, P.L.; Fuhrer, M.S.; Park, H. Single walled carbon nanotube electronics. IEEE Trans. Nanotechnol. 2002, 1, 78–85. [Google Scholar] [CrossRef]
- Liu, X.M.; Huang, Z.D.; Oh, S.W.; Zhang, B.; Ma, P.C.; Yuen, M.M.F.; Kim, J.K. Carbon nanotube (CNT)-based composites as electrode material for rechargeable Li-ion batteries: A review. Compos. Sci. Technol. 2012, 72, 121–144. [Google Scholar] [CrossRef]
- Cheung, C.L.; Kurtz, A.; Park, H.; Lieber, C.M. Diameter-controlled synthesis of carbon nanotubes. J. Phys. Chem. B 2002, 106, 2429–2433. [Google Scholar] [CrossRef]
- Orbaek, A.W.; Owens, A.C.; Crouse, C.C.; Pint, C.L.; Hauge, R.H.; Barron, A.R. Single walled carbon nanotube growth and chirality dependence on catalyst composition. Nanoscale 2013, 5, 9848–9859. [Google Scholar] [CrossRef] [PubMed]
- Nasibulin, A.G.; Pikhitsa, P.V.; Jiang, H.; Kauppinen, E.I. Correlation between catalyst particle and single-walled carbon nanotube diameters. Carbon 2005, 43, 2251–2257. [Google Scholar] [CrossRef]
- Schäffel, F.; Rümmeli, M.H.; Kramberger, C.; Queitsch, U.; Mohn, E.; Kaltofen, R.; Pichler, T.; Büchner, B.; Rellinghaus, B.; Schultz, L. Tailoring the diameter, density and number of walls of carbon nanotubes through predefined catalyst particles. Phys. Status Solidi A 2008, 205, 1382–1385. [Google Scholar] [CrossRef]
- Fiawoo, M.-F.C.; Bonnot, A.M.; Amara, H.; Bichara, C.; Thibault-Pénisson, J.; Loiseau, A. Evidence of correlation between catalyst particles and the single-wall carbon nanotube diameter: A first step towards chirality control. Phys. Rev. Lett. 2012, 108, 195503. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of ultralong and electrically uniform single-walled carbon nanotubes on clean substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Hu, Y.; Zhong, H.; Si, J.; Zhang, S.; Zhao, Q.; Lin, J.; Li, Q.; Zhang, Z.; Peng, L.; et al. Large-area growth of ultra-high-density single-walled carbon nanotube arrays on sapphire surface. Nano Res. 2015, 8, 3694–3703. [Google Scholar] [CrossRef]
- Youn, S.K.; Park, H.G. Morphological evolution of Fe–Mo bimetallic catalysts for diameter and density modulation of vertically aligned carbon nanotubes. J. Phys. Chem. C 2013, 117, 18657–18665. [Google Scholar] [CrossRef]
- Harutyunyan, A.R.; Pradhan, B.K.; Kim, U.J.; Chen, G.; Eklund, P.C. CVD synthesis of single wall carbon nanotubes under “soft” conditions. Nano Lett. 2002, 2, 525–530. [Google Scholar] [CrossRef]
- An, L.; Owens, J.M.; McNeil, L.E.; Liu, J. Synthesis of nearly uniform single-walled carbon nanotubes using identical metal-containing molecular nanoclusters as catalysts. J. Am. Chem. Soc. 2002, 124, 13688–13689. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.E.; Colorado, R., Jr.; Crouse, C.; Ogrin, D.; Maruyama, B.; Pender, M.J.; Edwards, C.L.; Whitsitt, E.; Moore, V.C.; Koveal, D.; et al. A study of the formation, purification and application as a SWNT growth catalyst of the nanocluster [HxPMo12O40⊂H4Mo72Fe30(O2CMe)15O254(H2O)98]. Dalton Trans. 2006, 3097–3107. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Rosén, A.; Bolton, K. Molecular dynamics study of the catalyst particle size dependence on carbon nanotube growth. J. Chem. Phys. 2004, 121, 2775–2779. [Google Scholar] [CrossRef] [PubMed]
- Cassell, A.M.; Raymakers, J.A.; Kong, J.; Dai, H. Large scale CVD synthesis of single-walled carbon nanotubes. J. Phys. Chem. B 1999, 103, 6484–6492. [Google Scholar] [CrossRef]
- Lamouroux, E.; Serp, P.; Kihn, Y.; Kalck, P. Identification of key parameters for the selective growth of single or double wall carbon nanotubes on FeMo/Al2O3 CVD catalysts. Appl. Catal. Gen. 2007, 323, 162–173. [Google Scholar] [CrossRef]
- Zhang, S.; Tong, L.; Hu, Y.; Kang, L.; Zhang, J. Diameter-specific growth of semiconducting SWNT arrays using uniform Mo2C solid catalyst. J. Am. Chem. Soc. 2015, 137, 8904–8907. [Google Scholar] [CrossRef] [PubMed]
- Harutyunyan, A.R.; Mora, E.; Tokune, T.; Bolton, K.; Rosén, A.; Jiang, A.; Awasthi, N.; Curtarolo, S. Hidden features of the catalyst nanoparticles favorable for single-walled carbon nanotube growth. Appl. Phys. Lett. 2007, 90, 163120. [Google Scholar] [CrossRef]
- Edgar, K.; Spencer, J.L. The synthesis of carbon nanotubes from Müller clusters. Curr. Appl. Phys. 2006, 6, 419–421. [Google Scholar] [CrossRef]
- Goss, K.; Kamra, A.; Spudat, C.; Meyer, C.; Kögerler, P.; Schneider, C.M. CVD growth of carbon nanotubes using molecular nanoclusters as catalyst. Phys. Status Solidi B 2009, 246, 2494–2497. [Google Scholar] [CrossRef]
- Esquenazi, G.L.; Barron, A.R. Reduction kinetics of the nanocluster [HxPMo12O40⊂H4Mo72Fe30(O2CMe)15O254(H2O)98-y (EtOH)y]. J. Clust. Sci. 2018, 29, 325–335. [Google Scholar] [CrossRef]
- Esquenazi, G.L.; Barron, A.R. Understanding the “activation” of the nanocluster [HxPMo12O40⊂H4Mo72Fe30(O2CMe)15O254(H2O)98-y (H2O)y] for low temperature growth of carbon nanotubes. J. Clust. Sci. 2018, in press. [Google Scholar] [CrossRef]
- Lee, C.J.; Park, J.; Huh, Y.; Lee, J.Y. Temperature effect on the growth of carbon nanotubes using thermal chemical vapor deposition. Chem. Phys. Lett. 2001, 343, 33–38. [Google Scholar] [CrossRef]
- Emmenegger, C.; Bonard, J.-M.; Mauron, P.; Sudan, P.; Lepora, A.; Grobety, B.; Züttel, A.; Schlapbach, L. Synthesis of carbon nanotubes over Fe catalyst on aluminium and suggested growth mechanism. Carbon 2003, 41, 539–547. [Google Scholar] [CrossRef]
- Young Lee, T.; Han, J.-H.; Hong Choi, S.; Yoo, J.-B.; Park, C.-Y.; Jung, T.; Yu, S.; Yi, W.K.; Han, I.T.; Kim, J.M. Effects of source gases on the growth of carbon nanotubes. Diam. Relat. Mater. 2003, 12, 851–855. [Google Scholar] [CrossRef]
- Orbaek, A.W.; Owens, A.C.; Barron, A.R. Increasing the efficiency of single walled carbon nanotube amplification by Fe–Co catalysts through the optimization of CH4/H2 partial pressures. Nano Lett. 2011, 11, 2871–2874. [Google Scholar] [CrossRef] [PubMed]
- Orbaek, A.W.; Aggarwal, N.; Barron, A.R. The development of a ‘process map’ for the growth of carbon nanomaterials from ferrocene by injection CVD. J. Mater. Chem. A 2013, 1, 14122–14132. [Google Scholar] [CrossRef]
- Müller, A.; Das, S.K.; Kögerler, P.; Bögge, H.; Schmidtmann, M.; Trautwein, A.X.; Schünemann, V.; Krickemeyer, E.; Preetz, W. A new type of supramolecular compound: Molybdenum-oxide-based composites consisting of magnetic nanocapsules with encapsulated keggin-ion electron reservoirs cross-linked to a two-dimensional network. Angew. Chem. Int. Ed. 2000, 39, 3413–3417. [Google Scholar] [CrossRef]
- Mekala, R.; Supriya, S.; Das, S.K. Fate of a giant {Mo72Fe30}-type polyoxometalate cluster in an aqueous solution at higher temperature: Understanding related keplerate chemistry, from molecule to material. Inorg. Chem. 2013, 52, 9708–9710. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wu, B.; Mamontov, E.; Daemen, L.L.; Cheng, Y.; Li, T.; Seifert, S.; Hong, K.; Bonnesen, P.V.; Keum, J.K.; Ramirez-Cuesta, A.J. X-ray and neutron scattering study of the formation of core–shell-type polyoxometalates. J. Am. Chem. Soc. 2016, 138, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Camacho-López, M.A.; Escobar-Alarcón, L.; Picquart, M.; Arroyo, R.; Córdoba, G.; Haro-Poniatowski, E. Micro-Raman study of the m-MoO2 to α-MoO3 transformation induced by cw-laser irradiation. Opt. Mater. 2011, 33, 480–484. [Google Scholar] [CrossRef]
- Müller, A.; Krickemeyer, E.; Das, S.K.; Kögerler, P.; Sarkar, S.; Bögge, H.; Schmidtmann, M.; Sarkar, S. Linking icosahedral, strong molecular magnets {Mo} to layers—A solid-state reaction at room temperature. Angew. Chem. Int. Ed. 2000, 39, 1612–1614. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Plata, D.L.; Meshot, E.R.; Reddy, C.M.; Hart, A.J.; Gschwend, P.M. Multiple alkynes react with ethylene to enhance carbon nanotube synthesis, suggesting a polymerization-like formation mechanism. ACS Nano 2010, 4, 7185–7192. [Google Scholar] [CrossRef] [PubMed]
- Nessim, G.D.; Seita, M.; Plata, D.L.; O’Brien, K.P.; Hart, A.J.; Meshot, E.R.; Reddy, C.M.; Gschwend, P.M.; Thompson, C.V. Precursor gas chemistry determines the crystallinity of carbon nanotubes synthesized at low temperature. Carbon 2011, 49, 804–810. [Google Scholar] [CrossRef]
- Bandow, S.; Asaka, S.; Saito, Y.; Rao, A.M.; Grigorian, L.; Richter, E.; Eklund, P.C. Effect of the growth temperature on the diameter distribution and chirality of single-wall carbon nanotubes. Phys. Rev. Lett. 1998, 80, 3779–3782. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Luo, D.; Sun, H.; Wang, J.; Yang, F.; Li, R.; Yang, J.; Li, Y. Diameter-controlled growth of aligned single-walled carbon nanotubes on quartz using molecular nanoclusters as catalyst precursors. Chin. Sci. Bull. 2013, 58, 433–439. [Google Scholar] [CrossRef]
- Kuwana, K.; Endo, H.; Saito, K.; Qian, D.; Andrews, R.; Grulke, E.A. Catalyst deactivation in CVD synthesis of carbon nanotubes. Carbon 2005, 43, 253–260. [Google Scholar] [CrossRef]
- Orbaek, A.W.; Barron, A.R. Towards a ‘catalyst activity map’ regarding the nucleation and growth of single walled carbon nanotubes. J. Exp. Nanosci. 2015, 10, 66–76. [Google Scholar] [CrossRef]
- Obrey, S.J.; Bott, S.G.; Barron, A.R. A Lewis base promoted alkyl/alkoxide ligand redistribution: Reaction of [Me2Al(μ-OCPh3)]2 with THF. Organometallics 2001, 20, 5119–5124. [Google Scholar] [CrossRef]
- Gomez-Ballesteros, J.L.; Burgos, J.C.; Lin, P.A.; Sharma, R.; Balbuena, P.B. Nanocatalyst shape and composition during nucleation of single-walled carbon nanotubes. RSC Adv. 2015, 5, 106377–106386. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, L.D.; Mueller-Markgraf, W.; Troe, J. Thermal decomposition of toluene: A comparison of thermal and laser-photochemical activation experiments. J. Phys. Chem. 1988, 92, 4905–4914. [Google Scholar] [CrossRef]
- Eng, R.A.; Gebert, A.; Goos, E.; Hippler, H.; Kachiani, C. Incubation times, fall-off and branching ratios in the thermal decomposition of toluene: Experiments and theory. Phys. Chem. Chem. Phys. 2002, 4, 3989–3996. [Google Scholar] [CrossRef]
- Li, J.; Kazakov, A.; Dryer, F.L. Experimental and numerical studies of ethanol decomposition reactions. J. Phys. Chem. A 2004, 108, 7671–7680. [Google Scholar] [CrossRef]
- Park, J.; Zhu, R.S.; Lin, M.C. Thermal decomposition of ethanol. I. ab Initio molecular orbital/Rice–Ramsperger–Kassel–Marcus prediction of rate constant and product branching ratios. J. Chem. Phys. 2002, 117, 3224–3231. [Google Scholar] [CrossRef]
- Gómez-Gualdrón, D.A.; Beetge, J.M.; Burgos, J.C.; Balbuena, P.B. Effects of precursor type on the CVD growth of single-walled carbon nanotubes. J. Phys. Chem. C 2013, 117, 10397–10409. [Google Scholar] [CrossRef]
- Zhang, G.; Mann, D.; Zhang, L.; Javey, A.; Li, Y.; Yenilmez, E.; Wang, Q.; McVittie, J.P.; Nishi, Y.; Gibbons, J.; et al. Ultra-high-yield growth of vertical single-walled carbon nanotubes: Hidden roles of hydrogen and oxygen. Proc. Natl. Acad. Sci. USA 2005, 102, 16141–16145. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ke, C.T.; Liu, K.; Li, P.; Liang, S.; Finkelstein, G.; Wang, F.; Liu, J. Importance of diameter control on selective synthesis of semiconducting single-walled carbon nanotubes. ACS Nano 2014, 8, 8564–8572. [Google Scholar] [CrossRef] [PubMed]
- Moon, C.Y.; Kim, Y.S.; Lee, E.C.; Jin, Y.G.; Chang, K.J. Mechanism for oxidative etching in carbon nanotubes. Phys. Rev. B 2002, 65, 155401. [Google Scholar] [CrossRef]












| Exp. | FeCl2 (mmol) | Na2MoO4 (mmol) | CH3CO2H (mmol) | H3[P(Mo3O10)4] (mmol) | pH a | Yield (g) b | Notes |
|---|---|---|---|---|---|---|---|
| 1 | 20.17 | 8.26 | 174 | 1.37 | 0.5 | 0.468 | 4× Fe, air |
| 2 | 20.13 | 8.28 | 174 | 1.37 | 1.5 | 0.685 | 4× Fe |
| 3 | 201.19 | 82.661 | 1740 | 13.70 | 1.4 | 18.180 | 10× Exp. 2 |
| 4 | 50.30 | 82.70 | 1740 | 13.70 | 2.0 | 6.480 | 10× Lit. |
| 5 | 20.12 | 8.27 | 174 | 1.37 | 2.5 | 2.500 | 4× Fe |
| 6 | 20.14 | 8.29 | 174 | 1.37 | 2.0 | 2.403 | 4× Fe, air |
| 7 | 50.55 | 82.66 | 1914 | 13.70 | 2.0 | 5.113 | 10× Lit. |
| 8 | 28.82 | 47.08 | 1091 | 7.815 | 2.0 | 2.717 | 6× Lit., air |
| CVD Run | Aspiration Temp. (°C) | Growth Temp. (°C) | Injection Rate (mL/h) | Catalyst Conc. (wt.%) | Carbon Source | Variable |
|---|---|---|---|---|---|---|
| 1 | 150 | 800 | 1 | 1.25 | EtOH | Aspiration temp. |
| 2 | 225 | 800 | 1 | 1.25 | EtOH | Aspiration temp. |
| 3 | 300 | 800 | 1 | 1.25 | EtOH | Aspiration temp. |
| 4 | 225 | 700 | 1 | 1.25 | EtOH | Growth temp. |
| 5 | 225 | 900 | 1 | 1.25 | EtOH | Growth temp. |
| 6 | 225 | 1000 | 1 | 1.25 | EtOH | Growth temp. |
| 7 | 225 | 1000 | 5 | 1.25 | EtOH | Injection rate |
| 8 | 225 | 1000 | 10 | 1.25 | EtOH | Injection rate |
| 9 | 225 | 800 | 1 | 0.25 | EtOH | Catalyst conc. |
| 10 | 225 | 800 | 1 | 5 | EtOH | Catalyst conc. |
| 11 | 225 | 800 | 1 | 5 | EtOH/toluene (1:1) | Carbon source |
| 12 | 225 | 800 | 1 | 5 | Toluene | Carbon source |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esquenazi, G.L.; Brinson, B.; Barron, A.R. Catalytic Growth of Carbon Nanotubes by Direct Liquid Injection CVD Using the Nanocluster [HxPMo12O40?H4Mo72Fe30(O2CMe)15O254(H2O)98-y(EtOH)y]. C 2018, 4, 17. https://doi.org/10.3390/c4010017
Esquenazi GL, Brinson B, Barron AR. Catalytic Growth of Carbon Nanotubes by Direct Liquid Injection CVD Using the Nanocluster [HxPMo12O40?H4Mo72Fe30(O2CMe)15O254(H2O)98-y(EtOH)y]. C. 2018; 4(1):17. https://doi.org/10.3390/c4010017
Chicago/Turabian StyleEsquenazi, Gibran L., Bruce Brinson, and Andrew R. Barron. 2018. "Catalytic Growth of Carbon Nanotubes by Direct Liquid Injection CVD Using the Nanocluster [HxPMo12O40?H4Mo72Fe30(O2CMe)15O254(H2O)98-y(EtOH)y]" C 4, no. 1: 17. https://doi.org/10.3390/c4010017
APA StyleEsquenazi, G. L., Brinson, B., & Barron, A. R. (2018). Catalytic Growth of Carbon Nanotubes by Direct Liquid Injection CVD Using the Nanocluster [HxPMo12O40?H4Mo72Fe30(O2CMe)15O254(H2O)98-y(EtOH)y]. C, 4(1), 17. https://doi.org/10.3390/c4010017