Microwave-Driven Plasma-Mediated Methane Cracking: Product Carbon Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor and Supporting Equipment
2.2. Solid Analysis
2.2.1. SEM and TEM
2.2.2. X-ray Diffraction
2.2.3. Thermogravimetric Analysis (TGA)
2.2.4. Elemental Composition and Surface Area
2.3. Optical Emission Spectroscopy
3. Results
3.1. Carbon Product: Physical and Chemical Characterization
3.2. Origin(s) of Nanostructure in Carbon Products
3.3. Radical Pool and Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Report of the Hydrogen Production Expert Panel: A Subcommittee of the Hydrogen & Fuel Cell Technical Advisory Committee, May 2013. Available online: https://www.hydrogen.energy.gov/pdfs/hpep_report_2013.pdf (accessed on 15 December 2017).
- Abbas, H.F.; Daud, W.W. Hydrogen production by methane decomposition: A review. Int. J. Hydrog. Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrog. Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Spath, P.L.; Mann, M.K. Life Cycle Assessment of Hydrogen Production via Natural Gas Steam Reforming; Technical Report NREL/TP-570-27637; National Renewable Energy Laboratory: Golden, CO, USA, 2001.
- Lee, K.K.; Han, G.Y.; Yoon, K.J.; Lee, B.K. Thermocatalytic hydrogen production from the methane in a fluidized bed with activated carbon catalyst. Catal. Today 2004, 93, 81–86. [Google Scholar] [CrossRef]
- Abbas, H.F.; Daud, W.W. Hydrogen production by thermocatalytic decomposition of methane using a fixed bed activated carbon in a pilot scale unit: Apparent kinetic, deactivation and diffusional limitation studies. Int. J. Hydrog. Energy 2010, 35, 12268–12276. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrog. Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Abánades, A.; Ruiz, E.; Ferruelo, E.M.; Hernández, F.; Cabanillas, A.; Martínez-Val, J.M.; Rubio, J.A.; López, C.; Gavela, R.; Barrera, G.; et al. Experimental analysis of direct thermal methane cracking. Int. J. Hydrog. Energy 2011, 36, 12877–12886. [Google Scholar]
- Ashik, U.P.M.; Daud, W.W.; Abbas, H.F. Production of greenhouse gas free hydrogen by thermocatalytic decomposition of methane—A review. Renew. Sustain. Energy Rev. 2015, 44, 221–256. [Google Scholar] [CrossRef]
- Suelves, I.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R. Carbonaceous materials as catalysts for decomposition of methane. Chem. Eng. J. 2008, 140, 432–438. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroğlu, T.N. “Green” path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies. Int. J. Hydrog. Energy 2008, 33, 6804–6839. [Google Scholar] [CrossRef]
- Gaudernack, B.; Lynum, S. Hydrogen from natural gas without release of CO2 to the atmosphere. Int. J. Hydrog. Energy 1998, 23, 1087–1093. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chen, K.T. Production of hydrogen and nano carbon powders from direct plasmalysis of methane. Int. J. Hydrog. Energy 2009, 34, 833–838. [Google Scholar] [CrossRef]
- Jasiński, M.; Dors, M.; Mizeraczyk, J. Production of hydrogen via methane reforming using atmospheric pressure microwave plasma. J. Power Sources 2008, 181, 41–45. [Google Scholar] [CrossRef]
- Muradov, N. Thermocatalytic CO2-free production of hydrogen from hydrocarbon fuels. In Proceedings of the 2000 Hydrogen Program Review, NREL/CP-570-28890; NREL/CP-610-32405; U.S. Department of Energy (DOE): Washington, DC, USA, 2002. [Google Scholar]
- Muradov, N.; Smith, F.; Ali, T. Catalytic activity of carbons for methane decomposition reaction. Catal. Today 2005, 102, 225–233. [Google Scholar] [CrossRef]
- Fidalgo, B.; Fernández, Y.; Domínguez, A.; Pis, J.J.; Menéndez, J.A. Microwave-assisted pyrolysis of CH4/N 2 mixtures over activated carbon. J. Anal. Appl. Pyrolysis 2008, 82, 158–162. [Google Scholar] [CrossRef]
- Domínguez, A.; Fidalgo, B.; Fernández, Y.; Pis, J.J.; Menéndez, J.A. Microwave-assisted catalytic decomposition of methane over activated carbon for CO2-free hydrogen production. Int. J. Hydrog. Energy 2007, 32, 4792–4799. [Google Scholar] [CrossRef]
- Muradov, N.; Smith, F.; Bokerman, G. Methane activation by nonthermal plasma generated carbon aerosols. J. Phys. Chem. C 2009, 113, 9737–9747. [Google Scholar] [CrossRef]
- Koptsov, G.L.; Strohm, J.J.; Roberts, B.Q.; H Quest Partners, L.P.; Battelle Memorial Institute Inc. Wave Modes for the Microwave Induced Conversion of Coal. U.S. Patent 14/881,991, 13 October 2015. [Google Scholar]
- Skoptsov, G.L.; Johnson, A.A.; H Quest Partners, L.P. Method for Processing Hydrocarbon Fuels Using Microwave Energy. U.S. Patent 9,682,359, 20 June 2017. [Google Scholar]
- Strohm, J.J.; Linehan, J.C.; Roberts, B.Q.; McMakin, D.L.; Sheen, D.M.; Griffin, J.W.; Franz, J.A.; Battelle Memorial Institute. Heavy Fossil Hydrocarbon Conversion and Upgrading Using Radio-Frequency or Microwave Energy. U.S. Patent 13/401,216, 23 August 2012. [Google Scholar]
- Dato, A.; Radmilovic, V.; Lee, Z.; Phillips, J.; Frenklach, M. Substrate-free gas-phase synthesis of graphene sheets. Nano Lett. 2008, 8, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- Fridman, A. Plasma Chemistry; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Keipi, T.; Tolvanen, K.E.; Tolvanen, H.; Konttinen, J. Thermo-catalytic decomposition of methane: The effect of reaction parameters on process design and the utilization possibilities of the produced carbon. Energy Convers. Manag. 2016, 126, 923–934. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Vander Wal, R.L. Soot Nanostructure: Definition, Quantification and Implications; (No. 2005-01-0964). SAE Technical Paper; SAE: Warrendale, PA, USA, 2005. [Google Scholar]
- Gaddam, C.K.; Vander Wal, R.L.; Chen, X.; Yezerets, A.; Kamasamudram, K. Reconciliation of carbon oxidation rates and activation energies based on changing nanostructure. Carbon 2016, 98, 545–556. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Tomasek, A.J. Soot oxidation: Dependence upon initial nanostructure. Combust. Flame 2003, 134, 1–9. [Google Scholar] [CrossRef]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Electrical conductivity of conductive carbon blacks: Influence of surface chemistry and topology. Appl. Surf. Sci. 2003, 217, 181–193. [Google Scholar] [CrossRef]
- Oberlin, A. Carbonization and graphitization. Carbon 1984, 22, 521–541. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Tomasek, A.J.; Street, K.W.; Hull, D.R.; Thompson, W.K. Carbon Nanostructure Examined by Lattice Fringe Analysis of High Resolution Transmission Electron Microscopy Images. Appl. Spectrosc. 2004, 58, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Muradov, N.; Smith, F.; Bockerman, G.; Scammon, K. Thermocatalytic decomposition of natural gas over plasma-generated carbon aerosols for sustainable production of hydrogen and carbon. Appl. Catal. A Gen. 2009, 365, 292–300. [Google Scholar] [CrossRef]
- Parigger, C.G.; Woods, A.C.; Surmick, D.M.; Gautam, G.; Witte, M.J.; Hornkohl, J.O. Computation of diatomic molecular spectra for selected transitions of aluminum monoxide, cyanide, diatomic carbon, and titanium monoxide. Spectrochim. Acta Part B Atom. Spectrosc. 2015, 107, 132–138. [Google Scholar] [CrossRef]
- Jasiński, M.; Dors, M.; Nowakowska, H.; Mizeraczyk, J. Hydrogen production via methane reforming using various microwave plasma sources. Chem. Listy 2008, 102, 1332–1337. [Google Scholar]
- Pellerin, S.; Musiol, K.; Motret, O.; Pokrzywka, B.; Chapelle, J. Application of the (0, 0) Swan band spectrum for temperature measurements. J. Phys. D Appl. Phys. 1996, 29, 2850. [Google Scholar] [CrossRef]
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037. [Google Scholar] [CrossRef]
- Yan, B.; Cheng, Y.; Li, T.; Cheng, Y. Detailed kinetic modeling of acetylene decomposition/soot formation during quenching of coal pyrolysis in thermal plasma. Energy 2017, 121, 10–20. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Appel, J.; Bockhorn, H.; Frenklach, M. Kinetic modeling of soot formation with detailed chemistry and physics: Laminar premixed flames of C2 hydrocarbons. Combust. Flame 2000, 121, 122–136. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Farsani, M.H.; Darbani, S.M.R.; Mousaviazar, A.; Soltanolkotabi, M.; Majd, A.E. CN and C2 vibrational spectra analysis in molecular LIBS of organic materials. Appl. Phys. B 2016, 122, 106. [Google Scholar] [CrossRef]
- Dato, A.; Lee, Z.; Jeon, K.J.; Erni, R.; Radmilovic, V.; Richardson, T.J.; Frenklach, M. Clean and highly ordered graphene synthesized in the gas phase. Chem. Commun. 2009, 40, 6095–6097. [Google Scholar] [CrossRef] [PubMed]
- McEnally, C.S.; Köylü, Ü.Ö.; Pfefferle, L.D.; Rosner, D.E. Soot volume fraction and temperature measurements in laminar nonpremixed flames using thermocouples. Combust. Flame 1997, 109, 701–720. [Google Scholar] [CrossRef]
- Sirignano, W.A. Fuel droplet vaporization and spray combustion theory. Prog. Energy Combust. Sci. 1983, 9, 291–322. [Google Scholar] [CrossRef]
- Kholghy, M.R.; Veshkini, A.; Thomson, M.J. The core–shell internal nanostructure of soot–A criterion to model soot maturity. Carbon 2016, 100, 508–536. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Strzelec, A.; Toops, T.J.; Stuart Daw, C.; Genzale, C.L. Forensics of soot: C5-related nanostructure as a diagnostic of in-cylinder chemistry. Fuel 2013, 113, 522–526. [Google Scholar] [CrossRef]
- Huang, C.H.; Vander Wal, R.L. Effect of soot structure evolution from commercial jet engine burning petroleum-based JP-8 and synthetic HRJ and FT fuels. Energy Fuels 2013, 27, 4946–4958. [Google Scholar] [CrossRef]
- Huang, C.H.; Vander Wal, R.L. Partial premixing effects upon soot nanostructure. Combust. Flame 2016, 168, 403–408. [Google Scholar] [CrossRef]
- Huang, C.H. Soot Nanostructure Evolution from Gas Turbine Engine, Premixed and Diffusion Flame: Equivalence Ratio, Flame Temperature, and Fuel Dependencies. Ph.D. Thesis, Pennsylvania State University, State College, PA, USA, 2016. [Google Scholar]





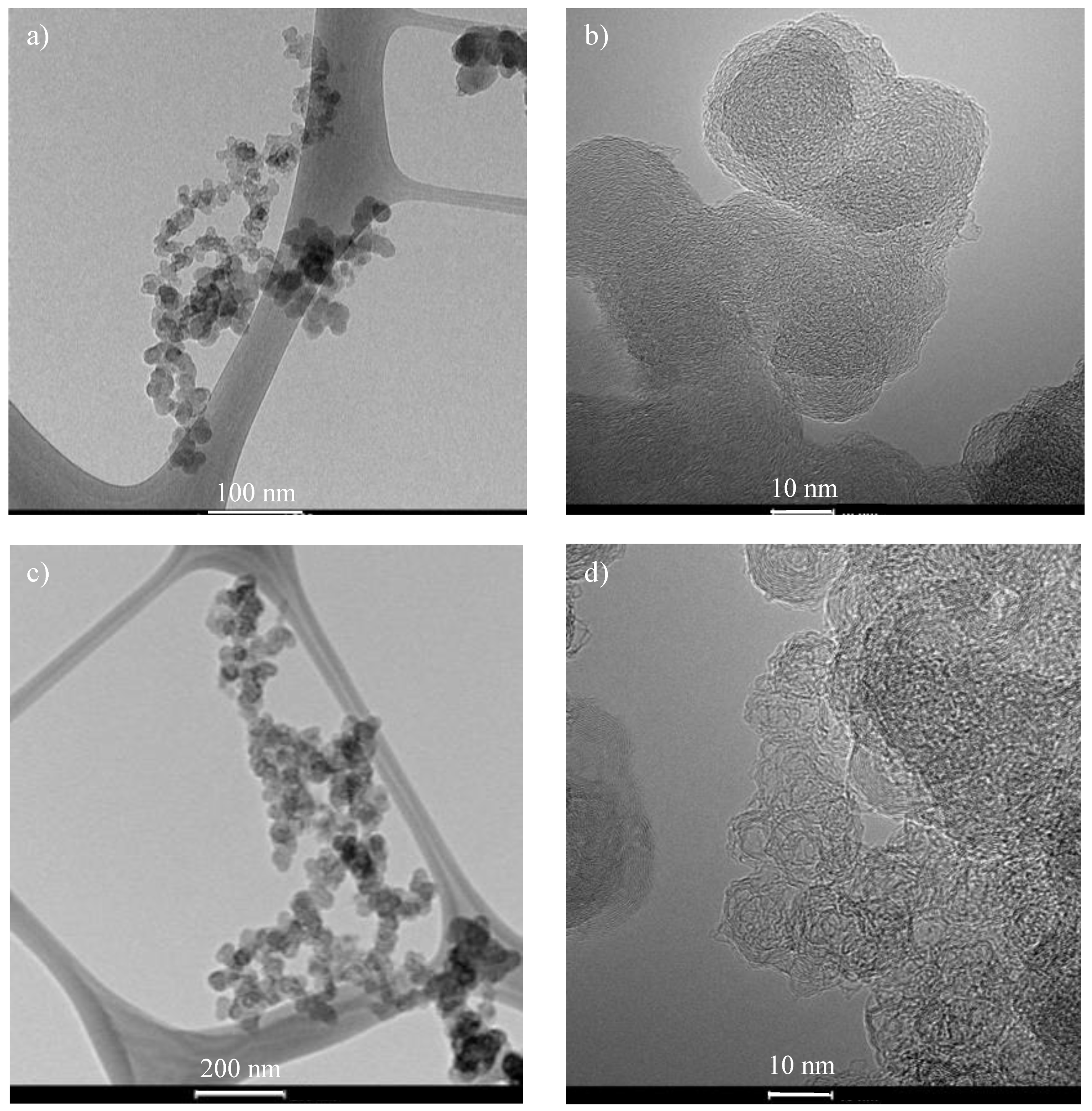
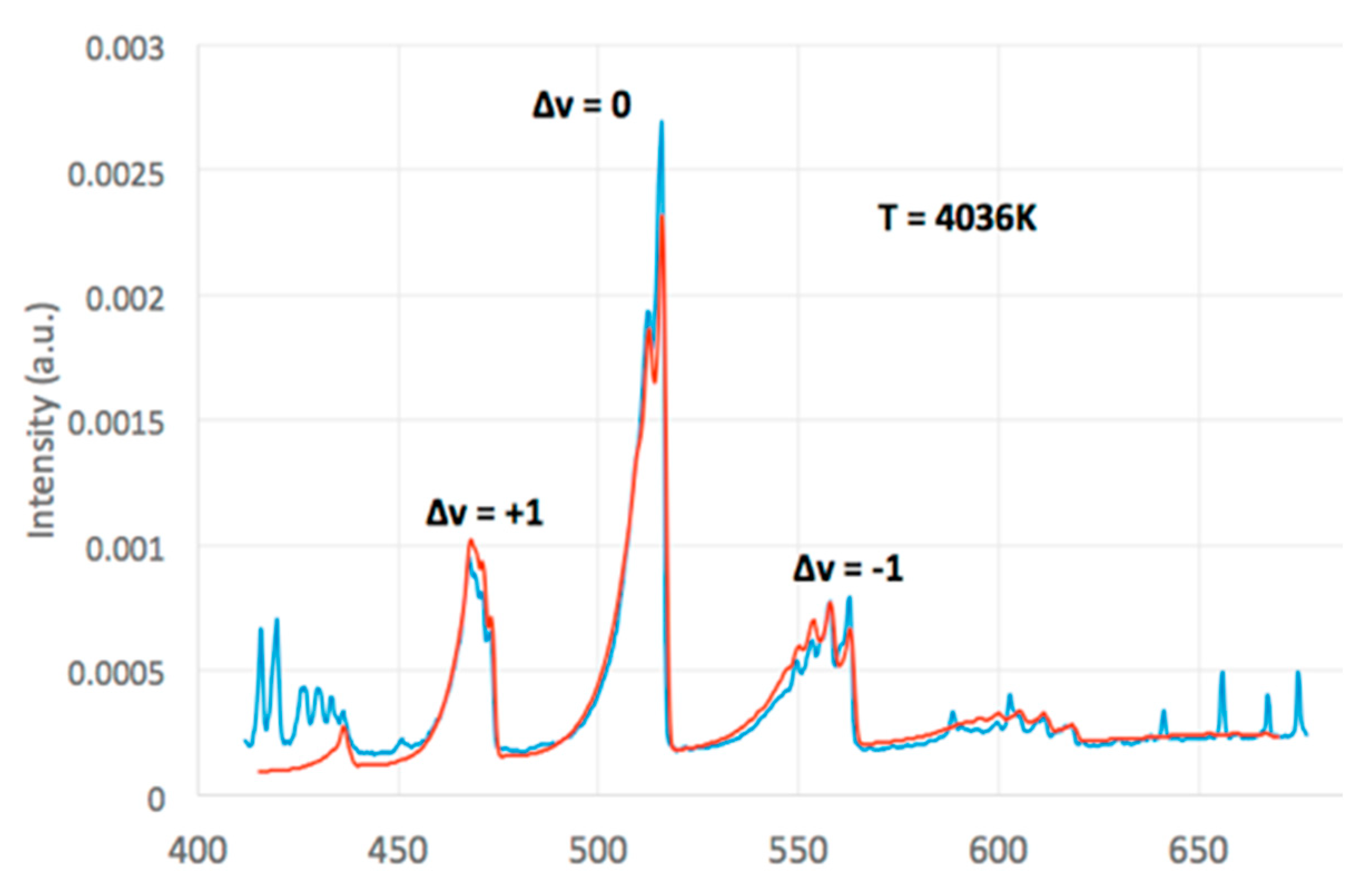
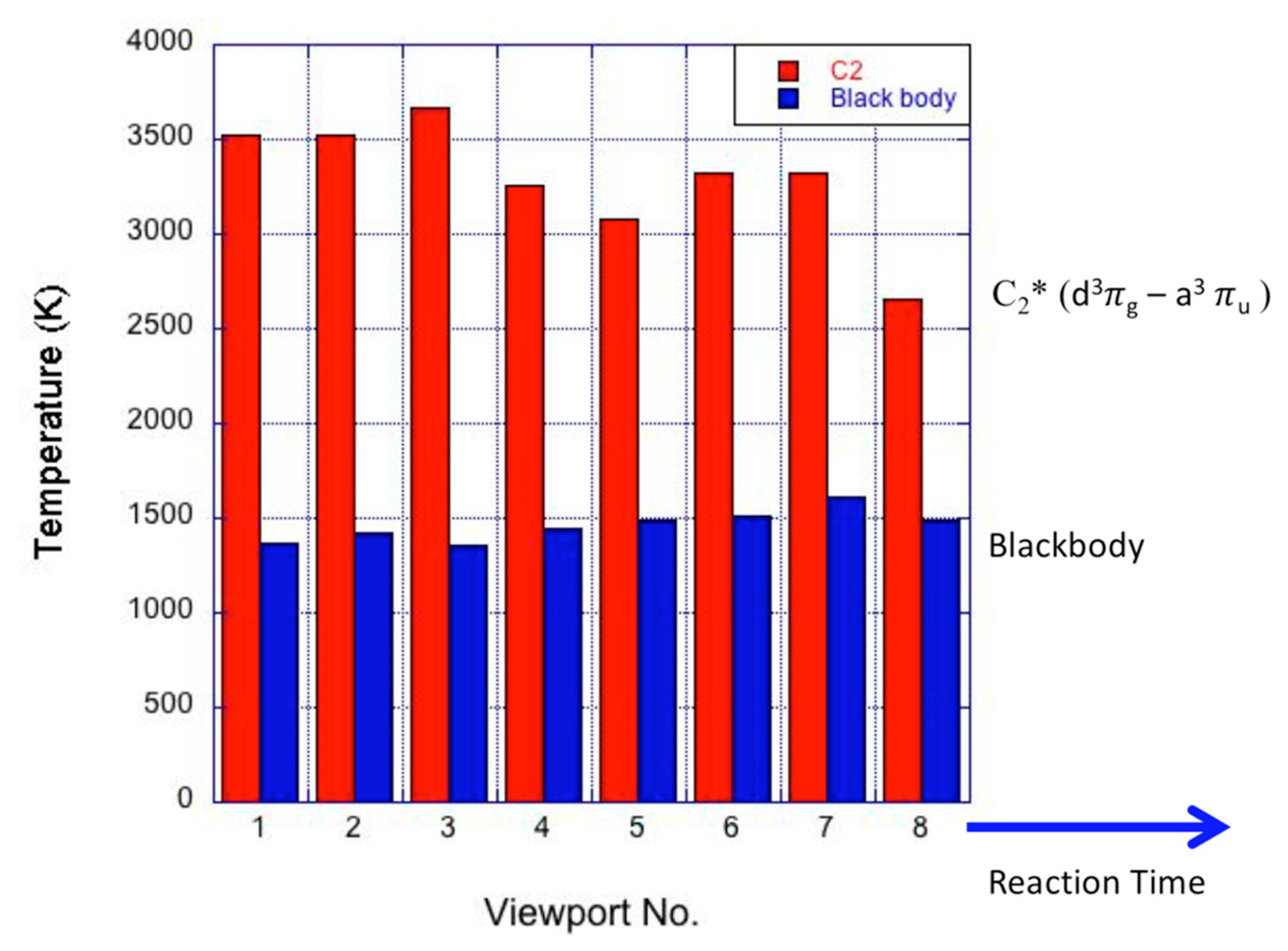
| Run | Lc (nm) | Stack Number | La (nm) |
|---|---|---|---|
| XC-72 (virgin) | 1.49 ± 0.05 | ~5 | 4.00 ± 0.52 |
| MW (Ar) XC-72 (7 pts) | 1.56 ± 0.06 | ~5 | 3.93 ± 0.42 |
| CH4/MW Carbon (5 pts) | 2.95 ± 0.5 | ~8 | 5.70 ± 0.58 |
| 2.49 ± 0.5 | ~7 | ||
| 6.58 ± 0.5 | ~19 |
| Material (CB) | Surface Area (m2/g) [Pore Volume (cm3/g)] | Elemental Composition (C, H) % |
|---|---|---|
| XC-72 (virgin) | 254 [0.174] | C: ~98 ± 0.2% H: ~1.5% S: ~0.5% |
| CH4/MW Carbon | 136 [0.241] | C: ~98 ± 0.2% H: 1 ± 0.1% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vander Wal, R.; Sengupta, A.; Musselman, E.; Skoptsov, G. Microwave-Driven Plasma-Mediated Methane Cracking: Product Carbon Characterization. C 2018, 4, 61. https://doi.org/10.3390/c4040061
Vander Wal R, Sengupta A, Musselman E, Skoptsov G. Microwave-Driven Plasma-Mediated Methane Cracking: Product Carbon Characterization. C. 2018; 4(4):61. https://doi.org/10.3390/c4040061
Chicago/Turabian StyleVander Wal, Randy, Arupananda Sengupta, Evan Musselman, and George Skoptsov. 2018. "Microwave-Driven Plasma-Mediated Methane Cracking: Product Carbon Characterization" C 4, no. 4: 61. https://doi.org/10.3390/c4040061
APA StyleVander Wal, R., Sengupta, A., Musselman, E., & Skoptsov, G. (2018). Microwave-Driven Plasma-Mediated Methane Cracking: Product Carbon Characterization. C, 4(4), 61. https://doi.org/10.3390/c4040061






