Mechanisms of Carbon Nanotubes and Graphene Growth: Kinetics versus Thermodynamics
Abstract
1. Introduction
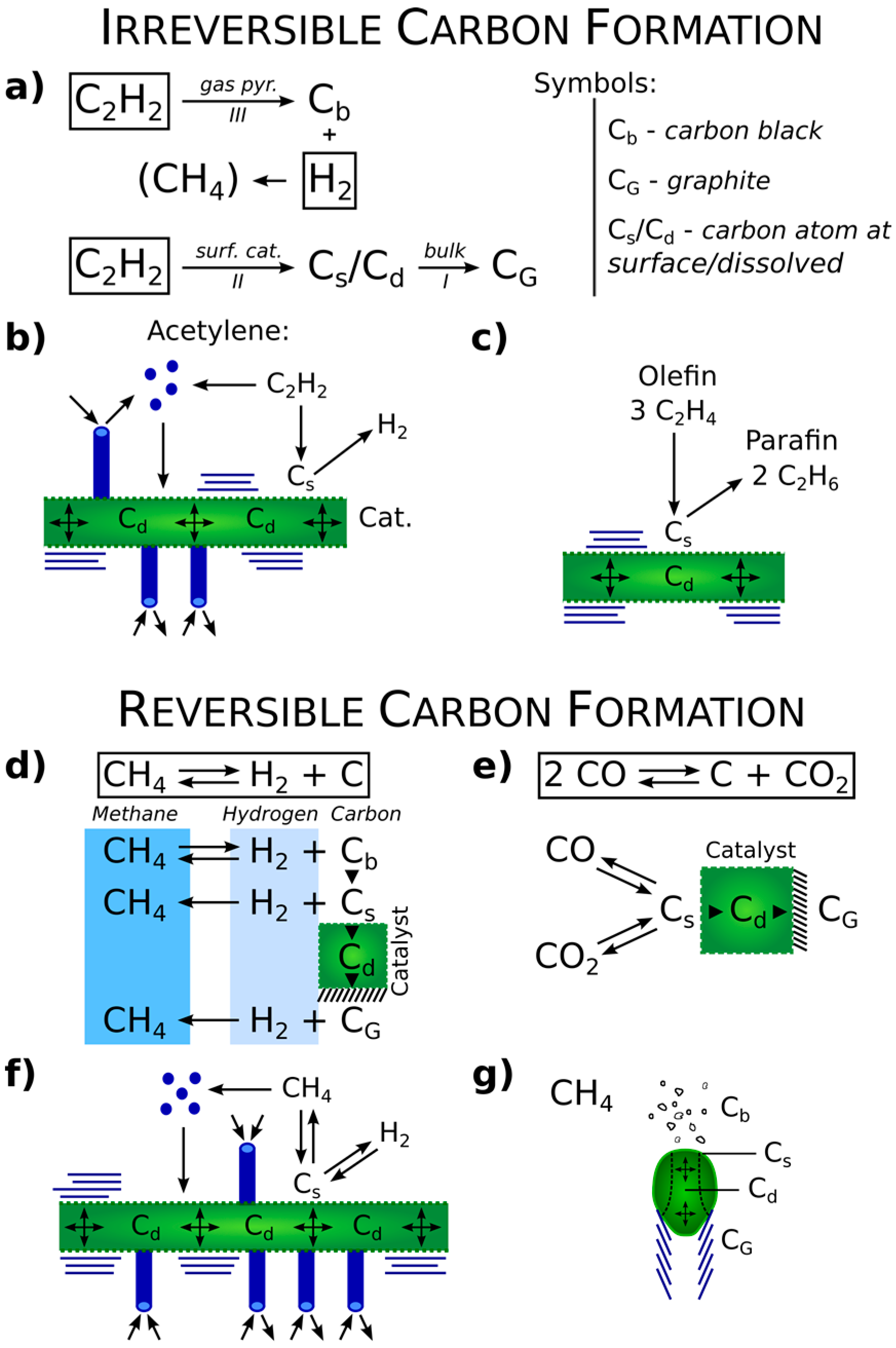
2. Thermodynamics versus Kinetics. Reversible versus Irreversible Carbon Formation
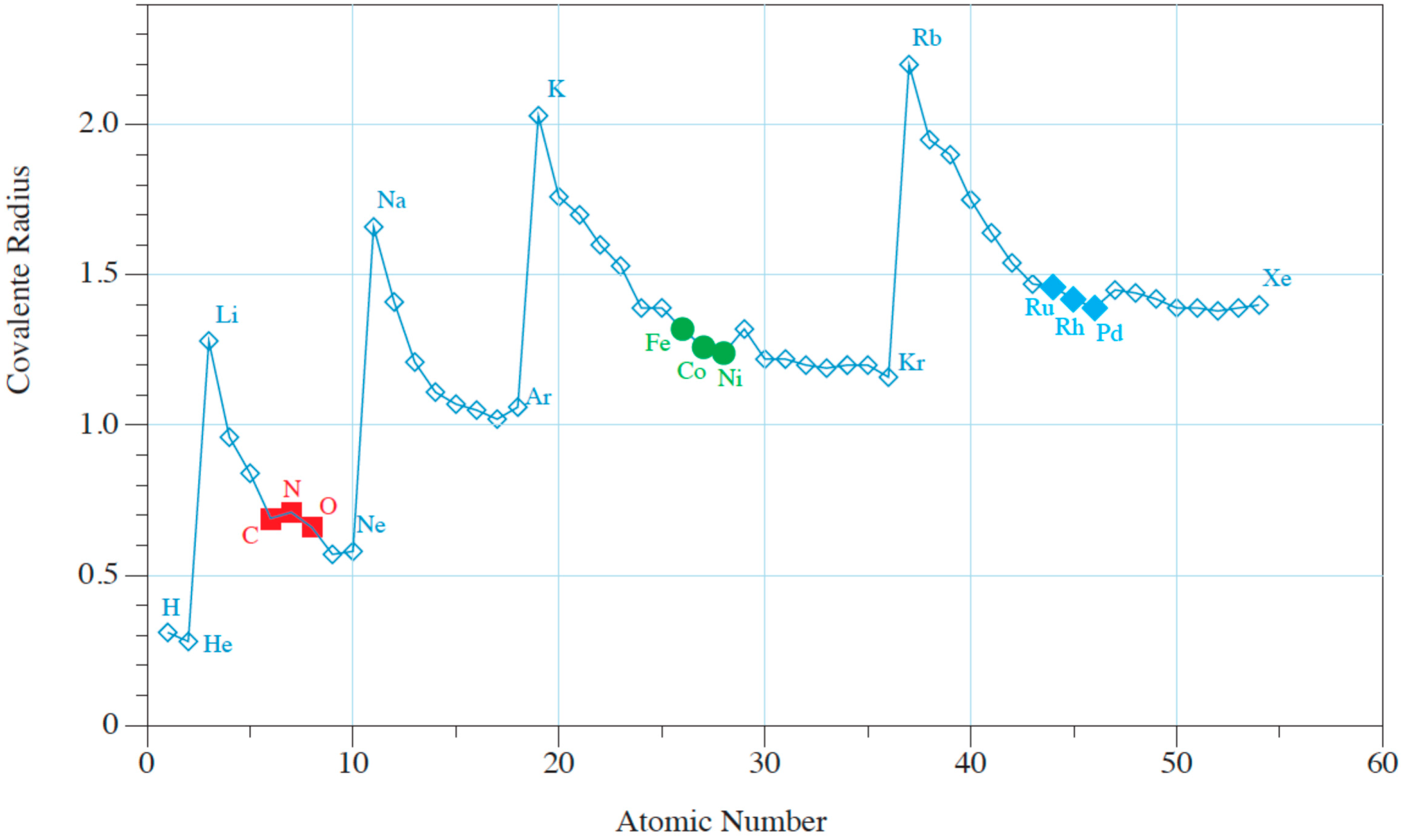
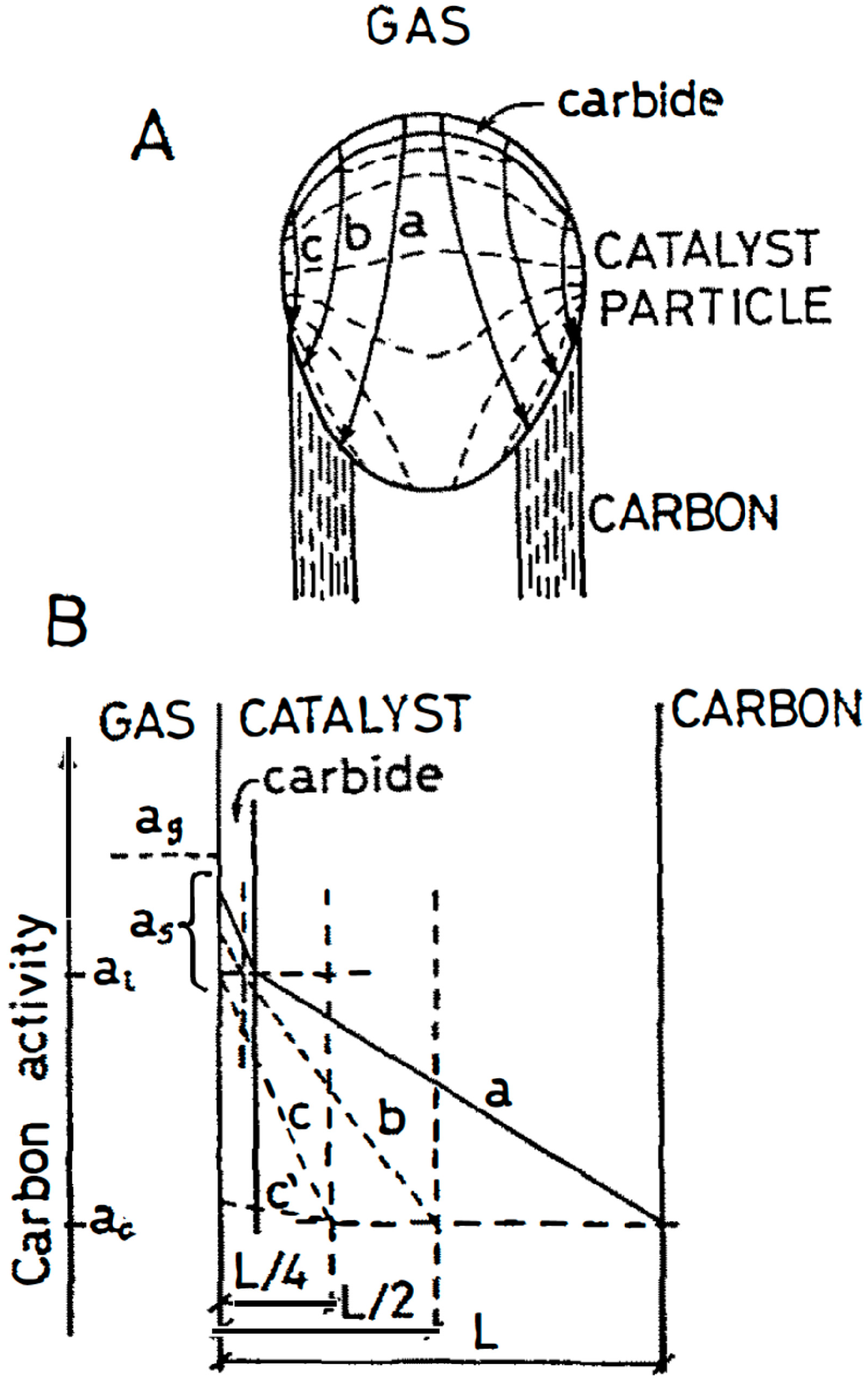
3. Interstitial Carbon Bulk Diffusion Operation
4. Recent Graphene Nucleation Studies
5. Comprehensive Kinetics, Activation Energies and Reaction Orders
6. Recent Kinetic Studies
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jourdain, V.; Bichara, C. Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Lobo, L.S. Mechanism of Catalytic CNTs Growth in 400–650 °C Range: Explaining Volcano Shape Arrhenius Plot and Catalytic Synergism Using both Pt (or Pd) and Ni, Co or Fe. C—J. Carbon Res. 2019, 5, 42. [Google Scholar] [CrossRef]
- Lobo, L.S.; Carabineiro, S.A.C. Carbon Formation at High Temperatures (550–1400 °C): Kinetics, alternative mechanisms and Growth Modes. Catalysis 2020, 10, 465. [Google Scholar] [CrossRef]
- Lobo, L.S.; Trimm, D.L. Studies of Carbon Formation on Metals Using a Vacuum Microbalance (1972). In Progress in Vacuum Microbalance Techniques; Heyden & Son: London, UK, 1973; Volume 2. [Google Scholar]
- Lobo, L.S.; Franco, M.D. Kinetics of catalytic carbon formation on steel surfaces from light hydrocarbons. Catal. Today 1990, 7, 247–256. [Google Scholar] [CrossRef]
- Bromley, J.; Strickland-Constable, R.F. Role of nucleation process in the reactions between CO and nickel. Trans. Faraday Soc. 1960, 10, 1492–1500. [Google Scholar] [CrossRef]
- Bernardo, C.; Trimm, D.L. Kinetics of Gasification of Carbon Deposited on Nickel Catalysts. Carbon 1979, 17, 115–120. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusion in and Through Solids; Cambridge UP: Cambridge, UK, 1941. [Google Scholar]
- Lobo, S.L.; Carabineiro, S.A.C. Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments. C—J. Carbon Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Safvi, S.A.; Bianchini, E.C.; Lund, C.R.F. The Dependence of Catalytic Carbon Filament Growth Kinetics upon Gas Phase Carbon Activity. Carbon 1991, 29, 1245–1250. [Google Scholar] [CrossRef]
- Yang, R.T.; Goethel, P.J.; Schwartz, J.M.; Lund, C.R.F. Solubility and Diffusivity of Carbon in Metals. J. Catal. 1990, 122, 206–210. [Google Scholar] [CrossRef]
- Picher, M.; Lin, P.A.; Gomez-Ballesteros, J.L.; Balbuena, P.B.; Sharma, R. Nucleation of Graphene and Its Conversion to Single-Walled Carbon Nanotubes. Nano Lett. 2014, 14, 6104–6108. [Google Scholar] [CrossRef]
- Cabrero-Vilatela, A.; Weatherup, R.S.; Braeuninger-Weimer, P.; Caneva, S.; Hofmann, S. Towards a general growth model for graphene CVD on transition metal catalysts. Nanoscale 2016, 8, 2149. [Google Scholar] [CrossRef] [PubMed]
- Olle, M.; Ceballos, G.; Serrate, D.; Gambardella, P. Yield and Shape Selection of Graphene nanoislands Grown on Ni(111). Nano Lett. 2012, 12, 4431–4436. [Google Scholar] [CrossRef] [PubMed]
- García-Lekue, A.; Ollé, M.; Sánchez-Portal, D.; Palacios, J.J.; Mugarza, A.; Ceballos, G.; Gambardella, P. Substrate-Induced Stabilization and Reconstruction of Zigzag Edges in Graphene Nanoislands on Ni(111). J. Phys. Chem. C 2015, 119, 4072–4078. [Google Scholar] [CrossRef]
- Mehl, R.F. Rate of Diffusion in Solid Alloys. J. Appl. Phys. 1937, 8, 174. [Google Scholar] [CrossRef]
- Budnikov, P.P.; Ginstling, A.M. Principles of solid state chemistry. In Reactions in Solids; Gordon and Breach Sci Pub: New York, NY, USA.
- Zaikovskii, V.I.; Chesnokov, V.V.; Buyanov, R.A. The Relationship between the State of Active Species in a Ni/Al2O3 Catalyst and the mechanism of Growth of Filamentous Carbon. Kinet. Catal. 2001, 42, 813–820. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Podyacheva, O.Y.; Shmakov, A.N.; Kibis, L.S.; Borinin, A.I.; Ismagilov, Z.R. Comparizon of growth mechanisms of undopped and nitrogen doped carbon nanofibers on nickel-containing catalysts. Chin. J. Catal. 2016, 37, 169–176. [Google Scholar] [CrossRef]
- Perez-Cabero, M.; Romeo, E.; Royo, C.; Monzón, A.; Guerrero Ruiz, A.; Rodriguez- Ramos, I. Growing Mechanism of CNTs: A kinetic approach. J. Catal. 2004, 224, 197–205. [Google Scholar] [CrossRef]
- Latorre, N.; Romeo, E.; Villacampa, J.I.; Cazana, F.; Royo, C.; Monzón, A. Kinetics of carbon nanotubes growth on a Ni-Mg-Al catalyst by CCVD of methane: Influence of catalytic deactivation. Catal. Today 2010, 154, 217–223. [Google Scholar] [CrossRef]
- Latorre, N.; Cazana, F.; Martinez-Hansen, V.; Royo, C.; Romeo, E.; Monzón, A. Ni.Co-Mg-Al catalysts for hydrogen and carbonaceous nanomaterials production by CCVD of methane. Catal. Today 2011, 172, 143–151. [Google Scholar] [CrossRef]
- Puretzky, A.A.; Geohegan, D.B.; Jesse, S.; Ivanov, I.N.; Eres, G. In situ measurements and modeling of carbon nanotube array growth kinetics during CVD. Appl. Phys. A 2005, 81, 223–240. [Google Scholar] [CrossRef]
- Geohegan, D.B.; Puretzky, A.A.; Ivanov, I.N.; Jesse, S.; Eres, G.; Howe, J.Y. In situ growth rate measurements and length control during CVD of VAMWCNTs. Appl. Phys. Lett. 2003, 83, 1851. [Google Scholar] [CrossRef]
- Kayastha, V.K.; Yap, Y.K.; Pan, Z.; Ivanov, I.N.; Puretzky, A.A.; Geohegan, D.B. High-density vertically aligned multiwalled carbon nanotubes with tubular structures. Appl. Phys. Lett. 2005, 86, 253105. [Google Scholar] [CrossRef]
- Lobo, L.S. Carbon Formation from Hydrocarbons on Metals. Ph.D. Thesis, Imperial College, London, UK, 1971. [Google Scholar]
- McKee, D.W. Metal Oxides as Catalysts for Oxidation of Graphite. Carbon 1970, 8, 623–635. [Google Scholar] [CrossRef]
- Lobo, L.S.; Carabineiro, S.A.C. Kinetics and mechanism of catalytic carbon gasification. Fuel 2016, 183, 457–469. [Google Scholar] [CrossRef]
- Carabineiro, S.A.C.; Lobo, L.S. Understanding the Reactions of CO2, NO, and N2O with Activated Carbon Catalyzed by Binary Mixtures. Energy Fuels 2016, 30, 6881–6891. [Google Scholar] [CrossRef]
- De Jong, K.; Geus, J.W. Carbon nanofibers: Catalytic synthesis and applications. Catal. Rev. Sci. Eng. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Chiashi, S. Temperature Dependence of Raman Scattering from SWCNTs: Undefined Radial Breading Mode Peaks at High Temperatures. Jpn. J. Appl. Phys. 2010, 47, 1956–1960. [Google Scholar]
- Molina-Duarte, J.; Espinosa-Vega, L.I.; Rodriguez, A.; Guirado Lopez, R.A. Raman spectra of SWCNTs at high temperatures: Pretreating samples in a nitrogen atmosphere improves their thermal stability in air. Phys. Chem. Chem. Phys. 2017, 19, 7215. [Google Scholar] [CrossRef]
- Bedewy, M.; Meshot, E.R.; Hart, A.J. Diameter-dependent kinetics of activation and deactivation in carbon nanotube population growth. Carbon 2012, 50, 5106–5116. [Google Scholar] [CrossRef]
- Salipira, K.; Coville, N.J.; Scurrell, M.S. Carbon produced by the catalytic decomposition of methane on Ni: Carbon yields and carbon structure as function of catalytic properties. J. Nat. Gas Sci. Eng. 2016, 32, 501. [Google Scholar] [CrossRef]

| Observed Behavior | Bulk Diffusion | Surface Diffusion |
|---|---|---|
| Zero order reaction kinetics | Yes | No |
| Temperature dependence, Ea~Cdif. | Yes | No |
| Various gases, same rates (route I, low T) | Yes | Yes |
| Ni3C recession | Yes | No |
| Nucleation inhibits further nucleation | Yes | No |
| Kinetic linearity | Yes | Yes |
| Smaller particles, faster growth | Yes | Yes |
| C bulk diffusion: covalent radius ratio | Yes | No |
| Metal | Melting (°C) | TTa (°C) | ARsolv (pm) | ARsolu/ARsolv |
|---|---|---|---|---|
| Fe | 1538 | 632 | 140 | 0.50 |
| Co | 1495 | 611 | 135 | 0.52 |
| Ni | 1455 | 590 | 135 | 0.52 |
| Cu | 1083 | 405 | 135 | 0.52 |
| Ru | 2334 | 1030 | 130 | 0.54 |
| Rh | 1964 | 845 | 135 | 0.52 |
| Pd | 1555 | 641 | 140 | 0.50 |
| Kinetic Routes | I—Catalytic Route | II—Hybrid Route | III—Pyrolytic Route (CVD) |
|---|---|---|---|
| Temperature Range and Growth Mode | 300–550 °C Surface catalysis, C diffuse & grows | 550–700 °C C black atoms dissolve & grow | 600–900 °C C black forming graphene layers |
| Carbon Formation Type | Geometry selective surface catalysis + growth | Selective nucleation and growth catalysis | Impingement, non-selective C2, C3… Cn |
| Surface Catalysis: C Atoms Formed? | Yes. Selective Ex: Ni(100) | No | No |
| Carbon Atoms Bulk Diffusion? | Yes | Yes | No |
| CNTs/CNFs Growth: Shape Catalysis | CNTs growth CNFs growth Selective Ex: Ni(111) | CNTs growth CNFs growth Selective | External surface layers on CNTs, other forms at very high T |
| How to Change Routes? Remarks | Higher T | Higher P | Carbon fouling on the walls |
| Active Catalysts | Ni, Co, Fe | Pt, Ru, Mo, Ni… | No catalysts, just shape interaction |
| Temperature Range and Growth Mode | 300–550 °C Surface catalysis, C diffuse and grows | 550–700 °C C black atoms dissolve and grow | 600–900 °C C black forming graphene layers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobo, L.S.; Carabineiro, S.A.C. Mechanisms of Carbon Nanotubes and Graphene Growth: Kinetics versus Thermodynamics. C 2020, 6, 67. https://doi.org/10.3390/c6040067
Lobo LS, Carabineiro SAC. Mechanisms of Carbon Nanotubes and Graphene Growth: Kinetics versus Thermodynamics. C. 2020; 6(4):67. https://doi.org/10.3390/c6040067
Chicago/Turabian StyleLobo, Luís Sousa, and Sónia A. C. Carabineiro. 2020. "Mechanisms of Carbon Nanotubes and Graphene Growth: Kinetics versus Thermodynamics" C 6, no. 4: 67. https://doi.org/10.3390/c6040067
APA StyleLobo, L. S., & Carabineiro, S. A. C. (2020). Mechanisms of Carbon Nanotubes and Graphene Growth: Kinetics versus Thermodynamics. C, 6(4), 67. https://doi.org/10.3390/c6040067






