The State of HiPco Single-Walled Carbon Nanotubes in 2019
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. NoPo HiPco Growth
2.3. Preparing Surfactant-Stabilized Dispersions
2.4. Characterization
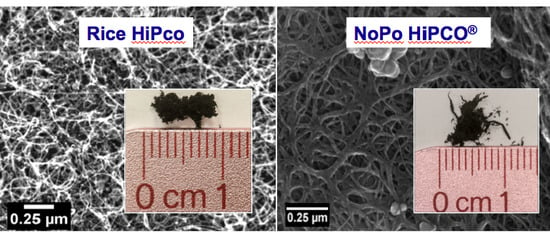
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, S.; Vosguerichian, M.; Bao, Z. A review of fabrication and applications of carbon nanotube film-based flexible electronics. Nanoscale 2013, 5, 1727–1752. [Google Scholar] [CrossRef] [PubMed]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Sikarwar, S.; Sonker, R.K.; Yadav, B.C. Carbon nanotube: Synthesis and application in solar cell. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1231–1242. [Google Scholar] [CrossRef]
- Luo, C.; Xie, H.; Wang, Q.; Luo, G.; Liu, C. A review of the application and performance of carbon nanotubes in fuel cells. J. Nanomater. 2015, 2015, 560392. [Google Scholar] [CrossRef]
- Das, R.; Ali, M.E.; Hamid, S.B.A.; Ramakrishna, S.; Chowdhury, Z.Z. Carbon nanotube membranes for water purification: A bright future in water desalination. Desalination 2014, 336, 97–109. [Google Scholar] [CrossRef]
- Lee, D.; Yoon, J.; Lee, J.; Lee, B.-H.; Seol, M.-L.; Bae, H.; Jeon, S.-B.; Seong, H.; Im, S.G.; Choi, S.-J.; et al. Logic circuits composed of flexible carbon nanotube thin-film transistor and ultra-thin polymer gate dielectric. Sci. Rep. 2016, 6, 26121. [Google Scholar] [CrossRef] [Green Version]
- Gangoli, V.S.; Azhang, J.; Willett, T.T.; Gelwick, S.A.; Haroz, E.H.; Kono, J.; Hauge, R.H.; Wong, M.S. Using nonionic surfactants for production of semiconductor-type carbon nanotubes by gel-based affinity chromatography. Nanomat. Nanotechnol. 2014, 4, 19. [Google Scholar] [CrossRef]
- Hornbostel, B.; Haluska, M.; Cech, J.; Dettlaff, U.; Roth, S. Arc discharge and laser ablation synthesis of singlewalled carbon nanotubes. In Carbon Nanotubes; Springer: Dordrecht, The Netherlands, 2006; pp. 1–18. [Google Scholar]
- Sharma, R.; Sharma, A.K.; Sharma, V. Synthesis of carbon nanotubes by arc-discharge and chemical vapor deposition method with analysis of its morphology, dispersion and functionalization characteristics. Cogent. Eng. 2015, 2, 1094017. [Google Scholar] [CrossRef]
- Arepalli, S. Laser ablation process for single-walled carbon nanotube production. J. Nanosci. Nanotechnol. 2004, 4, 317–325. [Google Scholar] [CrossRef]
- Chrzanowska, J.; Hoffman, J.; Małolepszy, A.; Mazurkiewicz, M.; Kowalewski, T.A.; Szymanski, Z.; Stobinski, L. Synthesis of carbon nanotubes by the laser ablation method: Effect of laser wavelength. Phys. Status Solidi B 2015, 252, 1860–1867. [Google Scholar] [CrossRef] [Green Version]
- Che, G.; Lakshmi, B.B.; Martin, C.R.; Fisher, E.R.; Ruoff, R.S. Chemical vapor deposition based synthesis of carbon nanotubes and nanofibers using a template method. Chem. Mater. 1998, 10, 260–267. [Google Scholar] [CrossRef]
- Cantoro, M.; Hofmann, S.; Pisana, S.; Scardaci, V.; Parvez, A.; Ducati, C.; Ferrari, A.C.; Blackburn, A.M.; Wang, K.-Y.; Robertson, J. Catalytic chemical vapor deposition of single-wall carbon nanotubes at low temperatures. Nano Lett. 2006, 6, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Orbaek, A.W.; Aggarwal, N.; Barron, A.R. The development of a ‘process map’ for the growth of carbon nanomaterials from ferrocene by injection CVD. J. Mater. Chem. A 2013, 1, 14122–14132. [Google Scholar] [CrossRef]
- Bronikowski, M.J.; Willis, P.A.; Colbert, D.T.; Smith, K.A.; Smalley, R.E. Gas-phase production of carbon single-walled nanotubes from carbon monoxide via the HiPco process: A parametric study. J. Vac. Sci. Technol. A 2001, 19, 1800. [Google Scholar] [CrossRef]
- Atom Optoelectronics, Inc. Available online: http://www.atomoe.com/hipco.html (accessed on 24 October 2019).
- Zhang, K.S.; Pham, D.; Lawal, O.; Ghosh, S.; Gangoli, V.S.; Smalley, P.; Kennedy, K.; Brinson, B.; Billups, W.E.; Hauge, R.; et al. Overcoming catalyst residue inhibition of the functionalization of single-walled carbon nanotubes via the Billups-Birch reduction. ACS Appl. Mater. Interfaces 2017, 9, 37972–37980. [Google Scholar] [CrossRef]
- Gangoli, V.S.; Raja, P.M.V.; Esquenazi, G.L.; Barron, A.R. The safe handling of bulk low-density nanomaterials. SN Appl. Sci. 2019, 1, 644. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-specific enhancement of the carbon–carbon dioxide (Boudouard) reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Ogrin, D.; Chattopadhyay, J.; Sadana, A.K.; Billups, E.; Barron, A.R. Epoxidation and deepoxidation of single-walled carbon nanotubes: Quantification of epoxide defects. J. Am. Chem. Soc. 2006, 128, 11322–11323. [Google Scholar] [CrossRef]
- Wright, K.D.; Barron, A.R. Catalyst residue and oxygen species inhibition of the formation of hexahapto-metal complexes of group 6 metals on single-walled carbon nanotubes. C 2017, 3, 17. [Google Scholar] [CrossRef]
- Pham, D.; Zhang, K.S.; Lawal, O.; Ghosh, S.; Gangoli, V.S.; Ainscough, T.; Hauge, R.H.; Adams, W.W.; Barron, A.R. Apparatus for scalable functionalization of single-walled carbon nanotubes via the billups-birch reduction. C 2017, 3, 19. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A. raman spectroscopy of carbon nanotubes in 1997 and 2007. J. Phys. Chem. C 2007, 111, 17887–17893. [Google Scholar] [CrossRef]







| Element | Rice University | NoPo |
|---|---|---|
| C | 64 ± 5.5 | 78 ± 5.3 |
| Fe | 24 ± 3.2 | 12 ± 2.6 |
| O | 10 ± 2.2 | 9 ± 2.2 |
| other | 2 ± 0.5 | 1 ± 0.25 |
| Excitation Wavelength (nm) | Rice University | NoPo |
|---|---|---|
| 514 | 4.6 ± 0.5 | 9.5 ± 1.7 |
| 633 | 7.1 ± 0.9 | 15.4 ± 1.5 |
| 785 | 11.4 ± 3.1 | 17.8 ± 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangoli, V.S.; Godwin, M.A.; Reddy, G.; Bradley, R.K.; Barron, A.R. The State of HiPco Single-Walled Carbon Nanotubes in 2019. C 2019, 5, 65. https://doi.org/10.3390/c5040065
Gangoli VS, Godwin MA, Reddy G, Bradley RK, Barron AR. The State of HiPco Single-Walled Carbon Nanotubes in 2019. C. 2019; 5(4):65. https://doi.org/10.3390/c5040065
Chicago/Turabian StyleGangoli, Varun Shenoy, M. Anto Godwin, Gadhadar Reddy, Robert Kelley Bradley, and Andrew R. Barron. 2019. "The State of HiPco Single-Walled Carbon Nanotubes in 2019" C 5, no. 4: 65. https://doi.org/10.3390/c5040065
APA StyleGangoli, V. S., Godwin, M. A., Reddy, G., Bradley, R. K., & Barron, A. R. (2019). The State of HiPco Single-Walled Carbon Nanotubes in 2019. C, 5(4), 65. https://doi.org/10.3390/c5040065