Abstract
Over the past decade, carbon nanostructures (CNSs) have been widely used in a variety of biomedical applications. Examples are the use of CNSs for drug and protein delivery or in tools to locally dispense nucleic acids to fight tumor affections. CNSs were successfully utilized in diagnostics and in noninvasive and highly sensitive imaging devices thanks to their optical properties in the near infrared region. However, biomedical applications require a complete biocompatibility to avoid adverse reactions of the immune system and CNSs potentials for biodegradability. Water is one of the main constituents of the living matter. Unfortunately, one of the disadvantages of CNSs is their poor solubility. Surface functionalization of CNSs is commonly utilized as an efficient solution to both tune the surface wettability of CNSs and impart biocompatible properties. Grafting functional groups onto the CNSs surface consists in bonding the desired chemical species on the carbon nanoparticles via wet or dry processes leading to the formation of a stable interaction. This latter may be of different nature as the van Der Waals, the electrostatic or the covalent, the π-π interaction, the hydrogen bond etc. depending on the process and on the functional molecule at play. Grafting is utilized for multiple purposes including bonding mimetic agents such as polyethylene glycol, drug/protein adsorption, attaching nanostructures to increase the CNSs opacity to selected wavelengths or provide magnetic properties. This makes the CNSs a very versatile tool for a broad selection of applications as medicinal biochips, new high-performance platforms for magnetic resonance (MR), photothermal therapy, molecular imaging, tissue engineering, and neuroscience. The scope of this work is to highlight up-to-date using of the functionalized carbon materials such as graphene, carbon fibers, carbon nanotubes, fullerene and nanodiamonds in biomedical applications.
1. Introduction
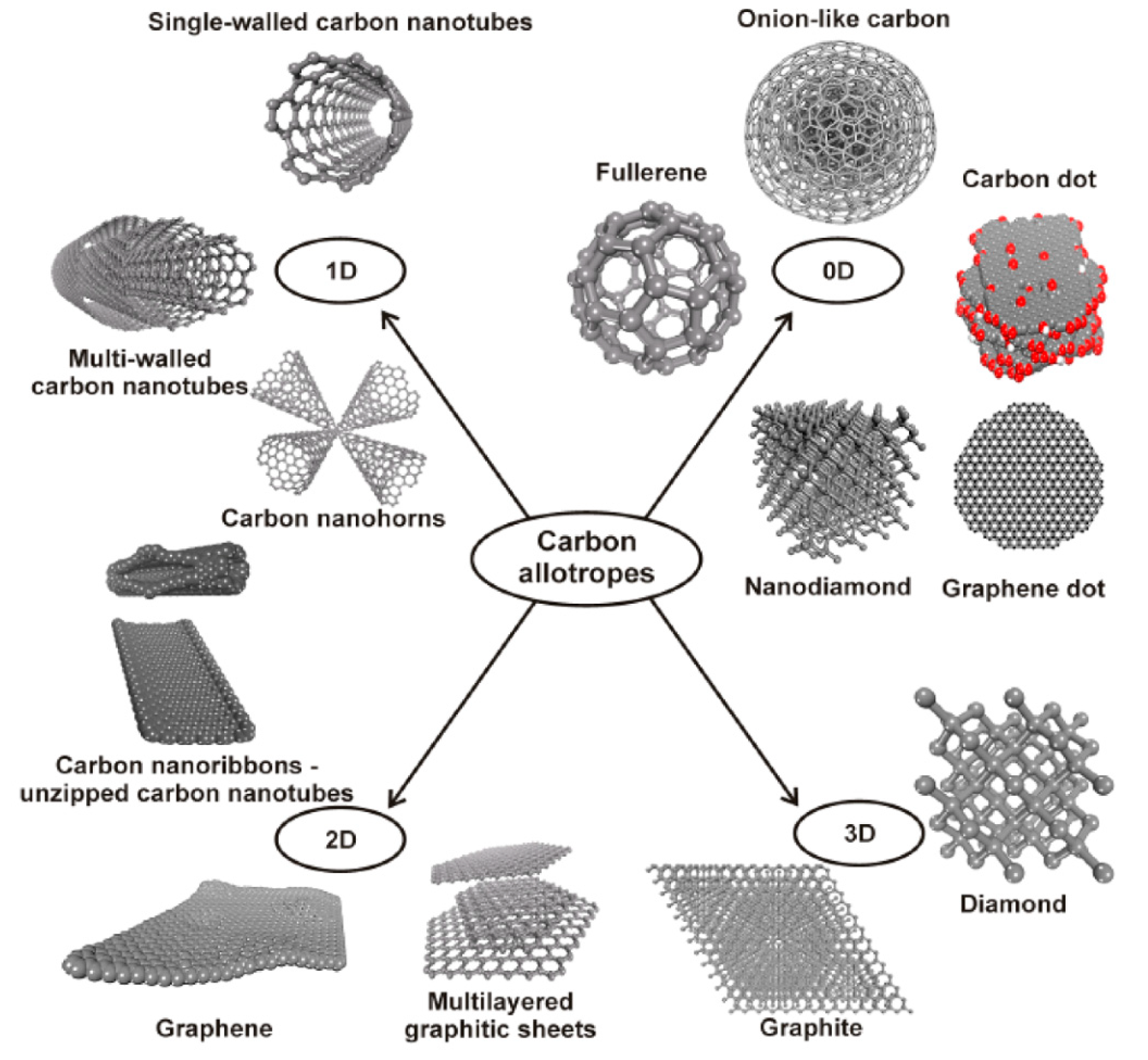
Difficulties in overcoming the limited control over biophysical and biochemical characteristics in traditional biomaterials have hampered their use in biomedical applications. This pushed the research of novel materials showing multiple properties that allow advanced functionalities. In this respect, carbon nanostructures (CNSs) thanks to the multiple uncommon properties, have had an impressive impact on scientific research with important technological implications. Carbon is one of the prototypal elements showing an organization in different allotropic forms (as sketched in Figure 1): from the zero-dimension fullerenes [1] and carbon quantum dots [2,3], to the one dimensional carbon nanotubes (CNTs) [4], to the two-dimensional graphene atomic sheet [5], to the 3D bulk graphite or diamond crystals where atoms are pure sp2 or sp3 hybrids organized in the hexagonal or cubic lattice, respectively [6].
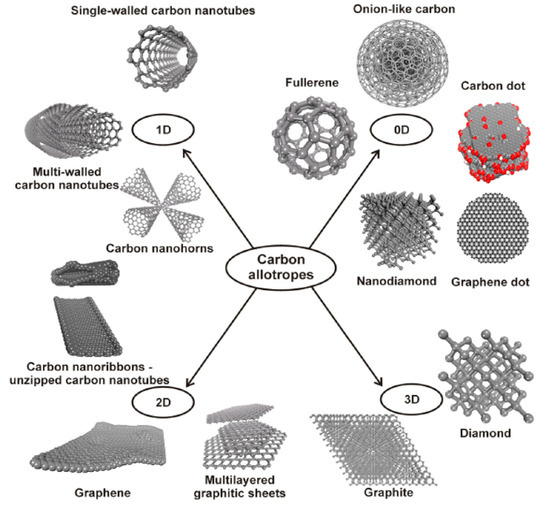
Figure 1.
The different forms of the carbon allotropes (Reprinted with permission from [7]).
The interesting electrical, chemical, and mechanical properties of these objects, have led to a number of different types of applications. Concerning biology and biomedicine, the big potential of carbon nanomaterials is the possibility to tune the CNS dimensions, the capability to functionalize the surface, and the high chemical stability coupled to the optical and biomimetic properties. As an example, CNTs and fullerenes soon after their discovery have been studied for biosensing, drug delivery, and bioimaging [8,9]. Recently they experienced a revival for their application in the regenerative medicine [10], cancer therapy, and theranostic applications [11].
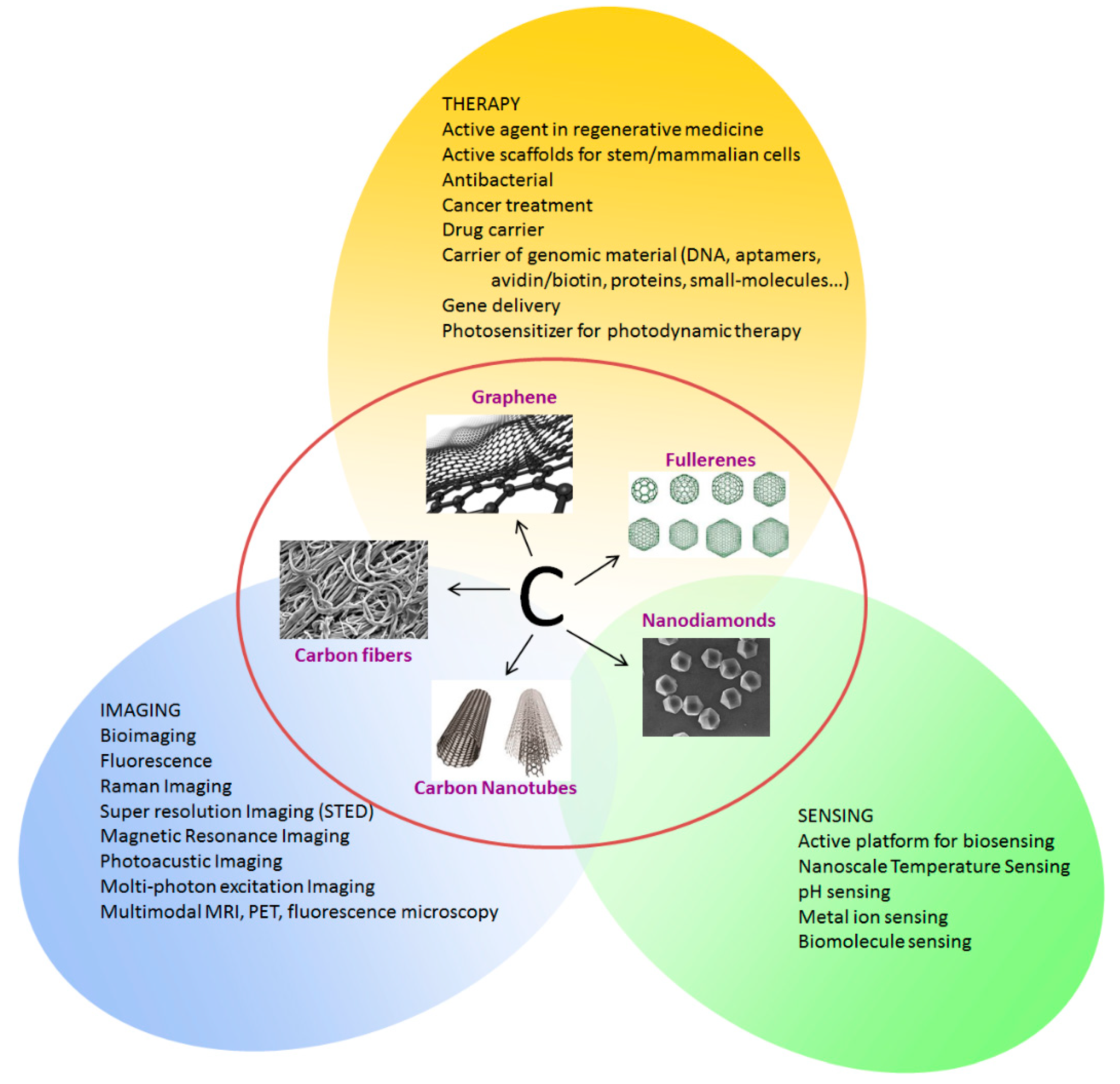
Figure 2 summarizes the possible applications of CNSs in biomedicine. The possibility to easily functionalizing the CNS surface is coupled with their unique optical properties such as intrinsic fluorescence, high photostability and the possibility to tune their emission, which are open important perspectives for their use in the imaging and diagnosis of cells and tissues. The possibility of modifying the CNS surface with oxygen or nitrogen based functional groups, enhances their properties and broadens the ensemble of applications facilitating the interaction with the hosting entity (cell, tissue, human body).
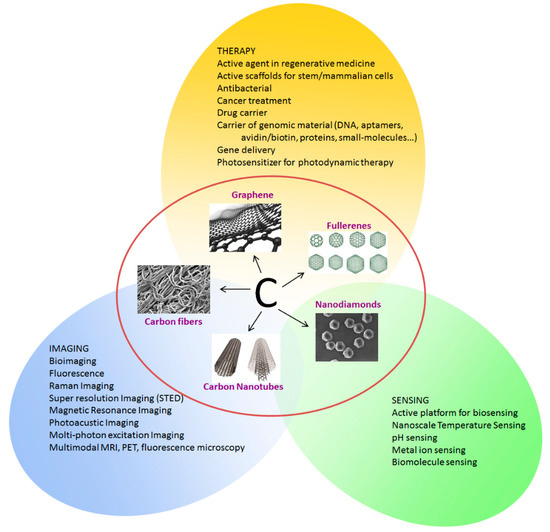
Figure 2.
Carbon nanostructures and their applications in biomedicine.
The high surface area, and good mechanical and electrical properties render the CNSs as optimal platforms for theranostic applications. However, in dealing with therapies, stability in aqueous suspensions have become one of the major issues for their practical utilization in biomedicine [12,13]. This problem is generally overcome by activating the CNS surface using covalent approaches, such as oxidation, ozonolysis, plasma treatments, and dehydronation [13]. Coming to the applications in biomedicine, some studies have pointed out the anti-inflammatory properties of treated CNSs [14] although mechanisms behind this behavior has still not fully understood. More defined is the role of CNSs in fighting cancer diseases [15] as fluorescent markers for tumor tissue detection [16,17,18], sensors for early cancer detection [19,20,21], drug carriers [22,23], cancer phototherapy [24] and theranostics in general [25,26]. Recently, CNSs have been used to directly influence the biological activities of living matter. As an example, CNSs can stimulate the production of reactive oxygen species (ROS) when internalized in cancer cells, interfering with their biological functions. Graphene and fullerenes are proposed as potential candidates for cancer therapy thanks to their ability to activate molecular oxygen to ROS upon light irradiation [27,28]. CNSs and ROS production are also utilized to control microbial proliferation [29]. ROS at high concentrations causes a series of mitochondrial damages including DNA mutations, change in the membrane permeability, change of the respiratory functions, affects the Ca2+ homeostasis, and the mitochondrial defense systems [30]. These adverse effects easily induce cell death by apoptosis. Regarding therapy, CNS are widely utilized for drug delivery, thanks to the possibility of funtionalizing their surface to enhance the targeting functions and to impart mimetic properties toward the immune system or cross biological barriers [31]. CNSs have been successfully applied in the delivery of diverse active principles such as small molecules, peptides, proteins, or nucleic acids [32], significantly increase the solubility of poorly soluble drugs, reducing cytotoxicity toward normal tissues, and improving therapeutic efficacy [32]. Considering applications in biomedicine, the CNS optical properties are utilized in several modalities. They are utilized in one-photon and two-photon imaging, photoacoustic imaging, Raman and near-infrared imaging and their fluorescence properties have been deeply investigated for sensing applications in cell labelling and diagnosis [33,34,35,36,37,38,39,40].
Functionalization is a necessary unavoidable step to make CNSs a multifunctional, multimodal, high-performancetool for biomedicine. Modification of the surface chemical properties of CNSs, by grafting selected functional groups and proceed with a surface engineering with multi-step processing is used to lower toxicity, enhance water solubility, and add various specific functions [41,42,43]. This review aims at illustrating the state-of-the-art methods of carbon functionalization and their characterization, providing an overview of recent applications of CNSs in biomedicine. Also discuss the concerns regarding the degree of biocompatibility and potential toxicity of CNS illustrating the possible solutions.
2. Graphene
Graphene is composed of single-layer carbon atoms which are packed into a two-dimensional planar structure with a honeycomb lattice. Carbon atoms in graphene are sp2 hybridized and connected with three neighboring carbon atoms via σ bonds, while π bonds are formed with an additional unhybridized pz-orbital perpendicular to the planar structure [44]. The in-planar σ bond in graphene has a length about 1.42 Å, even shorter than the sp3 bond distance of 1.54 Å in diamond, which gives graphene great mechanical strength, with a breaking strength of 42 N m−1 and Young’s modulus of 1.0 TPa [45]. The conjugated out-of-planar π bond network allows electron dislocation, providing graphene with high charge mobility (200,000 cm2 V−1 s−1 in suspended graphene [46]) and good electrical conductivity (6300 S cm−1 of graphene film [46]). Graphene is also highly thermal conductive and a thermal conductivity of about 5000 Wm K−1 was measured on single layer graphene [47]. Thanks to its single-atom thickness and 2D structure, graphene has an exceptional high theoretical specific surface area of 2630 m2 g−1 [48] and superior optical transparency of 97.7% to white light [49].
In addition to this set of unique chemical and physical properties, graphene also has derivatives, graphene oxide (GO) and reduced graphene oxide (rGO), which increase its functionalization capability and broaden its applications. GO can be considered as the oxidized form of graphene and there are abundant oxygen-containing function groups on their honeycomb structure, while rGO is the product of GO after a reduction process that partially restores the graphene structure whilst leaving some oxygen functionalities and defects. Graphene and its derivatives have been making essential changes in the field of energy technology [49], electronics, photonics [50] and catalysis [51] since the successfully isolation of single graphene layers from graphite using scotch tape method in 2004 [5]. The biomedical application is an emerging frontier to graphene and its derivatives. According to their attributes, graphene has been investigated as potential material in biosensors, drug delivery, bioimaging, and tissue engineering.
Accurate sensing and detection of biomolecules are crucial since biomolecules play critical roles in all life processes. Biosensors are designed and built for this purpose. Generally, biosensors are constructed from two structural parts: a receptor and a transducer. The former is usually a bioactive molecule which recognizes one or a group of analytes through a specific interaction with analytes, while the latter transfers the chemical information from the recognition event to a readable signal [52]. The unique properties of graphene make it an outstanding material for biosensors. Its high surface-to-volume ratio facilitates the attachments of molecules or particles to enhance a biosensor’s sensitivity. The quick electron mobility, high and surface-dependent electrical conductivity provide the device a low Johnson and 1/f noises [53]. The easy functionalization can be used to further enhance the response current. Furthermore, its strong mechanical properties give biosensor stability and flexibility, for example, biosensors based on graphene nanowalls for lactate measurement remained highly uniform in response after 250 bending process and 100 twisting process [54]. Biosensors built from graphene based material has been developed for detection of H2O2 [55], glucose [56], dopamine [57], DNA [58,59,60], and RNA [61,62]. We will take glucose as an example to discuss graphene application in biosensors.
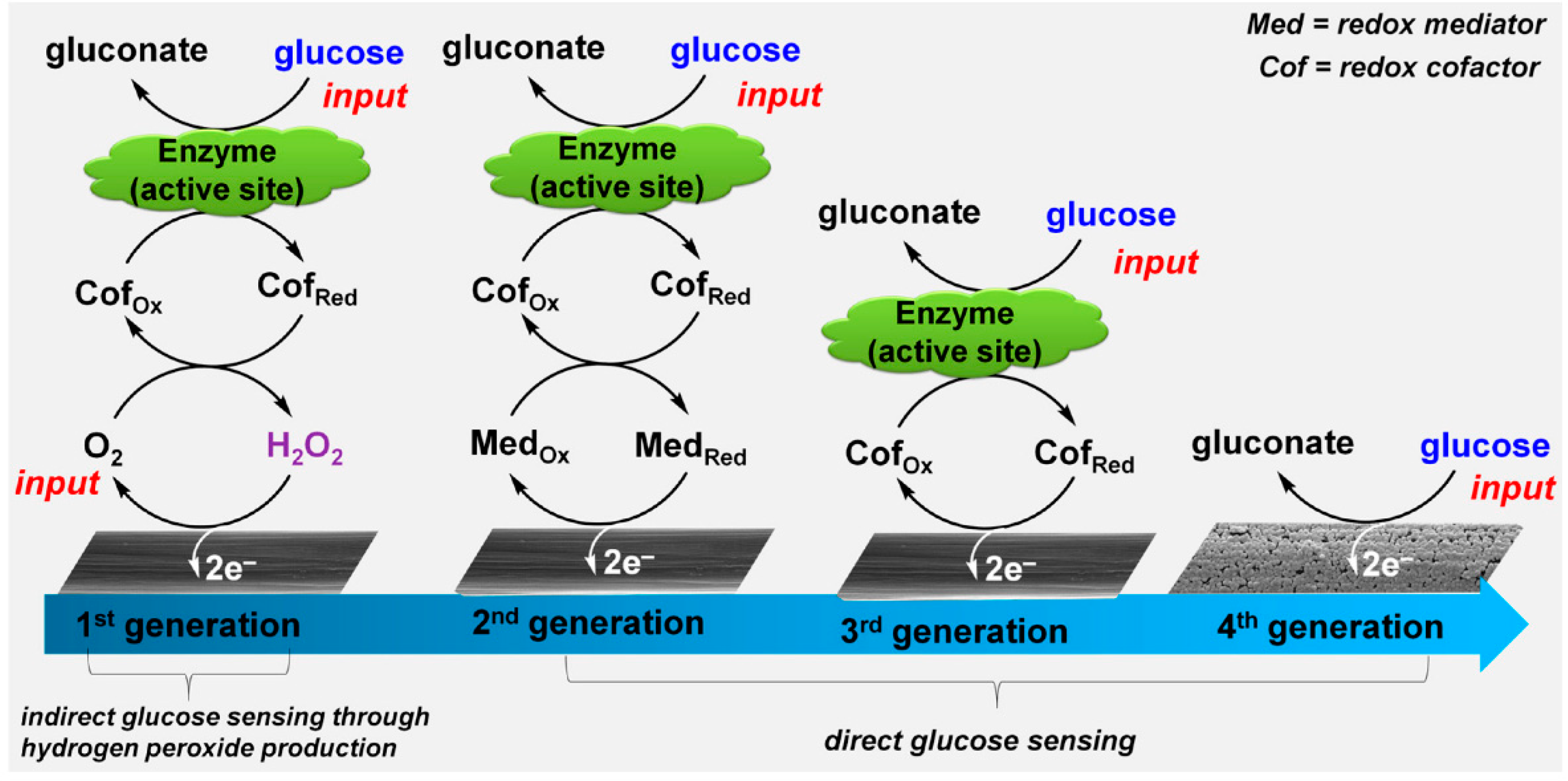
One of the most important biosensors is for the detection of glucose because it plays very important role in the diagnosis and therapy of diabetes. In recent years, great effort has been devoted to the third-generation glucose sensor, which can overcome the sensing dependence on dissolved oxygen in the first-generation sensors and the need of mediates in the second-generation sensors [63]. Same as the former two generations, glucose oxidase (GOx) with a flavin adenine dinucleotide (FAD) as cofactor is used to react with glucose in biological samples, with glucose oxidized by GOx into gluconic acid while GOx(FAD) reduced into GOx(FADH2). Then GOx is directly recycled at the electrode surface by electrochemical oxidation of GOx(FADH2) through a two electron process. Graphene materials have been widely used to make the direct electron transfer (DET) possible. For example, Kang et al. fabricated GOx-graphene-chitosan film on a glassy carbon electrode. A much higher GOx loading was obtained as compared with a glassy carbon surface (GCE), and DET wasobserved by studying the electrochemical behavior of GOx. The sensor exhibited a wide linearity range from 0.08 to 12 mM glucose with a detection limit of 0.02 mM and a sensitivity of 37.93 μA mM−1 cm−2 [64]. Although many graphene-containing glucose sensors were claimed to promote DET, there is a doubt about this process since they were tested in ambient conditions, where oxygen can act as a natural mediator of oxidase. The test a in N2-saturated solution of GOx adsorbed on electrochemical-reduced GO on GCE showed that GOx with enzyme activity could not direct transfer electrons to the electrode since as FAD is deeply embedded within aprotective protein shell and there is a long distance between the active GOx and the electrode surface [65] Therefore, it is still a challenge to obtain DET for third generation glucose sensors. The incoming fourth generation non-enzymatic glucose sensor is possibly one solution, which uses the principle of electrocatalytic oxidation of glucose on metal nanoparticle surfaces, as depicted in Figure 3. Graphene-based material can be used to anchor the metal nanoparticles. Pd/GO [66], Pt/GO [67], Cu/graphene [68], gold nanosheets/graphene fiber [69], and Co-Ni/N-doped graphene [70] have been investigated as materials for glucose sensors.
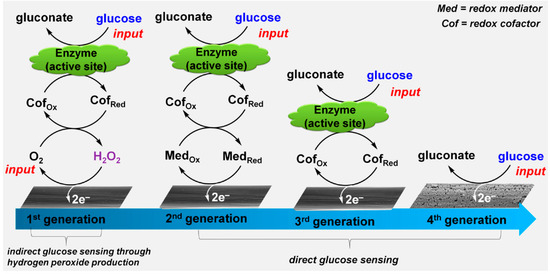
Figure 3.
Sensor development for glucose detection. Reprinted with permission from [63].
Graphene and its derivatives are also interesting materials for drug delivery because the two sides of graphene-based material are accessible to load drug molecules. The feasibility for further functionalization can provide pathways to obtain stable dispersion in physiological buffers and improve their biocompatibility. The loading of drug can be through π-π stacking interaction, hydrogen bonding, or hydrophobic interactions [71], while the drug release can be accomplished due to the pH difference of the tumor microenvironment, the cellular redox environment, the specific biomolecules, or external physical stimuli, e.g., light, magnetic field, temperature [72].
The pioneer work using graphene based material for drug delivery was done by Dai’s group in 2008 [73]. They adsorbed SN38, a FDA approved prodrug for colon cancer treatment, onto polyethylene glycol (PEG)-functionalized GO. They observed that the loading amount of SN38 increased to 0.1 g on 1 g PEG-GO from no loading on pure PEG polymer. The SN38-PEG-GO was water soluble and stable in phosphate buffer saline and mouse serum and showed enhanced cell killing ability compared with a water-soluble drug CPT-11. This pioneer study has stimulated the reach on the delivery of various drugs, e.g., doxorubicin hydrochloride (DOX) [74,75,76,77,78], chlorin e6 (Ce6) [79,80], camptothecin (CPT) [81,82], methotrexate (MTX) [83,84,85], and ibuprofen [86]. As the most effective drug for cancer treatment, DOX is widely investigated. For example, GO was successfully functionalized using chitosan and dextran by a layer-by-layer self-assembly technique. DOX was loaded into the nanocomposite via π-π stacking and electrostatic attraction. The functionalization not only improved the dispersion the DOX-loaded GO nanosheets in physiological conditions, but also decreased the non-specific protein adsorption of GO nanosheets. The nanocomposites were able to be untaken by MCF-7 cells and DOX loaded counterpart had a strong cytotoxicity to cancer cells [87]. When DOX was loaded onto chitosan modified Ag-GO hybrid particles, an antibacterial activity was added in addition to anti-cancer performance [88]. To increase cancer treatment efficiency, it is necessary to develop a delivery carrier which can bond with multiple drugs simultaneously. By grafting GO with poly(N-isopropylacrylamide) via covalent interaction, the functionalized GO proved to be an efficient carrier for both hydrophilic DOX and hydrophobic indomethacin (IMC). The release of both of the loaded drugs can be trigged by a pH change of the microenvironment [89]. Two anticancer drugs, quercetin and gefitinib, were also reported to be successfully loaded on and released from polyvinylpyrrolidone (PVP) modified GO, exhibiting higher cytotoxicity to PA-1 ovarian cancer cells in comparison to the individual drugs loaded onto a GO polymer composite [90].
Another important field of graphene biomedical application is bioimaging. Bioimaging enable us to understand biological process happing in the living cells, tissues and organs using an imaging probe. The versatile surface functionalization coupled with high surface area, good biocompatibility and unique optical properties of graphene-based materials make them good materials for improving the currently used imaging probes for exploring new probes. Currently, there are several main imaging techniques, that is, fluorescence imaging, Raman imaging, magnetic resonance imaging etc. [91]. Electron paramagnetic resonance imaging (EPRI) is a relatively new and developing technique for imaging, in which radicals are used as imaging agents. Carbon blacks was also reported as an EPRI medium for localized measurements of tissue oxygenation [92]. Recently, graphene nanoribbons have shown to be promising material for oxygen detection using the EPRI technique [93]. However, here, we will focus mainly on the mostly used techniques.
For fluorescence imaging, Sun et al. prepared single-layer GO sheets down to less than 10 nm, covalently grafted with PEG and used as a probe for imaging. Since the prepared GO and PEG-GO showed photoluminescence in the IR and NIR regions, NIR photoluminescence was selectively detected on positive Raji B-cell surface after PEG-GO was conjugated with a B-cell specific antibody Rituxan (anti-CD20) and incubated with B-cells and T-cells [94]. To explore the possibility of in vivo imaging, fluorescent dyes co-conjugated onto GO to enhance the fluorescent signal. Cy7, a common NIR fluorescent dye is used to label PEG-nanographene sheets. In vivo fluorescence imaging revealed high tumor uptake in several xenograft tumor mouse models. Coupled with strong optical absorbance of the nanographene sheet in the NIR region, this material proved to be an excellent in vivo imaging probe as well as photothermal therapy agent [95]. However, due to the potential risk of photobleaching of the fluorescent dyes, upconversion nanoparticles have been used to anchor on graphene-based material to obtain fluorescent imaging. GO quantum dots functionalized with NaYF4:Yb3+, Er3+ upconversion nanoparticles and hypocrellin A (a commonly used chemotherapy drug and a photo-sensitizer) were taken up by tumor cells and improved upconversion signal properties. The HeLa cells can be detected and imaged when treated with 5 g mL−1 of the functionalized GO quantum dots [96].
Raman mapping is also a powerful tool for bioimaging since both graphene and GO show unique Raman signala. However, since the Raman signals from graphene-based material are weak, Au nanoparticles are usually decorated on the graphene surface to enhance the intensity of the Raman signal through surface enhanced Raman scattering (SERS). By the decoration of GO and rGO, Raman imaging for HeLa 229 and HepG2 hepatocarcinoma cells was realized, respectively [97,98]. In another study, Au nanoparticles were wrapped by graphene oxide and this material showed enhanced Raman signal compared with GO only. HeLa cells were clearly imaged after a treatment with Au@GO nanoparticles for 24 h [99].
Magnetic resonance imaging (MRI) is a widely used technique for medical diagnosis in hospitals and clinics. In order to obtain good diagnosis quality, contrast agents are usually used to increase the contrast between the examined part and normal parts. Gd3+ chelates are the most used T1 contrast agents to get brighter images, but they suffer from the release of free Gd3+ ions which are very toxic because they block calcium channels since they have similar ionic size as Ca2+. A new T1 contrast agent based on water dispersible Gd2O3/GO nanocomposites was synthesized by Wang et al. using a facile solvent evaporation method. Gd2O3/GO exhibited better biocompatibility and higher relaxivity value than the typical commercial MRI T1 contrast agent Gd-DTPA [100]. Iron containing magnetic nanomaterials, e.g., Fe3O4, and CoFeO4, are important components of the T2 contrast agent, through which much darker images can be obtained. However, these nanomaterials tend to aggregate and precipitate in practical application. This problem was solved by using Fe3O4 functionalized graphene oxide as report by Zhou et al. [101]. The prepared Fe3O4-GO hybrid demonstrated good hydrophilicity, less cytotoxicity, high NRI enhancement with the relaxivity of 493 mM−1 s−1 as well as an MRI contrast effect of BxPC-3 cells [101].
Last but not the least, is graphene’s biological application to tissue engineering. Tissue engineering is an interdisciplinary field that involves the knowledge of bioengineering, the life sciences, and the clinical sciences towards solving the critical medical problems of tissue loss and organ failure [102]. Graphene-based material has attracted great attention for application in tissue engineering due to their strong mechanical strength, excellent electrical conductivity, and versatile surface modification properties. Their applications in cardiac, neural, bone, cartilage, skeletal, and skin/adipose tissue engineering and regeneration have been investigated [103]. For example, Kim et al. prepared graphene-incorporated chitosan substrata and studied their effects on the adhesion and differentiation of human mesenchymal stem cells. Their results showed that the adhesion and differentiation of human mesenchymal stem cells were greatly enhanced due to the asymmetrical nanotopology environment of the RGO-chitosan substrata and the enhancement of cell-substrate interaction and cell-cell contacts [104].
2.1. Synthesis
The interactions between biomolecules and graphene-based material depend greatly on the properties of graphene-based materials, e.g., layer number, lateral dimension, chemical residual, surface charge and surface functional groups, while synthesis methods of graphene-based materials have led to large difference in their properties. There have several synthesis methods developed for graphene synthesis along with the intense study on their applications. These synthesis methods can be generally divided into two categories: the bottom-up approach and the top-down approach.
2.1.1. Bottom-Up Synthesis
The bottom-up synthesis approach builds graphene from atom levels and it includes three main synthesis methods: epitaxial growth on silicon carbide (SiC), the chemical vapor deposition method (CVD) and plasma-enhanced chemical vapor deposition (PECVD). Epitaxial graphene growth on SiC uses the thermal decomposition of SiC at high temperature under vacuum or in inert gas. Since carbon has neglectable vapor pressure compared to silicon, graphene layers are formed on the SiC surface after silicon sublimation. This method was firstly reported in 1965 by Badani. In this study, SiC crystals were annealed in vacuum in an induction furnace up to 2280 °C for an hour and the development of a graphite lattice was found on SiC crystals [105]. Later research by Van Bommel et al. suggested that a monolayer of graphene had already formed at temperatures as low as 800 °C under ultra-high vacuum. They also observed that graphite layers were formed on both the C-face and Si-face of the SiC crystals. The C-face generated a polycrystalline graphite layer, while Si-face generated a monocrystalline graphite layer [106]. However, this method suffered from low quality graphene with varied thickness of small graphene grains due to the surface morphology change of SiC from high temperature annealing under vacuum. To slow down the sublimation rate, annealing SiC in an argon atmosphere [107] or in an external Si flux [108], or depositing a nickel [109] or cobalt [110] layer on SiC, were proved effective in controlling the resulted graphene quality. Epitaxial graphene growth on SiC is a promising method to produce graphene on semi-insulating material which can then be directly used as electronic materials without transferring, but for biomedical application, it may hinder its application because in most cases graphene transfers are required.
One of the most studied methods for producing high quality, large area graphene is the CVD method. During the CVD process, a precursor gas containing hydrocarbon molecules is charged into the reactor, in which hydrocarbon molecules are catalytically decomposed into carbon radicals and arranged into a graphene structure on the catalytic layer of the substrate. Methane is the most used precursor for graphene production using the CVD method, but there are also plenty of other gas precursors used, e.g., acetylene [111], ethylene [112], propene [113], or liquid precursors like methanol [114], ethanol [115], propanol [114], hexane [116], benzene [117], or even solid precursors like poly(methyl methacrylate) (PMMA) [118], and amorphous carbon [119]. The catalysts investigated include nickel (Ni) [120], copper (Cu) [120], Rhodium (Rh) [121], cobalt (Co) [122] or alloys [123,124,125]. The catalyst layer was proved not only to lower the activation energy for precursor decomposition, but also led to different graphene formation mechanisms. Graphene growth on Ni is a carbon segregation and precipitation process since Ni has a high carbon solubility at elevated temperatures. During the cooling down process, carbon solubility in Ni decreases and carbon atoms diffuse out from the Ni-C solid solution and precipitate on the Ni surface to form graphene films. However, Cu has much lower carbon solubility than Ni and there is only a small amount of carbon dissolved in Cu, thus the carbon source of graphene growth on Cu is directly from precursor decomposition. Furthermore, graphene growth on Cu is a self-limiting process, which ispreferable to form single layer graphene than Ni [120,126]. The properties of the obtained graphene, e.g., layer number, crystal size and layer number, are affected by several factors such as presursors, assistant gases, catalyst layer, and temperature.
PECVD has been developed to synthesize graphene at a much lower temperature than CVD with a shorter deposition time. This method can overcome the evaporation problem of catalyst layer at high temperature. For example, Malesevic prepared free standing 4–6-layer graphene at a temperature of 700 °C using a microwave PECVD method. However, one problem of the PECVD method is the generation of plenty of defects in the graphene structure due to the interaction of high energetic particles with the growing surface [127].
2.1.2. Top-Down Synthesis
The top-down synthesis separates graphene sheets from high-quality graphite which contains stacked multi-layer graphene using a mechanical or chemical approach. It includes the mechanical exfoliation method, the chemical exfoliation method and the GO reduction method.
The mechanical exfoliation method is the first documented method to successfully separate graphene from graphite. It used scotch adhesive tape to break the van de Waals force between layers of graphite by a repeated sticking and lifting process [5]. This method can produce high quality graphene, but suffers from the problem of scalability which hinders its commercialization. Ultrasonic force is also used to produce graphene in solvents, e.g., N-methylpyrrolidone (NMP), utilizing the strong interactions between solvents and graphene [128]. Another kind of force used is the shear force in the ball-milling process. The ball-milling can be processed in both wet [129] and dry form [130]. It is a problem to separate graphene for both the ultrasonic and ball-milling methods since some un-exfoliated graphite is mixed with graphene sheets. The ball-milling method also has shortcomings of the formation of amorphous carbon and defect generation on the produced graphene.
One versatile method for the large-scale synthesis of graphene, to be exact rGO, is the reduction of GO. This method generally includes two steps: GO production and GO reduction to rGO. To generate GO, the Hummers method is usually used to oxidize graphite. In the oxidation process, graphite reacts with sodium nitrate, concentrated sulfuric acid, and potassium permanganate, which introduces oxygen-containing functional groups onto graphite sheets [131]. The graphene oxide layer can be separated by ultrasonic treatment in polar solvent, especially in water, due to the enlarged layer spacing as the functional groups are introduced. As already discussed, GO itself can be used for biomedical applications because of their easy functionalization as a result of the presence of functional groups. It can, as well, restore its graphene structure through electrochemical reduction [132], thermal reduction [133], chemical reduction [134] or hydro/solvothermal method [135,136]. Chemical reduction is widely used for GO reduction and a variety of reductants have been studied. There are several reductants which are effective for GO reduction to rGO, e.g., hydrazine [137], sodium borohydride [138], hydrohalic acid [139], and ascorbic acid [140]. For biomedical applications, chemical resides from reductants will affect rGO performance and its biocompatibility since most of them are toxic. Recently, green reductants such as plant extracts, sugars, microorganisms, and amino acids have been exploited to remove this barrier [134]. For example, baker’s yeast containing nicotinamide adenine dinucleotide phosphate (NADPH) has been used as a reducing agent and functionalizing agent. The amine functional group of NADPH can easily couple with the epoxy functionalities of GO and forms stable water dispersion of yeast-rGO [141]. These effects aimed at preparing biocompatible rGO through chemical reduction.
2.2. Functionalized Graphene-Based Materials
For biomedical applications, the surface functionalization of graphene-based material is essential. Firstly, surface functionalization with PEG [142] or dextran [143], was reported to largely decrease the toxicity and improve the biocompatibility of graphene. Secondly, a good dispersion of graphene-based materials is critical. However, graphene and rGO with low oxygen content are hydrophobic in polar solvents and they tend to aggregate in aqueous solution. Even water-soluble GO, which is rich with oxygen-containing functional groups, is liable to aggregate in physiological buffers with salts due to a charge-screening effect [144,145]. More importantly, the surface functionalization brings functional centers important for biological applications, for example, the Au nanoparticle for Raman imaging.
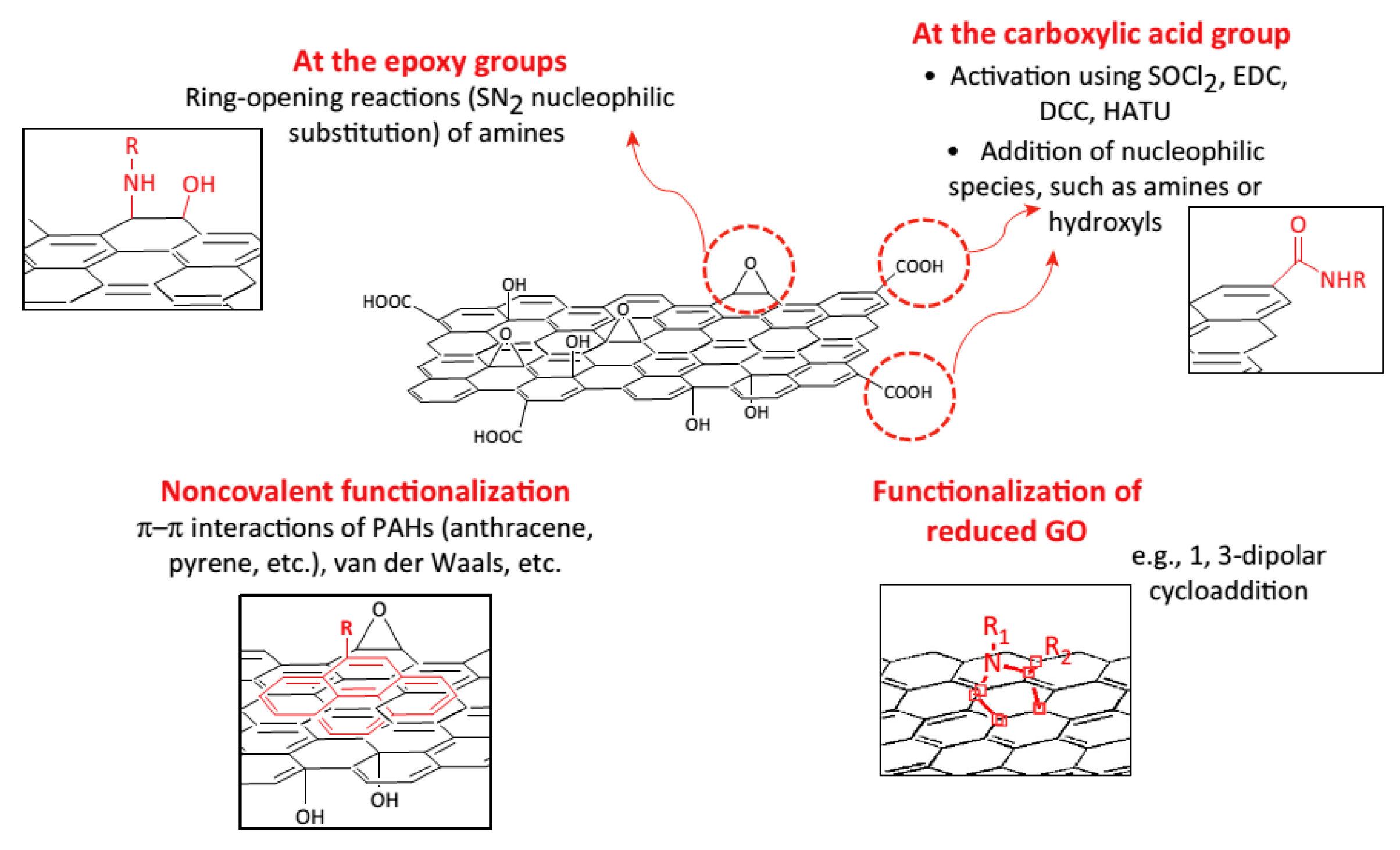
Up to date, surface functionalization of graphene and its derivatives can be processed via two strategies: non-covalent binding and covalent binding. As shown in Figure 4, noncovalent functionalization can proceed through the adsorption of biomolecules via the van der Waals force, π-π interactions, while covalent functionalization can be performed by grafting biomolecules using chemical reactions between biomolecules and oxygen-containing functional groups of GO or dipolar cycloaddition reaction on graphene.
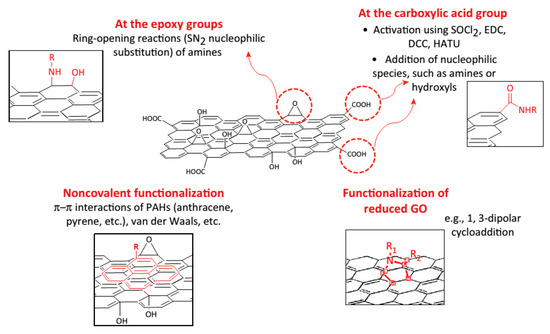
Figure 4.
Different approaches for the surface functionalization of graphene-based materials. Reprinted with permission from [146].
2.2.1. Non-Covalent Functionalized Graphene-Based Materials
We will firstly discuss the non-covalent functionalization of graphene-based materials with surfactants, polymers, or biomolecules. The forces involved for non-covalent functionalization include hydrophobic interactions, π–π stacking, or electrostatic binding.
The hydrophobic nature of graphene and rGO can be used to adsorb the aliphatic parts of surfactants through hydrophobic interactions. This process makes the polar parts of surfactants interact with the surrounding environment, enhancing the stability of the surfactant-functionalized graphene or rGO in polar solvents, especially in aqueous solution. Assali et al. [147] functionalized graphene sheets with three different surfactants, cetrimide, sodium dodecyl sulfate, and tween 80, in 1% w/v surfactant solution with 30 min sonication. After functionalization with surfactants, the graphene sheets became totally dispersed and all three dispersions kept good stability for more than three months without the formation of any precipitate. In other studies, rGO functionalized with tween 20 [148] and Pluronic F127 [149] were prepared by adding the surfactant during the GO reduction with hydrazine hydrate. After reduction, both functionalized rGO materials formed a stable homogeneous black solution.
Electrostatic interactions are also effectivein functionnalizing graphene-based material. Since GO sheets are negatively charged, a positively charged molecule can therefore bind to the GO surface via electrostatic force. Feng et al. prepared GO-polyethyleneimine (PEI) complexes by simply mixing PEI solution with GO solution, followed by separation and washing after 10 min sonication and overnight stirring. The obtained GO-PEI complexes are positively charged and negatively charged DNA was also loaded on it by electrostatic interactions [150]. Fang et al. prepared chitosan-mediated graphene suspensions by simply adding the GO suspension to the solution of chitosan under stirring. Since GO is negatively charged and chitosan is positively charged, when GO was added to the solution of chitosan, every GO sheet was immediately wrapped by large amounts of chitosan molecules through electrostatic attractions. Then, the GO sheets were isolated as individual ones and a uniform dispersion formed. The system is pH responsive and has potential for biomedicine application [151].
Thanks to the conjugated out-of-planar π bond network of graphene-based materials, molecules containing aromatic rings can attach to the surface of graphene-based materials by π–π interactions. Husale et al. [152] observed that ssDNA binded only to the graphene and not to the SiO2 substrate, confirming that the binding energy is mainly due to the π–π stacking interaction. This phenomenon was reported to be used to design DNA sensors [153]. The π–π interaction is widely investigated as aforce to load aromaticrings containing drugs on graphene-based materials for drug delivery. Anti-cancer drugs DOX, and quercetin together with gefitinib were successfully loaded and delivered with PEG-GO and PVP-GO, respectively [90,154].
2.2.2. Covalent Functionalized Graphene-Based Materials
As we discussed above, GO bears carboxyl, epoxy, and hydroxyl groups on its surface, which provide reactive sites for biomolecule covalent functionalization. The functionalization can be through the amidation reaction of the carboxylic acid group of GO with amine groups in polymers. In 2008, Dai’s group prepared PEG functionalized nano-graphene oxide (nGO) for the delivery of an insoluble cancer drug. The functionalization of nGO was performed in three steps. Firstly, nGO was synthesized using a modified Hummer’s method. Then the obtained nGO was carboxylic acid modified using 3M NaOH solution or chloroacetic acid in NaOH solution. Then six-armed PEG-amine stars were conjugated to the carboxylic acid group on nGO via a carbodiimide catalyzed amide formation [73,94]. This material showed superior stability in biological solution. This PEGylation method has been widely used to functionalize graphene-based material for varieties of biomedical applications [81,83,155,156].
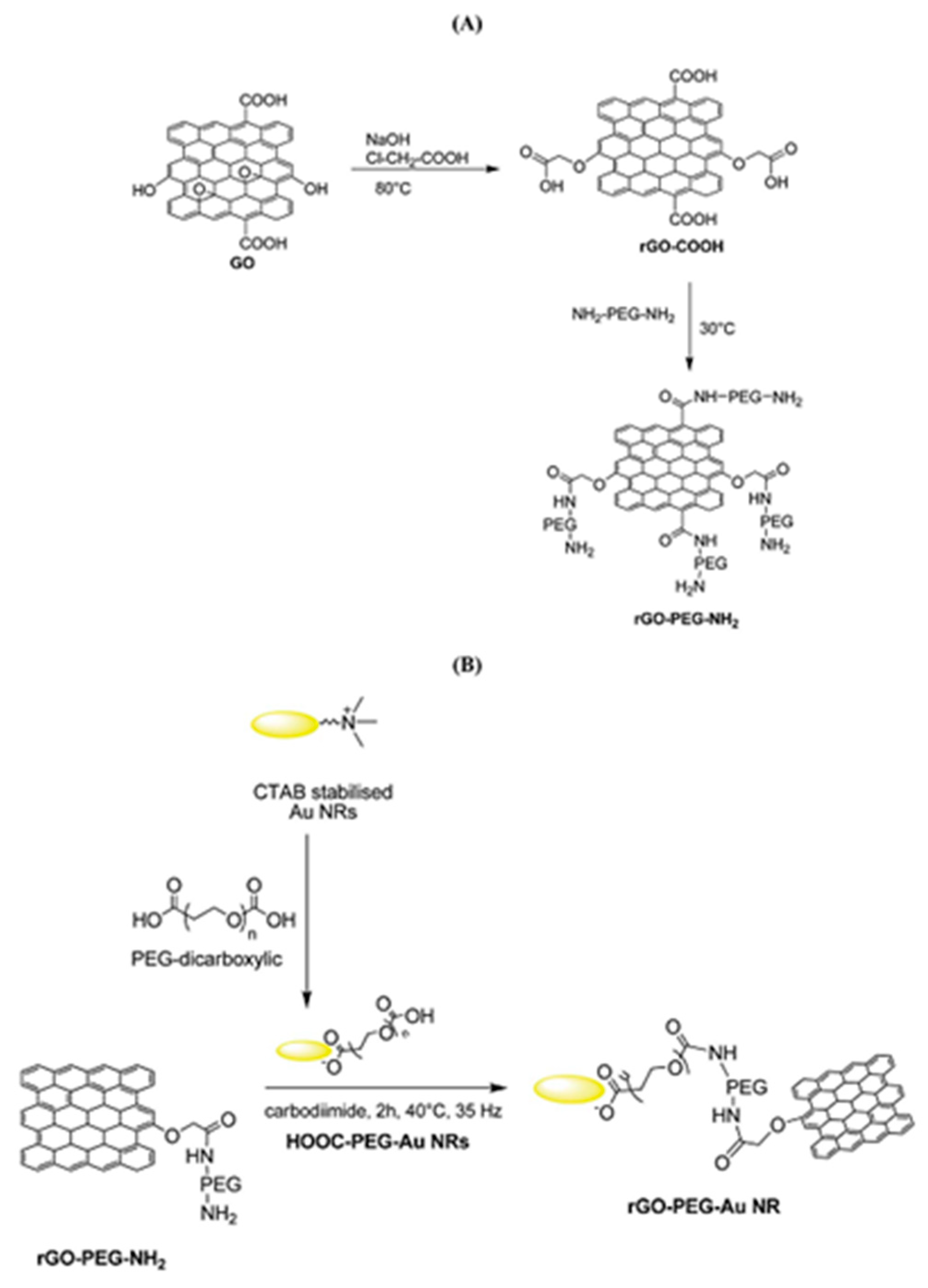
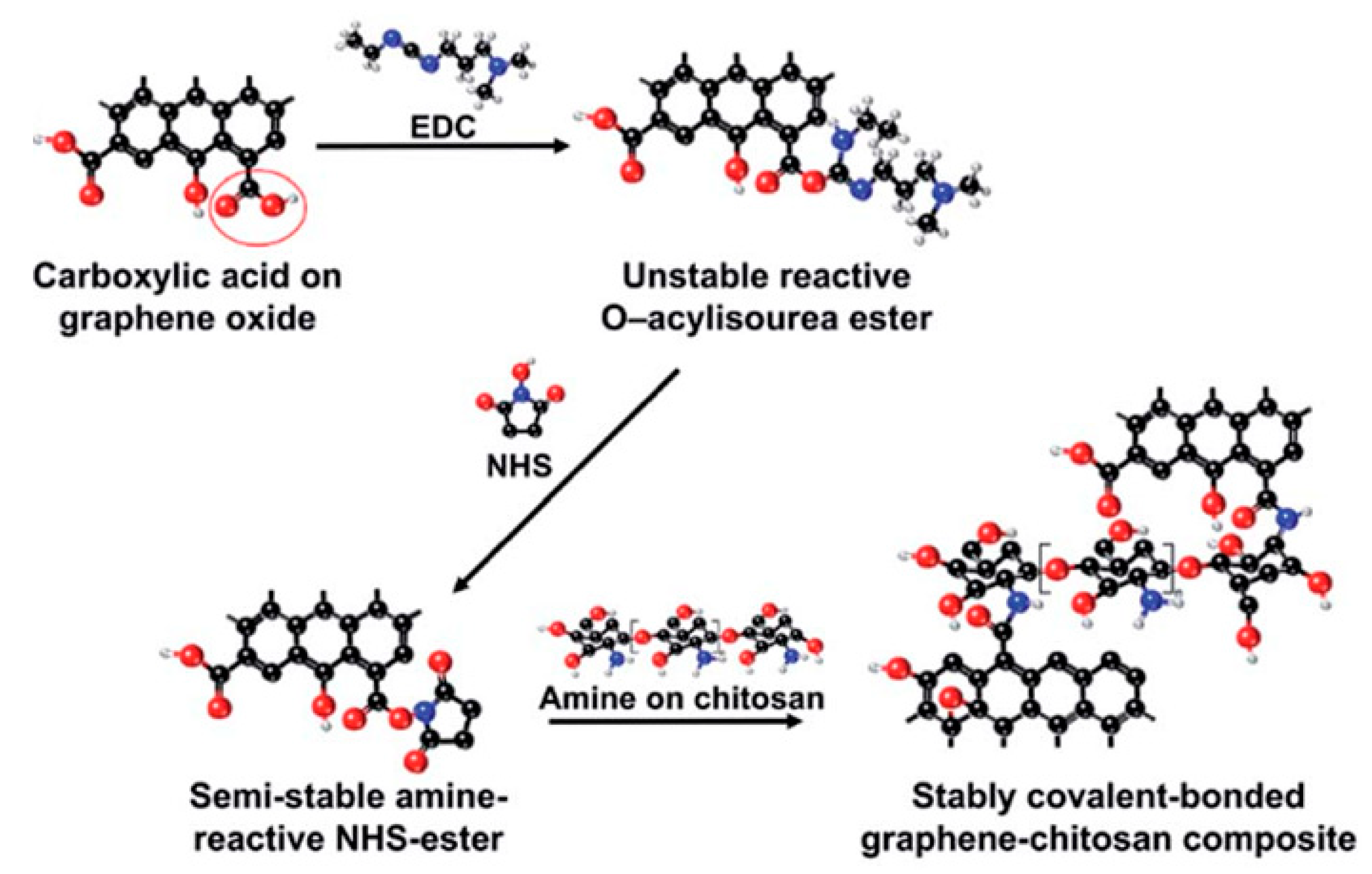
During the functionalization process, if PEG-diamine is used instead of PEG-amine, the functionalized GO has an amine functional group on the surface for further functionalization. For example, as shown in Figure 5, gold nanorods (Au NRs) coated with rGO-PEG (rGO-PEG-Au) were synthesized for the selective killing of uropathogenic E. coli UTI89 which is associated with urinary tract infection. The first step for rGO-PEG-NH2 synthesis followed the sample procedure from Dai’s group but replacing PEG-NH2 with NH2-PEG-NH2 (Figure 5A). In the second step, cetyltrimethylammonium bromide (CTAB) protected Au NRs were prepared using a seed-mediated procedure and pegylated with PEG-dicarbonylic to get HOOC-PEG-Au NRs. Then the reaction between rGO-PEG-NH2 and HOOC-PEG-Au NRs formed the final product (Figure 5B). This product was proven to be a powerful photothermal agent for the effective killing of the target bacteria with a 99% killing efficiency [157]. The carbodiimide catalyst during the amidation reaction forms an unstable reactive O-acylisourea ester which also reacts water. To improve the stability of the intermediate and promote the creation of the amide type linkage, another co-catalyst, e.g., N-hydroxysuccinimide (NHS), is usually added during the functionalization of GO with polymers. As demonstrated in Figure 6, a graphene-chitosan composite was formed by an unstable O-acylisourea ester and a semi-stable amine-reactive NHS-ester which reacted with amine on chitosan. Based on this composite, mechanically robust graphene oxide fibers were form using a wet spinning approach [158]. Poly(ethyleneimine) was also successfully covalently functionalized on poly(acrylic acid) modified GO after activating the carboxylic acid group using EDC/NHS. In vitro cell studies revealed that GO-PEI composites promoted proliferation and focal adhesions in hMSCs with potential use as tissue scaffolds [159].
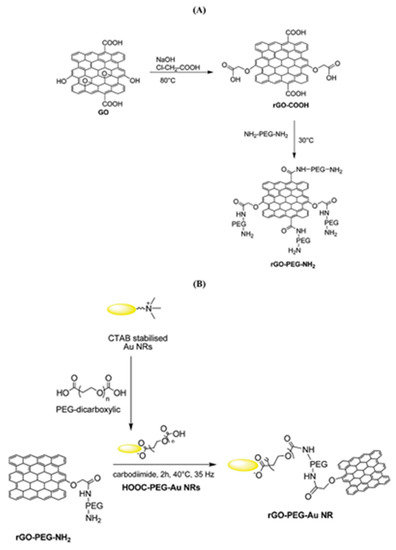
Figure 5.
(A) Formation of PEG-modified reduced graphene oxide (rGO-PEG-NH2) and (B) rGO-PEG-Au NRs coated through the covalent linking of rGO-PEG-NH2. Reprinted with permission from [157].
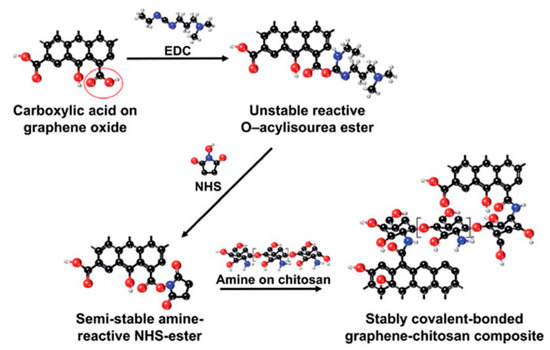
Figure 6.
Reaction pathways of EDC and NHS intermediated functionalization of GO with chitosan. Reprinted with permission from [158].
Esterification is another reaction which is frequently used to functionalize GO. Monica Veca et al. prepared PVA functionalized few-layer graphene material through the carbodiimide-activated esterification reaction in DMSO between the carboxylic acid moiety on the nanosheets and hydroxyl groups on PVA. After functionalization with PVA, a stable solution was formed in DMSO and hot water [160]. Salavagione et al. firstly converted the carboxylic acid groups of GO into acryl chlorides using SOCl2 in DMF. Then the acryl chloride-derivative graphite was reacted with PVA in DMSO to obtain PVA-functionalized GO. The obtained composite is soluble in DMSO and water with the aid of heat [161]. PVC-modified GO was also synthesized by same group [162].
Besides the reaction with the carboxylic groups of GO, it is found that the epoxy group can also be used for the functionalization of GO with polymers. Shan et al. [163] prepared poly-L-lysine (PLL) covalently functionalized graphene through the reaction of epoxy groups on GO and amino groups on PLL in the presence of KOH, followed by reduction by NaBH4 solution. PLL-functionalized graphene is water soluble and biocompatible and has the potential to be a promising material for biomedical applications.
For graphene and rGO which have no or less oxygen-containing functional groups, free radical addition and 1,3-dipolar cycloaddition are good choices for covalent functionalization. A good example of free radical addition is the diazotization reaction. This reaction proceeds as following: generation of the diazonium salt from the amino reagent using a nitrite species, single electron transfer between the diazonium salt and the graphene material, radical addition, formation of the phenol and adsorption of phenol onto the graphene sheet [41,164]. Wei et al. functionalized rGO with p-aminobenzoic acid, which formed the diazonium ions through diazotization with a wet chemical method. After loading with PEI and FA, it was proven to be a good carrier for DOX to arrest cancer cells [165]. 1,3-dipolar cycloaddition method was applied by Prato et al. using azomethine ylide to modify few-layer graphene. The obtained functionalized material can be further modified for use in the biomedical field [166].
2.2.3. Nanoparticle Functionalized Graphene-Based Materials
A large number of inorganic nanostructures such as Au [97,98,167], Ag [168], Pd [66], Pt [67,169], Cu [68], Fe3O4 [101,170], Gd2O3 [100], and NaYF4-based upconversion nanocrystals [96] have been anchored on graphene-based materials for a variety of biomedical applications. Au NRs/GO core-shell nanocomposites was loaded with DOX for a combined chemo- and photothermal targeted treatment of cancer cells [171]. In certain cases, the integration of Au into graphene nanosheets to efficiently improve the sensitivity and detection limit, as demonstrated by Shan et al.’s study on Au/rGO functionalized with DOX for glucose detection [172]. Au and Ag decorated graphene-based materials were also used for Raman imaging [145]. Li et al. prepared folic acid-Fe3O4@nGO-DOX using nGO encapsulated Fe3O4, which is very effective for simultaneous tumor MRI imaging and target therapy [173].
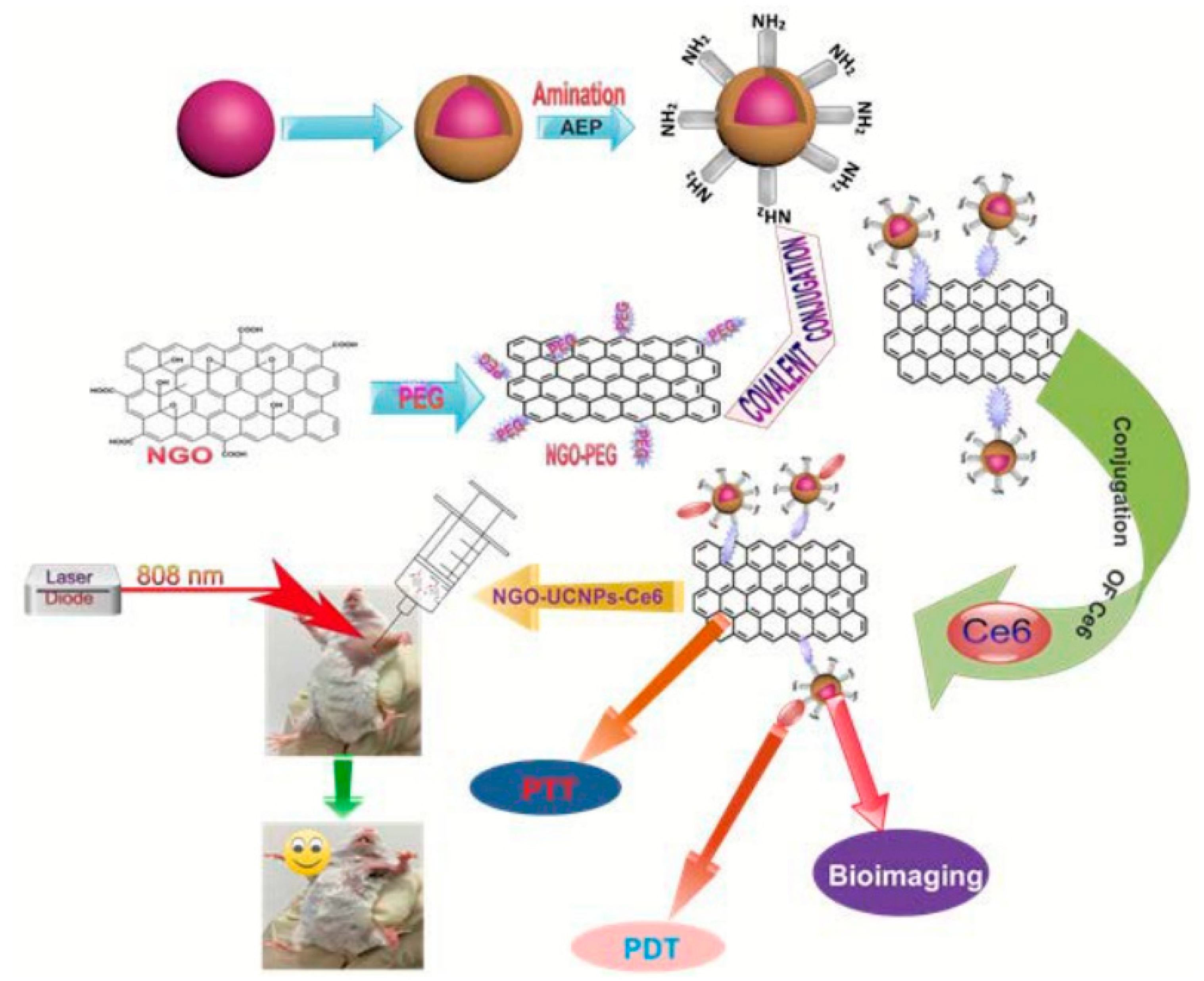
Utilizing upconversion nanocrystals is a promising method for NIR light-mediated bioimaging and photothermal and photodynamic therapy. Gulzar et al. [131,174] covalently functionalized nGO with core-shell structured unconversion nanoparticles in the steps as shown in Figure 7. Oleic acid stabilized NaGdF4: Yb3+, Er3+ was firstly synthesized followed by the formation of a NaGdF4: Nd3+, Yb3+ shell around it. The surface of the core-shell structure was then amino-modified via a phase transfer process using 2-aminoethl dihydrogen phosphate (AEP). GO was made using a modified Hummer’s method and PEGylated using the EDC intermediated synthesis method. Carbodiimide cross linking reaction between the amino group of amino-modified core-shell unconversion nanoparticles and the carboxyl group of NGO-PEG was employed for the covalent grafting of the unconversion core-shell structure. After loading with Ce6, cytotoxicity assays revealed decent biocompatibility and minor toxicity. This multifunctional cancer therapy platform has achieved superior therapeutic efficacy for in vitro and in vivo cancer therapy.
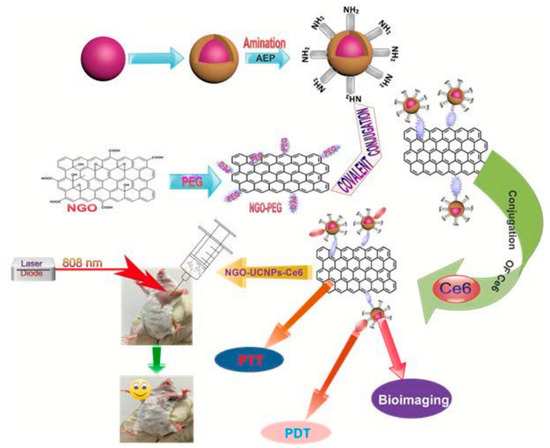
Figure 7.
Covalent functionalization of nGO with core-shell upconversion nanoparticles. Reprinted with permission from [174].
3. Carbon Nanotubes
Carbon Nanotubes (CNTs) are one kind of carbon allotrope which has a seamless hollow cylindrical shape. Like carbons in graphene, carbon atoms in CNTs are also bonded with three neighboring carbon atoms in a sp2 configuration forming the hexagonal units. Conceptually, CNTs are considered as rolled-up graphene sheets in certain directions. The rolling up of single-layer graphene and multi-layer graphene form single-wall CNTs (SWCNTs) and multi-wall CNTs (MWCNTs), respectively. Therefore, CNTs and graphene share many of the interesting properties. For example, SWCNTs have strong mechanical strength, with a Young’s modulus value range from 320 to 1470 GPa and breaking forces ranging from 13 to 52 GPa [175]. The electrical conductivity of the CNTs along the axis of a single CNT was reported to be as high as 2 × 107 S m−1 [176], while for MWCNTs the electrical conductivity is about 2 × 105 S/m [177]. The thermal conductivity of individual SWCNT was reported to be 3500 W m−1 K−1 [178], above that of bulk graphite (about 2000 W m−1 K−1), for individual MWCNT the value is about 3000 W m−1 K−1 [179]. SWCNTs have a high theoretical surface area of 1315 m2 g−1 [180], although only about half that of graphene; however, the experimental values depend greatly on the quality of the SWCNTs and the highest value reported is 1587 m2 g−1 [181]. The one-dimensional tubular morphology of CNTs also bring them unique properties. CNTs have super high length-to-diameter aspect ratio with a diameter of 0.4–2.5 nm and a length of 20–1000 nm for SWCNTs, and a diameter of 1.4–100 nm and a length of 1–500 μm for MWCNTs, respectively [182]. Individual CNT can be either metallic or semiconducting depending on the orientation of the lattice with respect to the tube axis, which is called chirality [183]. The tubular shape provides CNTs with a certain internal volume which can be applied to house specific functionalities. Furthermore, the curvature of CNTs makes CNTs generally much easier than pristine graphene for chemical functionalization.
Since the report of MWCNTs in 1991 by Iijima’s group [4] and the reports of SWCNTs in 1993 by the same group [184] and Bethune’s group [185], scientific research has boomed afterwards for the methodology development of CNT synthesis and exploration of their potential application in various fields because of their unique physicochemical properties. Their applications include but are not limited to electronics [186], energy [187], environment [188], atomic force microscopes [189] and nanomedicine [190]. Biomedical applications are one of the most important fields for CNTs. We will discuss below how CNTs work as materials for biosensors, drug delivery, bioimaging and tissue engineering.
Because of their high aspect ratio, high mechanical strength, and high electron transfer rates, CNTs have been considered as excellent material for biosensors. As we discussed in the graphene section, biosensors include two components, an acceptor and a transducer. When analytes contact the sensor, the reaction between analytes and acceptor generate an electrochemical or photo signal which is then transfered to the processing part to accomplish the sensing aim. With a tubular structure and superior electrical conductivity, CNTs are expected to be versatile material to enhance the direct electron transfer so as to increase the sensitivity of the sensor. Zhang et al. reported the use of functionalized SWCNTs as electrical connectors between the enzyme acceptor, GOx, and the Au electrodes for the glucose detector. A clear dependence of the electrical communication on the length of the CNTs was observed. The sensor with a 25 nm CNT revealed an approximate 1.5-fold current signal as compared with the electrode with 50 nm CNT. The author assumed that the electrical communication controlled by the length of CNTs might be due to the wall defect sites which act as local barriers to charge transport [191]. Recently, Rernglit et al. soaked GOx into porous CNT film on a Pt disk electrode and trapped it beneath a topcoat of electrodeposition paint. The resulting sensors produced a glucose signal that was linear up to 40 mM, with a 50 μM detection limit. This glucose sensor proved to have a signal stability over 100 h of continuous operation [192]. Enzyme-based biosensors with CNTs were also designed for the detection of various other analytes, e.g., tyrosinase on polypyrrole-SWCNTs for dopamine detection [193], glutamate dehydrogenase-nicotinamide adenine dinucleotide on chitosan-MWCNTs for glutamate sensing [194], D-fructose dehydrogenase on 3,4-dihydroxybenzaldehyde on CNTs for fructose determination [195], anti-NS1antibodies on CNTs electrode for virus NS1 protein detection [196], and DNA probes on MWCNTs modified glass carbon electrode for miRNA sensing [197]. Recently, the research on CNT application in biosensors transited to non-enzyme based sensors. For example, a glucose biosensor was constructed using NiCoO2 nanosheets on CNTs. This sensor displayed good performance for glucose with a response sensitivity of 1424.41 μA mM−1, and a low detection limit of 1.14 μM [198]. A dendrimer-encapsulated Pt nanoparticle-CNT composite-based electrochemical biosensor was fabricated for the detection of hydrogen peroxide, ascorbic acid, and acetaminophen in the concentration ranges of 10 μM–10mM, 50 μM–8mM and 20 μM–1 mM, respectively, with rapid current response and good reproducibility [199].
CNTs have been considered an excellent potential vehicle for drug delivery since their discovery. Their high surface area and internal volume determines the high loading of drugs not only by the outer wall surface but also the internal cavity. Their 1D needle-like morphology facilitate CNT penetration through the cell membranes of targeted cells and the CNTs have already proven to have the capability to smartly deliver to desired sites after appropriate functionalization [200]. In 2007, Dai and coworkers reported the preparation of water-soluble phospholipid (PL)-PEG modified SWCNTs and loaded the DOX drug on them. An extremely high drug loading of about 50–60 w.t% was obtained, which is remarkably higher than the about 8–10 wt.% for conventional liposomes. The SWCNT-DOX complex remained stable in normal physiological environments and released fast in acidic tumor cells [201]. In their later research, paclitaxel (PTX), a widely used cancer chemotherapy drug was conjugated onto PEGylated SWCNTs and obtained a water-soluble SWCNT-PTX conjugate. A 10-fold higher PTX uptake by SWCNT and prolonged blood circulation were observed. Thus, it had a higher efficacy in suppressing tumor growth than clinical Taxol in a murine 4T1 breast cancer model. Furthermore, this SWCNT-PTX caused no obvious toxic effects to normal organs [202]. Recently, Karthika et al. used TiO2-Au coated MWCNTs to deliver DOX. A drug loading capacity of 0.45 mg mL−1 was achieved. The delivered drug showed great efficiency in treating A549 and MCF7 cancer cells, with a releasing capacity of about 91% for 10 h [203]. Other drugs were also reported as delivered using SWCNTs or MWCNTs as carriers, for example, gemcitabine on FA-MWCNTs for breast cancer cells [204], daunorubicin on aptamer-SWCNTS for leukemia T-cells [205], 10-hydroxycamptothecin (HCPT) on diaminotriethylene glycol-MWCNTs for H22 tumor cells [206], curcumin on polysaccharides-SWCNTs for human lung adenocarcinoma cells [207], lobaplatin on PEG-CNTs for liver cancer cells [208], and formononetin on hydroxypropyl-cyclodextrin modified SWCNTs for MCF-7 cells and Hela cells [209].
Bioimaging is another important application for CNTs. In 2002, the observation of band-gap fluorescence from individual semiconducting SWCNTS in sodium dodecyl sulfate (SDS) opened the possibility to of using bad-gap fluorescence for bioimaging [210]. There are several advantages to the use of band gap fluorescence of CNTs for bioimaging. First of all, in the NIR emission band of semiconducting CNTs, cells, tissues, and other biological molecules show much less autofluorescence and thus minimize the background signals. Furthermore, NIR light can penetrate the biological tissues, ensuring the non-invasive imaging of cells under skin. Dai and coworkers reported the development of antibody functionalized SWCNTs as NIR fluorescent labels for probing cell surface receptors with high specificity and high sensitivity. The authors prepared highly water soluble and biologically inert phospholipid (PL)-PEG modified SWCNTs. Then Rituxan and Herceptin antibodies were conjugated onto the functionalized SWCNTs for the recognition of CD20 cell surface and HER2/new receptors, respectively. By detecting the intrinsic NIR photoluminescence of semiconducting SWCNTs, the authors observed the selective binding of SWCNTs-antibody conjugates to cell surface receptors [211]. Later on, they studied the in vivo NIR photoluminescence imaging in live mice. Firstly, PL-PEG modified SWCNTs solution was prepared by exchange with sodium cholate with PL-PEG. The exchange SWCNTs demonstrated the ability to achieve a high image contrast at a relatively low dose (17 mg L−1) for whole-mouse imaging [212]. Recently, Hirata used NIR fluorescence imaging to monitor the SWCNTs locally implanted in mice. SWCNTs were implanted between the periosteum and parietal bone of mice. Fluorescence was observed in the cranial region, not in other organs, demonstrating that the implanted MWCNTs remain at the site of implantation and did not accumulate in detectable quantities in other organs [213]. Interestingly, visible photoluminescence was also observed on functionalized SWCNTs and MWCNTs due to the trapping of excitation energy by the defect sites in the CNT structure [214]. The defects-and-functionalization dependent photoluminescence has also been used for bioimaging. For example, Lin et al. created fluorescent quantum defects in SWCNTs using NaClO and UV irradiation. With injection of only about 100 ng of the prepared SWCNTs in mice, high contrast images displayed clearly vascular and lymphatic structures under 980 nm excitation [215]. Raman is also a commonly used imaging technique and CNTs have been reported in this application. DNA wrapped SWCNTs have been used for Raman scattering and fluorescence spectra and they showed continued emission and staining and spectra changes upon uptake [216]. Surface-enhanced Raman scattering (SERS) has recently applied to enhance the Raman signal for bioimaging; for example, Au functionalized with DNA on SWCNTs for Hela cell imaging [217]. The last imaging technique we will mention here is MRI imaging, in which CNTs have been used as a carrier for contrast agents to improve the imaging quality, e.g., Fe3O4/MWCNTs [218], Gd-DTPA/SWCNT [219].
With respect to tissue engineering, CNTs have been reported to replace conventional materials because of their strong mechanical strength and excellent electrical conductivity. CNTs were reported as fillers to reinforce polymeric biomaterials for the strengthening of their structural integrity and to achieve better biomechanical properties. A 77% higher elastic modulus and 60% increase in mechanical stress were obtained with 5% MWCNT addition to chitosan films in comparison with pure chitosan films. It was also found that cell viability and proliferation succeeded after MWCNT reinforcing, and at the same time, no toxic effects were detected [220]. To fully utilize the mechanical and electrical properties of CNTs, CNT based scaffolds were built for tissue engineering. Rajesh et al. developed a CNT-alginate-hydroxyapatite tricomponent composite scaffold used for bone tissue engineering. In vivo studies showed that the composite scaffold showed good cell proliferation, cell differentiation, and cell attachment, which proved to be a promising candidate for bone tissue engineering [220]. Functionalized CNTs was also studied for nerve tissue engineering. Polypyrrole on chitosan-polyurethane with functionalized MWCNTs were reported to accelerate the regrowth, proliferation and migration of Schwann cells and the differentiation of rat pheochromocytoma cells [221].
3.1. Synthesis
A large number of methods have been developed CNT synthesis. Hereafter, we will briefly introduce the most used synthesis methods—the arc discharge method, laser ablation method and the chemical vapor deposition method.
The arc discharge method a is well established method for CNT synthesis. In the synthesis process, helium gas is filled into the reaction chamber, where two electrodes are separated in a distance of 1–2 mm. The anode is usually made of high purity graphite and metal catalyst, while the cathode is made of high purity graphite. Once an arc current is triggered between the anode and cathode, graphite and metal catalyst in anode evaporates and condenses onto the cathode surface or reactor inner wall to form CNTs and other impurities. Products from the arc discharge method usually contain both SWCNTs and MWCNTs. The reactor atmosphere, pressure, and arc current are important parameters for the process to control the yield and quality of CNTs [222].
Laser ablation is an important method for CNT synthesis, with a more controlled manner than the arc discharge method. The synthesis process proceeds in a quartz tube heated in a tubular furnace. Inside the quartz tube, a target made of high-purity graphite with small amount of metallic catalyst is used as a carbon source. A scanning laser beam is focused onto the target at a high temperature under an inert gas flow, which causes the evaporation of the carbon and metallic catalyst. The re-condensation of the carbon and catalyst vapor on a cooled collector along the downstream flow forms CNTs with impurities [223]. This process is cost consumable since it uses high energy power to vaporize the target, but this process has a high yield and produces primarily SWCNTs.
CVD is a widely used method for low temperature, low-cost bulk production of CNTs. The CVD process proceeds in a flow furnace. The carbon source is usually hydrocarbons such as methane, acetylene, ethane, ethylene or alcohols. The metallic catalyst, e.g., Ni, Co, Fe, can be used in a very fine powder or coated onto a substrate. When the reaction gas passes through the flow furnace at a high temperature about 1000 °C, hydrocarbon molecules decomposed into active carbon species on the catalyst surface and diffuse into the metal nanoparticles. When the carbon atoms are saturated in the metal nanoparticles, carbon precipitates out and forms tubular carbon solids with a sp2 structure. The characteristics of CNTs by the CVD method are dependent on the operation pressure, temperature, hydrocarbon concentration, the nature of the support and the time of the reaction [224]. To lower the reaction temperature for the CVD method, plasma-enhanced CVD (PECVD) has been developed [225].
3.2. Functionalized CNTs
With their unique properties, CNTs are expected to be versatile materials for biomedical application. However, there is one problem that hinders their practical application. Due to the hydrophobic nature of the walls of CNTs, the freshly prepared CNTs tend to aggregate into bundles via the van der Waals force. Therefore, it is difficult to separate single CNTs and make stable biocompatible solutions in most solvents, especially not possible in water. Surface functionalization has provided efficient solutions to solve this problem. Furthermore, surface functionalization could also provide efficient intracellular uptake and increase the possibility of the attachment of different functional groups onto the surface of CNTs for biomedical applications [226]. CNTs functionalization can be generally divided into two groups: noncovalent functionalization, and covalent functionalization.
3.2.1. Noncovalent Functionalization
Noncovalent functionalization is the process of adsorption or wrapping of surfactants, polymers or biopolymers onto the surface of CNTs. The noncovalent functionalization processes offer several advantages. Firstly, they are usually quick and it is not difficult to achieve effective de-bundling of CNTs and get good dispersion in water. Secondly, they are expected to bring less disturbance to the sp2 structure of CNTs and subsequently preserve, to most extent, their mechanical and electrical properties. Furthermore, most surfactants are easy to obtain and have already been used in pharmaceutical products [227]. In principle, the nonpolar part will adsorbed on the CNT surface with the polar end sticking out to contact with the solvent used. Moore et al. tested a series of anionic, cationic and nonionic surfactants for their ability to suspend individual SWCNTs. For the ionic surfactants, sodium dodecylbenzene sulfonate (SDBS) showed the best result. For the nonionic systems, surfactant with higher molecular weight suspend more SWCNTs [228]. Ciofani et al. found that pretreatment with stirring at 70 °C followed by a sonication procedure doubled the MWCNTs concentrations, reaching 160 μg mL−1 in 0.1% pluronic solution [229]. Good dispersion was also obtained with surfactants of sodium dodecyl sulfate [230], sodium dodecylbenzene sulfonate [231], lgepal [232], and more.
Polymers, especially conjugated polymers, are extensively used to wrap CNTs as a result of π–π stacking and van der Waals interactions between the conjugated polymer chains containing aromatic rings and the surface of CNTs [233]. For example, Petrov investigated pyrene containing polymer for the dispersion of CNTs. They synthesized copolymer, poly(MMA-co-PyMMP), with methyl methacrylate (MMA) and (1-pyrene)methyl-2-propenoate (PyMMP) by atom transfer radical polymerization. The copolymer was wrapped around MWCNTs by mixing copolymer and CNTs with a certain weight ratio. After functionalization, MWCNTs formed stable dispersion in THF, chloroform and toluene [234]. Non-covalent functionalization and dispersion were also achieved through polymer wrapping using sulfonated polyaniline [235], poly-L-lysine [236], Zn-porphyrin polymer [237], and oligothiophene-terminated poly(ethylene glycol) [238].
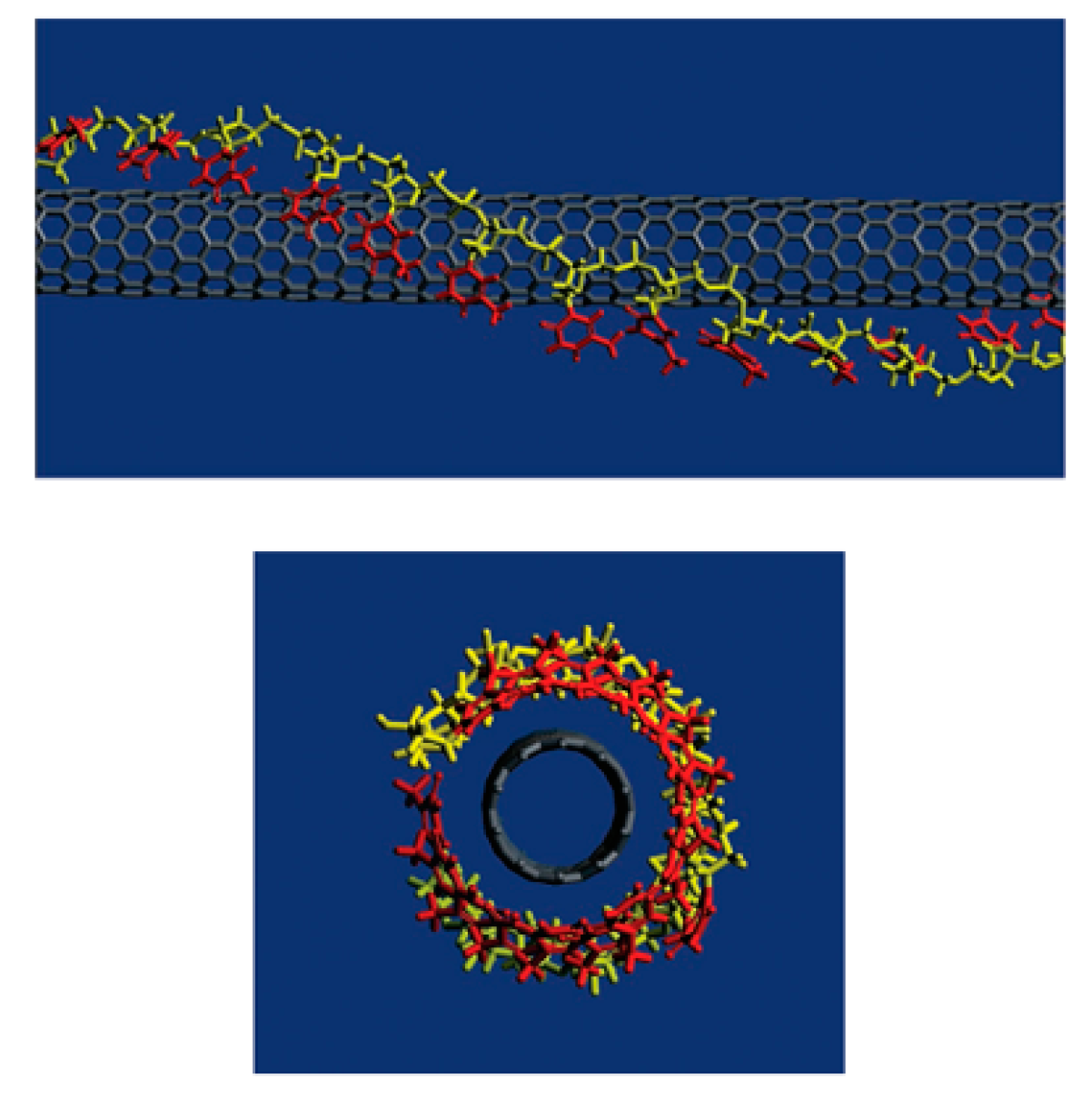
For biomedical applications, the functionalization with biomolecules is preferred. DNA-assisted dispersion and separation of carbon nanotubes were discovered by Zheng et al. [239]. Bundled CNTs were successfully separated into individual CNTs under sonication with the addition of single-stranded DNA (ssDNA). Theoretical calculation revealed that, as shown in Figure 8, ssDNA wrapped the CNT with the bases stack into nanotubes, while leaving the sugar-phosphate backbone exposed, which makes the functionalized CNT easy to solvate. Their later study showed that the ssDNA wrapping of CNTs is sequence dependent. When the n value of a sequence d(GT)n is between 10 to 45, the electrostatics between the ssDNA and CNT are suitable for the CNTs to be separated by anion exchange chromatography [240]. Sanz et al. optimized DNA binding to CNTs by a non-covalent method and developed a bilayer functionalization method that utilized RNA-wrapping to solubilize the nanotubes and a cationic polymer as a bridge between the RNA-nanotube and the DNA. Their method showed important potential for gene delivery [241]. Taeger et al.’s study revealed that protein, such as hydrophobin, can work in the same role as ssDNA to successfully disperse CNTs and it can suspend CNTs as efficiently as ssDNA [242].
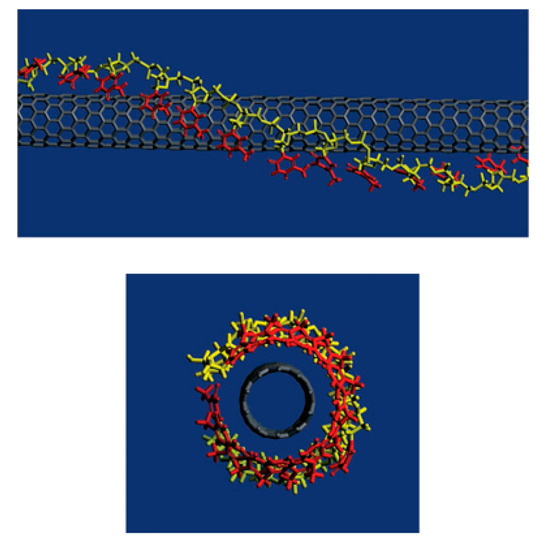
Figure 8.
Theoretical calculation results showing ssDNA converted CNTs are water soluble. Reprinted with permission from [239].
3.2.2. Covalent Functionalization
Covalent functionalization is widely used to disperse CNTs, to improve CNT biocompatibility and to bring biomedical functionality to CNTs. The covalent functionalization process involves several chemical reactions, through which covalent chemical bonds are formed between CNTs and the entities for functionalization. They can be divided into two categories: (i) oxidation and end/defect functionalization, and (ii) side wall covalent functionalization.
The main purpose of the oxidation of CNTs is to purify the CNTs. This process cuts tubes into short pieces and removes impurities inside CNTs, such as amorphous carbon and metallic catalysts. At the same time, it opens the tube ends of CNTs and brings in tube-end oxygen functional groups, such as carboxylic acid, ketone, alcohol, and ester groups. Meanwhile the oxidation process generates defects on the tube wall, for example holes with oxygenated functional groups [70,243].
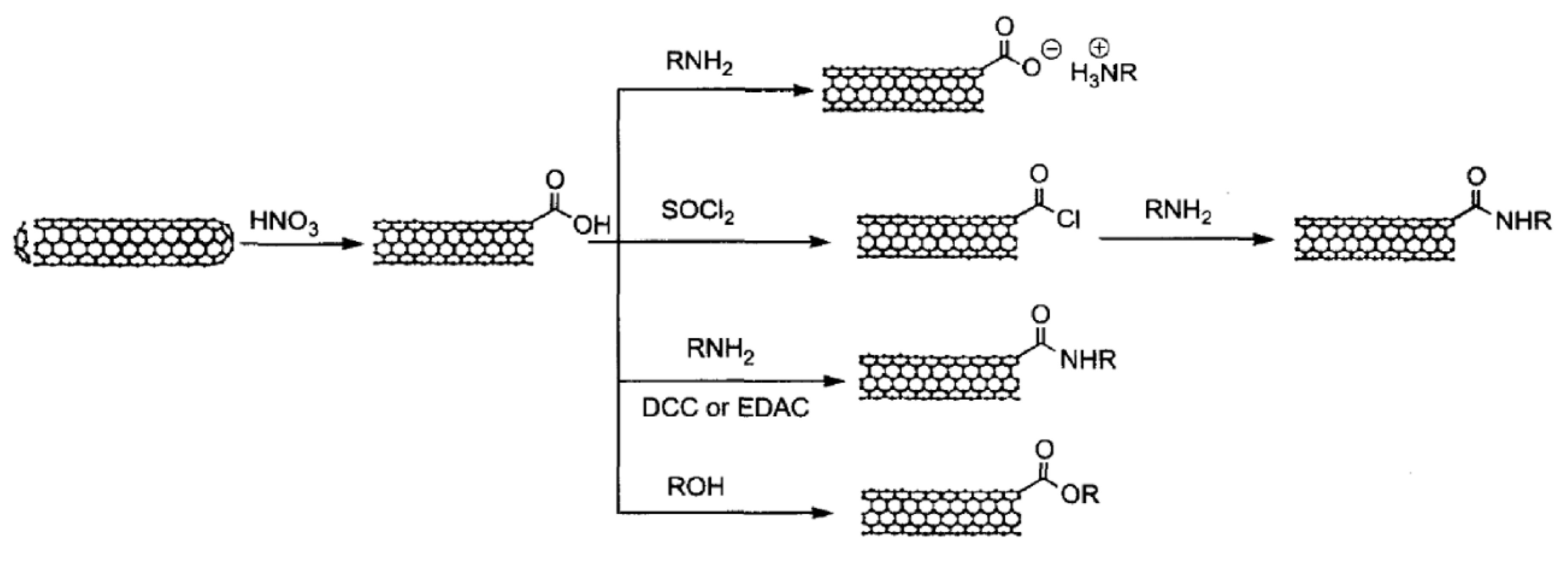
The widely used method for CNT oxidation is solution oxidation in acid solution using HNO3 [244], HNO3/H2SO4 [245], and KMnO4/H2SO4 [246].
The total acidic sites in oxidation purified SWCNTs are about 1–3% determined by acid-base titration [248]. These acidic sites play important roles in the further functionalization of CNTs, as shown in Figure 9. Further modification can be accomplished through four reaction pathways. The first reaction with the carboxylic group on end (or defects), as shown in Figure 9, is through simple acid-based reaction forming CNT-carboxylate zwitterion. Octadecylamine (ODA) has been reported to solubilize CNTs using the CNT-carboxylate zwitterion formation reaction [249,250]. The second reaction is the amidation reaction which is widely used to graft RNH2 on to CNTs as well as graphene through the formation of –CO–NH– bonding. During this reaction, the carboxylic group needs to be activated by the formation of acyl chlorides by reacting with thionyl chloride, or through the formation of unstable and sub-stable intermediates by reacting with EDC/NHS, as we have discussed in the graphene section. These reactions have been extensively used for further functionalization with PEG-NH2 to improve biocompatibility and to provide sites for biomedical functionalities [251,252,253,254]. Another functionalization pathway of CNT end and defects is through the esterification reaction between the surface carboxylic group on CNTs with ROH. One example is the grafting of poly(hexamethylene carbonate-co-caprolactone)diol, a biodegradable polyol, onto MWCNTs [255].
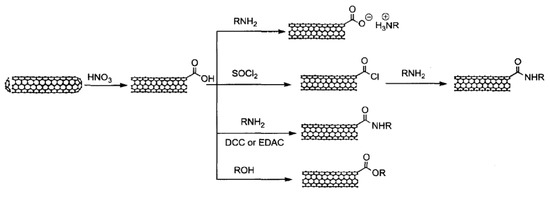
Figure 9.
Different chemical reactions for end functionalization of CNTs. Reprinted with permission from [247].
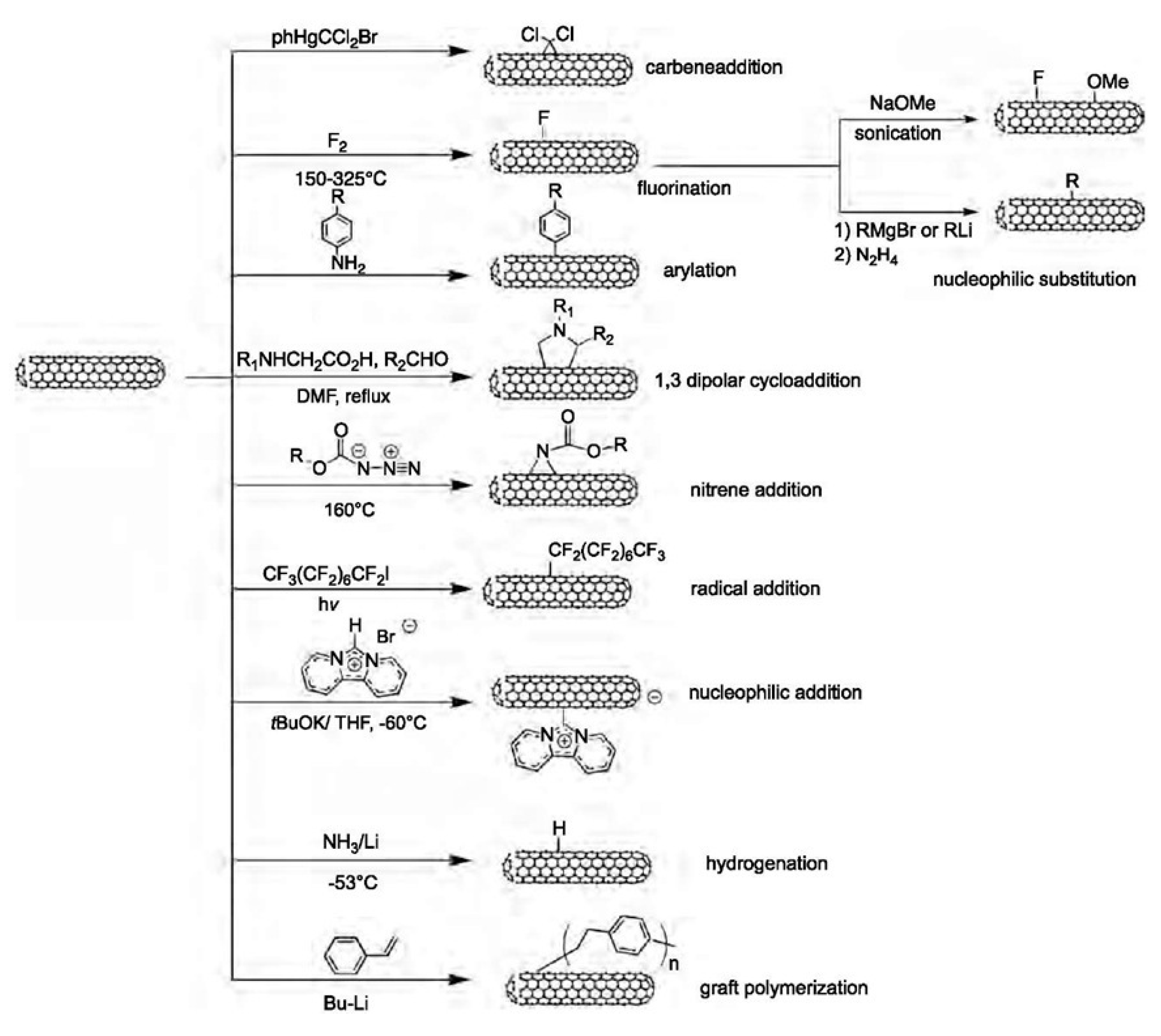
Side wall covalent functionalization of CNTs usually involves the breaking of sp2 bonding networks to form sp3 binding on CNT walls. Therefore, it generally requires harsh reaction conditions and leads to extreme changes in the properties of the CNTs. The chemical reaction for this purpose includes: carbene addition, fluorination, arylation, 1,3 dipolar cycloaddition, nitrene addition, radical addition, nucleophilic addition, hydrogenation, and grafting polymerization. Carbene addition was reported to have been carried out firstly by Chen et al. using dichlorocarbene, which was produced from phenyl(bromodichloromethyl)mercury. The author’s Raman results proved the addition of dichlorocarbon to the wall of the SWCNTs [256,257]. The fluorination functionalization of CNTs is by direct reaction with fluorine at about 400 °C, thanks to the high reactivity, oxidizing properties, and highest electronegativity of fluorine. The fluorination method is very useful since it provides an opportunity to introduce the alkyl group by replacing the fluorine atom using a Grignard agent or organolithium [258]. Covalent functionalization of CNTs via an arylation reaction involves the reaction of CNTs with aniline or its derivative in DMF. This reaction can proceed with a radical initiator. A dipolar reaction mechanism was proposed for this reaction. The functionalized CNTs dispersed well in acetonitrile, toluene and water and the dispersion was stable for more than one month [259]. The covalent functionalization of CNTs based on a 1,3-dipolar cycloaddition of azomethine ylides, generated by the condensation of an α-amino and an aldehyde, was reported by Georgakilas et al. The modified nanotubes are remarkable soluble in most organic solvents and even in water [260]. This is a versatile functionalization method and it has been reported to prepare functionalized CNTs for biomedical applications. For example, Samori et al. functionalized oxidized CNTs with amine moieties through the 1,3-dipolar cycloaddition of azomethine ylides. The introduced amine moieties were used as a cleavable linker to conjugate the anticancer drug methotrexate. The hybrid conjugate showed enhanced anticancer activities to human breast cancer cells [261]. The various functionalization pathways are summarized in Figure 10.
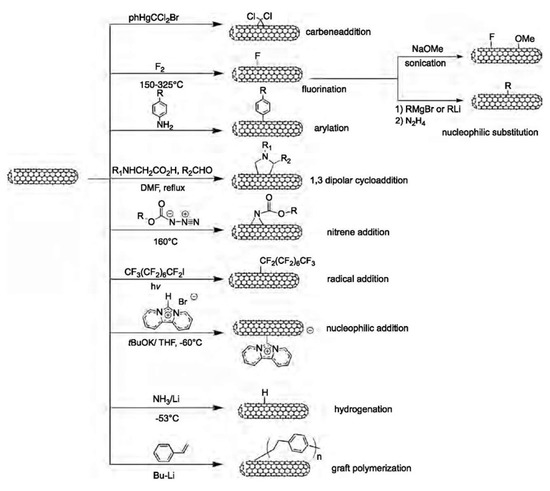
Figure 10.
Different chemical reactions for side wall covalent functionalization of CNTs. Reprinted with permission from [247].
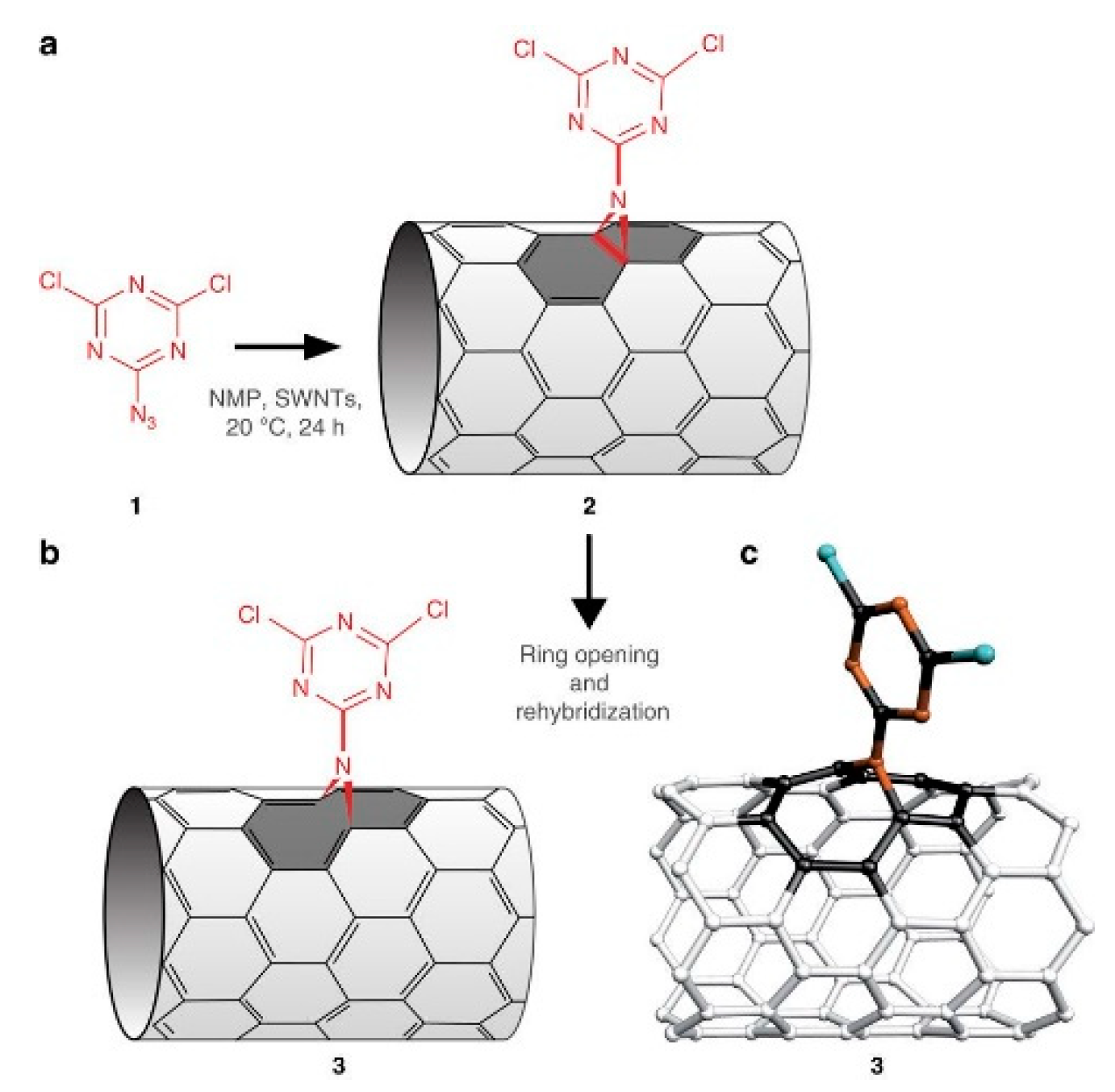
Nitrene addition is another cycloaddition reaction which is used for CNTs covalent functionalization to add a variety of different groups such as alkyl chains, aromatic groups, dendrimers and more. Holzinger et al. used oxycarbonyl nitrenes to react with SWCNTs in 1,2-dichorobenzene under a nitrogen atmosphere. After thermally induced N2 extrusion, nitrene addition resulted in the formation of alkoxycarbonylaziridino-SWCNTs. Such functionalization increased the solubility of SWCNTs in organic solvents [262]. Recently, interesting research on nitrene cycloaddition reported the preservation of π-conjugation in covalently functionalized CNTs. In CNT side wall functionalization, some sp2 carbons is changed into sp3 carbons which break the π-conjugation of the sp2 network. The quenching of the fluorescence of CNTs has been observed after exposure to different reactants [263]. The authors developed a [2+1] cycloaddition reaction based on electron-poor aromatic nitrene, monoazidodichloro-triazine. The electron-poor aromatic nitrene conjugated onto SWCNTs through [2+1] cycloaddition to form an intermediate adduct with high strain. Then the ring of intermediate adducts opened the ring to re-hybrid to fully conjugated hetero-bridged nanotubes (Figure 11) [91].
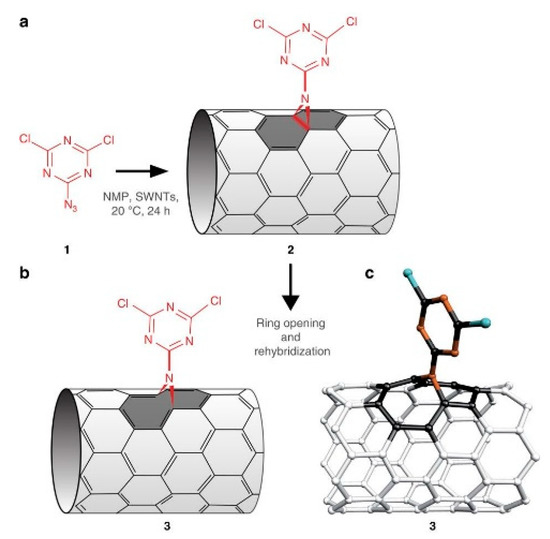
Figure 11.
Synthesis of functionalized CNTs which preserve π conjugation. Reprinted with permission from [264].
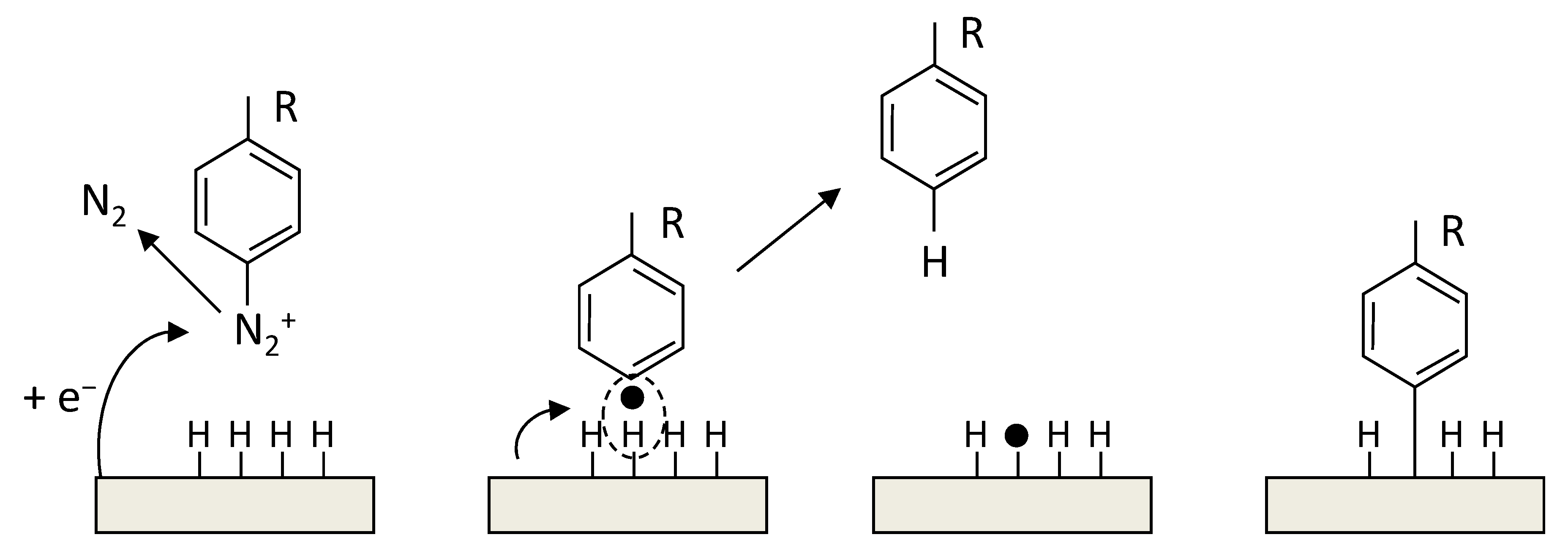
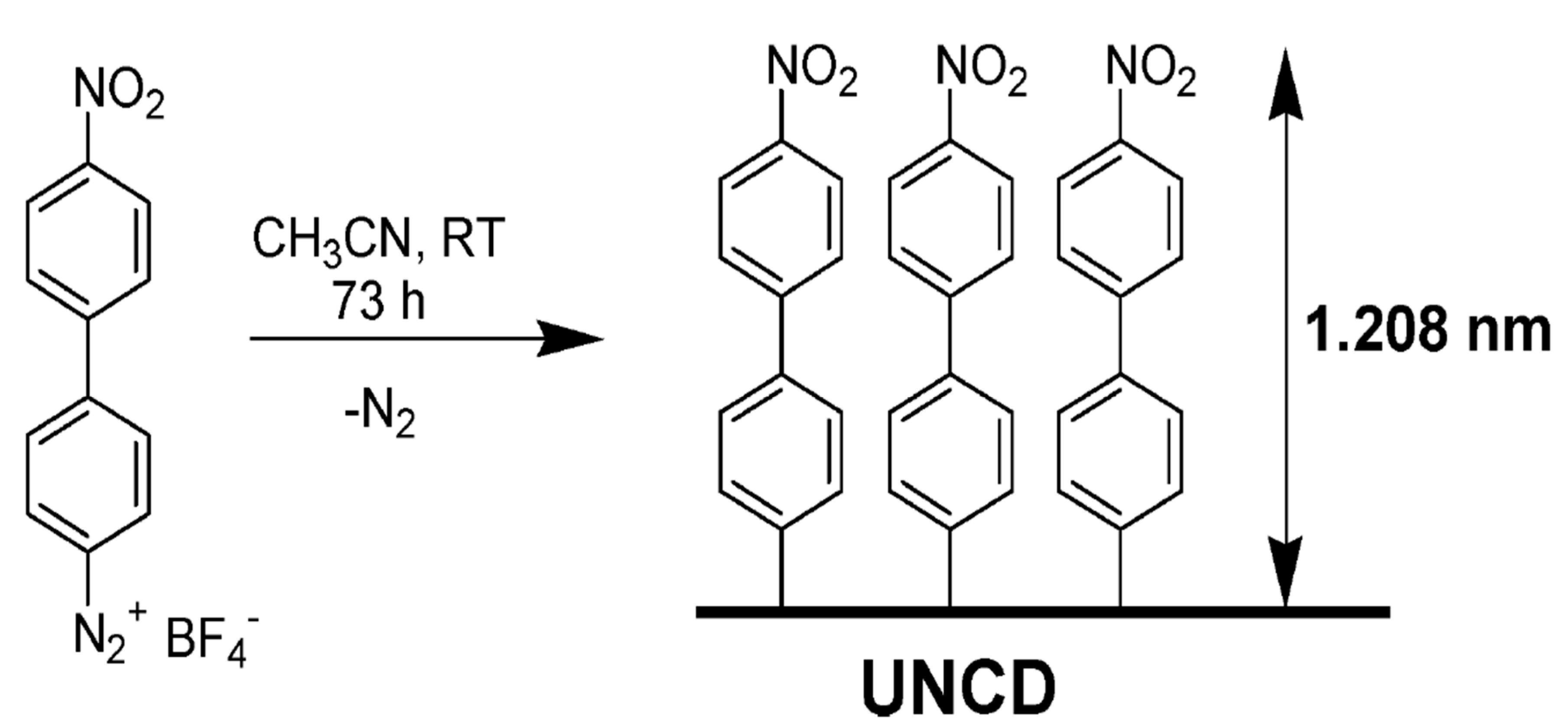
The radical functionalization of CNTs was reported with the use of perfluorinated alkyl iodides under UV light to generate radicals which functionalized SWCNTs. The products showed enhanced dispersion in CHCl3 [265]. In another study, photolysis of perfluoroazooctane was performed to modify the sidewall of SWCNTs based on radical reactions [266]. A simple approach to covalently functionalize CNTs is through diazonium salts (R-N2+X−). In the reaction, the reactive species are radicals, which forms through electron transfer between CNTs and the aryl diazonium salts. This reaction can proceed in organic or water media, or through electrochemical ways [267].
Nucleophilic addition is another method for side wall modification. Holzinger et al. used nucleophilic carbene, dipyridyl imidazolidine to react with electrophilic π systems to give zwitterionic adducts. In the addition reaction, one negative charge per carbene is transferred to the tube surface, thus offering a further parameter for modifying tube properties [268]. Other covalent functionalization methods for side walls include hydrogenation using carbanion complexes of lithium in liquid ammonia [269] and graft polymerization of styrene monomer using butyllithium as an initiator [270].
4. NanoDiamonds and Diamond Films
As with conventional diamond crystals, nanodiamonds (NDs) are, also pure sp3 hybrids with the only difference being that the crystal size now is in the nanometer range.
In diamond, the sp3 hybrization leads to a tetrahedral symmetry in which carbon atoms are bonded through strong covalent bonds. This perfect symmetric arrangement of the four electron orbitals explains why diamond possesses a density higher than that of graphite (3.514 g per cubic cm). Thanks to the tetrahedral structure, diamond also shows an unpaired resistance to compression, and hardness which is the highest with respect to all other substances on both the Vickers and Mohs scales. It is also the reason for the diamond’s extraordinary strength. There are no precise measurements of diamond tensile strength. Theoretical estimations have been calculated to be between 90 and 225 GPa, depending on the crystal orientation. These properties have been known from antiquity and were utilized to scratch other materials. Diamond is an insulating crystal classified as wide-bandgap material. Its remarkable resistivity from1011 to 1018 Ω·m, results from the high stability of the electronic structure and makes it the material of choice for high powered devices. On the contrary, diamond shows a very prominent phonon mobility leading to the best heat conductivity, 3320 W/(m·K) at RT, up to five times the amount of that of copper. High stability/strength of molecular bonds, the absence of reactive sites and free electron pairs make diamond chemically very inert even in contact with strong acids. Diamond can react with the oxygen of an air atmosphere at a temperature of ~700 °C [271], leading to decomposition in CO, CO2. Finally, diamond has a high refracting index varying from 2.465 in the violet to 2.409 in the red. This generates the prismatic colors of gemstones. Diamond absorption depends mainly on the defects contained in the crystal. There are several different extrinsic defects in diamond such as nitrogen, boron, phosphorous, hydrogen, nickel, cobalt, silicon, germanium and sulphur. Among them, nitrogen is the more common color center of diamond leading to a variety of defects called A-, B-, C- N2, N3 centers. In the visible, their characteristic absorption transitions fall at 575, 527, 478, 465, 452, 435, and 423 nm [272,273,274]. Concentration of nitrogen defects is used to classify diamond in type Ia with about 95% of all natural diamonds where the nitrogen impurities are ~0.3% (3000 ppm). Type Ib diamonds, which are about 0.1% of all natural diamonds, the nitrogen impurities are up to 0.05% (500 ppm). About 1–2% of all natural diamonds are type IIa. They are almost or entirely impurity free, and this explains why they are colourless and possesses the highest thermal conductivity. Finally, type IIb diamonds constitute ~0.1% of all natural diamonds, making them very rare and precious. They have the lowest level of nitrogen impurities but contain significant boron impurities.
The advent of HPHT and CVD technologies, with the possibility of synthesizing diamonds at reasonable costs, have fostered the use of diamond based technologies with outcomes in a variety of ordinary commercial products. Diamond lenses for optical applications for high-power high-energy radiations or harsh envorinments is an example [275,276]. Diamond with its outstanding thermal conductivity coupled with the high isolating power [277] makes it desirable in high power electrical devices [278,279]. Diamond hardness [280] and the capability to efficiently dissipate heat [281] is exploited in diamond coatings for processing hard materials [282] and for surgical blades [283]. Diamond in form of nanoparticles replays the properties of bulk diamonds in terms of biocompatibility and mechanical, optical, thermal, and electrical properties. These nanostructures are utilized in a variety of applications such as energy storage, catalysis, electroanalysis, tribology and lubrication, chromatography, and mass spectrometry [284].
Although NDs are widely utilized in industry, applications of diamond in biology and medicine has only started to appear a decade ago. It has been demonstrated that NDs do not influence cell metabolic activity, cell differentiation, growth and proliferation [285,286]. The NDs special biocompatibility and safety can be coupled to the rich chemistry provided by the diamond surface [287] which enables the grafting of a wide variety of molecules [288,289]. This renders diamond an extraordinary platform useful for different kinds of applications [284,290] spanning from quantum sensing [291,292], to fluorescent probes and tracking [293,294], bioimaging [295,296], drug delivery [297,298] and nano-biomedicine [299,300].
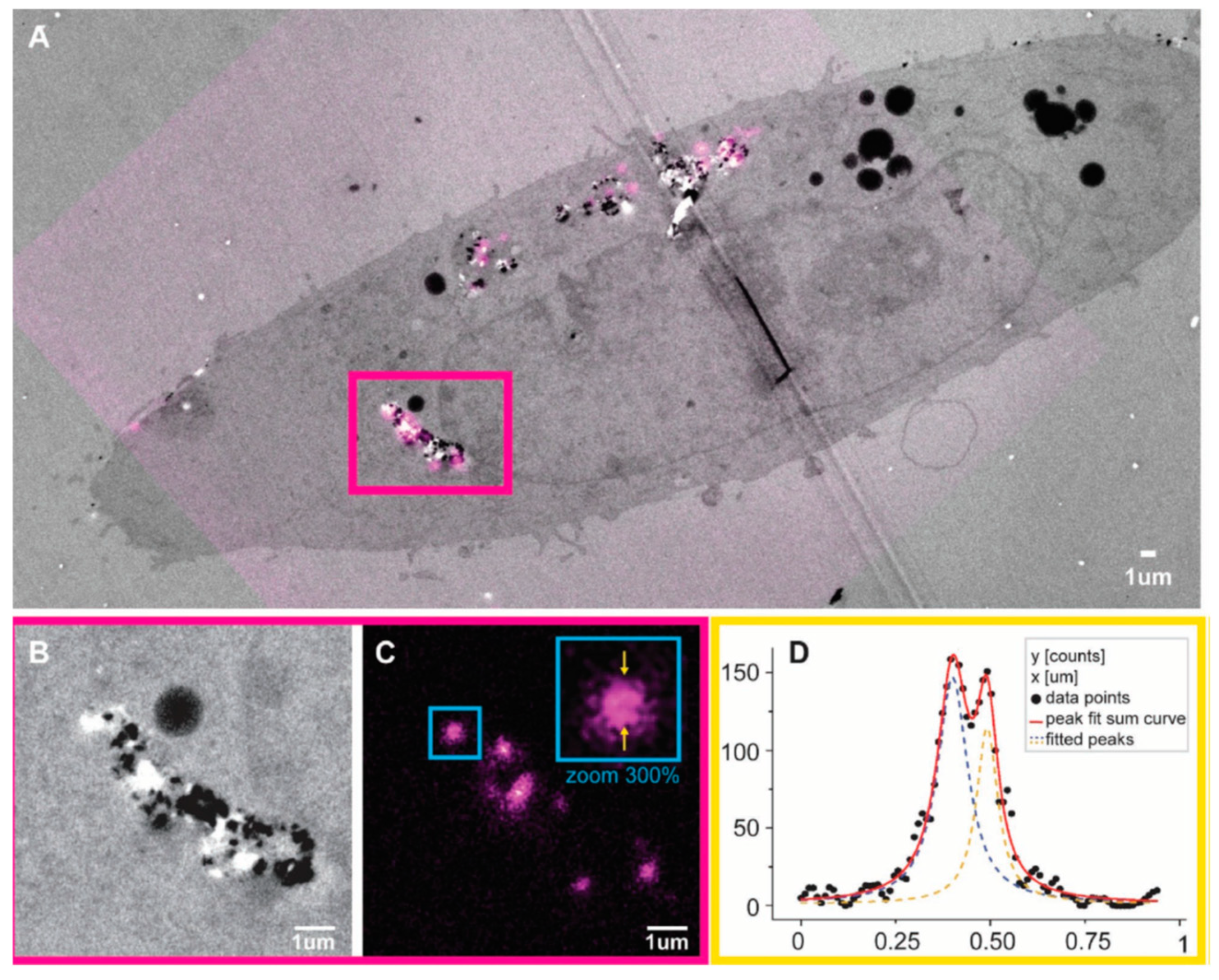
Lattice defects impart photoluminescent properties to NDs which can be used as biolabels or as biomarkers in lifesciences. Among the defects the nitrogen vacancy can be optically interrogated in high sensitivity magnetometry experiments and in quantum detection of temperature gradients in living cells [301,302]. For example, the optically detected magnetic resonance (ODMR) of negatively charged nitrogen vacancy centres in diamond has applications in temperature mapping, and the measurement of magnetic fields in single cells. However, color centers of NDs, in particular nitrogen and silicon vacancies, are of great interest also for imaging applications because they possess a strong absorption at 560 nm while they emit at ~700 nm. They are very stable under irradiation which makes them attractive for long-term, three-dimensional imaging and tracking in live cells [303,304]. The intense emission from NDs is currently utilized in super resolution stimulated emission depletion microscopy (STEM) and correlative scanning electron and confocal microscopy. An example is shown in Figure 12.
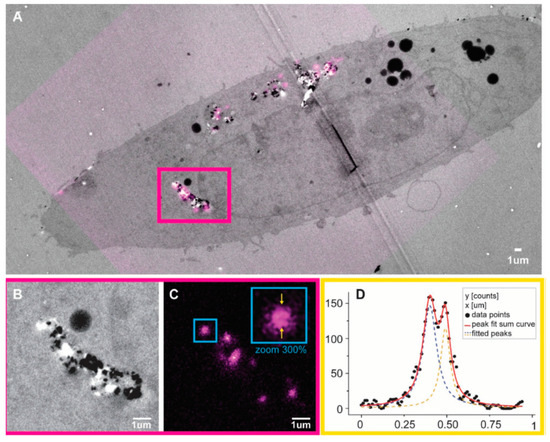
Figure 12.
Stimulated emission depletion microscopy (STED-TEM) correlative imaging of intracellular FNDs in TEM sections. (A) A correlation result on a single cell is shown, with TEM in gray and fluorescence signal from FNDs in magenta. (B,C) A zoomed section of the correlation result is shown for TEM and STED, respectively. (C) It is shown that it is possible to use the STED depletion to improve resolution with FNDs. The visual estimation is confirmed in a line profile measurement over a FND cluster, highlighted in (C). (D) The line profile values, and a two-peak Lorentzian fit of the data: the two distinct peaks are ≈90 nm apart. Reproduced with permission from [305].
Apart from fluorescent labelling, another possibility is to attach antibodies to the ND surface to target specific structures of the cells. As an example, in [306] the authors functionalized NDs with a bifunctional peptide enabling cell penetration and recognition of the amyloid-β aggregates, which are markers for the Alzheimer’s disease. Diamond also opens perspectives for the detection of multiple parameters. Raman spectra of diamond and cells peak in rather different energy regions namely 1332 cm−1 and 2800–3200 cm−1. This was exploited to visualize how lysozyme interacts with bacteria [307]. Diamond nanoparticles are utilized in drug delivery where the possibility of modifying the surface with a wide selection of functional groups allows both the bonding of antibodies and electrostatic drug fixation. This enables the ability to carry a large variety of therapeutics and environment-dependent release [308]. This coupled with the high biocompatibility and the possibility of performing imaging and sensing, opens the way to theranostics [295,299,303,309]. In the era of genomics, different types of nanoparticles were studied as nanocarriers for gene delivery. NDs are also attractive because their ample surface functionalization possibilities helping cell penetration and localized gene release [310]. In [311] authors were able to functionalize DNDs with two silanes enabling the generation of DND conjugates with fluorescein isothiocyanate (FITC) dyes and single-stranded DNA oligomers. Among the therapeutic use of ND, it has been demonstrated that NDs are useful in radiotherapy. When cancer cells are infiltrated with hydrogenated NDs, irradiation is more efficient [312], also in tissue normally resistant to radiotherapy. Authors attribute this effect to increased reactivity towards oxygen groups and to electron emission from irradiated NDs. Diamond and nanodiamond are superior platforms for tissue scaffolds, bone regeneration, and in surgical implants [313]. Thanks to the superior hardness and Young’s modulus NDs are beneficial in enhancing the mechanical and chemical properties of polymeric scaffolds [314]. In the following, we present a survey of the current research regarding the synthesis, and physicochemical properties of the NDs in relation to their surface functionalization, with the potential applications in biomedicine.
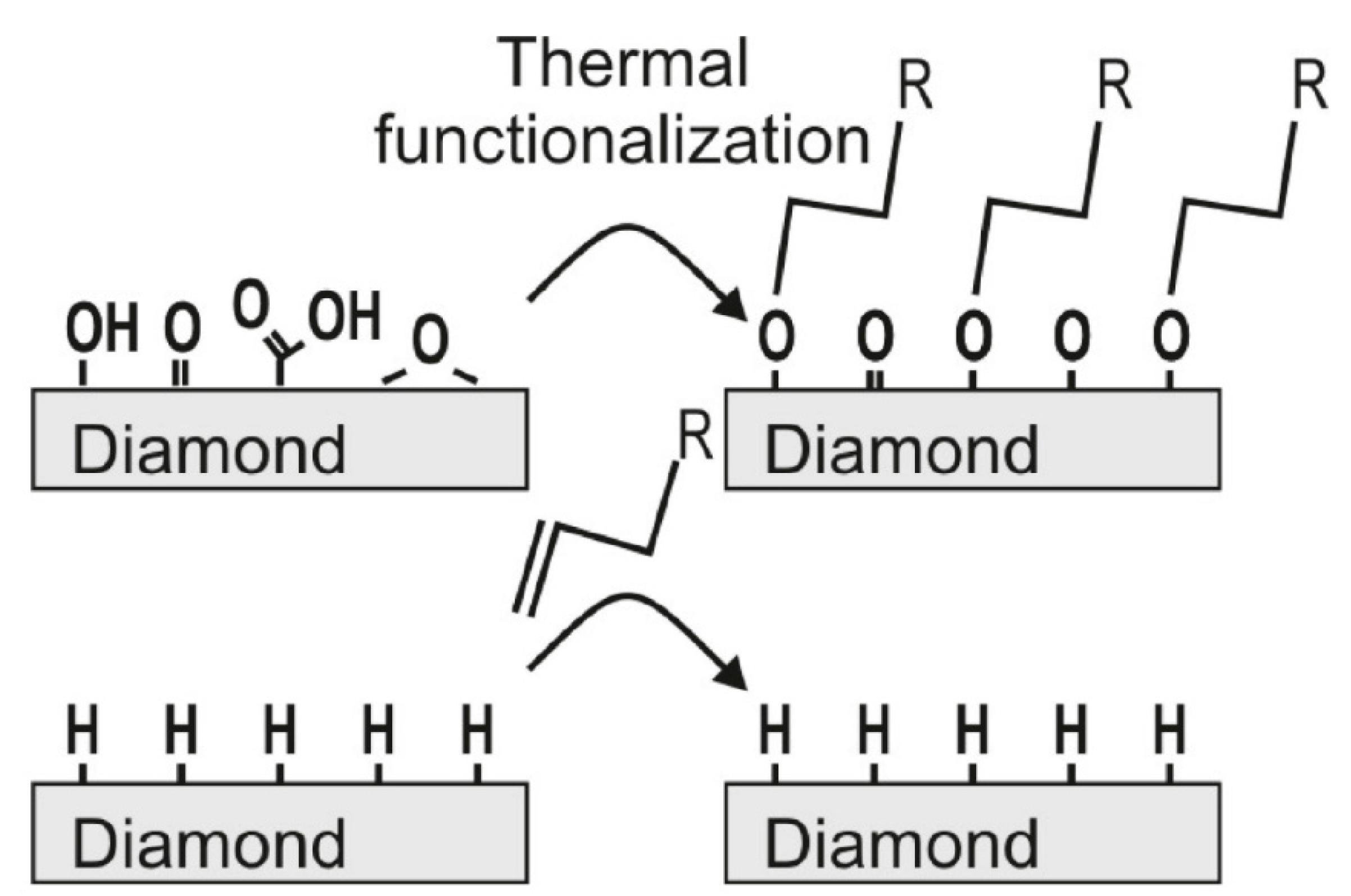
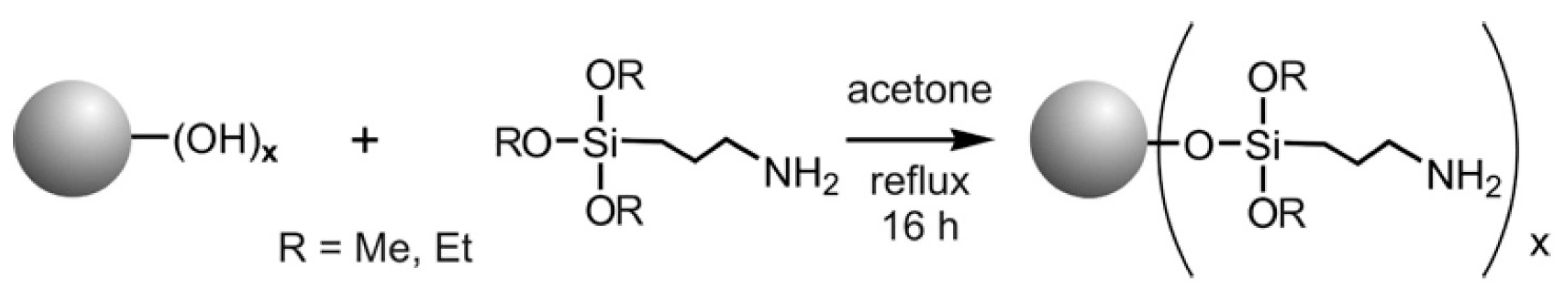
4.1. Synthesis
The name “nanodiamond” is used to indicate crystals which can be distinguished on the basis of their size. In particular, the NDs are considered as nanocrystalline when the crystal size is <100 nm or ultrananocrystalline if <10 nm [315]. NDs can also be classified considering the synthesis process: detonation NDs (DNDs) [284,316], chemical vapor deposited NDs (CVD-NDs) [317,318], pulsed laser ablation NDs (PLA-NDs) [319,320] and high pressure high temperature NDs (HPHT-NDs) [321] followed by grinding [322]. Examples of the NDs produced using these techniques are shown in Figure 13. Other less common techniques are production of NDs by electron [323] or ion [324] irradiation of graphite or carbon onions [325]. DNDs were firstly produced in USSR about 50 years ago using detonation processes [326]. DNDs are produced by an explosion of an explosive mixture made of trinitrotoluene (TNT) and cyclotrimethylenetrinitramine (RDX) in a closed chamber. The high temperature and pressure reached in the chamber led to the formation of a suspension of diamond crystals with a typical average size of about 5 nm [284]. The production method leads to the formation of a graphitic layer on the surface and a certain amount of impurities and structural defects [327]. Although the small dimensions lead to a high surface to volume ratio, they make impracticable any manipulation if well defined defects are needed, as quantum sensing requires. Much higher quality is obtained using CVD to produce diamonds. In a typical CVD process, a mixture of hydrogen and methane is utilized for diamond film growth [318]. While methane or other hydrocarbon precursors are utilized to provide the carbon atoms, hydrogen is utilized to saturate the dangling bonds on the diamond surface. In addition hydrogen can help cleave the neutral hydrocarbon molecules with the generation of radicals such as CH2. These radicals can react with the diamond surface and form amorphous graphite or sp3 diamond like structures [318]. The presence of hydrogen helps form pure diamond crystals since it etches the graphitic phase [315]. In this way it is possible to synthesize polycrystalline ultranano-, nano-, and micro-crystalline diamond films where the grain boundaries are composed by sp2-bonded amorphous carbon atoms. Nanocrystalline diamond films display a typical surface roughness in the range of 30–50 nm. PECVD is a good method to prepare specifically fluorescing diamond film using a relatively low temperature [328]. However, CVD processes have been optimized for the production of single-crystalline possessing electronic grade quality with defect densities of less than 102 cm−2 and atomically smooth surfaces.
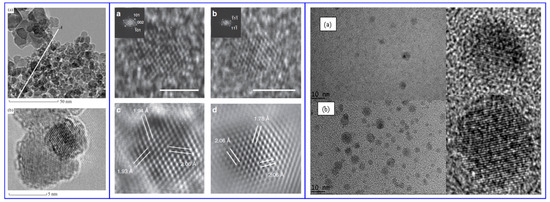
Figure 13.
Panel 1: (a) low resolution TEM image of detonation ND powder; (b) high resolution TEM scan along the AA direction shown in (a) ND nanocrystals with their lattice orientation (Reprinted with permission from [329]); Panel 2: high-resolution TEM of two nanodiamonds nanodiamonds produced by a microplasma in ethanol vapors imaged along the (010) (a) and (110) (b) zone axes. Inverse FFT images (c,d) confirm that the particles are lonsdaleite (a,c) and n-diamond (b,d), respectively. Scale bars, 2 nm (reprinted with permission from [330]). Panel 3: high resolution TEM of nanodiamonds grown by pulsed laser ablation in liquid media (PLAL) with different laser energy: (a) 135 μJ for 3 h; (b) 1 h 180 μJ. (adapted with permission from [331]).
Pulsed laser deposition (PLD) is an alternative method to produce films possessing complex stoichiometry. PLD is performed under vacuum and uses high power femtosecond lasers to locally vaporize a target and is followed by the deposition of the vaporized species on a substrate. An evolution of PLD is the laser ablation in liquid media (PLAL). In this case a laser with power exceeding the 108 W/cm2 causes a violent ejection of material from the target. The presence of a liquid environment confines the expansion leading to a significant increase of the pressure. This effect has been exploited to synthesize materials which, as in the case of diamond, require high pressure and high temperatures [320]. In particular, the shocks generated by the pressure waves and temperature quenching due to the liquid create favorable conditions for nanodiamond formation. Using PLAL in water, NDs of 25–250 nm can be produced in agreement with growth kinetics models [332,333]. In addition, the PLAL is able to remove the graphitic amorphous phase even if purification of the NDs is still required. A high pressure high temperature method is utilized to synthesize high quality type Ib, IIa and IIb diamond single crystals that are important in quantum sensing, which is out of the scope of this review. Finally, electron or ion irradiation of graphite or carbon onions has also been proven to induce nanodiamond formation. The transformation of the graphite to diamond requires overcoming the kinetic barrier between the two phases. This occurs only at extreme pressures and high temperatures. These conditions may be obtained by irradiating carbon onions with an electron beam. In these conditions, carbon onions undergo heavy self-compression leading to the nucleation of diamond crystals in their cores [325,334]. This process can be extended to convert graphite to diamond using high-temperature electron irradiation even without the application of pressure [335]. However, irradiation based methods cannot be used to produce large amounts of material and the interest in these synthesis procedure is essentially for base research.
4.2. Functionalized NanoDiamond
The utilization of NDs for biomedicine needs the particulate be suspended in a stable colloidal dispersion. However, NDs easily undergo agglomeration which renders them useless as fluorescent probes, or drug delivery carriers, and in other applications. This is related to the ζ potential which reflects the surface charge on these nanoparticles. Generally a colloidal suspension is considered stable when the ζ potential is lower than −30 mV or higher than 30 mV. The ζ potential strongly depends on the ND surface treatment and on the pH of the dispersing liquid. At pH = 7, vacuum annealed NDs show a ζ~−30mV due to the presence of surface oxidation [336]. The use of strong acids to remove the graphitic shell covering the diamond core, induces strong surface oxidation thus reducing the value of ζ and increasing the colloidal suspension stability [337]. The behavior of the NDs in a colloidal suspension depends on both the characteristics of the liquid environment and on the ND surface properties. It is then important to establish exact protocols for surface homogenization to obtain reproducible and uniform surface chemistry. Some synthesis techniques give the possibility of defining the diamond surface termination. As an example, in CVD processes generally the surface is H terminated. Differently, the DNDs possess rather variable surface chemistry composed by hydroxyl, carbonyl, ether and carboxyl functional groups in different proportions requiring homogenization. Due to the production process, DNDs are covered by a graphitic shell which must be removed. The process utilized to this scope determines the nature of the chemical groups on surface and thus the value of the ζ potential [337,338]. Purification of DNDs is made using highly oxidizing agents such as strong acids, singlet oxygen in NaOH, strong ozone, and air treated in the presence of a catalyst [338]. These processes also lead to a more reproducible surface composition. DND carboxylation is obtained in strong acids as mixtures of H2SO4/HNO3, or with H2SO4/HNO3/H2O2 which are both effective methods to remove non-diamond carbon and terminate the surface with –(COOH) [339]. Another possibility is DND treatment in supercritical water [340]. Surface carboxylation may also be obtained in vapor phases in air at a temperature ranging between 350 and 450 °C [341] or in ozone at 150–200 °C [342]. Other chemical processes are applied to obtain hydroxyl homogenization. Depending on the initial surface composition of the NDs, oxidative, reductive or mechanical techniques are selected. In the case of DNDs where a graphitic shell is present, an efficient hydroxylation is performed in a mixture of FeSO4 and H2O2 (Fenton). The reagents readily attack the non-diamond impurities producing OH radicals which will terminate the ND surface [343]. Another possible route is the use of borane which can reduce carbonyl groups of DNDs apart from lactones and esters [344]. Finally hydroxylation of the DND surface may also be obtained via mechanical methods such as milling and ultrasounds in water which induce radical reactions leading to the –(OH) termination [345]. Another possibility is to surface terminate the diamond surface with hydrogen. This may be accomplished in a hydrogen atmosphere at 900 °C [346]. However, this method can be applied only to films due to the etching properties of H. For diamond nanoparticles hydrogen plasma at low temperatures is performed [347].
Generally, the use of nanodiamonds in biomedicine requires functionalization with biological molecules to gain mimetic properties or the stable attachment of enzymes, proteins, and peptides for biosensing, targeting. One of the most viable approaches for grafting is the formation of extremely robust covalent bonds also leading to biointerface stability at relatively high temperatures. The proverbial stability and inertness of diamond has been considered for a long time as an obstacle to the surface functionalization. However, during the last 15 years a broad list of different processes have been developed to modify the diamond surface. The electrochemical reduction of aryldiazonium salts proposed by Kuo long time ago [348] offers a useful method to derivatize diamond [349] and nanodiamond surfaces [350]. During the functionalization process, the aryldiazonium cations draw electrons away from a reaction center while rather stable N2 molecules are released as sketched in the Figure 14. These two elements concur to facilitate the reduction of the aryldiazonium salts. Essentially the process proceeds with a charge transfer from the diamond to the dinitrogen groups of the salt ion inducing a release of a N2 molecule. Then the aryldiazonium cations are reduced and organic molecules bond to the diamond surface [345].
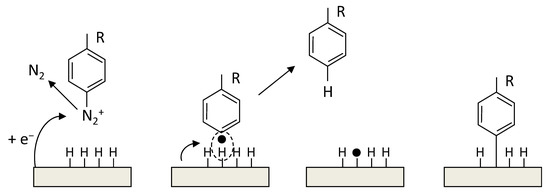
Figure 14.
Electrochemical reduction of aryl diazonium salts and linking of organic molecules via stable covalent bonds.
The popularity of this functionalization stems from the simplicity of the reaction requiring only hydrogen terminated diamond surfaces it provides a wide range of functional groups. However, a precise control over the density of the grafted molecules is difficult and the electrochemical reduction easily leads to the formation of multilayers. This problem was solved using an alternative organic molecule which was self assembled in a monolayer on a nanocrystalline diamond film [351]. In this work, authors dissolved dissolved 4-nitro-1,1-biphenyl-4-diazonium tetrafluoroborate in acetonitrile and stirred for 72 h.
The reaction scheme is sketched in Figure 15.
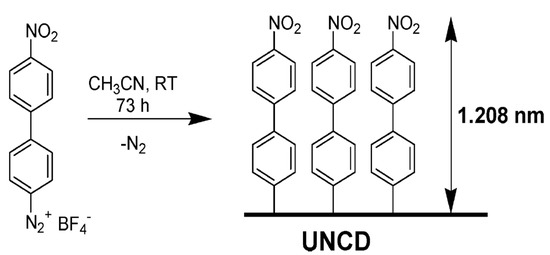
Figure 15.
Reaction scheme of the of the 4-nitro-1,1-biphenyl-4-diazonium tetrafluoroborate molecule with UNCD to form a self assembled monolayer with estimated thickness of 1.208 nm (reprinted with permission from [351]).
Grafting of chlorine-based, amine or carboxyl groups on hydrogen terminated diamond surfaces may be performed using photochemical processes. Originally, UV radiation from a low pressure mercury lamp was used to initiate the reaction between liquid solutions of fluorinated alkene and alkanes organic molecules and the immersed diamond surfaces [352]. Similar reactions can be obtained using non fluorinated compounds. In the proposed mechanism, UV light induces the photoemission of an electron from the H-terminated diamond due to its negative electron affinity. This leaves highly reactive sites on the diamond surface that then induce a nucleophilic attack by the double bond moieties of the organic molecules. The chemical process is shown in Figure 16. The possibility of selecting the desired alkane molecule termination renders this process very useful for further bonding of biological molecules such as DNA oligonucleotides. Initially the w-unsaturated amine of 10-aminodec-1-ene molecules was protected with the trifluoroacetamide functional groups. UV irradiation of these molecules in presence of a H-diamond substrate leads to the activation of the diamond surface and attachment of the 10-aminodec-1-ene molecules. Deprotection of the amine renders these groups available for interactions with DNA oligomers.
Figure 16.
UV- photografting of alkene molecules on H-terminated diamond surfaces.
Photochemical functionalization may also be performed using oxygen terminated diamond surfaces. The oxidation process leads to the presence of oxygen moieties as hydroxyl (–OH), ketone (C=O), and ether (C–O–C) groups [353]. The authors showed that when heating oxidized diamond surfaces at ~200 °C, they are able to selectively bond 1-octadecene molecules via covalent C–O–C bonds. The chemical process is sketched in Figure 17.
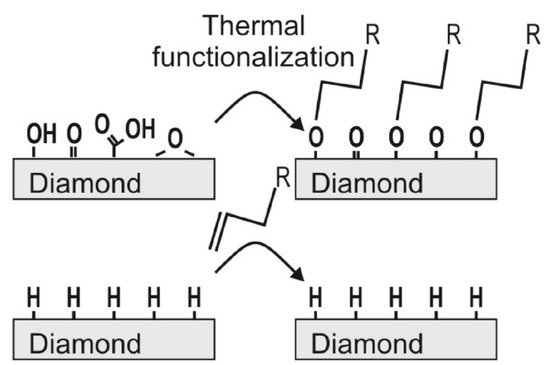
Figure 17.
The thermal functionalization scheme on oxygenated and hydrogenated diamond surfaces. Reproduced with permission from [353].
Specific attention requires the amination of the diamond surfaces since this termination is one of the most commonly used to attach biological molecules. Methylamine can react with chlorinated diamond surfaces leading to surface amination [354]. After, it was demonstrated that amination can also be performed on more common hydrogenated diamond surfaces under UV irradiation [355]. Diamond films were introduced in a chamber in a pure nitrogen atmosphere. Applying irradiation as a 253.7 nm wavelength, the authors were able to induce an amine functionalization. 21-mer probe DNA oligonucleotides were then bonded to the diamond amines and a solution-gate FET sensor was fabricated. The stability of the whole functionalization was made by controlling the shift of the gate potential in successive hybridization/denaturation cycles [355]. Another possibility is to use a cold NH3 plasma treatment of hydrogenated diamond substrate which is able to induce terminal amino groups [356].
DND functionalization requires the process be performed in a solution. In [357] aminated DNDs were produced by linking ethylenediamine to purified carboxylated nanocrystals to obtain covalently bonded epoxycomposites. In another work [358], bead assisted sonic disintegration was utilized to deagglomerate DNDs. Deagglomerated nanocrystals were then treated with mineral acids at high temperatures to induce surface oxidation. Then the chemistry of the diamond surface can be modified with a variety of reagents. For example, we used the reaction with various trialkoxy silanes such as 3-aminopropyl silanes with methoxy and ethoxy groups leading to the grafting of these silanes onto the diamond surface, as shown in Figure 18.
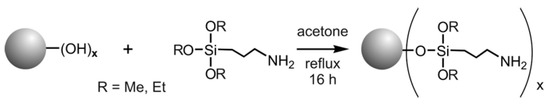
Figure 18.
Grafting of trialkoxy silanes homogenized DNDs (reprinted with permission from [358].
In [359], a very clean surface amination is performed using a one-step process consisting of UV irradiation of the diamond surface in an ammonia atmosphere. XPS analysis confirmed that only amino-groups were grafted on the diamond surfaces. This not only allows further surface engineering with a wide class of biological molecules, but also preserves the surface conductivity which is needed for sensing applications.
5. Carbon Fibers
Carbon fibers (CFs) are fibrous carbon materials that have a graphitic structure with strong crystallite covalent bonds that are highly anisotropic. Carbon fibers are polycrystalline materials containing at least 92% of carbon atoms. These fibers are characterized by a two-dimensional long-range order of carbon atoms arranged in planar hexagonal networks forming stakes in the third dimension without any regularity. As a consequence, they show outstanding mechanical properties along the axis direction but poor mechanical properties in the transverse direction due to the weak van der Waals forces between the layers typical of graphite [360]. Carbon fibers display rather different mechanical properties: tensile strengths from 5.65 to 1.5 GPa and specific modulus from 407 to 106 GPa [360] which may exceed the strength and modulus of steel, 2.39 and 26.6 GPa, respectively [361]. Different types of thermal and stretching treatments can be applied to improve the CF orientation thus increasing the Yang modulus [361]. However, because they are more expensive when compared with other competitors such as glass fibers or plastic fibers, they are used in high performance specialized materials. Typical applications are those requiring lightness and high mechanical properties, particularly in the aerospace and aircraft industries [362]. Besides mechanical properties, CFs display also chemical stability and good electrical, thermal, optical, and structural properties so they are applied in various fields including civil engineering, military, and competition sports [363]. In addition CFs after unloading, have a complete elastic recovery which makes them resistant to fatigue [364]. In industry, CF are added to other materials such as plastic, ceramic, and metals to make composites with superior strength and high specific modulus, low density, low thermal expansion, heat resistance and improved chemical stability [365].
Since the 1970s, CF have been investigated for use in biomedicine. The reason for this are the mechanical properties and chemical inertness which provide obvious benefits of using CFs as biomaterials. These characteristics are the prerequisite for biocompatibility and long-term in vivo stability. In addition, the CFs can be manufactured in various shapes, can be easily combined with conventional biomaterials, and display high radiolucency [365]. Since CFs have a local structure similar to graphite, they are good electrical conductors with resistivity in the range from 9.5·10−6 to 18·10−6 Ωm. They can be utilized in voltammetric recognition of biological molecules [366], to make electrodes for neural recording [367,368], or the stimulation of cardiomyocytes [369]. Concerning regeneration medicine, CFs are mainly utilized to fabricate scaffolds for tissue repair. In this respect, CFs are utilized to fabricate scaffolds to enhance tissue regeneration in both soft [370] and hard tissues [371]. Cartilage tissues are a complex environment because, differently from other tissues, they are not self-healing and are rather difficult to regenerate [372]. Nowadays, the majority of the scaffolds produced for cartilage repair fail because of insufficient mechanical strength and structural resilience [370]. In [373], additive 3D printing is utilized to sinter polyamide/CF composite in a computer designed complex structures. Human mesenchymal stem cells were cultivated to improve the scaffold performances in terms of promoting cell adhesion, proliferation, and viability. Images of stained cultured cells on these scaffolds showed a good degree of spreading, demonstrating that CFs promoted the colonization and viability of cells and the chondrogenic differentiation of mesenchymal stem cells. CFs are also utilized in orthoplasty. As an example, composite scaffolds were produced mixing cellulose-hydroxyapatite and functionalized CF to enhance flexural and compressive properties. Results showed that those scaffolds are capable of generating favorable biological properties, allowing preosteoblast cells to proliferate and differentiate [374]. Hip joint sockets and knee joint liners are already produced and applied to patients. A recent work summarized the outcomes of studies evaluating CFR-PEEK as an articulating surface in orthopedic implants [375]. This investigation showed that CF-PEEK based composites utilized for hip replacements, pins, plates, tibial nails, cups, stems, and spinal cages, possess significant mechanical benefits under a number of tests and higher durability and corrosion resistance while maintaining good biocompatibility. CFs are mixed with other polymers such as ultra high molecular weight polyethylene, epoxy resins, and polyamide, displaying good compatibility with the surrounding bones, and rigid fixation while improving the mechanical properties of these polymers [365]. The corrosion resistance and the exceptional strength of CFs are also utilized in regenerative medicine. The variety of surface chemistries and functionalities that can be grafted on the CF surface induce specific bioactivities in relation to their location; for example, improving the adhesion and proliferation of osteoblasts on CF based scaffolds [376]. Another interesting application regards muscles where local electrical stimuli enhance cell proliferation, differentiation, and tissue regeneration, favoring myoblast differentiation into myocytes [377]. CFs functionalized with carbon nanotubes showed remarkable high metabolic activity of myocytes. In addition, the possibility of fabricating fiber oriented scaffolds might be beneficial for a more efficient cell differentiation and higher myotube formation [378]. Poly(lactic-co-glycolic) acid/CF composites were used to generate a carbon nanofiber reinforced patch. The presence of CF induces a high protein adsorption increasing cardiomiocytes proliferation and growth [379], and an understanding of their functions [380]. Results showed that CF-based scaffolds have great potential in becoming the primary treatment in the case of end-stage heart failures [379].
CFs were also found to stimulate wound healing [381]. In the 1980s, carbon fibers were used in clinics to make an active scaffold, inducing tendon regeneration or ligament repair [382,383]. Unfortunately, the weak forces between graphitic planes induced shear stresses, causing formation of debris. Finally, CF-reinforced composite materials were successfully utilized for bone repair. In addition to the improvement of the mechanical properties, CFs added other characteristics such flexibility and improved osseointegration [365].
5.1. Synthesis of Carbon Fibers
First attempts to synthesize carbon fibers date back to 1879 with experiments by Edison to fabricate carbon filaments suitable for the electric lamp. He dissolved cellulosic materials and then extruded the solution in a spin bath to obtain the cellulose in form of fibers. Subsequent carbonization at high temperature in the absence of air led to the formation of weak filaments which were then reinforced by cracking a layer of pyrolytic carbon onto the surface. Nowadays the synthesis of CFs is made starting from three different precursors: polyacrylonitrile (PAN), rayon (regenerated cellulosic fibers), and petroleum pitch. An example is reported in Figure 19.
Figure 19.
SEM images of carbon fibers (CFs) synthesized from different precursors: (1) SEM image of polyacrylonitrile (PAN) nanofibers after carbonization at 850 °C (reprinted with permission from [388]); (2)Morphological changes of pitch carbon fibers having different textures before exfoliation top after exfoliation bottom; from left: radial, flat layer, and corrugate radial, (reprinted with permission from [389]); (3) SEM images of the stabilized lignin fibers at the heating rates of (a) 0.2 °C min−1 and (b) 2.0 °C min−1, and iodine stabilized lignin fibers at the heating rates of (c) 0.2 °C min−1 and (d) 2.0 °C min−1(reprinted with permission from [390]).
The original synthesis process of CFs from PAN fibers was firstly carried out by R.C. Houtz who heated a PAN fiber for 4–16 h in air at 200 °C [384]. During heating, the PAN fibers underwent a dehydrogenation reaction with the formation of heterocyclic fused rings. In more recent PAN processing, the synthesis proceeds with the polymerization of PAN and the production of fibers by spinning, then fibers are oxidized and carbonized. Finally, surface treatment is performed to allow better coupling with other materials. Production of CFs using PAN holds the main part of the CF market because PAN fibers allow a faster pyrolysis, PAN chains can be aligned, PAN fibers can be stretched up to 800% further improving the chain alignment, PAN decomposes before melting and provides a high amount of product when pyrolyzed at 1000 °C [385]. CFs produced from PAN are commonly utilized as reinforcement to produce high performance composites for applications in sporting goods, aerospace, and automotive industry [386]. To improve the CF performances, more aligned chains in PAN should be obtained. One possibility is to add comonomers to PAN to form a copolymer [387]. This is made by mixing methacrylate and itaconic comonomers. The first reduces the glass transition temperature while the second acts as an initiator for the formation of the ladder polymers. The final result is an improvement of the 2% to 5% of chain alignment in the final product.
Another commercially available CF was produced from rayon in the sixties, achieved strength of 2.76 GPa and a modulus of 345 GPa. Let us remember that pure graphite is characterized by a strength of 100 GPa and a modulus of ~1 TPa [385]. Rayon is a promising precursor for CF production since cellulosic precursors have high thermal conductivity, high purity, mechanical flexibility, and low cost. In addition, it forms strong carbon fibers with pyrolysis. The process of producing the precursor for the CF is rather complex and regards the cellulose purification and production of rayon filaments. These are produced by wet spinning from capillaries in an acid spin bath which induces cross linking of cellulose molecules and coagulation in rayon filaments. Slowing down the cellulose regeneration and stretching processes leads to greater crystalline domains within the fiber inducing higher rayon tenacity. Similar to PAN-based fibres, rayon fibers are thermally stabilized in inert atmosphere where the cellulose fibers undergo a decomposition in a range of temperatures between 200 and 380 °C. The mass loss with emission of volatiles in form of CO, CO2 explains why the carbon yield is only between 10% and 30%. Therefore, the selection of suitable stabilizer materials is essential for increasing the carbon yield and properties of the final products [391]. The final step regards the carbonization of rayon fibers into graphite-like layers through repolymerization. The conversion occurs in a temperature range between 300 up to 900 °C where semiordered carbonaceous structures are formed in inert atmosphere. Graphitization of the material at 900–3000 °C takes place and high-modulus fibers are produced with the formation of an enhanced order of graphene stacks [391].
An alternative method to synthesize carbon fibers developed in the 1970s is based on pitch [392]. The pitch can be derived from petroleum or coal but the first is preferred because coal-derived pitch is a more rough material requiring refining. Differently from PAN and rayon, the pitch is composed by low molecular weight components with minor thermal stability. Based on the optical properties, pitch precursors are classified as isotropic and anisotropic. The isotropic pitches are used to produce general purpose carbon fibers which are non-graphitic and have poorer properties. Anisotropic mesophase pitch produces high performance CFs containing a large amount of graphitic domains. Pitch mesophases can be produced either by the thermal polymerization of petroleum- or coal-pitches. Also, the fabrication of CFs from pitch consists of spinning, stabilization, and carbonization. In the melt spinning [393], the precursor is melted, extruded through capillaries to form the fibers which are drawn while they are cooling. The stabilization of the pitch fibers is made via oxidation in air. To keep the fiber structure orientated, oxidation must be performed at a temperature below the fiber softening point (~250–350 °C) for a duration ranging from 30 min to several h. The ketone, carbonyl, and carboxyl groups on the oxidized fiber surface induce strong hydrogen bonding between the adjacent molecules facilitating the three-dimensional cross-linking. Finally, the carbonization and graphitization of stabilized precursor fibers are performed initially at 700–900 °C to avoid formation of defects due to the excessive release of volatiles. Then carbonization is carried out at 1500–1800 °C. It has been shown that increasing the carbonization temperature, it is possible to increase the preferred alignment of the crystalline domains [394] and then graphitization may be performed up to 3000 °C.
5.2. Functionalization of Carbon Fibers
Among all the reinforcing fibers, CFs offer the highest specific modulus and highest specific strength. Thanks to their impressive mechanical properties, CFs are widely used as reinforcement of composite materials. Composites based on CFs are suited to applications where low weight, strength and outstanding fatigue characteristics such as stiffness are essential requirements. CFs can also be selected for their resistance to high temperature, the chemical inertness, the electrical and thermal conductivity and low linear coefficient of thermal expansion [395].
CFs are widely applied in aerospace, nuclear, and general engineering, in transportation, and all sectors requiring components with superior resistance to fatigue and degeneration (bearings, gears, cams, fan blades, etc.). However, emerging are also applications in biomedicine where novel materials display the capability to interface with a complex environment where corrosion resistance and tunable mechanical properties are desirable. One of the most important sector of CF application is the fiber/polymer composites where the polymeric materials are polyether ether ketone or epoxy resins polyurethane. They are utilized in prosthetic in hip joint, in spinal fixation, in bone fracture fixation. The advantage of using the CF/PEEK composite is that they have specific modulus similar to bone thereby avoiding interface disruption with risk of bone loss [396]. CFs have shown good biocompatibility by both in vivo and in vitro experiments [397]. CF/carbon composites, scaffolds made of CF find application in regenerative medicine, drug delivery, nerve stimulation ([367,370,397,398]; see Figure 20).
Figure 20.
SEM images of electrospun SF/P(LLA-CL): (A) random nanofibrous scaffold; aligned nanofibrous scaffold (B), and (C) nanofibrous reinforced scaffold with yarns twisted by aligned nanofibers (scale bars: 20 μm for (A,B); 100 μm for (C). Confocal microscopy fluorescence images show the actin filaments (red) and nuclei (blue) of MSCs on the random nanofibrous scaffold (A), aligned nanofibous scaffold (B) and nanofibrous reinforced scaffold (C) after 7-day culture. Magnifications of all images are 400x. Adapted with permission from [399].
In all these cases, the surface termination, which drives the interaction with the composite material and the living matter, is important. Regarding CFs, the carbonization process at high temperature generates a chemically inert graphitic surface which may be important for biocompatibility. However, this hinders the interaction with other matrix materials and likely behaves as a defect, degrading the overall mechanical properties. Generally, when CFs are utilized without any kind of surface treatment, the poor adhesion with the surrounding material leads to low interlaminar shear strength [400]. The surface treatments can be classified in two gross categories: (i) treatments using physical means thereby enhancing the surface roughness, the surface area, the number of micro-pores or surface pits leading thus increasing the contact points with the external matrix; (ii) the second type of treatments involves chemical reactions with grafting of reactive functional groups promoting good chemical bonding with the surrounding material and, thanks to the presence of polar groups, renders the CF surface wettable. Different kinds of functional groups can be grafted, increasing the interaction strength with the external matrix [401].
In the majority of the cases, CF are coupled to biomaterials such as polymers or metals to form composites. The main purpose of using CF based bio-composites is to improve the mechanical properties of the material or maintaining the same strength while reducing the weight.
A kind of surface treatment consists of surface oxidation which is carried out in a gas-phase, a liquid-phase or chemically and via catalytic processes [402]. The non-oxidative treatments consist of depositing active forms of carbon, plasma polymerization, and grafting of polymers onto the fiber surface [403]. The majority of surface treatments lead simultaneously to both the changes. However, it is important to consider that methods inducing surface etching have positive effects due to the reasons in (i) and (ii), and on the other side, induce a deterioration of the fiber strength thus adversely affecting the whole mechanical properties of the composite.
Let us turn our attention to CF functionalization and, in particular, to CF oxidation. One common method to perform CF oxidation is to utilize strong acids such as nitric acid [404], a mixture of nitric and sulphuric acids [405], or hydrochloric acid [406]. The treatment efficacy depends on the acid concentration, the temperature and process duration. The acid treatment leads to the formation of carboxylic acid, ester, lactone, phenol, and quinone structure moieties and the density of polar groups, leading to a surface acid character which increases linearly with process duration. This conclusion is confirmed by other works where the density of polar groups increased as a function of the process severity, bringing an overall enhancement of the surface energy. However, as already observed, acid treatment caused a surface etching with an increase of surface roughness and surface area but a loss in mechanical properties. In [407], authors performed a chemical and thermal attack using nitric, and sulphuric acids, hydrogen peroxide, or thermal treatment at increasing temperatures in atmosphere from 380 to 520 °C, or in nitrogen atmosphere from 500 to 600 °C. Results show that oxidation induces the formation of hydroxyl, carbonyl, carboxyl functional groups due to the chemical attack or the interaction of oxygen with the CF carbon atoms. Increasing the duration of the chemical treatment induces an increase of CF mass loss up to 2.5% for 120 min of processing. The mass loss is more pronounced when thermal treatment is applied. In this case, the temperature growing leads to a faster reaction kinetic of O with the CF carbon atoms. In particular, the thermal attack starts at temperatures around 400 °C and becomes massive at temperatures ~500–550 °C with a CF mass loss up to 70% in the case of treatments in air while it was 11% for treatments in nitrogen. It is confirmed that there is a linear growth of surface energy with the duration of the processes for both the chemical and thermal treatments [407]. In [406], the authors compared the efficiency of chemical and plasma treatments to oxidize the CF surface.
Nitric acid was utilized for chemical treatment while oxygen and argon precursors were used in two distinct plasma processes. Authors found that the nitric acid led to more pronounced changes of the CF surface, introducing a larger number of functional groups and augmenting the roughness. The oxygen plasma caused CF etching, removing carbon atoms with the generation of CO and CO2 molecules. Finally, the argon plasma treatment surprisingly eliminated fiber defects, reducing the size of critical faults, thereby increasing the CF tensile strength.
Plasma treatments may induce diverse surface changes: (i) removal of surface contaminations providing a better interaction of the fibers with the external matrix; (ii) enhanced fiber/matrix interaction due to increased surface roughness; (iii) increase of surface polarity/surface energy with better surface wettability; and (iv) grafting of desired functional groups depending on the plasma precursor used. In [408], an atmospheric oxygen plasma resulted in the formation of COOH, –C–OH and =C=O groups on the fiber surface, improving the adhesion of CF to epoxy composites, thus enhancing the overall mechanical properties. Similar results were obtained by other authors [409] who observed an increase of the interlaminar shear strength with an increase in the duration of the plasma treatment.
We dedicate a short note to the radiation induced surface modifications of CF. Surface roughening as well as grafting of chemical groups were observed in CF exposed to high-energy gamma-irradiation, UV or laser irradiation. It was found that gamma radiation is able to promote the reaction of CFs suspended in water with the oxygen dissolved in the solution [410]. In another work, similar reactions were induced by UV radiation in atmosphere, thanks to the ozone generated by the UV photons [411].
In general, oxidation treatments are desirable to induce a better coupling of CFs with the matrix of the polymeric or metallic or ceramic materials to produce materials with superior mechanical properties.
6. Fullerenes
Among the other allotropic forms, carbon atoms can arrange in cage-like stable structures. In 1970, this idea was substantiated by theoretical calculations on a C60 structure, describing a superaromatic π-systems and predicting its stability from Hückel calculations [412]. However only in 1985 was the first experimental evidence of fullerenes realized, using a mass spectrometer with spectra dominated by a peak at m/z 720 corresponding to C60 while a second peak at m/z 820 was assigned to C70. Spherical cages of carbon atoms can be obtained alternating hexagons and pentagons. In the case of the more common C60 structure, the bonding situation does not differ from that of the graphite single sheet, the only difference is the different angle at pentagon corners which force the σ-bonds is out of its usual angle of 120°, leading to surface curvature. Combining pentagons and hexagons, it is possible to generate fullerene structures with a “magic” number of carbon atoms n = 60, 70, 72, 76, 78, 84, … Cages with a smaller number of carbon atoms also exist. The smallest possible fullerene is C20, with a dodecahedral structure. Possible structures are C2n molecules with n = 12, 13, 14, … where the number of fullerenes grows with increasing n. Interestingly, there are no C22 fullerenes. The presence of pentagons forbid bond resonance thereby making C60 a non superaromatic structure with poor electron delocalization.
The stable fullerene structures constitute a family of molecules possessing unique properties. They are very strong, fullerenes can sustain pressure as high as 3000 atm. and return to their original shape without being damaged [413]. They possess tensile strength about 20 times higher than that of high-performing steel alloys but a density which is half that of aluminum.
Macroscopic amounts of fullerenes were produced in 1990 by the evaporation and recondensation of graphite [414]. This promoted the use of fullerenes in base and applied research. For example, delocalization of charges makes C60 an electron deficient system, reacting easily with electron rich species. This offers the possibility of stabilizing electron donor molecules. This property can be exploited in a donor-acceptor system where fullerenes promote charge separation and reduce the recombination, as for example photovoltaics [415], photochemistry [416], photobiology [417,418], and medicine [419]. Referring to this last sector, nanomedicine is based on the development of new nanosized multifunctional platforms [420]. However, fullerenes display a very poor solubility in aqueous solvents leading to aggregation. This makes these structures in their original form, an unattractive candidate in biological applications as already observed long time ago [421,422]. Functionalization of the fullerene surface may solve the problem, generating fullerenes with excellent solubility in both aqueous and organic solvents [423,424]. Platforms based on functionalized fullerenes are currently utilized as scavengers of reactive oxygen (ROS) and nitrogen species (RNS) produced by normal and abnormal cell metabolisms. Free radicals can induce oxidative stress damaging cells, leading to aging tissue abnormalities, and disease processes [425]. They are detrimental for biological molecules such as proteins, lipids, and DNA. As a whole, free ROS and RNS can induce different biological responses related to different degrees of disorders including neurodegenerative/neuropsychiatric and cardiovascular diseases [425], atherosclerosis and cancer [426]. As an electron deficient system where double bonds can react with free radicals, fullerenes are exceptional free radical interceptor, protecting cells from dysfunctions [427]. However, despite of the free radical scavenging properties, fullerenes can also promote the generation of ROS. The mechanism is shown in Figure 21.
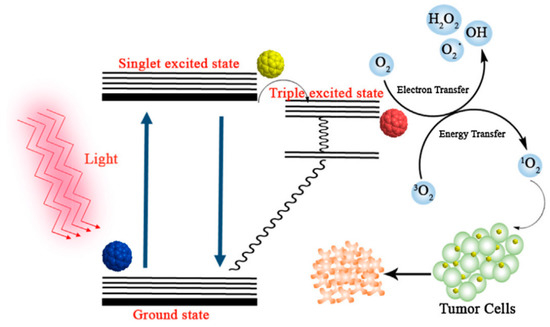
Figure 21.
Fullerene mediated generation of ROS and principle of the photodynamic therapy. Reproduced with permission from [428].
Essentially C60 is irradiated with visible light and excited from a S0 ground state to a singlet excited state S1. A fast decay follows in a more stable triplet T1 state which can react with oxygen dissolved in water, producing 1O2 ROS. If 1O2 ROS is produced inside tumor tissues, it can lead to cell necrosis and death. Fullerenes can be utilized also as carriers for bioactive elements such as drugs, proteins, and genomic material into the cell compartments [429]. Compared to other carriers, fullerenes show the possibility of carrying different payloads, thanks to their biocompatibility which can overcome resistance mechanisms, making the cancer treatment more effective [430]. Fullerenes are also useful to sense biomolecules, enzymes, antibodies or receptors, DNA, or sense the presence of microorganisms, when they are coupled to a transducer [431]. Essentially, fullerene deposited on an electrode can amplify the charge transfer induced by biochemical reactions occurring between an analyte and a target site [432]. When atomic nitrogen is encapsulated in fullerenes [433], because of the narrow EPR lines of nitrogen, it has the potential of being EPRI sensors for imaging. Other therapeutic use of fullerenes are as treatment for osteoporosis. It has been demonstrated that fullerenes conjugated with C(60)(OH)(16)AMBP. Crystal growth studies indicate that C(60)(OH)(16)AMBP reduces hydroxyapatite mineralization by 50% [434]. Crystal growth inhibition technology is highly desired because bone-vectored substances generally target bone areas undergoing formation and resorption processes. Fullerenes also display antimicrobial activity and promising bactericidal activity was observed on Bacillus subtilis, Candida albicans, and Escherichia coli colonies and immunodeficiency virus-reverse transcriptase inhibition was also proved [29,435]. The suppressing activity of fullerenes is ascribed to the generation of ROS under illumination. The fullerene absorbing properties of reactive oxygen/nitrogen species generated by the overexcitation of glutamic acid receptors can be exploited to protect from neurodegenerative diseases like Alzheimer’s and Parkinson’s diseases [436]. The unpaired antioxidant activity of fullerenes is used to reduce the apoptosis in cortical neurons and to block the receptors of glutamic acid which are accompanied by antiamyloid action [437]. The same antioxidant activity of fullerenes is exploited in cosmetics to decrease inflammatory lesions and to protect against ultraviolet absorption leading to oxidative cell stress and aging [438].
6.1. Synthesis of Fullerenes
The synthesis of fullerenes is made via pyrolysis of polycyclic aromatic hydrocarbon as naphthalene, corannulene, or higher polycyclic compounds. The molecules are treated at about 1000 °C in an inert atmosphere (usually argon). In these conditions, a decomposition of the molecules occurs with cleavage of the hydrogen bonds and mainly C60 and C70 are produced. Gas pressure is the most important parameter affecting the C70/C60 ratio, with a larger C70 fraction with increasing pressure.
A second method to synthesize fullerenes is an arc discharge produced between two graphite electrodes. When high voltage is applied to two approaching electrodes, an arc develops. The discharge induces vaporization of the graphite with the formation of a plasma. Fullerene are synthesized by condensation of the graphite plasma in particles which deposit on the reactor walls. The process yield is about 15% with C60 constituting about 80% of the fullerene material. Interestingly, small electrodes are normally utilized because vaporized molecules are sensitive to the UV radiation emitted by the arc discharge. Too intense a discharge causes too intense a UV emission, exciting the molecules in a reactive triplet state. These molecules readily interact with formed fullerenes, generating open shell structures and causing a drop in the fullerene yield. A similar synthesis process is the use of high density currents flowing graphite electrodes. If the contact point between the two electrodes is very small, the temperature rises up to 2500–3000 °C, thus vaporizing the graphite. The molecules then precipitate on the cool reactor walls or on a cool finger in the proximity. As for the arc discharge, this process also has to be carried out in inert atmosphere to avoid graphite burning. The process yield may be up to 15% and, after purification, mainly C60 is obtained (C70/C60 = 0.02:0.18).
6.2. Functionalization of Fullerenes
As already pointed out, one of the major problems hindering the utilization of fullerenes for biological applications is its insolubility in water and low solubility in many organic solvents [439]. However, this problem can be successfully solved by functionalizing the fullerene surface. The presence of double bonds in the fullerene chemical structure opens a large variety of possibilities to modify the surface chemistry [440]. Among others, addition reactions are commonly utilized to graft the desired functional groups [441]. Fullerene functionalization is made using two different routes: (i) use of solubilizing agent complexation to partially mask fullerene surface, and (ii) covalent functionalization of the fullerene surface [442,443]. With biomedical applications in mind, we will focus on four different routes utilized to render fullerenes hydrophilic.
The first two reactions regard surface hydroxylation and amination. The presence of these functional groups add a certain polarity to the fullerene thus rendering this molecule reasonably soluble in water. However, there is not a precise control over the density of functional groups grafted on the surface. This constitutes the major disadvantage for their use in the pharmaceutical industry, where reproducibility of each chemical reaction and precise chemical composition is required.
In the second kind of reactions, the tendency of C60 to behave as a reactive 2π component in cycloaddition is exploited [444]. This route is very popular and utilized to introduce a large number of complex molecules in a one-pot reaction.
As for other graphite-like systems (graphene, carbon nanotubes…), the introduction of oxygen based functional groups can be made using strong acids at high temperatures [445]. The reaction leads to the polyhydroxylated of fullerenes. An alternative and frequent method is the use of a basic solution. A very fast reaction is obtained adding a suspension of C60 in benzene to an excess NaOH in water. The reaction is carried out in the presence of a small amount of tetrabutylammonium hydroxide acting as a catalyzer [446]. These reactions bring to the formation of an indefinite number of addends. However, despite the lack of control, these reactions are widely utilized to render fullerenes hydro-soluble.
Regarding the amination of C60, in [447], the authors mixed C60 in a series of neat aliphatic primary amines (n-propylamine, t-butylamine7 and dodecylamine) for 16 to 24 h. In other reactions, smaller primary and secondary amine chains (methylamine, diethylamine) reacted with C60 and C70 fullerenes. The reaction mechanism for the amination of C60 with addition at the [6,6] bond is represented in Figure 22.
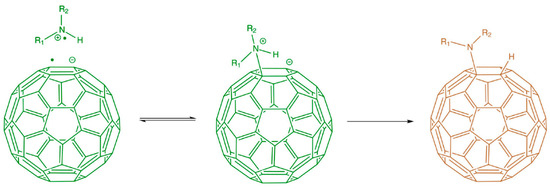
Figure 22.
Amination mechanism of C60. The first step is single-electron transfer (a fast process) to produce the C60 anion radical which has a characteristic green colour. Radical recombination gives a zwitterion which can be stabilised by proton transfer to give the final product (a slow process giving a brown solution). Reproduced with permission from [448].
Let us turn the attention to the second group of reactions. The 1,3-dipolar cycloaddition of an azomethine ylide to a C60 molecule produces a stable pyrrolidinofullerene complex. The reaction scheme is represented in Figure 23. The ylide(ylide is correct) is a reactive intermediate product obtained by the reaction of an α-amino acid with an aldehyde or ketone with subsequent decarboxylation [444]. The resulting compound is a C60 with a pyrollidine ring attached across the [6,6] bond. This reaction is very appealing since it is possible to attach different functionalities since, as it appears in Figure 23, the R1, R2, and R3 groups can be tailored to the desired needs.
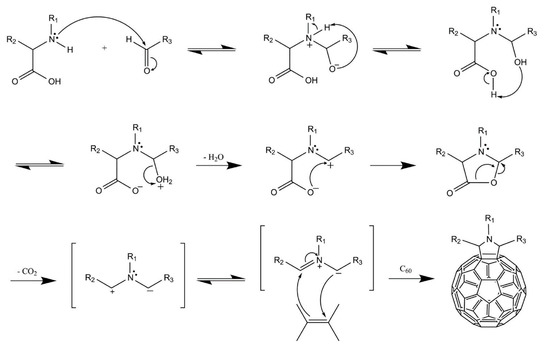
Figure 23.
Initial attack of the aldehyde/ketone’s polar carbonyl group by the nitrogen lone pair of the amino acid leads to the expulsion of water. Subsequent decarboxylation with loss of CO2 gives the reactive ylide intermediate. Reproduced with permission from [448].
Differently from the previous amination and hydroxylation processes, this reaction leads to a controlled density of functional groups by fixing the reagent stoichiometry and the reaction time. Because of the symmetry of the fullerene molecules, there are identical [6,6] bonds available for coordination, giving origin to regioisomerism and a lack of control on the adduct position [449]. This could have some implications in biomedicine since regioisomerism may determine the pharmaco-kinetics and dynamics [450].
An alternative to this process is the cyclopropanation which consists of the bonding of an α-halo ester/ketone to the cage under strongly basic conditions, resulting in a methanofullerene [451]. The reaction scheme is reported in Figure 24 where the strong basic environment causes the deprotonation of the malonate and a nucleophilic attack at the [6,6] position of the fullerene. As for the previous reaction, also in this case, different groups R1 and R2 of the bromomalonate may be selected for specific purposes. The popularity of this reaction stems from the possibility to easily modify the original reaction to produce halogenated intermediates.
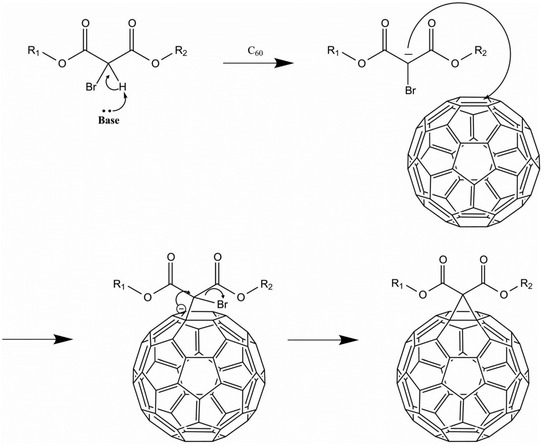
Figure 24.
Bingel reaction mechanism. Deprotonation by the strong base gives the nucleophilic malonate anion which then attacks the [6,6] bond. Bromine is then expelled as cyclisation is completed.
An example is given in [452] where C60 hexakis-adducts were produced with a cyclopropanation reaction. The presence of a high number of polar groups of these hexakis-adducts make them particularly soluble, which is important for biomedical applications.
The aforementioned four processes are successfully utilized to produce a vast class of products such as the dendro [60] fullerene [453] in which the dendrimers masking the hydrophobic character of fullerenes induce the highest solubility of the product. Pyrrolidine biscarboxylic acid derivatization of C60 allows subsequent PEGylation or reaction with the peptide sequence, GABA-GPLGVRGA [454].
Metallo fullerenes are another functionalization process where one or more metal atoms are inserted in the fullerene cage. This imparts different reactivities to and properties useful for biomedical applications [455]. Examples are the Gd@C60 @ IS CORRECT complex utilized as a contrasting agent for MRI imaging.
Sugars are important components for the biological activity of mammalian cells [456], for their interactions with proteins. There are a series of possible reactions which can be utilized to coordinate sugars with fullerenes. Sugars are firstly bonded to fullerenes via diazoniums [457], azide reactions [458], penta addition [459], cyclopropanation [460], and the Diels-Alder reaction [461]. It is important to observe that in these hybrids, the intrinsic properties of sugar molecules and C60 can be preserved despite the mutual interactionsthanks to the high flexibility in molecular design which can be utilized to synthesize multifunctional glycofullerenes acting as fluorescent drug carriers and with sensing functions. As an example, fullerenes are ideal electron collectors which can be released to a transducer. They can be located closer to an enzyme active site to detect its catalytic activity [462]. This idea was utilized in a sensor measuring glucose levels via the oxidation of glucose to gluconic acid catalyzed by the glucose oxidase enzyme/C60 complex [463].
7. Conclusions
Carbon nanomaterials, have been used as multifunctional potent platforms for a number of different biological and biomedical applications. These nanomaterials have been utilized as drug and gene delivery carriers, as fluorescent biolabelling probes, as active elements in photoacustics, in Raman multiphoton and NIR imaging, as contrasting agents, as electron transfer units in biosensors, as constituents of scaffolds for tissue engineering, and therapeutic principles. The contributions of these carbon nanostructures to the diverse applications derive from their unique physico-chemical properties. Among others, we quote their biocompatibility and the varied routes of surface derivatization. The flexibility offered by these carbon nanomaterials will likely determine a continue utilization of these nanostructures in an ever expanding number of biological applications. Still, a deeper comprehension of the physics behind the behavior of these fascinating objects is need as well as technical and conceptual challenges to be addressed for a better and more efficient application in biomedicine.
Further work should be focused on the following points with respect to their biomedical application. The first and most important aspect is a systematic investigation of their toxicity to the human body, especially in the long-term. Although there has already been some research work carried out to investigate the toxicity of carbon nanomaterials, some controversial conclusions were obtained. The long-term effects of carbon nanomaterials are less reported since these investigations are time-consuming. Secondly, more work on a more precisely controlled synthesis method is still needed for some of these carbon nanomaterials. Usually, materials from different synthesis methods show very different properties and, thus, different biomedical properties. For example, graphene with a single or a few layers is usually required for biomedical applications, which need a more controlled route for their synthesis. With more threats to human health from cancers, medicines based on carbon nanomaterials for cancer treatment has become a hot research area. Carbon materials with versatile functionalization capability provides the possibility of having multifunctional drugs in just one dose. Considering the long period needed for the discovery of a new drug, more effort and more government support is needed to promote their development in this area.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Pan, D.; Zhang, J.; Wu, M. Hydrothermal Route for Cutting Graphene Sheets intoBlue-Luminescent Graphene Quantum Dots. Adv. Mater. 2010, 22, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Pierson, H.O. Handbook of Carbon, Graphite, Diamonds and Fullerene, 1st ed.; Noyes Publications: Park Ridge, NJ, USA, 1994. [Google Scholar]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Bekyarova, E.; Ni, Y.; Malarkey, E.B.; Montana, V.; McWilliams, J.L.; Haddon, R.C.; Parpura, V. Applications of Carbon Nanotubes in Biotechnology and Biomedicine. J. Biomed. Nanotechnol. 2005, 1, 3–17. [Google Scholar] [CrossRef]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar]
- Perkins, B.L.; Naderi, N. Carbon Nanostructures in Bone Tissue Engineering. Open Orthop. J. 2016, 10, 877–899. [Google Scholar] [CrossRef]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2433–2454. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, S.I.; Song, G.Y.; Kim, I. Non-Covalently Functionalized Carbon Nanostructures for Synthesizing Carbon-Based Hybrid Nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Czerniak-Reczulska, M.; Niedzielski, P.; Balcerczyk, A.; Bartosz, G.; Karowicz-Bilinska, A.; Mitura, K. Biological properties of different type carbon particles. In vitro study on primary culture of endothelial cells. J. Nanosci. Nanotechnol. 2010, 10, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Lee, Y.K.; Lee, J.Y.; Hong, J.H.; Khang, D. PEGylated anticancer-carbon nanotubes complex targeting mitochondria of lung cancer cells. Nanotechnology 2017, 28, 465102. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Zhao, M. Target-directed functionalized ferrous phosphate-carbon dots fluorescent nanostructures as peroxidase mimetics for cancer cell detection and ROS-mediated therapy. Sens. Actuators B 2019, 297, 126739. [Google Scholar] [CrossRef]
- Gopi, K.; Murugan, V.; Gnanasekaran, J.; Krishnaswamy, B.; James, J. Amygdalin-Functionalized Carbon Quantum Dots for Probing β-Glucosidase Activity for Cancer Diagnosis and Therapeutics. ACS Biomater. Sci. Eng. 2019, 5, 3089–3099. [Google Scholar]
- Pirsaheb, M.; Mohammadi, S.; Salimi, A.; Payandeh, M. Functionalized fluorescent carbon nanostructures for targeted imaging of cancer cells: A review. Microchim. Acta 2019, 186, 231. [Google Scholar] [CrossRef]
- Meng, Z.; Song, Y.; Guo, C.; Cui, B.; Ji, H.; Ma, Z. Tailoring the dimensionality of carbon nanostructures as highly electrochemical supports for detection of carcinoembryonic antigens. RSC Adv. 2019, 9, 13431–13443. [Google Scholar] [CrossRef]
- Ibáñez-Redína, G.; Furuta, R.H.M.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.M.R.B.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Chaur, M.N.; et al. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C 2019, 99, 1502–1508. [Google Scholar] [CrossRef]
- Cui, L.; Wang, M.; Sun, B.; Ai, S.; Wang, S.; Zhang, C.Y. Substrate-free and label-free electrocatalysis-assisted biosensor for sensitive detection of microRNA in lung cancer cells. Chem. Commun. 2019, 55, 1172–1175. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.H. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, e1802368. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.J.; Sim, M.; Oh, L.; Lim, K.; Park, H. Carbon-based drug delivery carriers for cancer therapy. Arch. Pharm. Res. 2014, 37, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jing, L.; Liu, Z.; Gao, D.; Chen, H.; Song, W.; Wang, T.; Fang, X.; Qin, W.; Zhen, Y.; et al. Mesoporous carbon nanospheres as a multifunctional carrier for cancer theranostics. Theranostics 2018, 8, 663–675. [Google Scholar] [CrossRef]
- Wang, H.; Bi, J.; Zhu, B.W.; Tan, M. Multicolorful Carbon Dots for Tumor Theranostics. Curr. Med. Chem. 2018, 25, 2894–2909. [Google Scholar] [CrossRef]
- Tabish, T.A.; Zhang, S.; Winyard, P.G. Developing the next generation of graphene-based platforms for cancer therapeutics: The potential role of reactive oxygen species. Redox Biol. 2018, 15, 34–40. [Google Scholar] [CrossRef]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generationand quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef]
- Al-Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M.V. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013, 8, 2003–2014. [Google Scholar]
- Mendes, R.G.; Bachmatiuk, A.; Büchner, B.; Cuniberti, G.; Rümmeli, M.H. Carbon nanostructures as multi-functional drug delivery platforms. J. Mater. Chem. B 2013, 1, 401–428. [Google Scholar] [CrossRef]
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016, 2016, 5762431. [Google Scholar] [CrossRef]
- Ma, J.; Huang, J.; Song, S.; Chen, H.; Zhang, Z. Cancer-Targeted Nanotheranostics Recent Advances and Perspectives. Small 2016, 12, 4936–4954. [Google Scholar] [CrossRef] [PubMed]
- Bartelmess, J.; Quinn, S.J.; Giordani, S. Carbon nanomaterials: Multi-functional agents for biomedical fluorescence and Raman imaging. Chem. Soc. Rev. 2015, 44, 4672–4698. [Google Scholar] [CrossRef]
- Wang, H.; Sun, Y.; Yi, J.; Fu, J.; Di, J.; del Carmen Alonso, A.; Zhou, S. Fluorescent porous carbon nanocapsules for two-photon imaging, NIR/pH dual-responsive drug carrier, and photothermal therapy. Biomaterials 2015, 53, 117–126. [Google Scholar] [CrossRef]
- Li, M.; Teh, C.; Ang, C.Y.; Tan, S.Y.; Luo, Z.; Qu, Q.; Zhang, Y.; Korzh, V.; Zhao, Y. Near-Infrared Light-Absorptive Stealth Liposomes for Localized Photothermal Ablation of Tumors Combined with Chemotherapy. Adv. Funct. Mater. 2015, 25, 5602–5610. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, Z.; Zhong, H.; Qin, X.; Wan, M.; Yang, B. Graphene oxide based surface-enhanced Raman scattering probes for cancer cell imaging. Phys. Chem. Chem. Phys. 2013, 15, 2961–2966. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Torres Andón, F.; Feliu, N. Carbon Nanotubes as Optical Sensors in Biomedicine. ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef]
- Baldrighi, M.; Trusel, M.; Tonini, R.; Giordani, S. Carbon Nanomaterials Interfacing with Neurons: An In vivo Perspective. Front. Neurosci. 2016, 10, 250. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, A.; Wang, X.; Zhu, J.; Fan, Y.; Yu, H.; Yang, Z. The Advances of Carbon Nanotubes in Cancer Diagnostics and Therapeutics. J. Nanomater. 2017, 2017, 3418932. [Google Scholar] [CrossRef]
- Bardhan, N.M. 30 years of advances in functionalization of carbon nanomaterials for biomedical applications: A practical review. J. Mater. Res. 2017, 32, 107–127. [Google Scholar] [CrossRef]
- Atta Editor, N.F. Designing Nanosensors for Chemical and Biological Applications; International Frequency Sensor Association (IFSA): Barcelona, Spain, 2017; ISBN 978-84-697-3290-8. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Chemical Functionalization of Carbon Nanomaterials: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4822-5396-2. [Google Scholar]
- Aliofkhazraei, M.; Ali, N.; Milne, W.I.; Ozkan, C.S.; Mitura, S.; Gervasoni, J.L. Graphene Science Handbook: Nanostructure and Atomic Arrangement; CRC Press: Boca Raton, FL, USA, 2016; ISBN 0-429-16937-X. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Sudan, P.; Mauron, P.; Wenger, P. Model for the hydrogen adsorption on carbon nanostructures. Appl. Phys. A 2004, 78, 941–946. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Uz, M.; Jackson, K.; Donta, M.S.; Jung, J.; Lentner, M.T.; Hondred, J.A.; Claussen, J.C.; Mallapragada, S.K. Fabrication of High-resolution Graphene-based Flexible Electronics via Polymer Casting. Sci. Rep. 2019, 9, 10595. [Google Scholar] [CrossRef]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218. [Google Scholar] [CrossRef]
- Peña-Bahamonde, J.; Nguyen, H.N.; Fanourakis, S.K.; Rodrigues, D.F. Recent advances in graphene-based biosensor technology with applications in life sciences. J. Nanobiotechnol. 2018, 16, 75. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, T.; Song, X.; Ran, Q.; Yu, C.; Yang, J.; Feng, H.; Yu, L.; Wei, D. Flexible electrochemical biosensors based on graphene nanowalls for the real-time measurement of lactate. Nanotechnology 2017, 28, 315501. [Google Scholar] [CrossRef]
- Waifalkar, P.P.; Chougale, A.D.; Kollu, P.; Patil, P.S.; Patil, P.B. Magnetic nanoparticle decorated graphene based electrochemical nanobiosensor for H2O2 sensing using HRP. Colloids Surf. B Biointerfaces 2018, 167, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bharath, G.; Madhu, R.; Chen, S.-M.; Veeramani, V.; Balamurugan, A.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Enzymatic electrochemical glucose biosensors by mesoporous 1D hydroxyapatite-on-2D reduced graphene oxide. J. Mater. Chem. B 2015, 3, 1360–1370. [Google Scholar] [CrossRef]
- Thanh, T.D.; Balamurugan, J.; Lee, S.H.; Kim, N.H.; Lee, J.H. Effective seed-assisted synthesis of gold nanoparticles anchored nitrogen-doped graphene for electrochemical detection of glucose and dopamine. Biosens. Bioelectron. 2016, 81, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An ultrasensitive electrochemical DNA biosensor based on graphene/Au nanorod/polythionine for human papillomavirus DNA detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef]
- Chen, M.; Hou, C.; Huo, D.; Fa, H.; Zhao, Y.; Shen, C. A sensitive electrochemical DNA biosensor based on three-dimensional nitrogen-doped graphene and Fe3O4 nanoparticles. Sens. Actuators B Chem. 2017, 239, 421–429. [Google Scholar] [CrossRef]
- Wang, Y.; Sauriat-Dorizon, H.; Korri-Youssoufi, H. Direct electrochemical DNA biosensor based on reduced graphene oxide and metalloporphyrin nanocomposite. Sens. Actuators B Chem. 2017, 251, 40–48. [Google Scholar] [CrossRef]
- Tian, M.; Xu, S.; Zhang, J.; Wang, X.; Li, Z.; Liu, H.; Song, R.; Yu, Z.; Wang, J. RNA Detection Based on Graphene Field-Effect Transistor Biosensor. Adv. Condens. Matter Phys. 2018, 2018, 8146765. [Google Scholar] [CrossRef]
- Eksin, E.; Bikkarolla, S.K.; Erdem, A.; Papakonstantinou, P. Chitosan/Nitrogen Doped Reduced Graphene Oxide Modified Biosensor for Impedimetric Detection of microRNA. Electroanalysis 2018, 30, 551–560. [Google Scholar] [CrossRef]
- Holade, Y.; Tingry, S.; Servat, K.; Napporn, T.W.; Cornu, D.; Kokoh, K.B. Nanostructured Inorganic Materials at Work in Electrochemical Sensing and Biofuel Cells. Catalysts 2017, 7, 31. [Google Scholar] [CrossRef]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose Oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Liang, B.; Guo, X.; Fang, L.; Hu, Y.; Yang, G.; Zhu, Q.; Wei, J.; Ye, X. Study of direct electron transfer and enzyme activity of glucose oxidase on graphene surface. Electrochem. Commun. 2015, 50, 1–5. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Chen, J.; Zheng, X.; Liu, C.; Xue, T.; Wang, H.; Jin, Z.; Qiao, L.; Zheng, W. Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv. 2012, 2, 6245–6249. [Google Scholar] [CrossRef]
- Wu, G.; Song, X.; Wu, Y.-F.; Chen, X.; Luo, F.; Chen, X. Non-enzymatic electrochemical glucose sensor based on platinum nanoflowers supported on graphene oxide. Talanta 2013, 105, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Baby, T.T.; Aravind, S.S.J.; Arockiadoss, T.; Rakhi, R.B.; Ramaprabhu, S. Metal decorated graphene nanosheets as immobilization matrix for amperometric glucose biosensor. Sens. Actuators B Chem. 2010, 145, 71–77. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, D.; Justin, G.J.; Xue, Y.; Dai, L. Flexible fiber-shaped non-enzymatic sensors with a graphene-metal heterostructure based on graphene fibres decorated with gold nanosheets. Carbon 2018, 136, 329–336. [Google Scholar] [CrossRef]
- Janyasupab, M.; Promptmas, C. Development of Non-Enzymatic N-doped Graphene supported Cobalt/Iron Amperometric based Sensor for Glucose Detection in Urine. In Proceedings of the IEEE-EMBS Conference on Biomedical Engineering and Sciences (IECBES), Sarawak, Malaysia, 3–6 December 2018; pp. 577–582. [Google Scholar]
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Sun, X.; Dai, H. PEGylated Nanographene Oxide for Delivery of Water-Insoluble Cancer Drugs. J. Am. Chem. Soc. 2008, 130, 10876–10877. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Liu, Z.; Ma, Y.; Huang, Y.; Chen, Y. High-Efficiency Loading and Controlled Release of Doxorubicin Hydrochloride on Graphene Oxide. J. Phys. Chem. C 2008, 112, 17554–17558. [Google Scholar] [CrossRef]
- Zhou, T.; Zhou, X.; Xing, D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials 2014, 35, 4185–4194. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. C 2015, 48, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Ge, H.; Liu, L.; Zhu, C.; Min, L.; Liu, M.; Fan, L.; Li, D. Carboxymethyl cellulose modified graphene oxide as pH-sensitive drug delivery system. Int. J. Biol. Macromol. 2018, 107, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Kazempour, M.; Namazi, H.; Akbarzadeh, A.; Kabiri, R. Synthesis and characterization of PEG-functionalized graphene oxide as an effective pH-sensitive drug carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef]
- Kim, D.J.; Kim, J.; Lee, H.L.; Lee, S.; Choi, J.S.; Kim, S.J.; Jeong, Y.-I.; Kang, D.H. Redox-Responsive Nanocomposites Composed of Graphene Oxide and Chlorin e6 for Photodynamic Treatment of Cholangiocarcinoma. Bull. Korean Chem. Soc. 2018, 39, 1073–1082. [Google Scholar] [CrossRef]
- Deb, A.; Vimala, R. Camptothecin loaded graphene oxide nanoparticle functionalized with polyethylene glycol and folic acid for anticancer drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 333–342. [Google Scholar] [CrossRef]
- Deb, A.; Andrews, N.G.; Raghavan, V. Natural polymer functionalized graphene oxide for co-delivery of anticancer drugs: In-vitro and in-vivo. Int. J. Biol. Macromol. 2018, 113, 515–525. [Google Scholar] [CrossRef]
- Chai, D.; Hao, B.; Hu, R.; Zhang, F.; Yan, J.; Sun, Y.; Huang, X.; Zhang, Q.; Jiang, H. Delivery of Oridonin and Methotrexate via PEGylated Graphene Oxide. ACS Appl. Mater. Interfaces 2019, 11, 22915–22924. [Google Scholar] [CrossRef]
- Karimi Shervedani, R.; Mirhosseini, H.; Samiei Foroushani, M.; Torabi, M.; Rahsepar, F.R.; Norouzi-Barough, L. Immobilization of methotrexate anticancer drug onto the graphene surface and interaction with calf thymus DNA and 4T1 cancer cells. Bioelectrochemistry 2018, 119, 1–9. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, B.; Li, C.; Kuang, W.; Zhang, J.; Xiong, Y.; Tan, S.; Cai, X.; Huang, L. Carboxymethyl cellulose-grafted graphene oxide for efficient antitumor drug delivery. Nanotechnol. Rev. 2018, 7, 291. [Google Scholar] [CrossRef]
- Luo, H.; Ao, H.; Li, G.; Li, W.; Xiong, G.; Zhu, Y.; Wan, Y. Bacterial cellulose/graphene oxide nanocomposite as a novel drug delivery system. Curr. Appl. Phys. 2017, 17, 249–254. [Google Scholar] [CrossRef]
- Xie, M.; Lei, H.; Zhang, Y.; Xu, Y.; Shen, S.; Ge, Y.; Li, H.; Xie, J. Non-covalent modification of graphene oxide nanocomposites with chitosan/dextran and its application in drug delivery. RSC Adv. 2016, 6, 9328–9337. [Google Scholar] [CrossRef]
- Rasoulzadehzali, M.; Namazi, H. Facile preparation of antibacterial chitosan/graphene oxide-Ag bio-nanocomposite hydrogel beads for controlled release of doxorubicin. Int. J. Biol. Macromol. 2018, 116, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Nandi, S.; Das, P.; Nandi, A.K. Fluorescent Graphene Oxide via Polymer Grafting: An Efficient Nanocarrier for Both Hydrophilic and Hydrophobic Drugs. ACS Appl. Mater. Interfaces 2015, 7, 3512–3523. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef]
- Gong, H.; Peng, R.; Liu, Z. Carbon nanotubes for biomedical imaging: The recent advances. Adv. Drug Deliv. Rev. 2013, 65, 1951–1963. [Google Scholar] [CrossRef]
- Lan, M.; Beghein, N.; Charlier, N.; Gallez, B. Carbon Blacks as EPR Sensors for Localized Measurements of Tissue Oxygenation. Magn. Reson. Med. 2004, 51, 1272–1278. [Google Scholar] [CrossRef]
- Rao, S.S.; Stesmans, A.; Keunen, K.; Kosynkin, D.V.; Higginbotham, A.; Tour, J.M. Unzipped graphene nanoribbons as sensitive O2 sensors: Electron spin resonance probing and dissociation kinetics. Appl. Phys. Lett. 2011, 98, 083116. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.-T.; Liu, Z. Graphene in Mice: Ultrahigh In Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Choi, S.Y.; Baek, S.H.; Chang, S.-J.; Song, Y.; Rafique, R.; Lee, K.T.; Park, T.J. Synthesis of upconversion nanoparticles conjugated with graphene oxide quantum dots and their use against cancer cell imaging and photodynamic therapy. Biosens. Bioelectron. 2017, 93, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wei, L.; Wang, J.; Peng, F.; Luo, D.; Cui, R.; Niu, Y.; Qin, X.; Liu, Y.; Sun, H.; et al. Cell imaging by graphene oxide based on surface enhanced Raman scattering. Nanoscale 2012, 4, 7084–7089. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Amatore, C.; Chen, Y.; Jiang, H.; Wang, X.-M. Gold Nanoclusters and Graphene Nanocomposites for Drug Delivery and Imaging of Cancer Cells. Angew. Chem. Int. Ed. 2011, 50, 11644–11648. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y.; Luo, Z.; Zhao, Y.; Ng, K.W.; Zhao, Y. Graphene oxide wrapped gold nanoparticles for intracellular Raman imaging and drug delivery. J. Mater. Chem. B 2013, 1, 6495–6500. [Google Scholar] [CrossRef]
- Wang, F.; Peng, E.; Zheng, B.; Li, S.F.Y.; Xue, J.M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T1 Relaxivity. J. Phys. Chem. C 2015, 119, 23735–23742. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, H.; Wang, M.; Huang, C.; Yang, D.; Jia, N. Functionalized graphene oxide/Fe3O4 hybrids for cellular magnetic resonance imaging and fluorescence labeling. Mater. Sci. Eng. C 2017, 78, 817–825. [Google Scholar] [CrossRef]
- Patrick, C.W.; Mikos, A.G.; McIntire, L.V. Chapter I—Prospectus of Tissue Engineering. In Frontiers in Tissue Engineering; Patrick, C.W., Mikos, A.G., McIntire, L.V., Langer, R.S., Eds.; Pergamon: Oxford, UK, 1998; pp. 3–11. ISBN 978-0-08-042689-1. [Google Scholar]
- Shin, S.R.; Li, Y.-C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S.; Cho, S.-P.; Hong, B.H.; Choung, P.-H.; Chung, T.D.; et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef]
- Badami, D.V. X-ray studies of graphite formed by decomposing silicon carbide. Carbon 1965, 3, 53–57. [Google Scholar] [CrossRef]
- Van Bommel, A.J.; Crombeen, J.E.; Van Tooren, A. LEED and Auger electron observations of the SiC (0001) surface. Surf. Sci. 1975, 48, 463–472. [Google Scholar] [CrossRef]
- Emtsev, K.V.; Bostwick, A.; Horn, K.; Jobst, J.; Kellogg, G.L.; Ley, L.; McChesney, J.L.; Ohta, T.; Reshanov, S.A.; Röhrl, J.; et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat. Mater. 2009, 8, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Tromp, R.M.; Hannon, J.B. Thermodynamics and Kinetics of Graphene Growth on SiC (0001). Phys. Rev. Lett. 2009, 102, 106104. [Google Scholar] [CrossRef] [PubMed]
- Juang, Z.-Y.; Wu, C.-Y.; Lo, C.-W.; Chen, W.-Y.; Huang, C.-F.; Hwang, J.-C.; Chen, F.-R.; Leou, K.-C.; Tsai, C.-H. Synthesis of graphene on silicon carbide substrates at low temperature. Carbon 2009, 47, 2026–2031. [Google Scholar] [CrossRef]
- Li, C.; Li, D.; Yang, J.; Zeng, X.; Yuan, W. Preparation of single- and few-layer graphene sheets using co deposition on SiC substrate. J Nanomater. 2011, 2011, 44. [Google Scholar] [CrossRef]
- Osikoya, A.O.; Parlak, O.; Murugan, N.A.; Dikio, E.D.; Moloto, H.; Uzun, L.; Turner, A.P.F.; Tiwari, A. Acetylene-sourced CVD-synthesised catalytically active graphene for electrochemical biosensing. Biosens. Bioelectron. 2017, 89, 496–504. [Google Scholar] [CrossRef]
- Cushing, G.W.; Johánek, V.; Navin, J.K.; Harrison, I. Graphene Growth on Pt (111) by Ethylene Chemical Vapor Deposition at Surface Temperatures near 1000 K. J. Phys. Chem. C 2015, 119, 4759–4768. [Google Scholar] [CrossRef]
- Gotterbarm, K.; Zhao, W.; Höfert, O.; Gleichweit, C.; Papp, C.; Steinrück, H.-P. Growth and oxidation of graphene on Rh (111). Phys. Chem. Chem. Phys. 2013, 15, 19625–19631. [Google Scholar] [CrossRef]
- Guermoune, A.; Chari, T.; Popescu, F.; Sabri, S.S.; Guillemette, J.; Skulason, H.S.; Szkopek, T.; Siaj, M. Chemical vapor deposition synthesis of graphene on copper with methanol, ethanol, and propanol precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- Zhao, P.; Kumamoto, A.; Kim, S.; Chen, X.; Hou, B.; Chiashi, S.; Einarsson, E.; Ikuhara, Y.; Maruyama, S. Self-Limiting Chemical Vapor Deposition Growth of Monolayer Graphene from Ethanol. J. Phys. Chem. C 2013, 117, 10755–10763. [Google Scholar] [CrossRef]
- Bautista, C.; Mendoza, D. Multilayer graphene synthesized by CVD using liquid hexane as the carbon precursor. arXiv 2011, arXiv:1109.1318. [Google Scholar]
- Jang, J.; Son, M.; Chung, S.; Kim, K.; Cho, C.; Lee, B.H.; Ham, M.-H. Low-temperature-grown continuous graphene films from benzene by chemical vapor deposition at ambient pressure. Sci. Rep. 2015, 5, 17955. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hao, Y.; Ren, Y.; Charlton, M.; Lee, W.H.; Wu, Q.; Li, H.; Zhu, Y.; Wu, Y.; Piner, R.; et al. Graphene Growth Using a Solid Carbon Feedstock and Hydrogen. ACS Nano 2011, 5, 7656–7661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Zhou, C. Review of Chemical Vapor Deposition of Graphene and Related Applications. Acc. Chem. Res. 2013, 46, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. Controllable Synthesis of Graphene on Rh. In Controlled Synthesis and Scanning Tunneling Microscopy Study of Graphene and Graphene-Based Heterostructures; Springer: Singapore, 2018; pp. 19–35. ISBN 978-981-10-5181-4. [Google Scholar]
- Wang, S.M.; Pei, Y.H.; Wang, X.; Wang, H.; Meng, Q.N.; Tian, H.W.; Zheng, X.L.; Zheng, W.T.; Liu, Y.C. Synthesis of graphene on a polycrystalline Co film by radio-frequency plasma-enhanced chemical vapour deposition. J. Phys. Appl. Phys. 2010, 43, 455402. [Google Scholar] [CrossRef]
- Dai, B.; Fu, L.; Zou, Z.; Wang, M.; Xu, H.; Wang, S.; Liu, Z. Rational design of a binary metal alloy for chemical vapour deposition growth of uniform single-layer graphene. Nat. Commun. 2011, 2, 522. [Google Scholar] [CrossRef]
- Choi, H.; Lim, Y.; Park, M.; Lee, S.; Kang, Y.; Kim, M.S.; Kim, J.; Jeon, M. Precise control of chemical vapor deposition graphene layer thickness using NixCu1−x alloys. J. Mater. Chem. C 2015, 3, 1463–1467. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, X.; Yuan, Q.; Xue, J.; Lu, G.; Liu, Z.; Wang, H.; Wang, H.; Ding, F.; Yu, Q.; et al. Fast growth of inch-sized single-crystalline graphene from a controlled single nucleus on Cu–Ni alloys. Nat. Mater. 2015, 15, 43. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, G.; Prakash, J.; Chen, Z.; Gauthier, M.; Sun, S. Chemical vapour deposition of graphene: Layer control, the transfer process, characterisation, and related applications. Int. Rev. Phys. Chem. 2019, 38, 149–199. [Google Scholar] [CrossRef]
- Woehrl, N.; Ochedowski, O.; Gottlieb, S.; Shibasaki, K.; Schulz, S. Plasma-enhanced chemical vapor deposition of graphene on copper substrates. AIP Adv. 2014, 4, 047128. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Knieke, C.; Berger, A.; Voigt, M.; Taylor, R.N.K.; Röhrl, J.; Peukert, W. Scalable production of graphene sheets by mechanical delamination. Carbon 2010, 48, 3196–3204. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, L.; Jiang, C.; Chen, S.; Nie, Z. Synthesis of graphene nanosheet powder with layer number control via a soluble salt-assisted route. RSC Adv. 2014, 4, 13350–13354. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Quezada-Renteria, J.A.; Ania, C.O.; Chazaro-Ruiz, L.F.; Rangel-Mendez, J.R. Influence of protons on reduction degree and defect formation in electrochemically reduced graphene oxide. Carbon 2019, 149, 722–732. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Mei, X.; Meng, X.; Wu, F. Hydrothermal method for the production of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 68, 81–86. [Google Scholar] [CrossRef]
- Dubin, S.; Gilje, S.; Wang, K.; Tung, V.C.; Cha, K.; Hall, A.S.; Farrar, J.; Varshneya, R.; Yang, Y.; Kaner, R.B. A One-Step, Solvothermal Reduction Method for Producing Reduced Graphene Oxide Dispersions in Organic Solvents. ACS Nano 2010, 4, 3845–3852. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite- and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Lee, H. Highly qualified reduced graphene oxides: The best chemical reduction. Chem. Commun. 2011, 47, 9681–9683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide vial-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Khanra, P.; Kuila, T.; Kim, N.H.; Bae, S.H.; Yu, D.; Lee, J.H. Simultaneous bio-functionalization and reduction of graphene oxide by baker’s yeast. Chem. Eng. J. 2012, 183, 526–533. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In Vivo Pharmacokinetics, Long-Term Biodistribution, and Toxicology of PEGylated Graphene in Mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Yang, K.; Gong, H.; Shi, X.; Wan, J.; Zhang, Y.; Liu, Z. In Vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration. Biomaterials 2013, 34, 2787–2795. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Na, H.-K.; Kim, S.; Jang, H.; Chang, S.-J.; Min, D.-H. One-Pot Synthesis of Multifunctional Au@Graphene Oxide Nanocolloid Core@Shell Nanoparticles for Raman Bioimaging, Photothermal, and Photodynamic Therapy. Small 2015, 11, 2527–2535. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Patila, M.; Bornscheuer, U.T.; Gournis, D.; Stamatis, H. Graphene-based nanobiocatalytic systems: Recent advances and future prospects. Trends Biotechnol. 2014, 32, 312–320. [Google Scholar] [CrossRef]
- Taha, A.A.; Mousa, A.; Al-ott, M.; Faroun, M.; Assali, M.; Gomez, P.R.; Thiab, S. Non-Covalent Functionalization of Graphene Sheets with Surfactants and their Antibacterial Activity. Palest. Med. Pharm. J. 2016, 1, 65–72. [Google Scholar]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Layek, R.K.; Uddin, M.E.; Kim, N.H.; Tak Lau, A.K.; Lee, J.H. Noncovalent functionalization of reduced graphene oxide with pluronic F127 and its nanocomposites with gum arabic. Compos. Part B Eng. 2017, 128, 155–163. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Liu, Z. Graphene based gene transfection. Nanoscale 2011, 3, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Long, J.; Zhao, W.; Wang, L.; Chen, G. pH-Responsive Chitosan-Mediated Graphene Dispersions. Langmuir 2010, 26, 16771–16774. [Google Scholar] [CrossRef] [PubMed]
- Husale, B.S.; Sahoo, S.; Radenovic, A.; Traversi, F.; Annibale, P.; Kis, A. ssDNA Binding Reveals the Atomic Structure of Graphene. Langmuir 2010, 26, 18078–18082. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, E.; Yang, Z.; Loh, K.P. Optimizing Label-Free DNA Electrical Detection on Graphene Platform. Anal. Chem. 2011, 83, 2452–2460. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Lee, D.-H.; Cho, H.-S.; Han, D.; Chand, R.; Yoon, T.-J.; Kim, Y.-S. Highly selective organic transistor biosensor with inkjet printed graphene oxide support system. J. Mater. Chem. B 2017, 5, 3580–3585. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Hage, C.-H.; Spadavecchia, J.; Serrano, A.Y.; Larroulet, I.; Pesquera, A.; Zurutuza, A.; Pisfil, M.G.; Héliot, L.; Boukaert, J.; et al. Plasmonic photothermal destruction of uropathogenic E. coli with reduced graphene oxide and core/shell nanocomposites of gold nanorods/reduced graphene oxide. J. Mater. Chem. B 2015, 3, 375–386. [Google Scholar] [CrossRef]
- Kwon, Y.; Lee, B.-S.; Park, S.; Yu, W.-R. A facile route to mechanically robust graphene oxide fibers. RSC Adv. 2019, 9, 20248–20255. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Sarkar, K.; Chatterjee, K. Engineering a multi-biofunctional composite using poly(ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale 2016, 8, 6820–6836. [Google Scholar] [CrossRef] [PubMed]
- Veca, L.M.; Lu, F.; Meziani, M.J.; Cao, L.; Zhang, P.; Qi, G.; Qu, L.; Shrestha, M.; Sun, Y.-P. Polymer functionalization and solubilization of carbon nanosheets. Chem. Commun. 2009, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Gómez, M.A.; Martínez, G. Polymeric Modification of Graphene through Esterification of Graphite Oxide and Poly (vinyl alcohol). Macromolecules 2009, 42, 6331–6334. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Martínez, G. Importance of Covalent Linkages in the Preparation of Effective Reduced Graphene Oxide−Poly (vinyl chloride) Nanocomposites. Macromolecules 2011, 44, 2685–2692. [Google Scholar] [CrossRef]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Water-Soluble Graphene Covalently Functionalized by Biocompatible Poly-l-lysine. Langmuir 2009, 25, 12030–12033. [Google Scholar] [CrossRef]
- Kasprzak, A.; Zuchowska, A.; Poplawska, M. Functionalization of graphene: Does the organic chemistry matter? Beilstein J. Org. Chem. 2018, 14, 2018–2026. [Google Scholar] [CrossRef]
- Wei, G.; Yan, M.; Dong, R.; Wang, D.; Zhou, X.; Chen, J.; Hao, J. Covalent Modification of Reduced Graphene Oxide by Means of Diazonium Chemistry and Use as a Drug-Delivery System. Chem. Eur. J. 2012, 18, 14708–14716. [Google Scholar] [CrossRef]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3-Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Boukherroub, R.; Szunerits, S. Gold-graphene nanocomposites for sensing and biomedical applications. J. Mater. Chem. B 2015, 3, 4301–4324. [Google Scholar] [CrossRef]
- Shao, W.; Liu, X.; Min, H.; Dong, G.; Feng, Q.; Zuo, S. Preparation, Characterization, and Antibacterial Activity of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposite. ACS Appl. Mater. Interfaces 2015, 7, 6966–6973. [Google Scholar] [CrossRef] [PubMed]
- Dey, R.S.; Raj, C.R. Development of an Amperometric Cholesterol Biosensor Based on Graphene-Pt Nanoparticle Hybrid Material. J. Phys. Chem. C 2010, 114, 21427–21433. [Google Scholar] [CrossRef]
- Swain, A.K.; Pradhan, L.; Bahadur, D. Polymer Stabilized Fe3O4-Graphene as an Amphiphilic Drug Carrier for Thermo-Chemotherapy of Cancer. ACS Appl. Mater. Interfaces 2015, 7, 8013–8022. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yang, D.; Mei, L.; Li, Q.; Zhu, H.; Wang, T. Targeting Chemophotothermal Therapy of Hepatoma by Gold Nanorods/Graphene Oxide Core/Shell Nanocomposites. ACS Appl. Mater. Interfaces 2013, 5, 12911–12920. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Yang, H.; Han, D.; Zhang, Q.; Ivaska, A.; Niu, L. Graphene/AuNPs/chitosan nanocomposites film for glucose biosensing. Biosens. Bioelectron. 2010, 25, 1070–1074. [Google Scholar] [CrossRef]
- Li, D.; Deng, M.; Yu, Z.; Liu, W.; Zhou, G.; Li, W.; Wang, X.; Yang, D.-P.; Zhang, W. Biocompatible and Stable GO-Coated Fe3O4 Nanocomposite: A Robust Drug Delivery Carrier for Simultaneous Tumor MR Imaging and Targeted Therapy. ACS Biomater. Sci. Eng. 2018, 4, 2143–2154. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Yang, D.; Xu, L.; He, F.; Gai, S.; Yang, P. Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 2018, 47, 3931–3939. [Google Scholar] [CrossRef]
- Yu, M.-F.; Files, B.S.; Arepalli, S.; Ruoff, R.S. Tensile Loading of Ropes of Single Wall Carbon Nanotubes and their Mechanical Properties. Phys. Rev. Lett. 2000, 84, 5552–5555. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Hiura, H.; Bennett, J.W.; Ghaemi, H.F.; Thio, T. Electrical conductivity of individual carbon nanotubes. Nature 1996, 382, 54–56. [Google Scholar] [CrossRef]
- Li, Q.W.; Li, Y.; Zhang, X.F.; Chikkannanavar, S.B.; Zhao, Y.H.; Dangelewicz, A.M.; Zheng, L.X.; Doorn, S.K.; Jia, Q.X.; Peterson, D.E.; et al. Structure-Dependent Electrical Properties of Carbon Nanotube Fibers. Adv. Mater. 2007, 19, 3358–3363. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [PubMed]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Cinke, M.; Li, J.; Chen, B.; Cassell, A.; Delzeit, L.; Han, J.; Meyyappan, M. Pore structure of raw and purified HiPco single-walled carbon nanotubes. Chem. Phys. Lett. 2002, 365, 69–74. [Google Scholar] [CrossRef]
- Priyanka, S.; Neelesh Kumar, M.; Keerti, J.; Jain, N.K. Biomedical Applications of Carbon Nanotubes: A Critical Review. Curr. Drug Deliv. 2016, 13, 796–817. [Google Scholar]
- Joselevich, E.; Lieber, C.M. Vectorial Growth of Metallic and Semiconducting Single-Wall Carbon Nanotubes. Nano Lett. 2002, 2, 1137–1141. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; de Vries, M.S.; Gorman, G.; Savoy, R.; Vazquez, J.; Beyers, R. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Cao, Y.; Cong, S.; Cao, X.; Wu, F.; Liu, Q.; Amer, M.R.; Zhou, C. Review of Electronics Based on Single-Walled Carbon Nanotubes. In Single-Walled Carbon Nanotubes: Preparation, Properties and Applications; Li, Y., Maruyama, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 189–224. ISBN 978-3-030-12700-8. [Google Scholar]
- Yan, Y.; Li, C.; Liu, C.; Mutlu, Z.; Dong, B.; Liu, J.; Ozkan, C.S.; Ozkan, M. Bundled and dispersed carbon nanotube assemblies on graphite superstructures as free-standing lithium-ion battery anodes. Carbon 2019, 142, 238–244. [Google Scholar] [CrossRef]
- Song, B.; Xu, P.; Zeng, G.; Gong, J.; Zhang, P.; Feng, H.; Liu, Y.; Ren, X. Carbon nanotube-based environmental technologies: The adopted properties, primary mechanisms, and challenges. Rev. Environ. Sci. Biotechnol. 2018, 17, 571–590. [Google Scholar] [CrossRef]
- Slattery, A.D.; Shearer, C.J.; Shapter, J.G.; Blanch, A.J.; Quinton, J.S.; Gibson, C.T. Improved Application of Carbon Nanotube Atomic Force Microscopy Probes Using PeakForce Tapping Mode. Nanomaterials 2018, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Kaushik, A.; Vashist, A.; Sagar, V.; Ghosal, A.; Gupta, Y.K.; Ahmad, S.; Nair, M. Advances in Carbon Nanotubes–Hydrogel Hybrids in Nanomedicine for Therapeutics. Adv. Healthc. Mater. 2018, 7, 1701213. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Weizmann, Y.; Willner, I. Long-Range Electrical Contacting of Redox Enzymes by SWCNT Connectors. Angew. Chem. Int. Ed. 2004, 43, 2113–2117. [Google Scholar] [CrossRef]
- Rernglit, W.; Teanphonkrang, S.; Suginta, W.; Schulte, A. Amperometric enzymatic sensing of glucose using porous carbon nanotube films soaked with glucose oxidase. Microchim. Acta 2019, 186, 616. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yoo, Y.J. Amperometric detection of dopamine based on tyrosinase–SWNTs–Ppy composite electrode. Talanta 2009, 80, 1007–1011. [Google Scholar] [CrossRef]
- Hughes, G.; Pemberton, R.M.; Fielden, P.R.; Hart, J.P. Development of a novel reagentless, screen-printed amperometric biosensor based on glutamate dehydrogenase and NAD+, integrated with multi-walled carbon nanotubes for the determination of glutamate in food and clinical applications. Sens. Actuators B Chem. 2015, 216, 614–621. [Google Scholar] [CrossRef]
- Antiochia, R.; Lavagnini, I.; Magno, F. Amperometric Mediated Carbon Nanotube Paste Biosensor for Fructose Determination. Anal. Lett. 2004, 37, 1657–1669. [Google Scholar] [CrossRef]
- Dias, A.C.M.S.; Gomes-Filho, S.L.R.; Silva, M.M.S.; Dutra, R.F. A sensor tip based on carbon nanotube-ink printed electrode for the dengue virus NS1 protein. Biosens. Bioelectron. 2013, 44, 216–221. [Google Scholar] [CrossRef]
- Li, F.; Peng, J.; Wang, J.; Tang, H.; Tan, L.; Xie, Q.; Yao, S. Carbon nanotube-based label-free electrochemical biosensor for sensitive detection of miRNA-24. Biosens. Bioelectron. 2014, 54, 158–164. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Xiao, C.; Zhou, H.; Wang, X.; He, D. Carbon nanotube template synthesis of hierarchical NiCoO2 composite for non-enzyme glucose detection. Sens. Actuators B Chem. 2016, 222, 232–239. [Google Scholar] [CrossRef]
- Deb, A.K.; Das, S.C.; Saha, A.; Wayu, M.B.; Hensley Marksberry, M.; Baltz, R.J.; Chusuei, C.C. Ascorbic acid, acetaminophen, and hydrogen peroxide detection using a dendrimer-encapsulated Pt nanoparticle carbon nanotube composite. J. Appl. Electrochem. 2016, 46, 289–298. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Hasnain, M.S.; Swain, S.; Imam, S.; Kazmi, I.; Akhter, S. Emergence in the Functionalized Carbon Nanotubes as Smart Nanocarriers for Drug Delivery Applications. In Fullerens, Graphenes and Nanotubes; Elsevier BV: Amsterdam, The Netherlands, 2018; Chapter 4; pp. 105–133. [Google Scholar]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, K.; Davis, C.; Sherlock, S.; Cao, Q.; Chen, X.; Dai, H. Drug Delivery with Carbon Nanotubes for In vivo Cancer Treatment. Cancer Res. 2008, 68, 6652–6660. [Google Scholar] [CrossRef] [PubMed]
- Karthika, V.; Kaleeswarran, P.; Gopinath, K.; Arumugam, A.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-anbr, M.N.; Benelli, G. Biocompatible properties of nano-drug carriers using TiO2-Au embedded on multiwall carbon nanotubes for targeted drug delivery. Mater. Sci. Eng. C 2018, 90, 589–601. [Google Scholar] [CrossRef]
- Singh, R.; Mehra, N.K.; Jain, V.; Jain, N.K. Gemcitabine-loaded smart carbon nanotubes for effective targeting to cancer cells. J. Drug Target. 2013, 21, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Lavaee, P.; Ramezani, M.; Abnous, K. Reversible Targeting and controlled release delivery of daunorubicin to cancer cells by aptamer-wrapped carbon nanotubes. Eur. J. Pharm. Biopharm. 2011, 77, 200–206. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Bian, X.; Zhu, Z.; Ding, D.; Li, X.; Jia, Z.; Jiang, X.; Hu, Y. Covalently Combining Carbon Nanotubes with Anticancer Agent: Preparation and Antitumor Activity. ACS Nano 2009, 3, 2740–2750. [Google Scholar] [CrossRef]
- Singh, N.; Sachdev, A.; Gopinath, P. Polysaccharide Functionalized Single Walled Carbon Nanotubes as Nanocarriers for Delivery of Curcumin in Lung Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 1534–1541. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Wang, J.; Du, J.; Gao, Y.; Zhang, L.; Chen, L.; Yang, Y.; Liu, X. A targeted drug delivery system based on carbon nanotubes loaded with lobaplatin toward liver cancer cells. J. Mater. Res. 2018, 33, 2565–2575. [Google Scholar] [CrossRef]
- Liu, X.; Xu, D.; Liao, C.; Fang, Y.; Guo, B. Development of a promising drug delivery for formononetin: Cyclodextrin-modified single-walled carbon nanotubes. J. Drug Deliv. Sci. Technol. 2018, 43, 461–468. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band Gap Fluorescence from Individual Single-Walled Carbon Nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Liu, Z.; Daranciang, D.; Dai, H. Selective Probing and Imaging of Cells with Single Walled Carbon Nanotubes as Near-Infrared Fluorescent Molecules. Nano Lett. 2008, 8, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Welsher, K.; Liu, Z.; Sherlock, S.P.; Robinson, J.T.; Chen, Z.; Daranciang, D.; Dai, H. A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nat. Nanotechnol. 2009, 4, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Yudasaka, M.; Ushijima, N.; Sakaguchi, N.; Maeda, Y.; Tanaka, T.; Kataura, H.; Yokoyama, A. Fate of Carbon Nanotubes Locally Implanted in Mice Evaluated by Near-Infrared Fluorescence Imaging: Implications for Tissue Regeneration. ACS Appl. Nano Mater. 2019, 2, 1382–1390. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, B.; Martin, R.B.; Henbest, K.B.; Harruff, B.A.; Riggs, J.E.; Guo, Z.-X.; Allard, L.F.; Sun, Y.-P. Visible Luminescence of Carbon Nanotubes and Dependence on Functionalization. J. Phys. Chem. B 2005, 109, 14779–14782. [Google Scholar] [CrossRef]
- Lin, C.-W.; Bachilo, S.M.; Zheng, Y.; Tsedev, U.; Huang, S.; Weisman, R.B.; Belcher, A.M. Creating fluorescent quantum defects in carbon nanotubes using hypochlorite and light. Nat. Commun. 2019, 10, 2874. [Google Scholar] [CrossRef]
- Heller, D.A.; Baik, S.; Eurell, T.E.; Strano, M.S. Single-Walled Carbon Nanotube Spectroscopy in Live Cells: Towards Long-Term Labels and Optical Sensors. Adv. Mater. 2005, 17, 2793–2799. [Google Scholar] [CrossRef]
- Ursu, E.-L.; Doroftei, F.; Peptanariu, D.; Pinteala, M.; Rotaru, A. DNA-assisted decoration of single-walled carbon nanotubes with gold nanoparticles for applications in surface-enhanced Raman scattering imaging of cells. J. Nanopart. Res. 2017, 19, 181. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, H. Microwave assisted fast fabrication of Fe3O4-MWCNTs nanocomposites and their application as MRI contrast agents. Mater. Lett. 2012, 67, 49–51. [Google Scholar] [CrossRef]
- Yan, C.; Chen, C.; Hou, L.; Zhang, H.; Che, Y.; Qi, Y.; Zhang, X.; Cheng, J.; Zhang, Z. Single-walled carbon nanotube-loaded doxorubicin and Gd-DTPA for targeted drug delivery and magnetic resonance imaging. J. Drug Target. 2017, 25, 163–171. [Google Scholar] [CrossRef]
- Kroustalli, A.; Zisimopoulou, A.E.; Koch, S.; Rongen, L.; Deligianni, D.; Diamantouros, S.; Athanassiou, G.; Kokozidou, M.; Mavrilas, D.; Jockenhoevel, S. Carbon nanotubes reinforced chitosan films: Mechanical properties and cell response of a novel biomaterial for cardiovascular tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 2889–2896. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Shrestha, B.K.; Kim, J.I.; Won Ko, S.; Park, C.H.; Kim, C.S. Electrodeless coating polypyrrole on chitosan grafted polyurethane with functionalized multiwall carbon nanotubes electrospun scaffold for nerve tissue engineering. Carbon 2018, 136, 430–443. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N.N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic growth of single-walled manotubes by laser vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Rahman, G.; Najaf, Z.; Mehmood, A.; Bilal, S.; Shah, A.U.H.A.; Mian, S.A.; Ali, G.; Ali, G. An Overview of the Recent Progress in the Synthesis and Applications of Carbon Nanotubes. J. Carbon Res. 2019, 5, 3. [Google Scholar] [CrossRef]
- Lim, S.H.; Luo, Z.; Shen, Z.; Lin, J. Plasma-Assisted Synthesis of Carbon Nanotubes. Nanoscale Res. Lett. 2010, 5, 1377–1386. [Google Scholar] [CrossRef]
- Mehra, N.K.; Jain, A.K.; Lodhi, N.; Raj, R.; Dubey, V.; Mishra, D.; Nahar, M.; Jain, N.K. Challenges in the Use of Carbon Nanotubes for Biomedical Applications. Crit. Rev. Ther. Drug Carr. Syst. 2008, 25, 169–206. [Google Scholar] [CrossRef]
- Lamprecht, C.; Torin Huzil, J.; V Ivanova, M.; Foldvari, M. Non-Covalent Functionalization of Carbon Nanotubes with Surfactants for Pharmaceutical Applications—A Critical Mini-Review. Drug Deliv. Lett. 2011, 1, 45–57. [Google Scholar]
- Moore, V.C.; Strano, M.S.; Haroz, E.H.; Hauge, R.H.; Smalley, R.E.; Schmidt, J.; Talmon, Y. Individually Suspended Single-Walled Carbon Nanotubes in Various Surfactants. Nano Lett. 2003, 3, 1379–1382. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Pensabene, V.; Menciassi, A.; Dario, P. Dispersion of Multi-Walled Carbon Nanotubes in Aqueous Pluronic F127 Solutions for Biological Applications. Fuller. Nanotub. Carbon Nanostruct. 2009, 17, 11–25. [Google Scholar] [CrossRef]
- Angelikopoulos, P.; Gromov, A.; Leen, A.; Nerushev, O.; Bock, H.; Campbell, E.E.B. Dispersing Individual Single-Wall Carbon Nanotubes in Aqueous Surfactant Solutions below the cmc. J. Phys. Chem. C 2010, 114, 2–9. [Google Scholar] [CrossRef]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High Weight Fraction Surfactant Solubilization of Single-Wall Carbon Nanotubes in Water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Premkumar, T.; Geckeler, K.E. Dispersion of Single-Walled Carbon Nanotubes by Using Surfactants: Are the Type and Concentration Important? Chem. Eur. J. 2008, 14, 6044–6048. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.-Y.; Chang, D.W.; Nanjundan, A.K.; Baek, J.-B. Functionalization of Carbon Nanotubes. In Functional Molecular Nanostructures; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-953-307-498-6. [Google Scholar]
- Petrov, P.; Stassin, F.; Pagnoulle, C.; Jérôme, R. Noncovalent functionalization of multi-walled carbon nanotubes by pyrene containing polymers. Chem. Commun. 2003, 23, 2904–2905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, H.X.; Cheng, H.M. Water-Soluble Multiwalled Carbon Nanotubes Functionalized with Sulfonated Polyaniline. J. Phys. Chem. B 2006, 110, 9095–9099. [Google Scholar] [CrossRef]
- Ling, X.; Wei, Y.; Zou, L.; Xu, S. Functionalization and dispersion of multiwalled carbon nanotubes modified with poly-l-lysine. Colloids Surf. Physicochem. Eng. Asp. 2014, 443, 19–26. [Google Scholar] [CrossRef]
- Cheng, F.; Adronov, A. Noncovalent Functionalization and Solubilization of Carbon Nanotubes by Using a Conjugated Zn–Porphyrin Polymer. Chem. Eur. J. 2006, 12, 5053–5059. [Google Scholar] [CrossRef]
- Lee, J.U.; Huh, J.; Kim, K.H.; Park, C.; Jo, W.H. Aqueous suspension of carbon nanotubes via non-covalent functionalization with oligothiophene-terminated poly (ethylene glycol). Carbon 2007, 45, 1051–1057. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Semke, E.D.; Diner, B.A.; McLean, R.S.; Lustig, S.R.; Richardson, R.E.; Tassi, N.G. DNA-assisted dispersion and separation of carbon nanotubes. Nat. Mater. 2003, 2, 338–342. [Google Scholar] [CrossRef]
- Zheng, M.; Jagota, A.; Strano, M.S.; Santos, A.P.; Barone, P.; Chou, S.G.; Diner, B.A.; Dresselhaus, M.S.; Mclean, R.S.; Onoa, G.B.; et al. Structure-Based Carbon Nanotube Sorting by Sequence-Dependent DNA Assembly. Science 2003, 302, 1545–1548. [Google Scholar] [CrossRef]
- Sanz, V.; Borowiak, E.; Lukanov, P.; Galibert, A.M.; Flahaut, E.; Coley, H.M.; Silva, S.R.P.; McFadden, J. Optimising DNA binding to carbon nanotubes by non-covalent methods. Carbon 2011, 49, 1775–1781. [Google Scholar] [CrossRef]
- Taeger, S.; Xuang, L.Y.; Günther, K.; Mertig, M. Noncovalent Sidewall Functionalization of Carbon Nanotubes by Biomolecules: Single-stranded DNA and Hydrophobin. AIP Conf. Proc. 2005, 786, 262–265. [Google Scholar]
- Barinov, A.; Gregoratti, L.; Dudin, P.; La Rosa, S.; Kiskinova, M. Imaging and Spectroscopy of Multiwalled Carbon Nanotubes during Oxidation: Defects and Oxygen Bonding. Adv. Mater. 2009, 21, 1916–1920. [Google Scholar] [CrossRef]
- Saleh, T.A. The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3 or a mixture of HNO3/H2SO4. Appl. Surf. Sci. 2011, 257, 7746–7751. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Lin, W.-H.; Chang, Y.-C. The influence of treatment duration on multi-walled carbon nanotubes functionalized by H2SO4/HNO3 oxidation. Appl. Surf. Sci. 2011, 257, 2401–2410. [Google Scholar] [CrossRef]
- Hiura, H.; Ebbesen, T.W.; Tanigaki, K. Opening and purification of carbon nanotubes in high yields. Adv. Mater. 1995, 7, 275–276. [Google Scholar] [CrossRef]
- Moradi, O.; Yari, M.; Zare, K.; Mirza, B.; Najafi, F. Carbon Nanotubes: A Review of Chemistry Principles and Reactions. Fuller. Nanotub. Carbon Nanostruct. 2012, 20, 138–151. [Google Scholar] [CrossRef]
- Hu, H.; Bhowmik, P.; Zhao, B.; Hamon, M.A.; Itkis, M.E.; Haddon, R.C. Determination of the acidic sites of purified single-walled carbon nanotubes by acid-base titration. Chem. Phys. Lett. 2001, 345, 25–28. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Lastella, S.; Kim, S.; Papadimitrakopoulos, F. Length Separation of Zwitterion-Functionalized Single Wall Carbon Nanotubes by GPC. J. Am. Chem. Soc. 2002, 124, 728–729. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y. Large-scale aligned carbon nanotubes films. Phys. E Low-Dimens. Syst. Nanostruct. 2006, 33, 235–239. [Google Scholar] [CrossRef]
- Dinan, N.M.; Atyabi, F.; Rouini, M.-R.; Amini, M.; Golabchifar, A.-A.; Dinarvand, R. Doxorubicin loaded folate-targeted carbon nanotubes: Preparation, cellular internalization, in vitro cytotoxicity and disposition kinetic study in the isolated perfused rat liver. Mater. Sci. Eng. C 2014, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Khandare, J.J.; Jalota-Badhwar, A.; Satavalekar, S.D.; Bhansali, S.G.; Aher, N.D.; Kharas, F.; Banerjee, S.S. PEG-conjugated highly dispersive multifunctional magnetic multi-walled carbon nanotubes for cellular imaging. Nanoscale 2012, 4, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Negra, F.D.; Meneghetti, M.; Menna, E. Microwave-Assisted Synthesis of a Soluble Single Wall Carbon Nanotube Derivative. Fuller. Nanotub. Carbon Nanostruct. 2003, 11, 25–34. [Google Scholar] [CrossRef]
- Li, X.F.; Yan, H.; Peng, S.X. Tribological Behavior of Poly (ethylene Glycol)-Carbon Nanotubes. Adv. Mater. Res. 2011, 217–218, 688–691. [Google Scholar] [CrossRef]
- D’Arlas, B.F.; Goyanes, S.; Rubiolo, G.H.; Mondragon, I.; Corcuera, M.A.; Eceiza, A. Surface Modification of Multiwalled Carbon Nanotubes via Esterification Using a Biodegradable Polyol. J. Nanosci. Nanotechnol. 2009, 9, 6064–6071. [Google Scholar] [CrossRef]
- Chen, J.; Hamon, M.A.; Hu, H.; Chen, Y.; Rao, A.M.; Eklund, P.C.; Haddon, R.C. Solution Properties of Single-Walled Carbon Nanotubes. Science 1998, 282, 95–98. [Google Scholar] [CrossRef]
- Chen, Y.; Haddon, R.C.; Fang, S.; Rao, A.M.; Eklund, P.C.; Lee, W.H.; Dickey, E.C.; Grulke, E.A.; Pendergrass, J.C.; Chavan, A.; et al. Chemical Attachment of Organic Functional Groups to Single-walled Carbon Nanotube Material. J. Mater. Res. 1998, 13, 2423–2431. [Google Scholar] [CrossRef]
- Bettinger, H.F. Experimental and Computational Investigations of the Properties of Fluorinated Single-Walled Carbon Nanotubes. ChemPhysChem 2003, 4, 1283–1289. [Google Scholar] [CrossRef]
- Lipińska, M.E.; Rebelo, S.L.H.; Pereira, M.F.R.; Gomes, J.A.N.F.; Freire, C.; Figueiredo, J.L. New insights into the functionalization of multi-walled carbon nanotubes with aniline derivatives. Carbon 2012, 50, 3280–3294. [Google Scholar] [CrossRef]
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger, M.; Hirsch, A. Organic Functionalization of Carbon Nanotubes. J. Am. Chem. Soc. 2002, 124, 760–761. [Google Scholar] [CrossRef]
- Samorì, C.; Ali-Boucetta, H.; Sainz, R.; Guo, C.; Toma, F.M.; Fabbro, C.; da Ros, T.; Prato, M.; Kostarelos, K.; Bianco, A. Enhanced anticancer activity of multi-walled carbon nanotube—Methotrexate conjugates using cleavable linkers. Chem. Commun. 2010, 46, 1494–1496. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Abraham, J.; Whelan, P.; Graupner, R.; Ley, L.; Hennrich, F.; Kappes, M.; Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes with (R-)Oxycarbonyl Nitrenes. J. Am. Chem. Soc. 2003, 125, 8566–8580. [Google Scholar] [CrossRef] [PubMed]
- Cognet, L.; Tsyboulski, D.A.; Rocha, J.-D.R.; Doyle, C.D.; Tour, J.M.; Weisman, R.B. Stepwise Quenching of Exciton Fluorescence in Carbon Nanotubes by Single-Molecule Reactions. Science 2007, 316, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Setaro, A.; Adeli, M.; Glaeske, M.; Przyrembel, D.; Bisswanger, T.; Gordeev, G.; Maschietto, F.; Faghani, A.; Paulus, B.; Weinelt, M.; et al. Preserving π-conjugation in covalently functionalized carbon nanotubes for optoelectronic applications. Nat. Commun. 2017, 8, 14281. [Google Scholar] [CrossRef]
- Hamilton, C.E.; Lomeda, J.R.; Sun, Z.; Tour, J.M.; Barron, A.R. Radical addition of perfluorinated alkyl iodides to multi-layered graphene and single-walled carbon nanotubes. Nano Res. 2010, 3, 138–145. [Google Scholar] [CrossRef]
- Nakamura, T.; Ishihara, M.; Ohana, T.; Tanaka, A.; Koga, Y. Sidewall modification of single-walled carbon nanotubes using photolysis of perfluoroazooctane. Diam. Relat. Mater. 2004, 13, 1971–1974. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Holzinger, M.; Vostrowsky, O.; Hirsch, A.; Hennrich, F.; Kappes, M.; Weiss, R.; Jellen, F. Sidewall Functionalization of Carbon Nanotubes. Angew. Chem. Int. Ed. 2001, 40, 4002–4005. [Google Scholar] [CrossRef]
- Borondics, F.; Bokor, M.; Matus, P.; Tompa, K.; Pekker, S.; Jakab, E. Reductive Functionalization of Carbon Nanotubes. Fuller. Nanotub. Carbon Nanostruct. 2005, 13, 375–382. [Google Scholar] [CrossRef]
- Viswanathan, G.; Chakrapani, N.; Yang, H.; Wei, B.; Chung, H.; Cho, K.; Ryu, C.Y.; Ajayan, P.M. Single-Step in Situ Synthesis of Polymer-Grafted Single-Wall Nanotube Composites. J. Am. Chem. Soc. 2003, 125, 9258–9259. [Google Scholar] [CrossRef]
- Shahin, D.I.; Anderson, T.J.; Feygelson, T.I.; Pate, B.B.; Wheeler, V.D.; Greenlee, J.D.; Hite, J.K.; Tadjer, M.J.; Christou, A.; Hobart, K.D. Thermal etching of nanocrystalline diamond films. Diam. Relat. Mater. 2015, 59, 116–121. [Google Scholar] [CrossRef]
- Lu, H.C.; Lin, M.Y.; Chou, S.L.; Peng, Y.C.; Lo, J.I.; Cheng, B.M. Identification of Nitrogen Defects in Diamond with Photoluminescence Excited in the 160−240 nm Region. Anal. Chem. 2012, 84, 9596–9600. [Google Scholar] [CrossRef] [PubMed]
- Walker, J. Optical absorption and luminescence in diamond. Rep. Prog. Phys. 1979, 42, 1606–1659. [Google Scholar] [CrossRef]
- Dischler, B. Volume 1: Tables and Interpretations. In Handbook of Spectral Lines in Diamond; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Liu, H.; Reilly, S.; Herrnsdorf, J.; Xie, E.; Savitski, V.G.; Kemp, A.J.; Gu, E.; Dawson, M.D. Large radius of curvature micro-lenses on single crystal diamond for application in monolithic diamond Raman lasers. Diam. Relat. Mater. 2016, 65, 37–41. [Google Scholar] [CrossRef]
- Woerner, E.; Wild, C.; Mueller-Sebert, W.; Koidl, P. CVD-diamond optical lenses. Diam. Relat. Mater. 2001, 10, 557–560. [Google Scholar] [CrossRef]
- Koizumi, S.; Umezawa, H.; Pernot, J.; Suzuki, M. Power Electronics Device Applications of Diamond Semiconductors, 1st ed.; Woodhead Publishing: Cambridge, UK, 2018; ISBN 978-0-08-102183-5. [Google Scholar]
- Umezawa, H.; Nagase, M.; Kato, Y.; Shikata, S. High temperature application of diamond power device. Diam. Relat. Mater. 2012, 24, 201–205. [Google Scholar] [CrossRef]
- Shikata, S. Single crystal diamond wafers for high power electronics. Diam. Relat. Mater. 2016, 65, 168–175. [Google Scholar] [CrossRef]
- Blank, V.; Popov, M.; Pivovarov, G.; Lvova, N.; Gogolinsky, K.; Reshetov, V. Ultrahard and superhard phases of fullerite C60: Comparison with diamond on hardness and wear. Diam. Relat. Mater. 1998, 7, 427–431. [Google Scholar] [CrossRef]
- Wei, L.; Kuo, P.K.; Thomas, L.R.; Anthony, T.R.; Banholzer, W.F. Thermal conductivity of isotopically modified single crystal diamond. Phys. Rev. Lett. 1993, 70, 3764–3767. [Google Scholar] [CrossRef]
- Inspektor, A.; Oles, E.J.; Bauer, C.E. Theory and practice in diamond coated metal-cutting tools. Int. J. Refract. Met. Hard Mater 1997, 15, 49–56. [Google Scholar] [CrossRef]
- Vajpayee, R.B.; Maharana, P.K.; Sharma, N.; Agarwal, T.; Jhanji, V. Diamond knife–assisted deep anterior lamellar keratoplasty to manage keratoconus. J. Cataract Refract. Surg. 2014, 40, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Wu, K.T.; Perevedentseva, E.; Karmenyan, A.; Lin, M.D.; Cheng, C.L. Nanodiamond for biolabelling and toxicity evaluation in the zebrafish embryo in vivo. J. Biophotonics 2016, 9, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Zhang, Y.; Yang, X.; Chen, N.; Sun, Y.; Zhao, Y.; Fan, C.; Huang, Q. The Biocompatibility of Nanodiamonds and Their Application in Drug Delivery Systems. Theranostics 2012, 2, 302–312. [Google Scholar]
- Shergold, H.L.; Hartley, C.J. The surface chemistry of diamond. Int. J. Miner. Process. 1982, 9, 219–233. [Google Scholar] [CrossRef]
- Szunerits, S.; Nebel, C.E.; Hamers, R.J. Surface functionalization and biological applications of CVD diamond. MRS Bull. 2014, 39, 517–524. [Google Scholar] [CrossRef]
- Stavis, C.; Lasseter Clare, T.; Butler, J.E.; Radadia, A.D.; Carr, R.; Zeng, H.; King, W.P.; Carlisle, J.A.; Aksimentiev, A.; Bashir, R.; et al. Surface functionalization of thin-film diamond for highly stable and selective biological interfaces. Proc. Natl. Acad. Sci. USA 2011, 18, 983–988. [Google Scholar] [CrossRef]
- Krueger, A. Nanodiamond. In Carbon Materials and Nanotechnology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 978-3-527-31803-2. [Google Scholar]
- Awschalom, D.D.; Hanson, R.; Wrachtrup, J.; Zhou, B.B. Quantum technologies with optically interfaced solid-state spins. Nat. Photonics 2018, 12, 516–527. [Google Scholar] [CrossRef]
- Degen, C.L.; Reinhard, F.; Cappellaro, P. Quantum sensing. Rev. Mod. Phys. 2017, 89, 035002. [Google Scholar] [CrossRef]
- Hui, Y.Y.; Hsiao, W.W.; Haziza, S.; Simonneau, M.; Treussart, F.; Chang, H.C. Single particle tracking of fluorescent nanodiamonds in cells and organisms. Curr. Opin. Solid State Mater. Sci. 2017, 21, 35–42. [Google Scholar] [CrossRef]
- Alkahtani, M.H.; Alghannam, F.; Jiang, L.; Almethen, A.; Rampersaud, A.A.; Brick, R.; Gomes, C.L.; Scull, M.O.; Hemmer, P.R. Fluorescent nanodiamonds: Past, present, and future. Nanophotonics 2018, 7, 1423–1453. [Google Scholar] [CrossRef]
- Prabhakar, N.; Rosenholm, J.M. Nanodiamonds for advanced optical bioimaging and beyond. Curr. Opin. Coll. Interfaces Sci. 2019, 39, 220–231. [Google Scholar] [CrossRef]
- Torelli, M.D.; Nunn, N.A.; Shenderova, O.A. A Perspective on Fluorescent Nanodiamond Bioimaging. Small 2019, 21, 1902151. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Drozd, V.; Durygin, A.; Rodriguez, J.; Barber, P.; Atluri, V.; Liu, X.; Voss, T.G.; Saxena, S.; Nair, M. Characterization of Nanodiamond-based anti-HIV drug Delivery to the Brain. Sci. Rep. 2018, 8, 1603. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, A.E.; Youan, B.-B.C. Nanodiamonds for Drug Delivery Systems. In Diamond-Based Materials for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Chen, X.; Zhang, W. Diamond nanostructures for drug delivery, bioimaging, and biosensing. Chem. Soc. Rev. 2017, 46, 734–760. [Google Scholar] [CrossRef]
- Chipaux, M.; van der Laan, K.J.; Hemelaar, S.R.; Hasani, M.; Zheng, T.; Schirhagl, R. Nanodiamonds and Their Applications in Cells. Small 2018, 14, e1704263. [Google Scholar] [CrossRef]
- Dale, M.W.; Morley, G.W. Medical applications of diamondmagnetometry: Commercial viability. arXiv 2017, arXiv:17050.1994. [Google Scholar]
- Kucsko, G.; Maurer, P.C.; Yao, N.Y.; Kubo, M.; Noh, H.J.; Lo, P.K.; Park, H.; Lukin, M.D. Nanometre-scale thermometry in a living cell. Nature 2013, 500, 54–58. [Google Scholar] [CrossRef]
- Hui, Y.Y.; Cheng, C.-L.; Chang, H.-C. Nanodiamonds for optical bioimaging. J. Phys. D 2010, 43, 374021. [Google Scholar] [CrossRef]
- Aharonovich, I.; Neu, E. Diamond Nanophotonics. Adv. Opt. Mater. 2014, 2, 911–928. [Google Scholar] [CrossRef]
- Prabhakar, N.; Peurla, M.; Koho, S.; Deguchi, T.; Näreoja, T.; Chang, H.C.; Rosenholm, J.M.; Hänninen, P.E. STED-TEM correlative microscopy leveraging nanodiamonds as intracellular dual-contrast markers. Small 2017, 14, 1701807. [Google Scholar] [CrossRef] [PubMed]
- Morales-Zavala, F.; Casanova-Morales, N.; Gonzalez, B.R.; Chandía-Cristi, A.; Estrada, L.D.; Alvizú, I.; Waselowski, V.; Guzman, F.; Guerrero, S.; Oyarzún-Olave, M.; et al. Functionalization of stable fluorescent nanodiamonds towards reliable detection of biomarkers for Alzheimer’s disease. J. Nanobiotechnol. 2018, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.I.; Perevedentseva, E.; Chung, P.H.; Liu, K.K.; Cheng, C.Y.; Chang, C.C.; Cheng, C.L. Nanometer-Sized Diamond Particle as a Probe for Biolabeling. Biophys. J. 2007, 93, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Vaijayanthimala, V.; Lee, D.K.; Kim, S.V.; Yen, A.; Tsai, N.; Ho, D.; Chang, H.C.; Shenderova, O. Nanodiamond-mediated drug delivery and imaging: Challenges and opportunities. Exp. Opin. Drug Deliv. 2015, 12, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.Y.; Chang, H.-C.; Dong, H.; Zhang, X. Carbon Nanomaterials for Bioimaging, Bioanalysis, and Therapy, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Martin, R.; Alvaro, M.; Herance, J.R.; Garcia, H. Fenton-treated functionalized diamond nanoparticles as gene delivery system. ACS Nano 2010, 4, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Edgington, R.; Spillane, K.M.; Papageorgiou, G.; Wray, W.; Ishiwata, H.; Labarca, M.; Leal-Ortiz, S.; Reid, G.; Webb, M.; Foord, J.; et al. Functionalisation of Detonation Nanodiamond for Monodispersed, Soluble DNA-Nanodiamond Conjugates Using Mixed Silane Bead-Assisted Sonication Disintegration. Sci. Rep. 2018, 8, 728. [Google Scholar] [CrossRef]
- Grall, R.; Girard, H.; Saad, L.; Petit, T.; Gesset, C.; Combis-Schlumberger, M.; Paget, V.; Delic, J.; Arnault, J.C.; Chevillard, S. Impairing the radioresistance of cancer cells by hydrogenated nanodiamonds. Biomaterials 2015, 61, 290–298. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond (Review). Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef]
- Bacakova, L.; Broz, A.; Liskova, J.; Stankova, L.; Potocky, S.; Kromka, A. The Application of Nanodiamond in Biotechnology and Tissue Engineering; IntechOpen: London, UK, 2016. [Google Scholar]
- Williams, O.A. Nanocrystalline diamond. Diam. Relat. Mater. 2011, 20, 621–640. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Detonation-synthesis nanodiamonds: Synthesis, structure, properties and applications. Russ. Chem. Rev. 2007, 76, 339–360. [Google Scholar] [CrossRef]
- Angus, J.C. Diamond synthesis by chemical vapor deposition: The early years. Diam. Relat. Mater. 2014, 49, 77–96. [Google Scholar] [CrossRef]
- Schwander, M.; Partes, K. A review of diamond synthesis by CVD processes. Diam. Relat. Mater. 2011, 20, 1287–1301. [Google Scholar] [CrossRef]
- Yang, G.W.; Wang, J.B.; Liu, Q.X. Preparation of nancrystalline diamond using pulsed laser induced reactive quencing. J. Phys. Condens. Matter 1988, 10, 7923–7927. [Google Scholar] [CrossRef]
- Amans, D.; Chenus, A.C.; Ledoux, G.; Dujardin, C.; Reynaud, C.; Sublemontier, O.; Masenelli-Varlot, K.; Guillois, O. Nanodiamond synthesis by pulsed laser ablation in liquids. Diam. Relat. Mater. 2009, 18, 177–180. [Google Scholar] [CrossRef]
- Abbaschian, R.; Zhu, H.; Clarke, C. High pressure–high temperature growth of diamond crystals using split sphere apparatus. Diam. Relat. Mater. 2005, 14, 1916–1919. [Google Scholar] [CrossRef]
- Boudou, J.P.; Curmi, P.A.; Jelezko, F.; Wrachtrup, J.; Aubert, P.; Sennour, M.; Balasubramanian, G.; Reuter, R.; Thorel, A.; Gaffet, E. High yield fabrication of fluorescent nanodiamonds. Nanotechnology 2009, 20, 235602. [Google Scholar] [CrossRef]
- Zaiser, M.; Lyutovich, Y.; Banhart, F. Irradiation-induced transformation of graphite to diamond: A quantitative study. Phys. Rev. B 2000, 62, 3058–3064. [Google Scholar] [CrossRef]
- Daulton, T.L.; Kirk, M.A.; Lewis, R.S.; Rehn, L.E. Production of nanodiamonds by high-energy ion irradiation of graphite at room temperature. Nucl. Instrum. Methods Phys. Res. B 2001, 175, 12–20. [Google Scholar] [CrossRef]
- Banhart, F.; Ajayan, P.M. Carbon onions as nanoscopic pressure cells for diamond formation. Nature 1996, 382, 433–435. [Google Scholar] [CrossRef]
- Danilenko, V.V. On the history of the discovery of nanodiamond synthesis. Phys. Solid State 2004, 46, 595–599. [Google Scholar] [CrossRef]
- Iakoubovskii, K.; Baidakova, M.V.; Wouters, B.H.; Stesmans, A.; Adriaenssens, G.J.; Vul, A.Y.; Grobet, P.J. Structure and defects of detonation synthesis nanodiamond. Diam. Relat. Mater. 2000, 9, 861–865. [Google Scholar] [CrossRef]
- Railkar, T.A.; Kang, W.P.; Windischmann, H.; Malshe, A.P.; Neseem, H.A.; Devidson, J.L.; Brown, W.D. A Critical Review of Chemical Vapor-Deposited (CVD) Diamond for Electronic Applications. Crit. Rev. Solid State Mater. Sci. 2000, 25, 163–277. [Google Scholar] [CrossRef]
- Baitinger, E.M.; Belenkov, E.A.; Brzhezinskaya, M.M.; Greshnyakov, V.A. Specific Features of the Structure of Detonation Nanodiamonds from Results of Electron Microscopy Investigations. Phys. Solid State 2012, 54, 1715–1722. [Google Scholar] [CrossRef]
- Kumar, A.; Lin, P.A.; Xue, A.; Hao, B.; Yap, Y.K.; Sankaran, M. Formation of nanodiamonds at near-ambient conditions via microplasma dissociation of ethanol vapour. Nat. Commun. 2013, 4, 2618. [Google Scholar] [CrossRef]
- Nee, C.-N.; Yap, S.-L.; Tou, T.-Y.; Chang, H.-C.; Yap, S.-S. Direct synthesis of nanodiamonds by femtosecond laser irradiation of ethanol. Sci. Rep. 2016, 6, 33966. [Google Scholar] [CrossRef]
- Pearce, S.R.J.; Henley, S.J.; Claeyssens, F.; May, P.W.; Hallam, K.R.; Smith, J.A.; Rosser, K.N. Production of nanocrystalline diamond by laser ablation at the solid-liquid interface. Diam. Relat. Mater. 2004, 13, 661–665. [Google Scholar] [CrossRef]
- Basso, L.; Gorrini, F.; Bazzanella, N.; Cazzanelli, M.; Dorigoni, C.; Bifone, A.; Miotello, A. The modeling and synthesis of nanodiamonds by laser ablation of graphite and diamond-like carbon in liquid-confined ambient. Appl. Phys. A 2018, 124, 72. [Google Scholar] [CrossRef]
- Zaiser, M. Microstructural Processes in Irradiated Materials; Zinkle, S.J., Lucas, G.E., Ewing, R.C., Williams, J.S., Eds.; Materials Research Society: Warrendale, PA, USA, 1999. [Google Scholar]
- Lyutovich, Y.; Banhart, F. Low-pressure transformation of graphite to diamond under irradiation. Appl. Phys. Lett. 1999, 74, 659–660. [Google Scholar] [CrossRef]
- Ginés, L.; Mandal, S.; Ahmed, A.I.; Cheng, C.L.; Sow, M.; Williams, A. Positive zeta potential of nanodiamonds. Nanoscale 2017, 9, 12549–12555. [Google Scholar] [CrossRef]
- Gibson, N.M.; Luo, T.-J.M.; Shenderova, O.; Koscheev, P.A.; Brenner, D.W. Electrostatically mediated adsorption by nanodiamond and nanocarbon particles. J. Nanopart. Res. 2012, 14, 700. [Google Scholar] [CrossRef]
- Petrova, N.; Zhukov, A.; Gareeva, F.; Koscheev, A.; Petrov, I.; Shenderova, O. Interpretation of electrokinetic measurements of nanodiamond particles. Diam. Relat. Mater. 2012, 30, 62–69. [Google Scholar] [CrossRef]
- Mitev, D.; Dimitrova, R.; Spassova, M.; Minchev, R.; Stavrev, S. Surface peculiarities of detonation nanodiamonds in dependence of fabrication and purification methods. Diam. Relat. Mater. 2007, 16, 776–780. [Google Scholar] [CrossRef]
- Anikeev, V.I.; Zaikovskii, V.I. Treatment of detonation carbon in supercritical water. Russ. J Appl. Chem. 2010, 83, 1202–1208. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3 carbon ratio and surface chemistry of nanodiamond powders by selective oxidation in air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef]
- Shenderova, O.; Koscheev, A.; Zaripov, N.; Petrov, I.; Skryabin, Y.; Detkov, P.; Turner, S. Surface Chemistry and Properties of Ozone-Purified Detonation Nanodiamonds. J. Phys. Chem. C 2011, 115, 9827–9837. [Google Scholar] [CrossRef]
- Martín, R.; Heydorn, P.C.; Heydorn, M.; Garcia, H. General strategy for high-density covalent functionalization of diamond nanoparticles using Fenton chemistry. Chem. Mater. 2009, 21, 4505–4514. [Google Scholar] [CrossRef]
- Krueger, A.; Stegk, J.; Liang, Y.; Lu, L.; Jarre, G. Biotinylated nanodiamond: Simple and efficient functionalization of detonation diamond. Langmuir 2008, 24, 4200–4204. [Google Scholar] [CrossRef]
- Liang, Y.; Ozawa, M.; Krueger, A. A general procedure to functionalize agglomerating nanoparticles demonstrated on nanodiamond. ACS Nano 2009, 3, 2288–2296. [Google Scholar] [CrossRef]
- Whitlow, J.; Pacelli, S.; Arghya, P. Multifunctional nanodiamonds in regenerative medicine: Recent advances and future directions. J. Control. Release 2017, 261, 62–86. [Google Scholar] [CrossRef]
- Yeap, W.S.; Chen, S.; Loh, K.P. Detonation nanodiamond: An organic platform for the Suzuki coupling of organic molecules. Langmuir 2009, 25, 185–191. [Google Scholar] [CrossRef]
- Kuo, T.-C.; McCreery, R.L.; Swain, G.M. Electrochemical Modifi cation of boron doped chemical vapor deposited surfaces with covalent bonded monolayers. Electrochem. Solid State Lett. 1999, 2, 288–290. [Google Scholar] [CrossRef]
- Uetsuka, H.; Shin, D.; Tokuda, N.; Saeki, K.; Nebel, C.E. Electrochemical Grafting of Boron-Doped Single-Crystalline Chemical Vapor Deposition Diamond with Nitrophenyl Molecules. Langmuir 2007, 23, 3466–3472. [Google Scholar] [CrossRef]
- Wang, J.; Firestone, M.A.; Auciello, O.; Carlisle, J.A. Surface Functionalization of Ultrananocrystalline Diamond Films by Electrochemical Reduction of Aryldiazonium Salts. Langmuir 2004, 20, 11450–11456. [Google Scholar] [CrossRef]
- Lud, S.Q.; Steenackers, M.; Jordan, R.; Bruno, P.; Gruen, D.M.; Feulner, P.; Garrido, J.A.; Stutzmann, M. Chemical Grafting of Biphenyl Self-Assembled Monolayers on Ultrananocrystalline Diamond. J. Am. Chem. Soc. 2006, 128, 16884–16891. [Google Scholar] [CrossRef]
- Strother, T.; Knickerbocker, T.; Russell, J.N.; Butler, J.E.; Smith, L.M.; Hamers, R.J. Photochemical Functionalisation of Diamond Films. Langmuir 2002, 18, 968–971. [Google Scholar] [CrossRef]
- Hoeb, M.; Auernhammer, M.; Schoell, S.J.; Brandt, M.S.; Garrido, J.A.; Stutzmann, M.; Sharp, I.D. Thermally Induced Alkylation of Diamond. Langmuir 2010, 26, 18862–18867. [Google Scholar] [CrossRef]
- Miller, J.B.; Brown, D.W. Photochemical Modification of Diamond Surfaces. Langmuir 1996, 12, 5809–5817. [Google Scholar] [CrossRef]
- Song, K.-S.; Zhang, G.-J.; Nakamura, Y.; Furukawa, K.; Hiraki, T.; Yang, J.-H.; Funatsu, T.; Ohdomari, I.; Kawarada, H. Label-free DNA sensors using ultrasensitive diamond field-effect transistors in solution. Phys. Rev. E 2006, 74, 041919. [Google Scholar] [CrossRef]
- Szunerits, S.; Manesse, M.; Actis, P.; Marcus, B.; Denuault, G.; Jama, C.; Boukherroub, R. Investigation of the infl uence of the surface termination of boron-doped diamond electrodes on the oxygen reduction in basic medium. Electrochem. Solid State Lett. 2007, 10, 34–36. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.M.; Peterson, A.; Palmese, G.; Gogotsi, Y. Covalent Incorporation of Aminated Nanodiamond into an Epoxy Polymer Network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef]
- Krueger, A.; Ozawa, M.; Jarre, G.; Liang, Y.; Stegk, J.; Lu, L. Deagglomeration and functionalisation of detonation diamond. Phys. State Solid A 2007, 204, 2881–2887. [Google Scholar] [CrossRef]
- Torrengo, S.; Miotello, A.; Minati, L.; Bernagozzi, I.; Ferrari, M.; Dipalo, M.; Kohn, E.; Speranza, G. The role of oxygen in the one step amination process of nanocrystalline diamond surface. Diam. Relat. Mater. 2011, 20, 990–994. [Google Scholar] [CrossRef]
- Chawla, K.K. Carbon Fibers in Composite Materials, 2nd ed.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Petersen, R. Carbon Fiber Biocompatibility for Implants. Fibers 2016, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Soutis, C. Fibre reinforced composites in aircraft construction. Prog. Aeosp. Sci. 2005, 41, 143–451. [Google Scholar] [CrossRef]
- Pooja, B.; Alka, G. Carbon Fibres: Production, Properties and Potential Use. Mater. Sci. India 2017, 14, 52–57. [Google Scholar]
- Balasubramanian, M. Composite Materials and Processing; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014. [Google Scholar]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef]
- Sugawara, K.; Yugami, A.; Kojima, A. Voltammetric detection of biological molecules using chopped carbon fiber. Anal. Sci. 2010, 26, 1059–1063. [Google Scholar] [CrossRef]
- Guitchounts, G.; Markowitz, J.E.; Liberti, W.A.; Gardner, T.J. A carbon-fiber electrode array for long-term neural recording. J. Neural Eng. 2010, 10, 046016. [Google Scholar] [CrossRef]
- Gillis, W.F.; Lissandrello, C.A.; Shen, J.; Pearre, B.W.; Mertiri, A.; Deku, F.; Cogan, S.; Holinski, B.J.; Chew, D.J.; White, A.E. Carbon fiber on polyimide ultra-microelectrodes. J. Neural Eng. 2018, 15, 016010. [Google Scholar] [CrossRef]
- Stout, D.A.; Raimondo, E.; Webster, T.J. Improved cardiomyocyte functions of carbon nanofiber cardiac patches. Mater. Res. Soc. Symp. Proc. 2012, 6, 1417. [Google Scholar] [CrossRef]
- Pei, B.; Wang, W.; Fan, Y.; Wang, X.; Watari, F.; Li, X. Fiber-reinforced scaffolds in soft tissue engineering. Regen. Biomater. 2017, 4, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.J.; Rider, A.E.; Ishaq, M.; Kumar, S.; Kondyurin, A.; Bilek, M.M.M.; Levchenko, I.; Ostrikov, K. Carbon nanostructures for hard tissue engineering. RSC Adv. 2013, 3, 11058–11072. [Google Scholar] [CrossRef]
- Huey, D.; Hu, J.; Athanasiou, K. Unlike bone, cartilage regeneration remains elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed]
- García-Ruíz, J.P.; Lantada, A.D. 3D Printed Structures Filled with Carbon Fibers and Functionalized with Mesenchymal Stem Cell Conditioned Media as In Vitro Cell Niches for Promoting Chondrogenesis. Materials 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Sahu, S.K.; Sinha, A.; Chakraborty, J.; Garai, S. Facile synthesis of carbon fiber reinforced polymer-hydroxyapatite ternary composite: A mechanically strong bioactive bone graft. Mater. Sci. Eng. C 2019, 97, 388–396. [Google Scholar] [CrossRef]
- Li, C.S.; Vannabouathong, C.; Sprague, S.; Bhandari, M. The Use of Carbon-Fiber-Reinforced (CFR) PEEK Material in Orthopedic Implants: A Systematic Review. Clin. Med. 2015, 8, 33–45. [Google Scholar] [CrossRef]
- Elias, K.L.; Price, R.L.; Webster, T.J. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials 2002, 23, 3279–3287. [Google Scholar] [CrossRef]
- Chen, M.C.; Sun, Y.C.; Chen, Y.H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. [Google Scholar] [CrossRef]
- Patel, A.; Mukundan, S.; Wang, W.; Karumuri, A.; Sant, V.; Mukhopadhyay, S.M.; Sant, S. Carbon-based hierarchical scaffolds for myoblast differentiation: Synergy between nano-functionalization and alignment. Acta Biomater. 2016, 32, 77–88. [Google Scholar] [CrossRef]
- Amezcua, R.; Shirolkar, A.; Fraze, C.; Stout, D.A. Nanomaterials for Cardiac Myocyte Tissue Engineering. Nanomaterials 2016, 6, 133. [Google Scholar] [CrossRef]
- Asiri, A.M.; Marwani, H.M.; Khan, S.B.; Webster, T.J. Understanding greater cardiomyocyte functions on aligned compared to random carbon nanofibers in PLGA. Int. J. Nanomed. 2015, 10, 89–96. [Google Scholar]
- Lin, Y.-H.; Lin, J.-H.; Wang, S.-H.; Ko, T.-H.; Tseng, G.-C. Evaluation of silver-containing activated carbon fiber for wound healing study: In vitro and in vivo. J. Biomed. Mater. Res. B 2012, 100, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science, 2nd ed.; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Mendes, D.G.; Angel, D.; Grishkan, A.; Boss, J. Histological response to carbon fibre. J. Bone Jt. Surg. 1985, 67, 645–649. [Google Scholar] [CrossRef]
- Houtz, R.C. Orlon acrylic fibre: Chemistry and properties. J. Text Res. 1950, 20, 786–801. [Google Scholar] [CrossRef]
- Shamsuddin, S.R.; Ho, K.K.C.; Lee, K.-Y.; Hodgkinson, J.M.; Bismarck, A. Carbon Fiber: Properties, Testing, and Analysis. In Wiley Encyclopedia of Composites; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Johnson, D.J. Structure-property relationships in carbon fibres. J. Phys. Appl. Phys. 1987, 20, 286–291. [Google Scholar] [CrossRef]
- Bajaj, P.; Chavan, R.B.; Manjeet, B. Thermal Studies on the Saponification Products of Acryionitrile Terpolymer. J. Macromol. Sci. Chem. A 1986, 23, 335–347. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Khan, W.S.; Asmatulu, R. Synthesis of electrospun polyacrylonitrile derived carbon fibers and comparison of properties with bulk form. PLoS ONE 2018, 13, e0201345. [Google Scholar] [CrossRef]
- Toyoda, M.; Inagaki, M. Exfoliation of carbon fibers. J. Phys. Chem. Sol. 2004, 65, 109–117. [Google Scholar] [CrossRef]
- Dai, Z.; Shi, X.; Liu, H.; Li, H.; Han, Y.; Zhou, J. High-strength lignin-based carbon fibers via a lowenergy method. RSC Adv. 2018, 8, 1218–1224. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Singer, L.S.; Lewis, I.C. ESR study of the kinetics of carbonization. Carbon 1978, 16, 417–423. [Google Scholar] [CrossRef]
- Edie, D.D.; Dunham, M.G. Melt spinning pitch-based carbon fibers. Carbon 1989, 27, 647–655. [Google Scholar] [CrossRef]
- Barr, J.B.; Chwastiak, S.; Didchenko, R.; Lewis, I.C.; Lewis, R.T.; Singer, L.S. High Modulus Carbon Fibers from Pitch Precursor. Appl. Polym. Symp. 1975, 29, 161–173. [Google Scholar]
- Park, S.J. (Ed.) Carbon Fibers, 2nd ed.; Springer: Singapore, 2018. [Google Scholar]
- Pape, D.; Adam, F.; Fritsch, E.; Mueller, K.; Kohn, D. Primary lumbosacral stability after open posterior and endoscopic anterior fusion with interbody implants: A roentgen stereophotogrammetric analysis. Spine 2000, 25, 2514–2518. [Google Scholar] [CrossRef]
- Naveen, K.; Kumar, G.A.; Sangeeta, D.K. Carbon Fibers in Biomedical Applications. In Recent Development Field Carbon Fibers; IntechOpen: London, UK, 2018; pp. 83–102. [Google Scholar]
- Stout, D.A. Recent advancements in carbon nanofiber and carbon nanotube applications in drug delivery and tissue engineering. Curr. Pharm. Des. 2015, 21, 2037–2044. [Google Scholar] [CrossRef]
- Yang, C.; Deng, G.; Chen, W.; Ye, X.; Mo, X. A novel electrospun-aligned nanoyarn-reinforced nanofibrous scaffold for tendon tissue engineering. Colloids Surf. B 2014, 122, 270–276. [Google Scholar] [CrossRef]
- Chand, S. Review Carbon Fibers for Composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Tiwari, S.; Bijwe, J. Surface Treatment of Carbon Fibers—A Review. Procedia Technol. 2014, 14, 505–512. [Google Scholar] [CrossRef]
- Zhang, J. Different Surface Treatments of Carbon Fibers and Their Influence on the Interfacial Prop-Erties of Carbon Fiber/Epoxy Composites. Ph.D. Thesis, Ecole Centrale Paris, Paris, France, 2012. [Google Scholar]
- Morgan, P. Carbon Fibers and Their Composites, 1st ed.; CRC Press: Boca Raton, NJ, USA, 2005; ISBN 978-0-429-11682-7. [Google Scholar]
- Langston, T.A. Wettability of Nitric Acid OxidizedCarbon Fibers. J. Reinf. Plast. Comp. 2010, 29, 2156–2169. [Google Scholar] [CrossRef]
- Feng, A.; Wu, G.; Pan, G.; Pan, C.; Wang, Y. The Behavior of Acid Treating Carbon Fiber and the Mechanical Properties and Thermal Conductivity of Phenolic Resin Matrix Composites. J. Nanosci. Nanotechnol. 2017, 17, 3786–3791. [Google Scholar] [CrossRef]
- Burakowski Nohara, L.; Petraconi Filho, G.; Nohara, E.L.; Urban Kleinke, M.; Cerqueira Rezende, M. Evaluation of carbon fiber surface treated by chemical and cold plasma processes. Mater. Res. 2005, 8, 281–286. [Google Scholar] [CrossRef]
- Ahmed, J.K.; Hamzah, A.F.; Hamed, A. Thermal and Chemical Etching of Carbon Fiber. Int. J. Eng. Technol. 2017, 7, 519–526. [Google Scholar]
- Park, S.-J.; Oh, J.-S.; Rhee, K.-Y. Effect of Atmospheric Plasma Treatment of Carbon Fibers on Crack Resistance of Carbon Fibers-reinforced Epoxy Composites. Carbon Sci. 2005, 6, 106–110. [Google Scholar]
- Santos Lima, A.; Botelho Cocchieri, E.; Kostov, K.G.; Nascente, P.A.P.; da Silva, L.L.G. Atmospheric Plasma Treatment of Carbon Fibers forEnhancement of Their Adhesion Properties. IEEE Trans. Plasma Sci. 2013, 41, 319–324. [Google Scholar] [CrossRef]
- Xu, Z.; Huang, Y.; Chen, G. Influence of rare earth treatment on interfacial properties of CFs/epoxy composites. Mater. Sci. Eng. A 2007, 444, 170–177. [Google Scholar] [CrossRef]
- Osbeck, S.; Bradley, R.H.; Liu, C.; Idriss, H.; Warde, S. Effect of an ultraviolet/ozone treatment on the surface texture and functional groups on polyacrylonitrile carbon fibres. Carbon 2011, 49, 4322–4330. [Google Scholar] [CrossRef]
- Osawa, E. Superaromaticity. Kagaku 1970, 25, 854–863. [Google Scholar]
- Castro, E.; Hernandez Garcia, A.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar]
- Fukuzumi, S. Nanocarbons as Electron Donors and Acceptors in Photoinduced Electron-Transfer Reactions. ECS J. Solid State Sci. Technol. 2017, 6, 3055–3061. [Google Scholar] [CrossRef]
- Mikami, K.; Matsumoto, S.; Tonoi, T.; Okubo, Y.; Suenobu, T.; Fukuzumi, S. Solid state photochemistry for fullerene functionalization: Solid state photoinduced electron transfer in the Diels-Alder reaction with anthracenes. Tetrahedron Lett. 1998, 39, 3733–3736. [Google Scholar] [CrossRef]
- Tkachenko, N.V.; Rantala, L.; Tauber, A.Y.; Helaja, J.; Hynninen, P.H.; Lemmetyinen, H. Photoinduced Electron Transfer in Phytochlorin—[60] Fullerene Dyads. J. Am. Chem. Soc. 1999, 121, 9378–9387. [Google Scholar] [CrossRef]
- Koeppe, R.; Sariciftci, N.S. Photoinduced charge and energy transfer involving fullerene derivatives. Photochem. Photobiol. Sci. 2005, 5, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Mroz, P.; Tegos, G.P.; Gali, H.; Wharton, T.; Sarna, T.; Hamblin, M.R. Photodynamic therapy with fullerenes. Photochem. Photobiol. Sci. 2007, 6, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Torres, V.M.; Srdjenovic, B. Biological Aspects of Fullerenes. In Handbook on Fullerene: Synthesis, Properties and Applications; Kadish, K.M., Ruoff, R.S., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 2000; pp. 431–436. [Google Scholar]
- Malhotra, R.; Lorents, D.C. Solubility of fullerene (C60) in a variety of solvents. J. Phys. Chem. 1993, 97, 3379–3383. [Google Scholar]
- Sivaraman, N.; Dhamodaran, R.; Kaliappan, I.; Srinivassan, T.G.; Rao, P.R.V.; Mathews, C.K. Solubility of C60 in organic solvents. J. Org. Chem. 1992, 57, 6077–6079. [Google Scholar] [CrossRef]
- Wilson, S.R. Biological Aspects of Fullerenes. In Fullerenes: Chemistry, physics and technology; John Wiley and Sons, Inc.: New York, NY, USA, 2000; pp. 431–436. [Google Scholar]
- Da Ros, T.; Prato, M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999, 8, 663–669. [Google Scholar] [CrossRef]
- Roberts, R.A.; Smith, R.A.; Safe, S.; Szabo, C.; Tjalkens, R.B.; Robertson, F.M. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology 2010, 276, 85–94. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Z. Radical scavenging activities of alpha-alanine C60 adduct. Bioorg. Med. Chem. Lett. 2006, 16, 3731–3734. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Bolskar, R.D. Fullerenes for Drug Delivery. In Encyclopedia of Nanotechnology; Chemistry and Materials Science; Springer: Dordrecht, The Netherlands, 2016; pp. 1267–1281. [Google Scholar]
- Ashcroft, J.M.; Tsyboulski, D.A.; Hartman, K.B.; Zakharian, T.Y.; Marks, J.W.; Weisman, R.B.; Rosenblum, M.G.; Wilson, L.J. Fullerene (C60) immunoconjugates: Interaction of water-soluble C60 derivatives with the murine anti-gp240 melanoma antibody. Chem. Commun. 2006, 28, 3004–3006. [Google Scholar] [CrossRef] [PubMed]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [PubMed]
- Afreen, S.; Muthoosamy, K.; Manickam, S.; Hashim, U. Functionalized fullerene (C60) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 2015, 63, 354–364. [Google Scholar] [CrossRef]
- Dietel, E.; Hirsch, A.; Pietzak, B.; Waiblinger, M.; Lips, K.; Weidinger, A.; Gruss, A.; Dinse, K.P. Atomic Nitrogen Encapsulated in Fullerenes: Effects of Cage Variations. J. Am. Chem. Soc. 1999, 121, 2432–2437. [Google Scholar] [CrossRef]
- Gonzalez, K.A.; Wilson, L.J.; Wu, W.; Nancollas, G.H. Synthesis and in vitro characterization of a tissue-selective fullerene: Vectoring C (60) (OH) (16) AMBP to mineralized bone. Bioorg. Med. Chem. 2002, 10, 1991–1996. [Google Scholar] [CrossRef]
- Mashino, T.; Shimotohno, K.; Ikegami, N.; Nishikawa, D.; Okuda, K.; Takahashi, K.; Nakamura, S.; Mochizuki, M. Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 1107–1109. [Google Scholar] [CrossRef]
- Dugan, L.L.; Lovett, E.G.; Quick, K.L.; Lotharius, J.; Lin, T.T.; O’Malley, K.L. Fullerene-based antioxidants and neurodegenerative disorders. Park. Relat. Disord. 2001, 7, 243–246. [Google Scholar] [CrossRef]
- Podolski, I.Y.; Podlubnaya, Z.A.; Godukhin, O.V. Fullerenes C60, Antiamyloid Action, the Brain, and Cognitive Processes. Biophysics 2010, 55, 71–76. [Google Scholar] [CrossRef]
- Murakami, M.; Hyodo, S.; Fujikawa, Y.; Fujimoto, T.; Maeda, K. Photoprotective effects of inclusion complexes of fullerenes with polyvinylpyrrolidone. Photodermatol. Photoimmunol. Photomed. 2013, 29, 196–203. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H. Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Perez-Cordero, E.; Echegoyen, L. Electrochemical detection of C60 6- and C70 6-: enhanced stability of fullerides in solution. J. Am. Chem. Soc. 1992, 114, 3978–3980. [Google Scholar] [CrossRef]
- Weibo, Y.; Seifermann, S.M.; Pierrat, P.; Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar]
- Diederich, F.; Gomez-Lopez, M. Supramolecular fullerene chemistry. Chem. Soc. Rev. 1999, 28, 263–277. [Google Scholar] [CrossRef]
- Montellano, A.; Da Ros, T.; Bianco, A.; Prato, M. Fullerene C60 as a multifunctional system for drug and gene delivery. Nanoscale 2011, 3, 4035–4041. [Google Scholar] [CrossRef]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Chiang, L.Y.; Swirczewski, J.W.; Hsu, C.S.; Chowdhury, S.K.; Cameron, S.; Creegan, K. Multi-hydroxy additions onto C60 fullerenemolecules. J. Chem. Soc. Chem. Commun. 1992, 1791–1793. [Google Scholar] [CrossRef]
- Li, J.; Takeuchi, A.; Ozawa, M.; Li, X.; Saigo, K.; Kitazawa, K. C60 fullerol formation catalysed by quaternary ammonium hydroxides. J. Chem. Soc. Chem. Commun. 1993, 1784–1785. [Google Scholar] [CrossRef]
- Wudl, F.; Hirsch, A.; Khemani, K.C.; Suzuki, T.; Allemand, P.M.; Koch, A.; Eckert, H.; Srdanov, G.; Webb, H.M. Survey of Chemical Reactivity of C60, Electrophile, and Dienopolarophile Par Excellence. In Synthesis, Properties, and Chemsitry of Large Carbon Clusters; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. 2017, 33, 777–794. [Google Scholar] [CrossRef]
- Milic, D.; Prato, M. Fullerene unsymmetrical bis-adducts as models for novel peptidomimetics. Eur. J. Org. Chem. 2010, 2010, 476–483. [Google Scholar] [CrossRef]
- Chhabra, N.; Aseri, M.L.; Padmanabhan, D. A review of drug isomerism and its significance. J. Appl. Basic Med. Res. 2013, 3, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Bingel, C. Cyclopropanierung von fullerenen. Chem. Ber. 1993, 126, 1957–1959. [Google Scholar] [CrossRef]
- Li, H.; Haque, S.A.; Kitaygorodskiy, A.; Meziani, M.J.; Torres-Castillo, M.; Sun, Y.P. Alternatively modified Bingel reaction for efficient syntheses of C60 hexakis-adducts. Org. Lett. 2006, 8, 5641–5643. [Google Scholar] [CrossRef] [PubMed]
- Brettreich, M.; Hirsch, A. A highly water-soluble dendro [60]-fullerene. Tetrahedron Lett. 1998, 39, 2731–2734. [Google Scholar] [CrossRef]
- Aroua, S.; Schweizer, W.B.; Yamakoshi, Y. C60 pyrrolidine biscarboxylic acid derivative as a versatile precursor for biocompatible fullerenes. Org. Lett. 2014, 16, 1688–1691. [Google Scholar] [CrossRef]
- Dallas, P.; Rasovic, I.; Rogers, G.; Porfyrakis, K. Endohedral Fullerenes: Optical Properties and Biomedical Applications. In Carbon Nanomaterials Sourcebook: Graphene, Fullerenes, Nanotubes and Nanodiamonds; Taylor & Francis: Boca Raton, FL, USA, 2016; pp. 255–270. [Google Scholar]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
- Vasella, A.; Uhlmann, P.; Waldraff, C.A.A.; Diederich, F.; Thilgen, C. Fullerene sugars: Preparation of enantiomerically pure, spiro-linked Cglycosides of C60. Angew. Chem. Int. Ed. 1992, 31, 1388–1390. [Google Scholar] [CrossRef]
- Yashiro, A.; Nishida, Y.; Ohno, M.; Eguchi, S.; Kobayashi, K. Fullerene glycoconjugates: A general synthetic approach via cycloaddition of per-Oacetyl glycosyl azides to [60] fullerene. Tetrahedron Lett. 1998, 39, 9031–9034. [Google Scholar] [CrossRef]
- Isobe, H.; Cho, K.; Solin, N.; Werz, D.B.; Seeberger, P.H.; Nakamura, E. Synthesis of fullerene glycoconjugates via a copper-catalyzed Huisgen cycloaddition reaction. Org. Lett. 2007, 9, 4611–4614. [Google Scholar] [CrossRef]
- Enes, R.F.; Tomé, A.C.; Cavaleiro, J.A.; El-Agamey, A.; McGarvey, D.J. Synthesis and solvent dependence of the photophysical properties of [60] fullerene–sugar conjugates. Tetrahedron 2005, 61, 11873–11881. [Google Scholar] [CrossRef]
- Tanimoto, S.; Sakai, S.; Matsumura, S.; Takahashi, D.; Toshima, K. Target selective photo-degradation of HIV-1 protease by a fullerene-sugar hybrid. Chem. Commun. 2008, 44, 5767–5769. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Atalick, S.; Guldi, D.M.; Lanig, H.; Brettreich, M.; Burghardt, S.; Hatzimarinaki, M.; Ravanelli, E.; Prato, M.; van Eldik, R.; et al. Electrostatic complexation and photoinduced electron transfer between Zn-cytochrome c and polyanionic fullerene dendrimers. Chem. Eur. J. 2003, 9, 3867–3875. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.W.; Shih, J.S. Preparation and application of immobilized c60-glucose oxidase enzyme in fullerene C 60-coated piezoelectric quartz crystal glucose sensor. Sens. Actuators B 2001. Sens. Actuators B 2001, 81, 1–8. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).