Electrochemical Tuning of CO2 Reactivity in Ionic Liquids Using Different Cathodes: From Oxalate to Carboxylation Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cyclic Voltammetry
2.2.2. Electrocarboxylation Processes
2.2.3. Determination of CO2 Concentration
3. Results and Discussion
3.1. Electrochemical Behaviour of Nitro-Compounds under Inert Atmposphere
3.2. Electrochemical Behaviour of Nitro-Compounds under CO2 Atmposphere
3.3. Electrocarboxylation of Cyano-Compounds 6 and 7 under CO2 Atmposphere
Electrocatalytic Behaviour of Cyano-Compounds 6 and 7 under CO2 Atmposphere
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holtsmark, B. Quantifying the global warming potential of CO2 emissions from wood fuels. GCB Bioenergy 2015, 7, 195–206. [Google Scholar] [CrossRef]
- Hu, L.; Song, Y.; Jiao, S.; Liu, Y.; Ge, J.; Jiao, H.; Zhu, J.; Wang, J.; Zhu, H.; Fray, D.J. Direct Conversion of Greenhouse Gas CO2 into Graphene via Molten Salts Electrolysis. ChemSusChem 2016, 9, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Kramm, G.; Dlugi, R. Scrutinizing the atmospheric greenhouse effect and its climatic impact. Nat. Sci. 2011, 3, 971–998. [Google Scholar] [CrossRef][Green Version]
- Mohan, S.V.; Modestra, J.A.; Amulya, K.; Butti, S.K.; Velvizhi, G. A circular bioeconomy with biobased products from co2 sequestration. Trends Biotechnol. 2016, 34, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Schleussner, C.F.; Lissner, T.K.; Fischer, E.M.; Wohland, J.; Perrette, M.; Golly, A.; Rogelj, J.; Childers, K.; Schewe, J.; Frieler, K.; et al. Differential climate impacts for policy-relevant limits to global warming: The case of 1.5 °C and 2 °C. Earth Syst. Dyn. 2016, 7, 327–351. [Google Scholar] [CrossRef]
- Szulejko, J.E.; Kumar, P.; Deep, A.; Kim, K.H. Global warming projections to 2100 using simple CO2 greenhouse gas modeling and comments on CO2 climate sensitivity factor. Atmos. Pollut. Res. 2017, 8, 136–140. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Cuce, E.; Nachan, Z.; Cuce, P.M.; Sher, F.; Neighbour, G.B. Strategies for ideal indoor environments towards low/zero carbon buildings through a biomimetic approach. Int. J. Ambient Energy 2019, 40, 86–95. [Google Scholar] [CrossRef]
- De Guido, G.; Compagnoni, M.; Pellegrini, L.A.; Rossetti, I. Mature versus emerging technologies for CO2 capture in power plants: Key open issues in post-combustion amine scrubbing and in chemical looping combustion. Front. Chem. Sci. Eng. 2018, 12, 315–325. [Google Scholar] [CrossRef]
- Borhani, T.N.; Wang, M. Role of solvents in CO2 capture processes: The review of selection and design methods. Renew. Sustain. Energy Rev. 2019, 114, 109299. [Google Scholar] [CrossRef]
- Asif, M.; Suleman, M.; Haq, I.; Jamal, S.A. Post-combustion CO2 capture with chemical absorption and hybrid system: Current status and challenges. Greenh. Gases Sci. Technol. 2018, 8, 998–1031. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Shi, Y.; Cai, N. Recent advances in elevated-temperature pressure swing adsorption for carbon capture and hydrogen production. Prog. Energy Combust. Sci. 2019, 75, 100784. [Google Scholar] [CrossRef]
- Sambo, C.; Iferobi, C.C.; Babasafari, A.A.; Rezaei, S.; Akanni, O.A. The role of 4d time lapse seismic technology as reservoir monitoring and surveillance tool: A comprehensive review. J. Nat. Gas Sci. Eng. 2020, 103312. [Google Scholar] [CrossRef]
- Thompson, J.G.; Combs, M.; Abad, K.; Bhatnagar, S.; Pelgen, J.; Beaudry, M.; Rochelle, G.; Hume, S.; Link, D.; Figueroa, J.; et al. Pilot testing of a heat integrated 0.7 MWe CO2 capture system with two-stage air-stripping: Emission. Int. J. Greenh. Gas Control 2017, 64, 267–275. [Google Scholar] [CrossRef]
- Thompson, J.G.; Bhatnagar, S.; Combs, M.; Abad, K.; Onneweer, F.; Pelgen, J.; Link, D.; Figueroa, J.; Nikolic, H.; Liu, K. Pilot testing of a heat integrated 0.7 MWe CO2 capture system with two-stage air-stripping: Amine degradation and metal accumulation. Int. J. Greenh. Gas Control 2017, 64, 23–33. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- Hassan, M.H.A.; Sher, F.; Zarren, G.; Suleiman, N.; Tahir, A.A.; Snape, C.E. Kinetic and thermodynamic evaluation of effective combined promoters for CO2 hydrate formation. J. Nat. Gas Sci. Eng. 2020, 78, 103313. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Jeon, J.-M.; Kumar, G.; Yang, Y.-H. Carbon dioxide capture and bioenergy production using biological system—A review. Renew. Sustain. Energy Rev. 2019, 110, 143–158. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, Y.; Zhang, Z.; Yang, W. Modification and improvement of microalgae strains for strengthening CO2 fixation from coal-fired flue gas in power plants. Bioresour. Technol. 2019, 291, 121850. [Google Scholar] [CrossRef]
- Cheng, F.; Porter, M.D.; Colosi, L.M. Is hydrothermal treatment coupled with carbon capture and storage an energy-producing negative emissions technology? Energy Convers. Manag. 2020, 203, 112252. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, E.E. Photoconversion of carbon dioxide into fuels using semiconductors. J. CO2 Util. 2019, 33, 72–82. [Google Scholar] [CrossRef]
- Galli, F.; Compagnoni, M.; Vitali, D.; Pirola, C.; Bianchi, C.L.; Villa, A.; Prati, L.; Rossetti, I. CO2 photoreduction at high pressure to both gas and liquid products over titanium dioxide. Appl. Catal. B Environ. 2017, 200, 386–391. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Rossetti, I. High pressure photoreduction of CO2: Effect of catalyst formulation, hole scavenger addition and operating conditions. Catalysts 2018, 8, 430. [Google Scholar] [CrossRef]
- Compagnoni, M.; Ramis, G.; Freyria, F.S.; Armandi, M.; Bonelli, B.; Rossetti, I. Innovative photoreactors for unconventional photocatalytic processes: The photoreduction of CO2 and the photo-oxidation of ammonia. Rend. Lincei 2017, 28, 151–158. [Google Scholar] [CrossRef]
- Rossetti, I.; Bahadori, E.; Tripodi, A.; Villa, A.; Prati, L.; Ramis, G. Conceptual design and feasibility assessment of photoreactors for solar energy storage. Sol. Energy 2018, 172, 225–231. [Google Scholar] [CrossRef]
- Bahadori, E.; Tripodi, A.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G.; Dimitratos, N.; Wang, D.; Rossetti, I. High pressure CO2 photoreduction using Au/TiO2: Unravelling the effect of co-catalysts and of titania polymorphs. Catal. Sci. Technol. 2019, 9, 2253–2265. [Google Scholar] [CrossRef]
- Rossetti, I.; Villa, A.; Pirola, C.; Prati, L.; Ramis, G. A novel high-pressure photoreactor for CO2 photoconversion to fuels. RSC Adv. 2014, 4, 28883–28885. [Google Scholar] [CrossRef]
- Sharma, N.; Das, T.; Kumar, S.; Bhosale, R.; Kabir, M.; Ogale, S. Photocatalytic Activation and Reduction of CO2 to CH4 over Single Phase Nano Cu3SnS4: A Combined Experimental and Theoretical Study. ACS Appl. Energy Mater. 2019, 2, 5677–5685. [Google Scholar] [CrossRef]
- Zhou, R.; Guzman, M.I. CO2 reduction under periodic illumination of ZnS. J. Phys. Chem. C 2014, 118, 11649–11656. [Google Scholar] [CrossRef]
- Aguirre, M.E.; Zhou, R.; Eugene, A.J.; Guzman, M.I.; Grela, M.A. Cu2O/TiO2 heterostructures for CO2 reduction through a direct Z-scheme: Protecting Cu2O from photocorrosion. Appl. Catal. B Environ. 2017, 217, 485–493. [Google Scholar] [CrossRef]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Policies and motivations for the CO2 valorization through the sabatier reaction using structured catalysts. A review of the most recent advances. Catalysts 2018, 8, 578. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Yaqoob, A.; Chen, G.Z. Electrochemical investigation of novel reference electrode Ni/Ni(OH)2 in comparison with silver and platinum inert quasi-reference electrodes for electrolysis in eutectic molten hydroxide. Int. J. Hydrogen Energy 2019, 44, 27224–27236. [Google Scholar] [CrossRef]
- Hudlicky, T. Benefits of Unconventional Methods in the Total Synthesis of Natural Products. ACS Omega 2018, 3, 17326–17340. [Google Scholar] [CrossRef]
- Dummel, R.J.; Mosher, H.S. Some Nitropyridine Derivatives. J. Org. Chem. 1959, 24, 1007–1009. [Google Scholar] [CrossRef]
- Gennaro, A.; Sánchez-Sánchez, C.M.; Isse, A.A.; Montiel, V. Electrocatalytic synthesis of 6-aminonicotinic acid at silver cathodes under mild conditions. Electrochem. Commun. 2004, 6, 627–631. [Google Scholar] [CrossRef]
- Ramesh Raju, R.; Krishna Mohan, S.; Jayarama Reddy, S. Electroorganic synthesis of 6-aminonicotinic acid from 2-amino-5-chloropyridine. Tetrahedron Lett. 2003, 44, 4133–4135. [Google Scholar] [CrossRef]
- Amatore, C.; Savéant, J.M. Mechanism and Kinetic Characteristics of the Electrochemical Reduction of Carbon Dioxide in Media of Low Proton Availability. J. Am. Chem. Soc. 1981, 103, 5021–5023. [Google Scholar] [CrossRef]
- Frontana-Uribe, B.A.; Little, R.D.; Ibanez, J.G.; Palma, A.; Vasquez-Medrano, R. Organic electrosynthesis: A promising green methodology in organic chemistry. Green Chem. 2010, 12, 2099. [Google Scholar] [CrossRef]
- Gennaro, A.; Isse, A.A.; Severin, M.-G.; Vianello, E.; Bhugun, I.; Savéant, J.-M. Mechanism of the electrochemical reduction of carbon dioxide at inert electrodes in media of low proton availability. J. Chem. Soc. Faraday Trans. 1996, 92, 3963–3968. [Google Scholar] [CrossRef]
- Isse, A.A.; Galia, A.; Belfiore, C.; Silvestri, G.; Gennaro, A. Electrochemical reduction and carboxylation of halobenzophenones. J. Electroanal. Chem. 2002, 526, 41–52. [Google Scholar] [CrossRef]
- Machado, A.S.R.; Nunes, A.V.M.; da Ponte, M.N. Carbon dioxide utilization-Electrochemical reduction to fuels and synthesis of polycarbonates. J. Supercrit. Fluids 2018, 134, 150–156. [Google Scholar] [CrossRef]
- Mateos, R.; Escapa, A.; Vanbroekhoven, K.; Patil, S.A.; Moran, A.; Pant, D. Microbial Electrochemical Technologies for CO2 and Its Derived Products Valorization. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 777–796. ISBN 9780444640529. [Google Scholar]
- Matthessen, R.; Fransaer, J.; Binnemans, K.; De Vos, D.E. Electrocarboxylation: Towards sustainable and efficient synthesis of valuable carboxylic acids. Beilstein J. Org. Chem. 2014, 10, 2484–2500. [Google Scholar] [CrossRef]
- Senboku, H.; Katayama, A. Electrochemical carboxylation with carbon dioxide. Curr. Opin. Green Sustain. Chem. 2017, 3, 50–54. [Google Scholar] [CrossRef]
- Tokuda, M. Efficient fixation of carbon dioxide by electrolysis—Facile synthesis of useful carboxylic acids—. J. Nat. Gas. Chem. 2006, 15, 275–281. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Windle, C.D.; Perutz, R.N. Advances in molecular photocatalytic and electrocatalytic CO2 reduction. Coord. Chem. Rev. 2012, 256, 2562–2570. [Google Scholar] [CrossRef]
- Yuan, X.; Lu, B.; Liu, J.; You, X.; Zhao, J.; Cai, Q. Electrochemical conversion of methanol and carbon dioxide to dimethyl carbonate at graphite-Pt electrode system. J. Electrochem. Soc. 2012, 159, 183–186. [Google Scholar] [CrossRef]
- Zhang, W.; Lü, X. Synthesis of carboxylic acids and derivatives using CO2 as carboxylative reagent. Chin. J. Catal. 2012, 33, 745–756. [Google Scholar] [CrossRef]
- Damodar, J.; Krishna Mohan, S.; Khaja Lateef, S.K.; Jayarama Reddy, S. Electrosynthesis of 2-arylpropionic acids from α-methylbenzyl chlorides and carbon dioxide by [Co(Salen)]. Synth. Commun. 2005, 35, 1143–1150. [Google Scholar] [CrossRef]
- Correa, A.; Martin, R. ChemInform abstract: Palladium-catalyzed direct carboxylation of aryl bromides with carbon dioxide. ChemInform 2010, 41, 15974–15975. [Google Scholar] [CrossRef]
- Damodar, J.; Krishna Mohan, S.R.; Jayarama Reddy, S.R. Synthesis of 2-arylpropionic acids by electrocarboxylation of benzylchlorides catalysed by PdCl2(PPh3)2. Electrochem. Commun. 2001, 3, 762–766. [Google Scholar] [CrossRef]
- Durante, C.; Isse, A.A.; Todesco, F.; Gennaro, A. Electrocatalytic activation of aromatic carbon-bromine bonds toward carboxylation at silver and copper cathodes. J. Electrochem. Soc. 2013, 160, G3073–G3079. [Google Scholar] [CrossRef]
- Durante, C.; Isse, A.A.; Sandonà, G.; Gennaro, A. Electrochemical hydrodehalogenation of polychloromethanes at silver and carbon electrodes. Appl. Catal. B Environ. 2009, 88, 479–489. [Google Scholar] [CrossRef]
- Isse, A.A.; De Giusti, A.; Gennaro, A.; Falciola, L.; Mussini, P.R. Electrochemical reduction of benzyl halides at a silver electrode. Electrochim. Acta 2006, 51, 4956–4964. [Google Scholar] [CrossRef]
- Isse, A.A.; Durante, C.; Gennaro, A. One-pot synthesis of benzoic acid by electrocatalytic reduction of bromobenzene in the presence of CO2. Electrochem. Commun. 2011, 13, 810–813. [Google Scholar] [CrossRef]
- Isse, A.A.; Falciola, L.; Mussini, P.R.; Gennaro, A. Relevance of electron transfer mechanism in electrocatalysis: The reduction of organic halides at silver electrodes. Chem. Commun. 2006, 1, 344–346. [Google Scholar] [CrossRef]
- Isse, A.A.; Gennaro, A. Electrochemical synthesis of cyanoacetic acid from chloroacetonitrile and carbon dioxide. J. Electrochem. Soc. 2002, 149, D113. [Google Scholar] [CrossRef]
- Isse, A.A.; Gottardello, S.; Durante, C.; Gennaro, A. Dissociative electron transfer to organic chlorides: Electrocatalysis at metal cathodes. Phys. Chem. Chem. Phys. 2008, 10, 2409–2416. [Google Scholar] [CrossRef]
- Isse, A.A.; Ferlin, M.G.; Gennaro, A. Electrocatalytic reduction of arylethyl chlorides at silver cathodes in the presence of carbon dioxide: Synthesis of 2-arylpropanoic acids. J. Electroanal. Chem. 2005, 581, 38–45. [Google Scholar] [CrossRef]
- Korsager, S.; Taaning, R.H.; Skrydstrup, T. Effective palladium-catalyzed hydroxycarbonylation of aryl halides with substoichiometric carbon monoxide. J. Am. Chem. Soc. 2013, 135, 2891–2894. [Google Scholar] [CrossRef]
- Lugaresi, O.; Minguzzi, A.; Locatelli, C.; Vertova, A.; Rondinini, S.; Amatore, C. Benzyl chloride electroreduction on Ag cathodes in CH3CN in the presence of small amounts of water: Evidences of quantitative effects on reaction rates and mechanism. Electrocatalysis 2013, 4, 353–357. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Filardo, G.; Isse, A.A.; Gennaro, A. Electrocatalytic carboxylation of chloroacetonitrile at a silver cathode for the synthesis of cyanoacetic acid. Electrochim. Acta 2008, 54, 634–642. [Google Scholar] [CrossRef]
- Yoo, W.J.; Kondo, J.; Rodríguez-Santamaría, J.A.; Nguyen, T.V.Q.; Kobayashi, S. Efficient synthesis of α-trifluoromethyl carboxylic acids and esters through fluorocarboxylation of gem-difluoroalkenes. Angew. Chem. Int. Ed. 2019, 58, 6772–6775. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Hara, S.; Senboku, H. Synthesis of 2-aryl-3,3,3-trifluoropropanoic acids using electrochemical carboxylation of (1-bromo-2,2,2-trifluoroethyl)arenes and its application to the synthesis of β,β,β-trifluorinated non-steroidal anti-inflammatory drugs. Tetrahedron 2010, 66, 473–479. [Google Scholar] [CrossRef]
- Scibioh, M.A.; Viswanathan, B. Electrochemical reduction of carbon dioxide: A status report. Proc. Indian Natl. Sci. Acad. 2004, 70, 407–462. [Google Scholar]
- Schlager, S.; Dumitru, L.M.; Haberbauer, M.; Fuchsbauer, A.; Neugebauer, H.; Hiemetsberger, D.; Wagner, A.; Portenkirchner, E.; Sariciftci, N.S. Electrochemical reduction of carbon dioxide to methanol by direct injection of electrons into immobilized enzymes on a modified electrode. ChemSusChem 2016, 9, 631–635. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Alinajafi, H.A.; Rezaei, B. Pt-modified nitrogen doped reduced graphene oxide: A powerful electrocatalyst for direct CO2 reduction to methanol. J. Electroanal. Chem. 2016, 783, 82–89. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Q.; Luo, J.-L. In-situ exsolved alloy nanoparticles on perovskite for direct CO2 reduction. ECS Trans. 2017, 75, 1–6. [Google Scholar] [CrossRef]
- Savéant, J.M. Molecular catalysis of electrochemical reactions. Mechanistic aspects. Chem. Rev. 2008, 108, 2348–2378. [Google Scholar] [CrossRef]
- Feng, D.M.; Zhu, Y.P.; Chen, P.; Ma, T.Y. Recent advances in transition-metal-mediated electrocatalytic CO2 reduction: From homogeneous to heterogeneous systems. Catalysts 2017, 7, 373. [Google Scholar] [CrossRef]
- Kang, P.; Chen, Z.; Brookhart, M.; Meyer, T.J. Electrocatalytic Reduction of Carbon Dioxide: Let the molecules do the work. Top. Catal. 2015, 58, 30–45. [Google Scholar] [CrossRef]
- Hammouche, M.; Lexa, D.; Savêant, J.M.; Momenteau, M. Chemical catalysis of electrochemical reactions. Homogeneous catalysis of the electrochemical reduction of carbon dioxide by iron (“0”) porphyrins. Role of the addition of magnesium cations. J. Am. Chem. Soc. 1991, 113, 8455–8466. [Google Scholar] [CrossRef]
- Laitar, D.S.; Müller, P.; Sadighi, J.P. Efficient homogeneous catalysis in the reduction of CO2 to CO. J. Am. Chem. Soc. 2005, 127, 17196–17197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 reduction: From homogeneous to heterogeneous electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wang, H.; Chen, L.; Bian, Z. Photocatalytic and electrocatalytic reduction of CO2 to methanol by the homogeneous pyridine-based systems. Appl. Catal. A Gen. 2016, 520, 1–6. [Google Scholar] [CrossRef]
- Theaker, N.; Strain, J.M.; Kumar, B.; Brian, J.P.; Kumari, S.; Spurgeon, J.M. Heterogeneously catalyzed two-step cascade electrochemical reduction of CO2 to ethanol. Electrochim. Acta 2018, 274, 1–8. [Google Scholar] [CrossRef]
- Geri, J.B.; Ciatti, J.L.; Szymczak, N.K. Charge effects regulate reversible CO2 reduction catalysis. Chem. Commun. 2018, 54, 7790–7793. [Google Scholar] [CrossRef]
- Benson, E.E.; Kubiak, C.P.; Sathrum, A.J.; Smieja, J.M. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels. Chem. Soc. Rev. 2009, 38, 89–99. [Google Scholar] [CrossRef]
- Grills, D.C.; Ertem, M.Z.; McKinnon, M.; Ngo, K.T.; Rochford, J. Mechanistic aspects of CO2 reduction catalysis with manganese-based molecular catalysts. Coord. Chem. Rev. 2018, 374, 173–217. [Google Scholar] [CrossRef]
- Luan, Y.X.; Ye, M. Transition metal-mediated or catalyzed hydrocarboxylation of olefins with CO2. Tetrahedron Lett. 2018, 59, 853–861. [Google Scholar] [CrossRef]
- Xie, J.N.; Yu, B.; Zhou, Z.H.; Fu, H.C.; Wang, N.; He, L.N. Copper(I)-based ionic liquid-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure. Tetrahedron Lett. 2015, 56, 7059–7062. [Google Scholar] [CrossRef]
- Mizuno, H.; Takaya, J.; Iwasawa, N. Rhodium(I)-catalyzed direct carboxylation of arenes with CO2 via chelation-assisted C-H bond activation. J. Am. Chem. Soc. 2011, 133, 1251–1253. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Catalytic CO2 conversion to organic carbonates with alcohols in combination with dehydration system. Catal. Sci. Technol. 2014, 4, 2830–2845. [Google Scholar] [CrossRef]
- Tappe, N.A.; Reich, R.M.; D’Elia, V.; Kühn, F.E. Current advances in the catalytic conversion of carbon dioxide by molecular catalysts: An update. Dalt. Trans. 2018, 47, 13281–13313. [Google Scholar] [CrossRef]
- Kleij, A.W.; North, M.; Urakawa, A. CO2 Catalysis. ChemSusChem 2017, 10, 1036–1038. [Google Scholar] [CrossRef]
- Dey, G.R.; Belapurkar, A.D.; Kishore, K. Photo-catalytic reduction of carbon dioxide to methane using TiO2 as suspension in water. J. Photochem. Photobiol. A Chem. 2004, 163, 503–508. [Google Scholar] [CrossRef]
- Veselovskaya, J.V.; Parunin, P.D.; Netskina, O.V.; Kibis, L.S.; Lysikov, A.I.; Okunev, A.G. Catalytic methanation of carbon dioxide captured from ambient air. Energy 2018, 159, 766–773. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Wang, X.; Wang, X. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Dokania, A.; Ramirez, A.; Bavykina, A.; Gascon, J. Heterogeneous Catalysis for the Valorization of CO2: Role of Bifunctional Processes in the Production of Chemicals. ACS Energy Lett. 2018, 4, 167–176. [Google Scholar] [CrossRef]
- Gennaro, A.; Isse, A.A.; Savéant, J.M.; Severin, M.G.; Vianello, E. Homogeneous electron transfer catalysis of the electrochemical reduction of carbon dioxide. Do aromatic anion radicals react in an outer-sphere manner? J. Am. Chem. Soc. 1996, 118, 7190–7196. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.-M. Multielectron, multistep molecular catalysis of electrochemical reactions: Benchmarking of homogeneous catalysts. ChemElectroChem 2014, 1, 1226–1236. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.M. Homogeneous catalysis of electrochemical reactions: The steady-state and nonsteady-state statuses of intermediates. ACS Catal. 2018, 8, 5286–5297. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.M. Homogeneous molecular catalysis of electrochemical reactions: Catalyst benchmarking and optimization strategies. J. Am. Chem. Soc. 2017, 139, 8245–8250. [Google Scholar] [CrossRef]
- Zhang, B.A.; Ozel, T.; Elias, J.S.; Costentin, C.; Nocera, D.G. Interplay of homogeneous reactions, mass transport, and kinetics in determining selectivity of the reduction of CO2 on gold electrodes. ACS Cent. Sci. 2019, 5, 1097–1105. [Google Scholar] [CrossRef]
- Nielsen, I.M.B.; Leung, K. Cobalt-porphyrin catalyzed electrochemical reduction of carbon dioxide in water. 1. A density functional study of intermediates. J. Phys. Chem. A 2010, 114, 10166–10173. [Google Scholar] [CrossRef]
- Leung, K.; Nielsen, I.M.B.; Sai, N.; Medforth, C.; Shelnutt, J.A. Cobalt-porphyrin catalyzed electrochemical reduction of carbon dioxide in water. 2. Mechanism from first principles. J. Phys. Chem. A 2010, 114, 10174–10184. [Google Scholar] [CrossRef]
- Shen, J.; Kolb, M.J.; Göttle, A.J.; Koper, M.T.M. DFT Study on the mechanism of the electrochemical reduction of CO2 catalyzed by cobalt porphyrins. J. Phys. Chem. C 2016, 120, 15714–15721. [Google Scholar] [CrossRef]
- Yao, C.L.; Li, J.C.; Gao, W.; Jiang, Q. Cobalt-porphine catalyzed CO2 electro-reduction: A novel protonation mechanism. Phys. Chem. Chem. Phys. 2017, 19, 15067–15072. [Google Scholar] [CrossRef]
- Bard, J.A.; Faulkner, L.R. Fundamentals and Applications, 2nd ed.; Harris, D., Swain, E., Robey, C., Aiello, E., Eds.; Wiley: Austin, TX, USA, 1990; ISBN 0471043729. [Google Scholar]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.C.; Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Feng, J.; Zeng, S.; Feng, J.; Dong, H.; Zhang, X. CO2 electroreduction in ionic liquids: A review. Chin. J. Chem. 2018, 36, 961–970. [Google Scholar] [CrossRef]
- Gallardo, I.; Soler, S. Electrochemically promoted arylation of iodoaromatics. J. Electroanal. Chem. 2017, 799, 9–16. [Google Scholar] [CrossRef]
- Das, R.N.; Roy, K. Development of classification and regression models for Vibrio fischeri toxicity of ionic liquids: Green solvents for the future. Toxicol. Res. 2012, 1, 186–195. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; Mac Farlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater 2009, 8, 621–629. [Google Scholar]
- Allen, G.D.; Buzzeo, M.C.; Davies, I.G.; Villagrán, C.; Hardacre, C.; Compton, R.G. A comparative study on the reactivity of electrogenerated bromine with cyclohexene in acetonitrile and the room temperature ionic liquid, 1-Butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide. J. Phys. Chem. B 2004, 108, 16322–16327. [Google Scholar] [CrossRef]
- Maca, J.; Sedlarikova, M.; Libich, J.; Kazda, T.; Vondrak, J. Ionic Liquids as Electrolytes and Aging Process. ECS Trans. 2016, 74, 179–184. [Google Scholar] [CrossRef]
- Barrosse-Antle, L.E.; Bond, A.M.; Compton, R.G.; O’Mahony, A.M.; Rogers, E.I.; Silvester, D.S. Voltammetry in room temperature ionic liquids: Comparisons and contrasts with conventional electrochemical solvents. Chem. Asian J. 2010, 5, 202–230. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Gohil, K. Ion exchange synthesis and thermal characteristics of some [N2222]+ based ionic liquids. Bull. Mater. Sci. 2013, 36, 1121–1125. [Google Scholar] [CrossRef][Green Version]
- Cruz, H.; Gallardo, I.; Guirado, G. Understanding specific effects on the standard potential shifts of electrogenerated species in 1-butyl-3-methylimidazolium ionic liquids. Electrochim. Acta 2008, 53, 5968–5976. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R.; Shara, M. Predicting physical properties of ionic liquids. Phys. Chem. Chem. Phys. 2006, 8, 642–649. [Google Scholar] [CrossRef]
- Doherty, A.P.; Brooks, C.A. Organic Electrochemistry in Ionic Liquids; Rogers, R., Seddon, K.R., Eds.; American Chemical Society: Washington, DC, USA, 2003; pp. 410–420. ISBN 978084123856. [Google Scholar]
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The distillation and volatility of ionic liquids. Nature 2006, 439, 831–834. [Google Scholar] [CrossRef]
- Gutowski, K.E. Industrial uses and applications of ionic liquids 1. Phys. Sci. Rev. 2018, 3. [Google Scholar] [CrossRef]
- Hayyan, M.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M.; Mei, T.X. Investigating the electrochemical windows of ionic liquids. J. Ind. Eng. Chem. 2013, 19, 106–112. [Google Scholar] [CrossRef]
- Jensen, M.P.; Neuefeind, J.; Beitz, J.V.; Skanthakumar, S.; Soderholm, L. Mechanisms of metal ion transfer into room-temperature ionic liquids: The role of anion exchange. J. Am. Chem. Soc. 2003, 125, 15466–15473. [Google Scholar] [CrossRef]
- Picquet, M.; Tkatchenko, I.; Tommasi, I.; Wasserscheid, P.; Zimmermann, J. Ionic Liquids, 3. Synthesis and utilisation of protic imidazolium salts in homogeneous catalysis. Adv. Synth. Catal. 2003, 345, 959–962. [Google Scholar] [CrossRef]
- Reche, I.; Gallardo, I.; Guirado, G. The role of cations in the reduction of 9-fluorenone in bis (trifluoromethylsulfonyl) imide room temperature ionic liquids. New J. Chem. 2014, 38, 5030–5036. [Google Scholar] [CrossRef]
- Seddon, K.R. Ionic Liquids for Clean Technolog. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1997, 64, 351–356. [Google Scholar]
- Wasserscheid, P.; Welton, T. Ionic Liquids Ionic Liquids. Top. Curr. Chem. 1999, 1, 223–226. [Google Scholar]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, S.; Yang, B.; Wang, P.; Alshammari, A.S.; Deng, Y. Electrochemistry communications highly selective and stable electro-catalytic system with ionic liquids for the reduction of carbon dioxide to carbon monoxide. Electrochem. Commun. 2015, 55, 43–46. [Google Scholar] [CrossRef]
- Tateno, H.; Nakabayashi, K.; Kashiwagi, T.; Senboku, H.; Atobe, M. Electrochemical fixation of CO2 to organohalides in room-temperature ionic liquids under supercritical CO2. Electrochim. Acta 2015, 161, 212–218. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Batchelor-McAuley, C.; Compton, R.G. Carbon dioxide reduction in room-temperature ionic liquids: The effect of the choice of electrode material, cation, and anion. J. Phys. Chem. C 2016, 120, 26442–26447. [Google Scholar] [CrossRef]
- Sung, S.; Kumar, D.; Gil-Sepulcre, M.; Nippe, M. Electrocatalytic CO2 reduction by imidazolium-functionalized molecular catalysts. J. Am. Chem. Soc. 2017, 139, 13993–13996. [Google Scholar] [CrossRef]
- Sun, L.; Ramesha, G.K.; Kamat, P.V.; Brennecke, J.F. Switching the reaction course of electrochemical CO2 reduction with ionic liquids. Langmuir 2014, 30, 6302–6308. [Google Scholar] [CrossRef]
- Rosen, B.A.; Salehi-Khojin, A.; Thorson, M.R.; Zhu, W.; Whipple, D.T.; Kenis, P.J.A.; Masel, R.I. Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science 2011, 334, 643–644. [Google Scholar] [CrossRef]
- Reche, I.; Gallardo, I.; Guirado, G. Cyclic voltammetry using silver as cathode material: A simple method for determining electro and chemical features and solubility values of CO2 in ionic liquids. Phys. Chem. Chem. Phys. 2015, 17, 2339–2343. [Google Scholar] [CrossRef]
- Reche, I.; Gallardo, I.; Guirado, G. Electrochemical studies of CO2 in imidazolium ionic liquids using silver as a working electrode: A suitable approach for determining diffusion coefficients, solubility values, and electrocatalytic effects. RSC Adv. 2014, 4, 65176–65183. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, J.; Zhang, K.; Xue, T.; Lu, J. Electrocatalytic carboxylation of benzyl chloride at silver cathode in ionic liquid BMIMBF4. Chin. J. Chem. 2009, 27, 1041–1044. [Google Scholar] [CrossRef]
- Mena, S.; Sanchez, J.; Guirado, G. Electrocarboxylation of 1-cholor-(4-isobutylphenyl)ethane with a silver cathode in ionic liquids: An environmentally benign and efficient way to synthesize Ibuprofen. RSC Adv. 2019, 9, 15115–15123. [Google Scholar] [CrossRef]
- Mei, K.; He, X.; Chen, K.; Zhou, X.; Li, H.; Wang, C. Highly efficient CO2 capture by imidazolium ionic liquids through a reduction in the formation of the carbene−CO2 complex. Ind. Eng. Chem. Res. 2017, 56, 8066–8072. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, P.; Zhang, S.; Yan, T.; Xin, J.; Zhang, X. Ionic liquids and supercritical carbon dioxide: Green and alternative reaction media for chemical processes. Rev. Chem. Eng. 2016, 32, 587–609. [Google Scholar] [CrossRef]
- Lim, H.K.; Kim, H. The mechanism of room-Temperature ionic-liquid-based electrochemical CO2 reduction: A review. Molecules 2017, 22, 536. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, D.; Guo, Z.; Liu, Y.; Lyu, Y.; Zhou, Y.; Wang, J. Imidazolinium based porous hypercrosslinked ionic polymers for efficient CO2 capture and fixation with epoxides. Green Chem. 2017, 19, 2675–2686. [Google Scholar] [CrossRef]
- Lau, G.P.S.; Schreier, M.; Vasilyev, D.; Scopelliti, R.; Gra, M.; Dyson, P.J. New Insights Into the Role of Imidazolium-Based Promoters for the Electroreduction of CO2 on a Silver Electrode. J. Am. Chem. Soc. 2016, 138, 7820–7823. [Google Scholar] [CrossRef]
- Faggion, D., Jr.; Gonçalves, W.D.G.; Dupont, J. CO2 Electroreduction in Ionic Liquids. Front. Chem. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Strmcnik, D. When small is big: The role of impurities in electrocatalysis. Top. Catal. 2015, 58, 1174–1180. [Google Scholar] [CrossRef]
- Taylor, R.J. Electrochemical studies on glassy carbon electrodes. III. Oxygen reduction in solutions of low pH (pH <10). J. Electroanal. Chem. 1975, 64, 85. [Google Scholar]
- Saveant, J.M.; Tessier, D. Potential dependence of the electrochemical transfer coefficient. Reduction of some nitro compounds in aprotic media. J. Phys. Chem. 1977, 81, 2192–2197. [Google Scholar] [CrossRef]
- Brooks, C.A. Electrochemistry in Ionic Liquids; Torriero, A.A.J., Ed.; Springer: London, UK, 2002; Volume 2002–19, ISBN 9783319151311. [Google Scholar]
- Mena, S.; Santiago, S.; Gallardo, I.; Guirado, G. Sustainable and efficient electrosynthesis of naproxen using carbon dioxide and ionic liquids. Chemosphere 2020, 245, 125557. [Google Scholar] [CrossRef]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and photoelectrochemical reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef]
- Al-Omari, A.A.; Yamani, Z.H.; Nguyen, H.L. Electrocatalytic CO2 reduction: From homogeneous catalysts to heterogeneous-based reticular chemistry. Molecules 2018, 23, 2835. [Google Scholar] [CrossRef]
- Yang, N.; Waldvogel, S.R.; Jiang, X. Electrochemistry of carbon dioxide on carbon electrodes. ACS Appl. Mater. Interfaces 2016, 8, 28357–28371. [Google Scholar] [CrossRef]
- Mena, S.; Gallardo, I.; Guirado, G. Electrocatalytic Processes for the Valorization of CO2: Synthesis of cyanobenzoic acid using eco-friendly strategies. Catalysts 2019, 9, 413. [Google Scholar] [CrossRef]








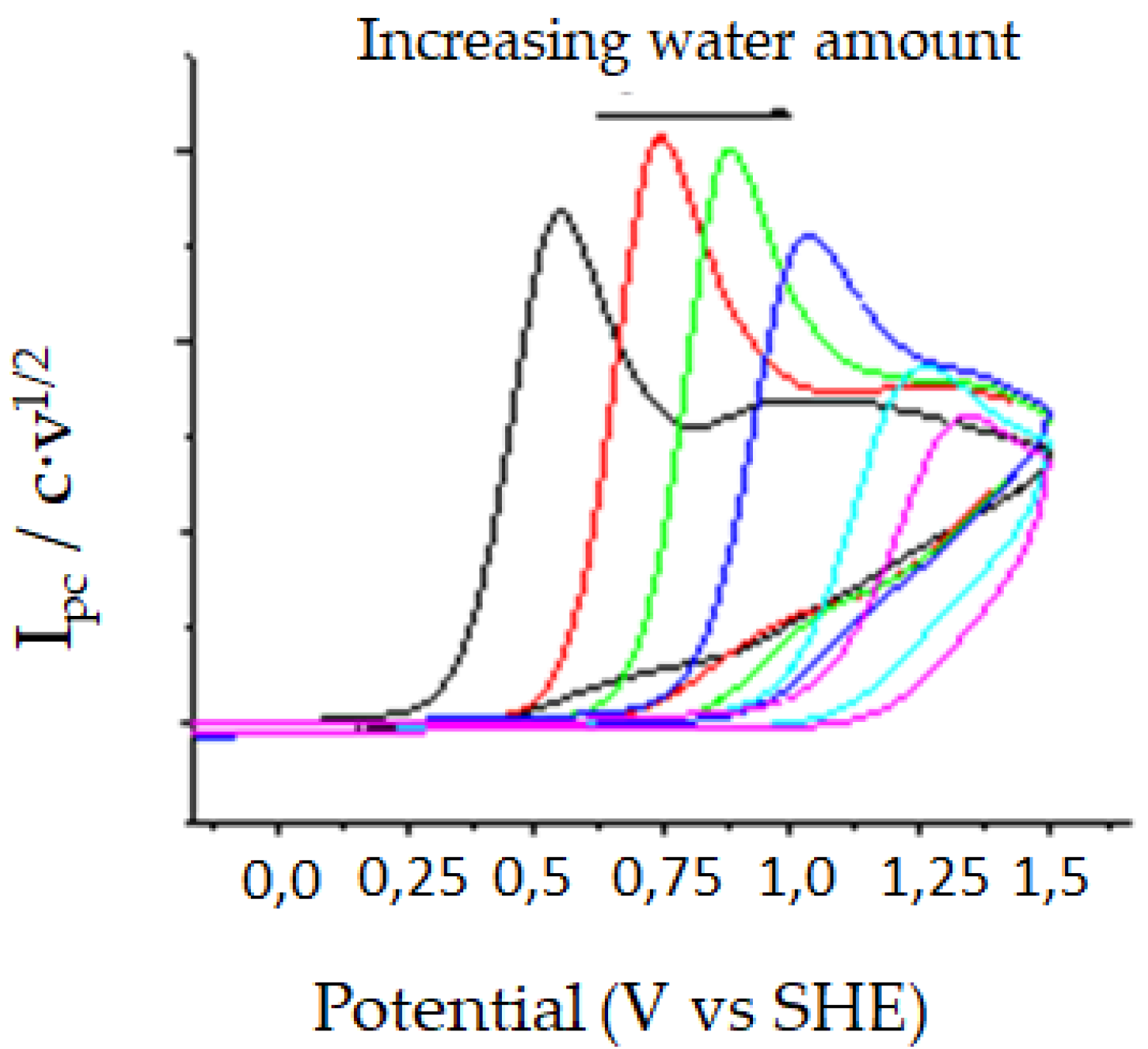




| DMF/0.1M TBA BF4 | |||||
| Entry | Nitro Derivative | WE | Epc (V vs. SHE) | E0 (V vs. SHE) | ΔEp (mV) |
| 1 | 1 | Ag | −0.969 | −0.919 | 72 |
| 2 | Cu | −0.989 | −0.889 | 70 | |
| 3 | C | −0.969 | −0.899 | 70 | |
| 4 | 2 | Ag | −0.869 | −0.839 | 60 |
| 5 | Cu | −0.869 | −0.839 | 72 | |
| 6 | C | −0.869 | −0.839 | 60 | |
| 7 | 3 | Ag | −0.779 | −0.699 | 67 |
| 8 | Cu | −0.779 | −0.689 | 68 | |
| 9 | C | −0.779 | −0.689 | 67 | |
| 10 | 4 | Ag | −0.819 | −0.719 | 83 |
| 11 | Cu | −0.869 | −0.699 | 90 | |
| 12 | C | −0.809 | −0.769 | 70 | |
| EMIM TFSI | |||||
| Entry | Nitro Derivative | WE | Epc (V vs. SHE) | E0 (V vs. SHE) | ΔEp (mV) |
| 13 | 1 | Ag | −0.949 | −0.919 | 58 |
| 14 | Cu | −0.949 | −0.919 | 58 | |
| 15 | C | −0.959 | −0.919 | 64 | |
| 16 | 2 | Ag | −0.889 | −0.859 | 59 |
| 17 | Cu | −0.899 | −0.859 | 64 | |
| 18 | C | −0.889 | −0.859 | 60 | |
| 19 | 3 | Ag | −0.859 | −0.829 | 58 |
| 20 | Cu | −0.859 | −0.819 | 64 | |
| 21 | C | −0.859 | −0.829 | 58 | |
| 22 | 4 | Ag | −0.889 | −0.829 | 60 |
| 23 | Cu | −0.919 | −0.839 | 70 | |
| 24 | C | −0.929 | −0.869 | 62 | |
| Entry | Cat. | WE | E0catalyst (V vs. SHE) | η (V) | TOF (s−1) | [C2O42-] (mM) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| DMF/0.1M TBA BF4 | EMIM TFSI | DMF/0.1M TBABF4 | EMIM TFSI | DMF/0.1M TBA BF4 | EMIM TFSI | DMF/0.1M TBA BF4 | EMIM TFSI | |||
| 1 | 1 | Ag | −0.919 | −0.919 | 0.90 | 0.92 | 7 | 6 | - | - |
| 2 | Cu | −0.889 | −0.919 | 0.88 | 0.92 | 24 | 11 | - | - | |
| 3 | C | −0.899 | −0.919 | 0.90 | 0.91 | 9 | 6 | 18.2 | 3.41 | |
| 4 | 2 | Ag | −0.839 | −0.859 | 1.00 | 0.98 | 10 | 7 | - | - |
| 5 | Cu | −0.80 | −0.859 | 1.00 | 0.97 | 8 | 13 | - | - | |
| 6 | C | −0.839 | −0.859 | 1.00 | 0.98 | 10 | 5 | 33.4 | 6.57 | |
| 7 | 3 | Ag | −0.699 | −0.829 | 1.09 | 1.01 | 29 | 7 | - | - |
| 8 | Cu | −0.689 | −0.819 | 1.09 | 1.01 | 22 | 5 | - | - | |
| 9 | C | −0.689 | −0.829 | 1.09 | 1.01 | 17 | 6 | 38.1 | 9.37 | |
| 10 | 4 | Ag | −0.719 | −0.829 | 1.05 | 0.98 | 8 | 7 | - | - |
| 11 | Cu | −0.699 | −0.839 | 1.00 | 0.95 | 14 | 6 | - | - | |
| 12 | C | −0.769 | −0.869 | 1.06 | 0.94 | 17 | 5 | 3.56 | 1.12 | |
| Entry | Solvent | Viscosity (mPa) a | Coefficient Diffusion (m2/s) b |
|---|---|---|---|
| 1 | DMF | 0.92 | 3.6 · 10−9 |
| 2 | EMIM TFSI | 37.3 | 9.7 · 10−11 |
| DMF/0.1M TBA BF4 | ||||
|---|---|---|---|---|
| Entries | Cyano Derivative | WE | Epc (V vs. SHE) | ΔEp (mV) |
| 1 | 6 | Ag | −1.73 | 85 |
| 2 | Cu | −1.80 | 78 | |
| 3 | C | −1.81 | 96 | |
| 4 | 7 | Ag | −1.47 | 201 |
| 5 | Cu | −1.87 | 140 | |
| 6 | C | −1.73 | 99 | |
| EMIM TFSI | ||||
| 7 | 6 | Ag | −1.80 | 52 |
| 8 | Cu | −1.80 | 65 | |
| 9 | C | −1.78 | 68 | |
| 10 | 7 | Ag | −1.61 | 70 |
| 11 | Cu | −1.85 | 80 | |
| 12 | C | −1.71 | 72 | |
| Entries | Reagent | Cathode | Solvent | Electrochemical Conditions | % Yield (Conversion Rate) | |||
|---|---|---|---|---|---|---|---|---|
| Carboxylated Products | Ar-H | Reagent | ||||||
| Eap (V vs. SHE) | 9 | 10 | 8 | |||||
| 1 | 6 | C | DMF | −1.86 | 20 (32%) | 10 (32%) | 32 | 38 |
| 2 | EMIM TFSI | −1.86 | 15 (26%) | 7 (26%) | 35 | 43 | ||
| 3 | 7 | C | DMF | −1.81 | 18 (28%) | 12 (28%) | 34 | 36 |
| 4 | EMIM TFSI | −1.81 | 16 (28%) | 10 (28%) | 31 | 43 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mena, S.; Guirado, G. Electrochemical Tuning of CO2 Reactivity in Ionic Liquids Using Different Cathodes: From Oxalate to Carboxylation Products. C 2020, 6, 34. https://doi.org/10.3390/c6020034
Mena S, Guirado G. Electrochemical Tuning of CO2 Reactivity in Ionic Liquids Using Different Cathodes: From Oxalate to Carboxylation Products. C. 2020; 6(2):34. https://doi.org/10.3390/c6020034
Chicago/Turabian StyleMena, Silvia, and Gonzalo Guirado. 2020. "Electrochemical Tuning of CO2 Reactivity in Ionic Liquids Using Different Cathodes: From Oxalate to Carboxylation Products" C 6, no. 2: 34. https://doi.org/10.3390/c6020034
APA StyleMena, S., & Guirado, G. (2020). Electrochemical Tuning of CO2 Reactivity in Ionic Liquids Using Different Cathodes: From Oxalate to Carboxylation Products. C, 6(2), 34. https://doi.org/10.3390/c6020034







