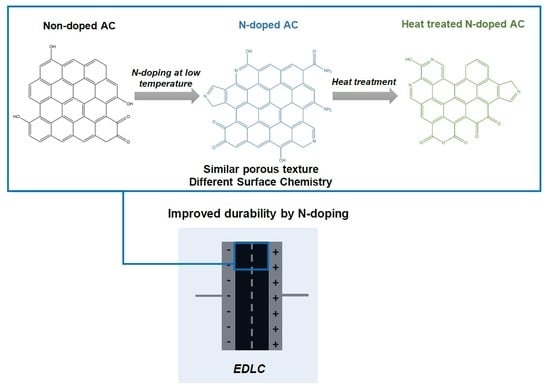
Nitrogen Doped Superactivated Carbons Prepared at Mild Conditions as Electrodes for Supercapacitors in Organic Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Carbon Materials
2.1.1. Pristine Carbon Material
2.1.2. N-Functionalization of Carbon Materials at Mild Conditions
- (i)
- Oxidation treatment. 1 g of activated carbon (KUA) was mixed with 40 mL of HNO3 65 wt % under stirring during 3 h at room temperature. After that, the activated carbon was filtrated and washed with Milli-Q water until the pH of elution was neutral. Finally, the sample (named KUA-COOH) was dried at 100 °C.
- (ii)
- Treatment with SOCl2. 1 g of KUA-COOH was introduced in a round bottom flask with 50 mL of toluene and 5 mL of SOCl2 (CAS number: 7719–09–7, 97%, Sigma-Aldrich, Saint Louis, MO, USA) was added to the flask. The mixture was refluxed at 120 °C for 5 h and finally washed with toluene and dried for 14 h.
- (iii)
- The activated carbon obtained in step (iii) was added into a 2M NH4NO3 diluted in N,N-dymethilformamide (CAS number: 68–12–2, Sigma-Aldrich, EEUU) in a round bottom flask, using an activated carbon to solution ratio of 1 g/150 mL. Then, 150 mL of pyridine (CAS number: 110–86–1, 99%, Acros Organics, Fisher Scientific, Waltham, MA, USA) were added slowly to the round bottom flask under continuous stirring at room temperature. The mixture was stirred at 70 °C for 65 h. The obtained amidated sample (KUA-CONH2) was washed with abundant water and ethanol, filtered, and dried at 100 °C overnight.
2.1.3. Heat Treatments
2.2. Physicochemical Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Physicochemical Characterization of Carbon Materials
3.1.1. Porous Texture
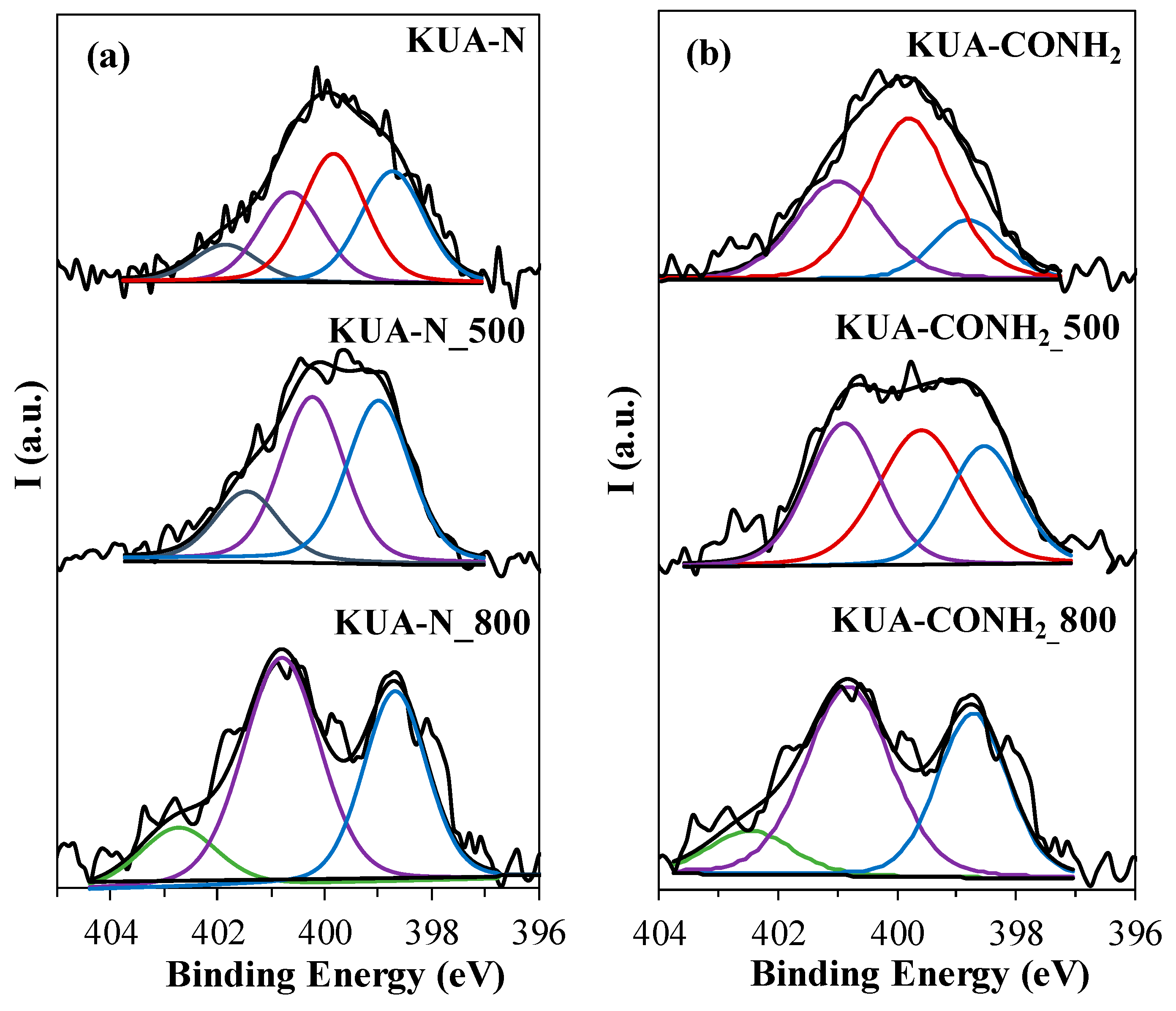
3.1.2. Surface Chemistry Characterization
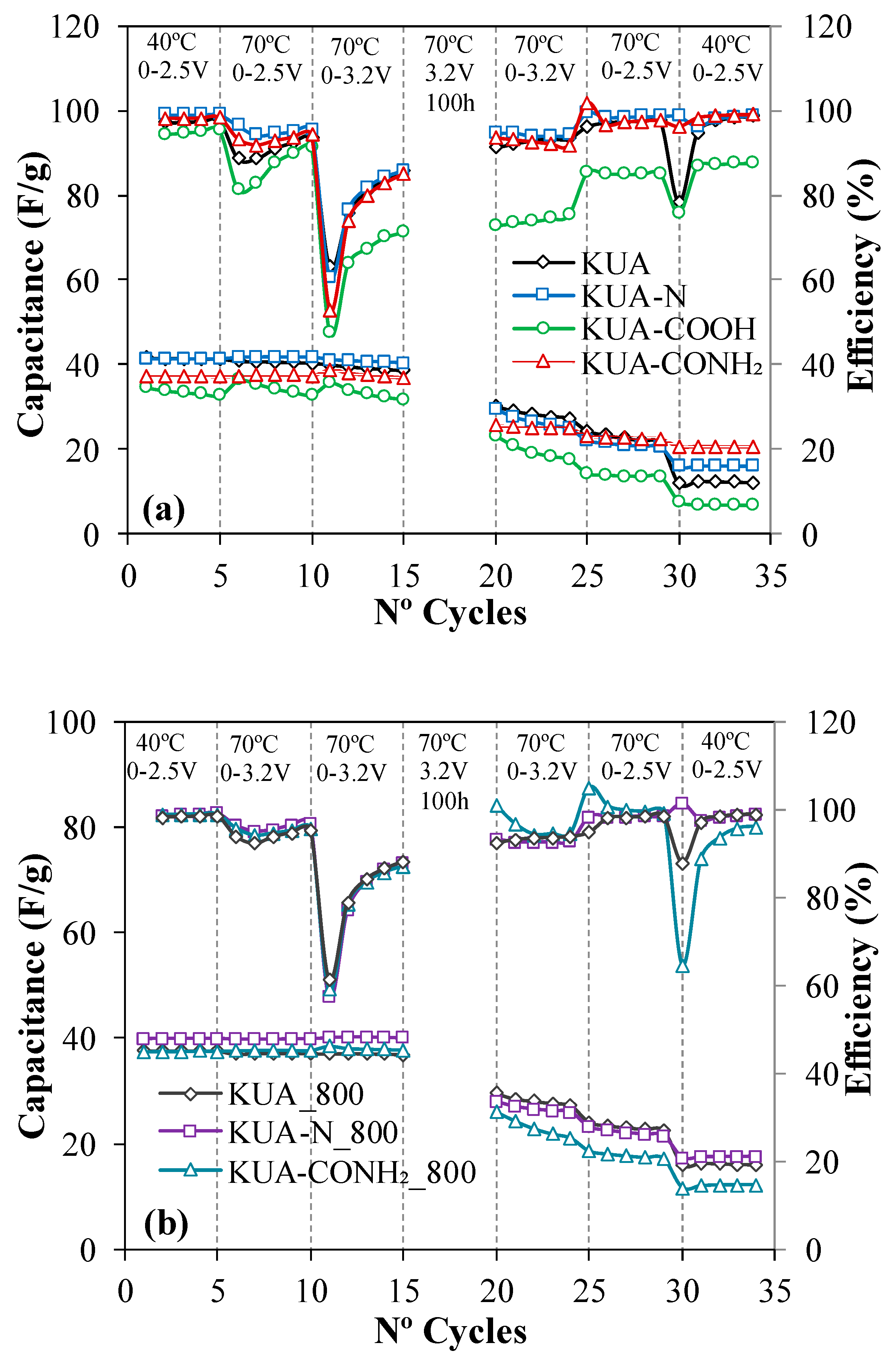
3.2. Electrochemical Characterization
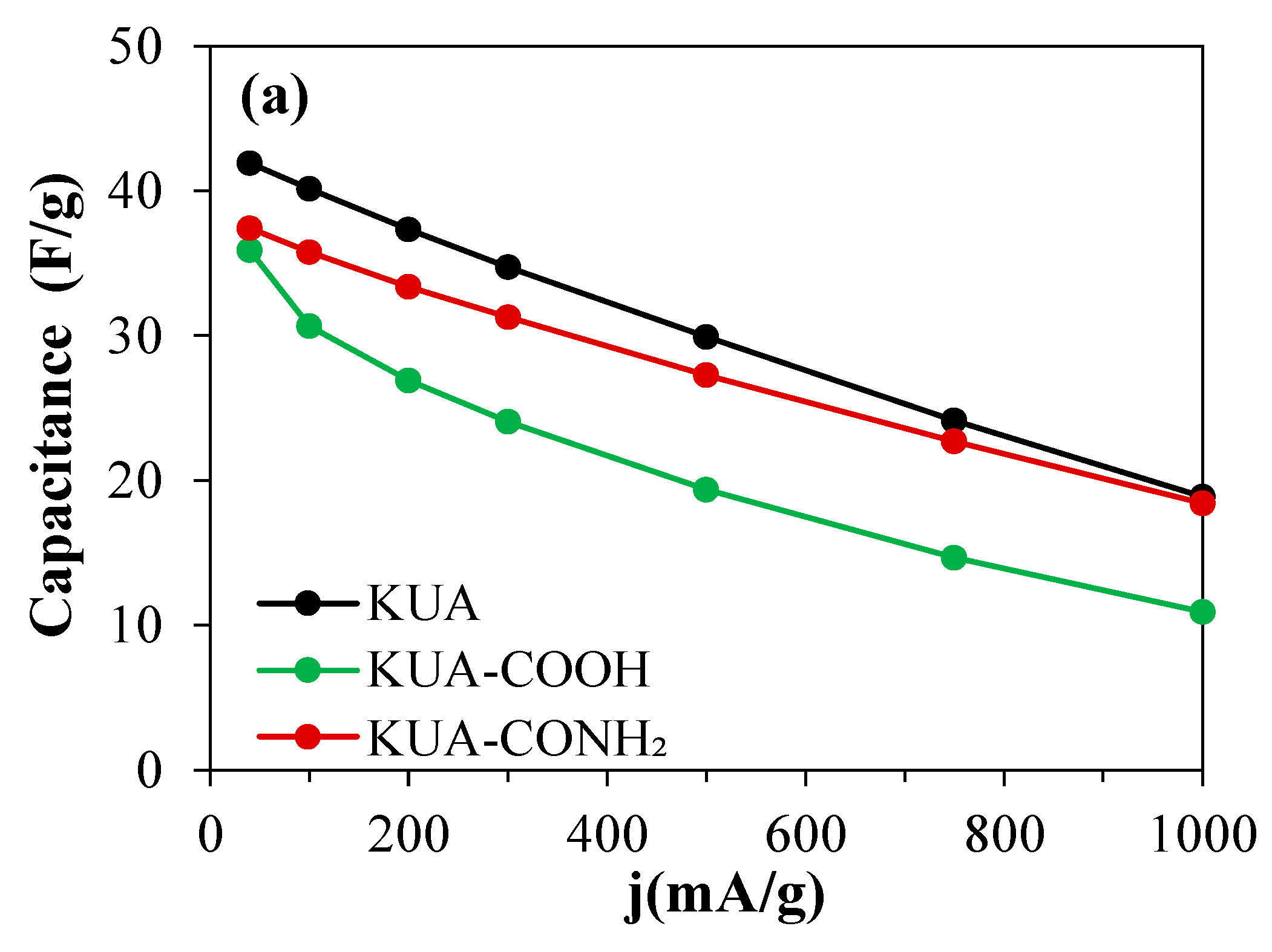
3.2.1. Effect of Surface Chemistry Modification at Mild Conditions
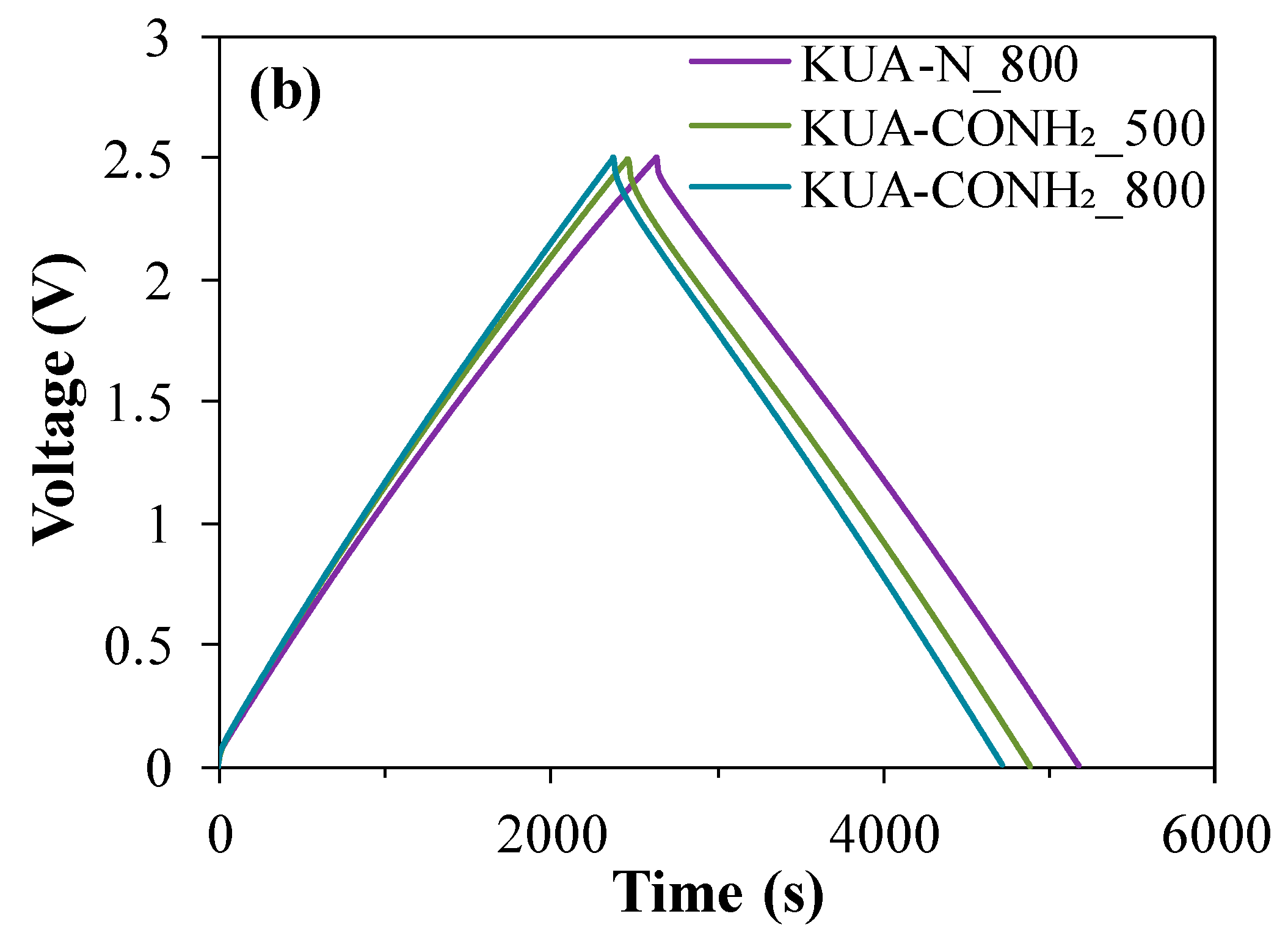
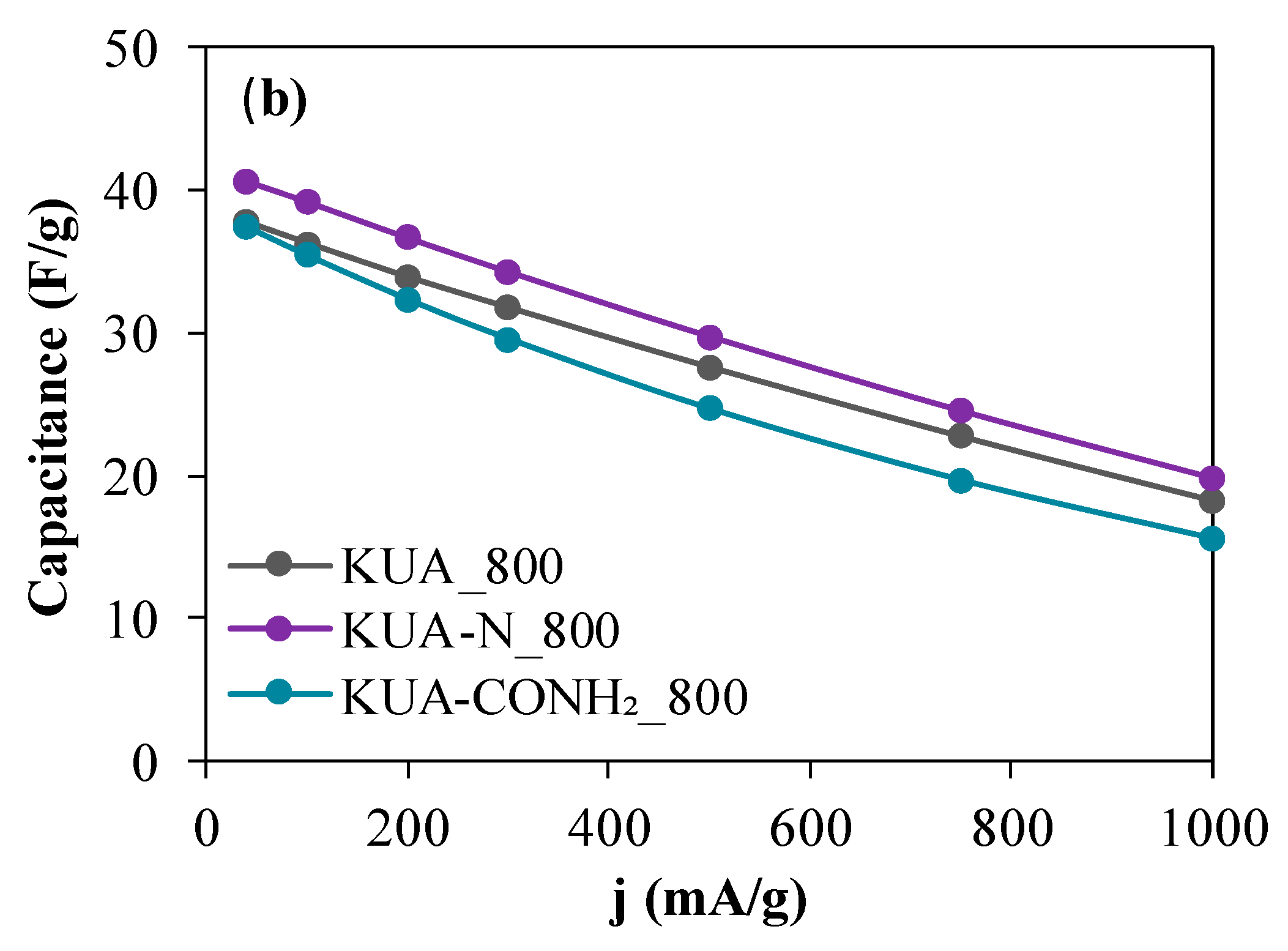
3.2.2. Effect of Surface Chemistry Modification by Heat Treatments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: New York, NY, USA, 1999. [Google Scholar]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumara, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Béguin, F.; Frackowiak, E. (Eds.) Carbons for Electrochemical Energy Storage and Conversion Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Le, T.X.H.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar] [CrossRef]
- Balducci, A. Electrolytes for high voltage electrochemical double layer capacitors: A perspective article. J. Power Sources 2016, 326, 534–540. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and Electrolytes for Advanced Supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Torres, D.; Shiraishi, S.; Morallón, E.; Cazorla-Amoros, D. Improvement of carbon materials performance by nitrogen functional groups in electrochemical capacitors in organic electrolyte at severe conditions. Carbon 2015, 82, 205–213. [Google Scholar] [CrossRef]
- Shiraishi, S. Heat-Treatment and Nitrogen-Doping of Activated Carbons for High Voltage Operation of Electric Double Layer Capacitor. Key Eng. Mater. 2011, 497, 80–86. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amoros, D. Nitrogen doped superporous carbon prepared by a mild method. Enhancement of supercapacitor performance. Int. J. Hydrogen Energy 2016, 41, 19691–19701. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Nie, H.; Chen, X.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Berenguer, R.; Ruiz-Rosas, R.; Gallardo, A.; Cazorla-Amoros, D.; Morallón, E.; Nishihara, H.; Kyotani, T.; Rodríguez-Mirasol, J.; Cordero, T. Enhanced electro-oxidation resistance of carbon electrodes induced by phosphorus surface groups. Carbon 2015, 95, 681–689. [Google Scholar] [CrossRef] [Green Version]
- González-Gaitán, C.; Ruiz-Rosas, R.; Nishihara, H.; Kyotani, T.; Morallón, E.; Cazorla-Amoros, D. Successful functionalization of superporous zeolite templated carbon using aminobenzene acids and electrochemical methods. Carbon 2016, 99, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Ornelas, O.; Sieben, J.M.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amoros, D.; Geng, J.; Soin, N.; Siores, E.; Johnson, B.F.G. On the origin of the high capacitance of nitrogen-containing carbon nanotubes in acidic and alkaline electrolytes. Chem. Commun. 2014, 50, 11343–11346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.-X.-H.; Esmilaire, R.; Drobek, M.; Bechelany, M.; Vallicari, C.; Cerneaux, S.; Julbe, A.; Cretin, M. Nitrogen-Doped Graphitized Carbon Electrodes for Biorefractory Pollutant Removal. J. Phys. Chem. C 2017, 121, 15188–15197. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Castro-Muñiz, A.; Nishihara, H.; Kyotani, T.; Morallón, E.; Cazorla-Amoros, D. Ultraporous nitrogen-doped zeolite-templated carbon for high power density aqueous-based supercapacitors. Carbon 2018, 129, 510–519. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Kodama, M.; Shiraishi, S.; Hatori, H.; Zhu, Z.; Lu, G. Nitrogen-Enriched Nonporous Carbon Electrodes with Extraordinary Supercapacitance. Adv. Funct. Mater. 2009, 19, 1800–1809. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Garcia, B.B.; Liu, D.; Cao, G. Nitrogen modification of highly porous carbon for improved supercapacitor performance. J. Mater. Chem. 2012, 22, 9884. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Yamanaka, T.; Hayashi, Y.; Oda, H. Preparation and Capacitive Properties of a Carbonaceous Material Containing Nitrogen. J. Electrochem. Soc. 2010, 157, A35. [Google Scholar] [CrossRef]
- Cazorla-Amoros, D.; Lozano-Castelló, D.; Morallón, E.; Bleda-Martínez, M.J.; Linares-Solano, A.; Shiraishi, S. Measuring cycle efficiency and capacitance of chemically activated carbons in propylene carbonate. Carbon 2010, 48, 1451–1456. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Shen, W.; Fan, W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Deng, Y.; Zou, K.; Xie, Y.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cazorla-Amoros, D.; Linares-Solano, A. The role of different nitrogen functional groups on the removal of SO2 from flue gases by N-doped activated carbon powders and fibres. Carbon 2003, 41, 1925–1932. [Google Scholar] [CrossRef]
- Itoi, H.; Nishihara, H.; Kyotani, T. Effect of Heteroatoms in Ordered Microporous Carbons on Their Electrochemical Capacitance. Langmuir 2016, 32, 11997–12004. [Google Scholar] [CrossRef]
- Nishihara, H.; Hou, P.-X.; Li, L.-X.; Ito, M.; Uchiyama, M.; Kaburagi, T.; Ikura, A.; Katamura, J.; Kawarada, T.; Mizuuchi, K.; et al. High-Pressure Hydrogen Storage in Zeolite-Templated Carbon. J. Phys. Chem. C 2009, 113, 3189–3196. [Google Scholar] [CrossRef]
- Mostazo-López, M.J.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amoros, D. Generation of nitrogen functionalities on activated carbons by amidation reactions and Hofmann rearrangement: Chemical and electrochemical characterization. Carbon 2015, 91, 252–265. [Google Scholar] [CrossRef] [Green Version]
- Tagaya, T.; Hatakeyama, Y.; Shiraishi, S.; Tsukada, H.; Mostazo-López, M.J.; Morallón, E.; Cazorla-Amorós, D. Nitrogen-Doped Seamless Activated Carbon Electrode with Excellent Durability for Electric Double Layer Capacitor. J. Electrochem. Soc. 2020, 167, 060523. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Lillo-Ródenas, M.A.; Cazorla-Amoros, D.; Linares-Solano, A. Preparation of activated carbons from Spanish anthracite. Carbon 2001, 39, 741–749. [Google Scholar] [CrossRef]
- Cazorla-Amoros, D.; Alcañiz-Monge, J.; de La de La Casa-Lillo, M.A.; Linares-Solano, A. CO2As an Adsorptive to Characterize Carbon Molecular Sieves and Activated Carbons. Langmuir 1998, 14, 4589–4596. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amoros, D.; Linares-Solano, A.; Shiraishi, S.; Kurihara, H.; Oya, A. Influence of pore structure and surface chemistry on electric double layer capacitance in non-aqueous electrolyte. Carbon 2003, 41, 1765–1775. [Google Scholar] [CrossRef]
- Bleda-Martínez, M.; Lozano-Castelló, D.; Morallón, E.; Cazorla-Amoros, D.; Linares-Solano, A. Chemical and electrochemical characterization of porous carbon materials. Carbon 2006, 44, 2642–2651. [Google Scholar] [CrossRef] [Green Version]
- Raymundo-Piñero, E.; Cazorla-Amoros, D.; Linares-Solano, A.; Find, J.; Wild, U.; Schlögl, R. Structural characterization of N-containing activated carbon fibers prepared from a low softening point petroleum pitch and a melamine resin. Carbon 2002, 40, 597–608. [Google Scholar] [CrossRef]
- Jansen, R.; van Bekkum, H. XPS of nitrogen-containing functional groups on activated carbon. Carbon 1995, 33, 1021–1027. [Google Scholar] [CrossRef]
- Yamada, Y.; Kim, J.; Matsuo, S.; Sato, S. Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy. Carbon 2014, 70, 59–74. [Google Scholar] [CrossRef]
- Figueiredo, J.; Pereira, M.F.R.; Freitas, M.M.; Órfão, J.J.D.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Arrigo, R.; Hävecker, M.; Wrabetz, S.; Blume, R.; Lerch, M.; McGregor, J.; Parrott, E.P.J.; Zeitler, J.A.; Gladden, L.; Knop-Gericke, A.; et al. Tuning the Acid/Base Properties of Nanocarbons by Functionalization via Amination. J. Am. Chem. Soc. 2010, 132, 9616–9630. [Google Scholar] [CrossRef]
- Strelko, V.V.; Kuts, V.S.; Thrower, P.A. On the mechanism of possible influence of heteroatoms of nitrogen, boron and phosphorus in a carbon matrix on the catalytic activity of carbons in electron transfer reactions. Carbon 2000, 38, 1499–1503. [Google Scholar] [CrossRef]
- Shiraishi, S. Electrochemical Performance. In Materials Science and Engineering of Carbon; Inagaki, M., Ed.; Tsinghua University Press Limited: Beijing, China; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 205–226. [Google Scholar]
- Weingarth, D.; Noh, H.; Foelske, A.; Wokaun, A.; Kötz, R. A reliable determination method of stability limits for electrochemical double layer capacitors. Electrochim. Acta 2013, 103, 119–124. [Google Scholar] [CrossRef]
- Weingarth, D.; Foelske, A.; Kötz, R. Cycle versus voltage hold–Which is the better stability test for electrochemical double layer capacitors? J. Power Sources 2013, 225, 84–88. [Google Scholar] [CrossRef]
- Ruch, P.W.; Cericola, D.; Foelske-Schmitz, A.; Kotz, R.; Wokaun, A. Aging of electrochemical double layer capacitors with acetonitrile-based electrolyte at elevated voltages. Electrochim. Acta 2010, 55, 4412–4420. [Google Scholar] [CrossRef]
- Ratajczak, P.; Jurewicz, K.; Béguin, F. Factors contributing to ageing of high voltage carbon/carbon supercapacitors in salt aqueous electrolyte. J. Appl. Electrochem. 2013, 44, 475–480. [Google Scholar] [CrossRef]
- Kotz, R.; Hahn, M.; Gallay, R. Temperature behavior and impedance fundamentals of supercapacitors. J. Power Sources 2006, 154, 550–555. [Google Scholar] [CrossRef]
- Bohlen, O.; Kowal, J.; Sauer, D.U. Ageing behaviour of electrochemical double layer capacitors. J. Power Sources 2007, 172, 468–475. [Google Scholar] [CrossRef]
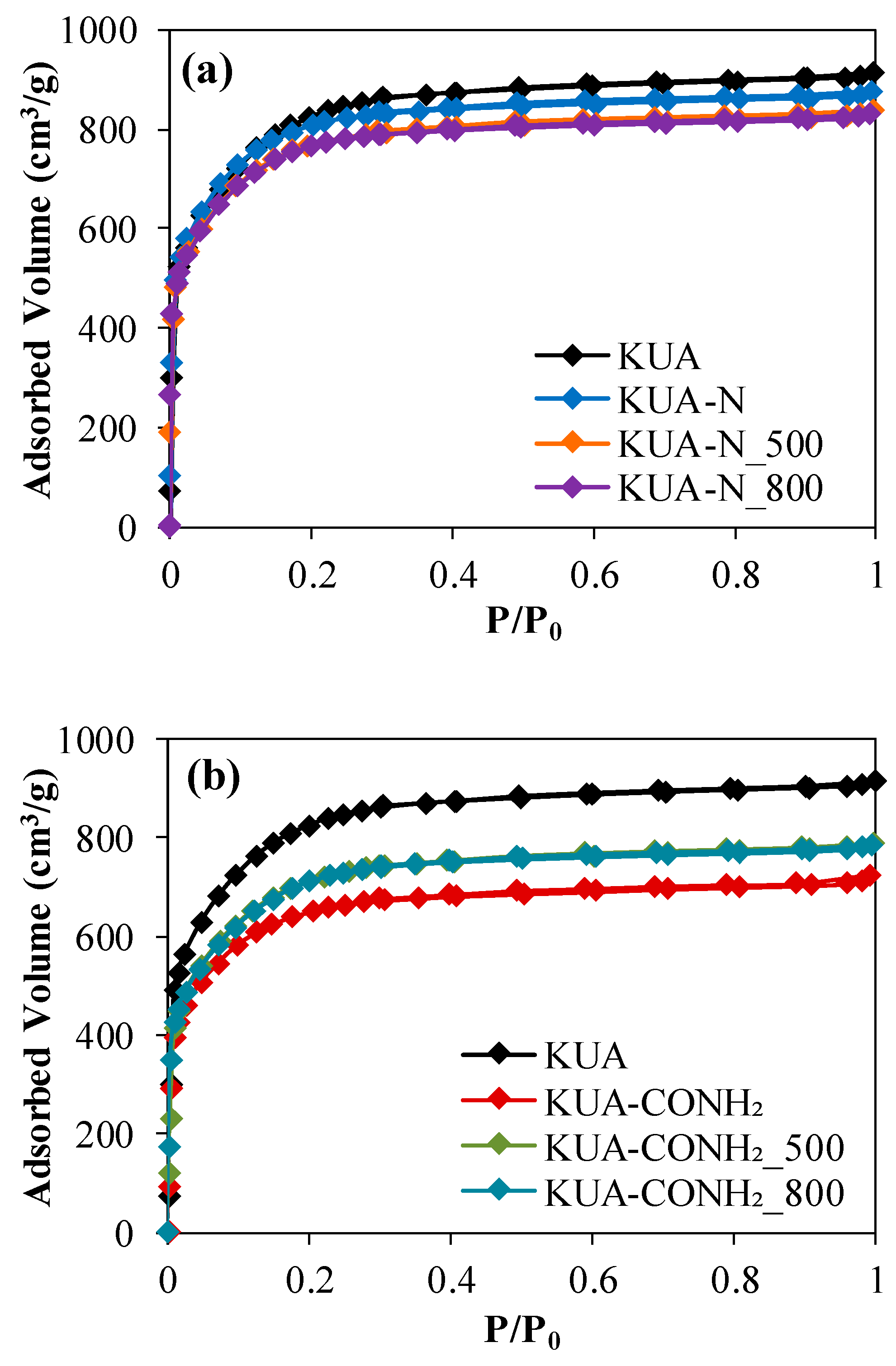





| Sample | SBET (m2/g) | VDRN2 (cm3/g) | VDRCO2 (cm3/g) |
|---|---|---|---|
| KUA | 3080 | 1.19 | 0.57 |
| KUA_800 | 2720 | 1.05 | 0.49 |
| KUA-N | 2960 | 1.18 | 0.52 |
| KUA-N_500 | 2800 | 1.11 | 0.49 |
| KUA-N_800 | 2770 | 1.09 | 0.48 |
| KUA-COOH | 2770 | 1.06 | 0.49 |
| KUA-CONH2 | 2390 | 0.97 | 0.45 |
| KUA-CONH2_500 | 2630 | 1.02 | 0.41 |
| KUA-CONH2_800 | 2630 | 1.0 | 0.43 |
| Sample | CO2 TPD (µmol/g) | COTPD (µmol/g) | OTPD (µmol/g) | OXPS (at. %) | NXPS (at. %) |
|---|---|---|---|---|---|
| KUA | 450 | 1970 | 2870 | 8.8 | 0.3 |
| KUA_800 | 170 | 620 | 960 | 2.3 | |
| KUA-N | 450 | 1750 | 2640 | 7.5 | 3.7 |
| KUA-N_500 | 250 | 1620 | 2120 | 5.1 | 2.3 |
| KUA-N_800 | 130 | 505 | 720 | 3.0 | 1.7 |
| KUA-COOH | 1790 | 3770 | 7360 | 15.8 | |
| KUA-CONH2 | 1140 | 2370 | 4650 | 10.2 | 4.2 |
| KUA-CONH2_500 | 250 | 1650 | 2140 | 8.0 | 3.4 |
| KUA-CONH2_800 | 140 | 570 | 830 | 4.5 | 2.1 |
| Sample | Binding Energy (eV) | Functional Group | N (at. %) | Ratio of N Species (%) |
|---|---|---|---|---|
| KUA-N | 401.9 ± 0.2 | Quaternary | 0.4 | 10 |
| 400.7 ± 0.2 | Pyrrole, Pyridone | 0.9 | 25 | |
| 399.8 ± 0.2 | Amide, Lactam, Amine, Imide | 1.3 | 35 | |
| 398.7 ± 0.2 | Pyridine, Imine | 1.1 | 30 | |
| KUA-N_500 | 401.5 ± 0.2 | Quaternary | 0.4 | 17 |
| 400.2 ± 0.2 | Pyrrole, pyridone | 0.9 | 42 | |
| 399.0 ± 0.2 | Pyridine, Imine | 1.0 | 41 | |
| KUA-N_800 | 402.7 ± 0.2 | Oxidized N | 0.3 | 14 |
| 400.8 ± 0.2 | Pyrrole, Pyridone | 1.2 | 51 | |
| 398.7 ± 0.2 | Pyridine | 0.8 | 35 | |
| KUA-CONH2 | 400.7 ± 0.2 | Pyrrole, pyridone | 0.7 | 19 |
| 399.8 ± 0.2 | Amide, lactam, amine, imide | 1.9 | 50 | |
| 398.8 ± 0.2 | Pyridine, imine | 1.2 | 31 | |
| KUA-CONH2_500 | 400.9 ± 0.2 | Pyrrole, pyridone | 1.7 | 34 |
| 399.6 ± 0.2 | Amide, lactam, amine, imide | 1.3 | 38 | |
| 398.5 ± 0.2 | Pyridine, imine | 1.0 | 28 | |
| KUA-CONH2_800 | 402.5 ± 0.2 | Oxidized N | 0.2 | 11 |
| 400.8 ± 0.2 | Pyrrole, pyridone | 0.9 | 52 | |
| 398.7 ± 0.2 | Pyridine, imine | 0.6 | 37 |
| Capacitor | C0 (F/g) | C0/SBET (μF/cm2) | E (Wh/kg) | C1/C0 (%) | Cf/C0 (%) | ΔR (Ω) | IL (mAh) |
|---|---|---|---|---|---|---|---|
| KUA | 41 | 13 | 37 | 45 | 29 | 33 | 37 |
| KUA_800 | 38 | 14 | 33 | 48 | 43 | 30 | 29 |
| KUA-N | 41 | 14 | 36 | 42 | 39 | 20 | 32 |
| KUA-N_500 | 41 | 15 | 35 | 45 | 39 | 32 | 36 |
| KUA-N_800 | 40 | 14 | 35 | 49 | 44 | 28 | 31 |
| KUA-COOH | 36 | 13 | 31 | 30 | 20 | 50 | 26 |
| KUA-CONH2 | 37 | 15 | 32 | 49 | 55 | 13 | 22 |
| KUA-CONH2_500 | 39 | 15 | 33 | 37 | 22 | 86 | 33 |
| KUA-CONH2_800 | 38 | 15 | 32 | 42 | 33 | 34 | 41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostazo-López, M.J.; Ruiz-Rosas, R.; Tagaya, T.; Hatakeyama, Y.; Shiraishi, S.; Morallón, E.; Cazorla-Amorós, D. Nitrogen Doped Superactivated Carbons Prepared at Mild Conditions as Electrodes for Supercapacitors in Organic Electrolyte. C 2020, 6, 56. https://doi.org/10.3390/c6030056
Mostazo-López MJ, Ruiz-Rosas R, Tagaya T, Hatakeyama Y, Shiraishi S, Morallón E, Cazorla-Amorós D. Nitrogen Doped Superactivated Carbons Prepared at Mild Conditions as Electrodes for Supercapacitors in Organic Electrolyte. C. 2020; 6(3):56. https://doi.org/10.3390/c6030056
Chicago/Turabian StyleMostazo-López, María José, Ramiro Ruiz-Rosas, Tomomi Tagaya, Yoshikiyo Hatakeyama, Soshi Shiraishi, Emilia Morallón, and Diego Cazorla-Amorós. 2020. "Nitrogen Doped Superactivated Carbons Prepared at Mild Conditions as Electrodes for Supercapacitors in Organic Electrolyte" C 6, no. 3: 56. https://doi.org/10.3390/c6030056
APA StyleMostazo-López, M. J., Ruiz-Rosas, R., Tagaya, T., Hatakeyama, Y., Shiraishi, S., Morallón, E., & Cazorla-Amorós, D. (2020). Nitrogen Doped Superactivated Carbons Prepared at Mild Conditions as Electrodes for Supercapacitors in Organic Electrolyte. C, 6(3), 56. https://doi.org/10.3390/c6030056