Performing Quality Assurance of Carbon Dioxide for Carbon Capture and Storage
Abstract
:1. Introduction
2. Purity across the CCS Process
2.1. Production
2.1.1. Pre-Combustion
2.1.2. Post-Combustion
2.1.3. Oxy-Fuel
2.1.4. Steam Methane Reforming
2.2. Capture or Separation and Transport
2.3. Storage
2.3.1. Deep Saline Aquifers
2.3.2. Unmineable Coal Seams
2.3.3. Depleted Oil and Gas Reservoirs
2.4. Utilisation
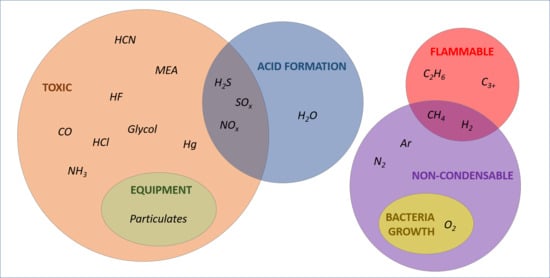
3. Quality Requirements for CCS
3.1. Water (H2O)
3.2. Hydrogen Sulphide (H2S), Sulphur Oxides (SOx), and Nitrogen Oxides (NOx)
3.3. Carbon Monoxide (CO)
3.4. Oxygen (O2), Methane (CH4), Nitrogen (N2), Argon (Ar), and Hydrogen (H2)
3.5. Ammonia (NH3) and Amines
3.6. Ethane (C2H6) and Hydrocarbons (C3+)
3.7. Particulates
3.8. Other Impurities
4. Recommendations
4.1. Assessing Effects of Carbon Dioxide Quality
4.2. Primary Reference Materials
4.3. Sampling
4.4. Gas Analysis Methods
4.5. Impact of Gas Quality on Other Measurements
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shell Carbon Capture and Storage Projects. Available online: https://www.shell.com/sustainability/environment/climate-change/carbon-capture-and-storage-projects.html (accessed on 1 June 2020).
- Northern Lights about the Project. Available online: https://northernlightsccs.com/en/about (accessed on 1 June 2020).
- Wetenhall, B.; Aghajani, H.; Chalmers, H.; Benson, S.D.; Ferrari, M.C.; Li, J.; Race, J.M.; Singh, P.; Davison, J. Impact of CO2 impurity on CO2 compression, liquefaction and transportation. Energy Procedia 2014, 63, 2764–2778. [Google Scholar] [CrossRef] [Green Version]
- DYNAMIS. Dynamis CO2 Quality Recommendations; DYNAMIS: Oslo, Norway, 2007. [Google Scholar]
- Abdelaziz, O.; Gadalla, M.; Ashour, F. Simulation of biomethanol production from green syngas through sustainable process design. In Proceedings of the 4th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH), Vienna, Austria, 28–30 August 2014. [Google Scholar]
- Raibhole, V.; Sapali, S. Simulation and Parametric Analysis of Cryogenic Oxygen Plant for Biomass Gasification. Mech. Eng. Res. 2012, 2, 97. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Chen, C.-Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- De Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; de Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 quality recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Global CCS Institute. CO2 Capture Techologies—Oxy Combustion with CO2 Capture; Global CCS Institute: Melbourne, Australia, 2012. [Google Scholar]
- Kather, A. CO2 Quality and Other Relevant Issues. In Proceedings of the 2nd Working Group Meeting on CO2 Quality and Other Relevant Issues, Cottbus, Germany, 7 September 2009. [Google Scholar]
- White, V.; Torrente-Murciano, L.; Sturgeon, D.; Chadwick, D. Purification of oxyfuel-derived CO2. Energy Procedia 2009, 1, 399–406. [Google Scholar] [CrossRef]
- Bert Metz, O.D.; de Coninck, H.; Loos, M.; Meyer, L. Carbon Dioxide Capture and Storage; IPCC: Cambridge, UK, 2005. [Google Scholar]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Rubin, E.S. The Outlook for Power Plant CO2 Capture. In International Seminar on Nuclear War and Planetary Emergencies—42nd Session; World Scientific: Singapore, 2009; pp. 157–173. [Google Scholar]
- Walspurger, S.; Dijk, H.A.J. EDGAR CO2 Purity: Type and Quantities of Impurities Related to CO2 Point Source and Capture Technology: A Literature Study; ECN: Petten, The Netherlands, 2012. [Google Scholar]
- World Resources Institute. Guidelines for Carbon Dioxide Capture, Transport, and Storage; World Resources Institute: Washington, DC, USA, 2008. [Google Scholar]
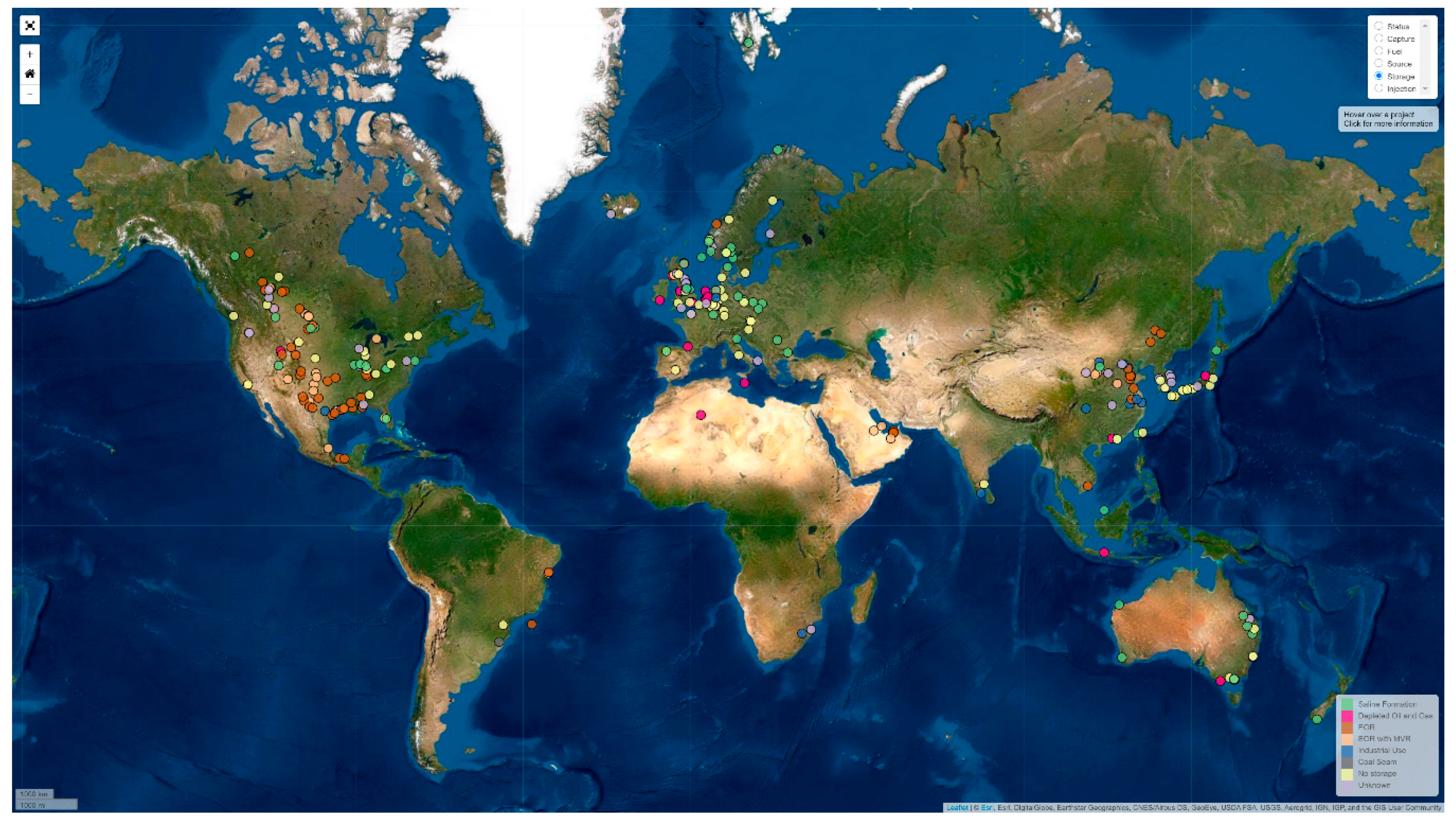
- SCCS Global CCS Map. Available online: https://www.sccs.org.uk/expertise/global-ccs-map (accessed on 1 June 2020).
- IEAGHG. Effects of Impurities on Geological Storage of CO2; IEAGHG: Cheltenham, UK, 2011. [Google Scholar]
- Das, S.; Dutta, P. Preliminary Understanding of CO2 Sequestration and Enhanced Methane Recovery in Raniganj Coalfield of India by Reservoir Simulation. Energy Procedia 2017, 114, 4643–4657. [Google Scholar] [CrossRef]
- Mazzotti, M.; Pini, R.; Storti, G.; Burlini, L. Carbon dioxide (CO2) sequestration in unmineable coal seams and use for enhanced coalbed methane recovery (ECBM). In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Maroto-Valer, M.M., Ed.; Woodhead Publishing: Cambridge, UK, 2010; Volume 2, pp. 127–165. [Google Scholar]
- Corum, M.D.; Jones, K.B.; Warwick, P.D. CO2 Sequestration Potential of Unmineable Coal—State of Knowledge. Energy Procedia 2013, 37, 5134–5140. [Google Scholar] [CrossRef] [Green Version]
- Godec, M.; Kuuskraa, V.; Van Leeuwen, T.; Stephen Melzer, L.; Wildgust, N. CO2 storage in depleted oil fields: The worldwide potential for carbon dioxide enhanced oil recovery. Energy Procedia 2011, 4, 2162–2169. [Google Scholar] [CrossRef] [Green Version]
- Sonnichsen, N. Largest Global Carbon Sequestration Projects in Operation 2019; Statistica: New York, NY, USA, 2020. [Google Scholar]
- EIGA. Carbon Dioxide Food and Beverages Grade, source Qualification, Quality Standards and Verification; EIGA: Brussels, Belgium, 2017. [Google Scholar]
- SNC Lavalin. Impact of Impurities on CO2 Capture, Transport and Storage; SNC Lavalin: Montreal, QC, Canada, 2004. [Google Scholar]
- Anheden, M.; Andersson, A.; Bernstone, C.; Eriksson, S.; Yan, J.; Liljemark, S.; Wall, C. CO2 quality requirement for a system with CO2 capture, transport and storage. In Greenhouse Gas Control Technologies 7; Elsevier Science Ltd.: Oxford, UK, 2005; pp. 2559–2564. [Google Scholar]
- White, V.; Allam, R.; Miller, E. Purification of Oxyfuel-Derived CO2 for Sequestration or EOR; IEAGHG: Cheltenham, UK, 2007. [Google Scholar]
- Veritas, D.N. Design and Operation of CO2 Pipelines. In GEnergy Procedia 4; Elsevier Science Ltd.: Oxford, UK, 2011; pp. 3032–3039. [Google Scholar]
- NETL. CO2 Impurity Design Parameters; NETL: Pittsburgh, PA, USA, 2012.
- Harkin, T.; Filby, I.; Sick, H.; Manderson, D.; Ashton, R. Development of a CO2 Specification for a CCS Hub Network. Energy Procedia 2017, 114, 6708–6720. [Google Scholar] [CrossRef]
- Health and Safety Executive. EH40/2005 Workplace Exposure Limits, 3rd ed.; Health and Safety Executive: Buxton, UK, 2018.
- ISO/TR 27921:2020. Carbon Dioxide Capture, Transportation, and Geological Storage—Cross Cutting Issues—CO2 Stream Composition; International Organisation of Standardisation: Geneva, Switzerland, 2020. [Google Scholar]
- Wetenhall, B.; Race, J.M.; Downie, M.J. The Effect of CO2 Purity on the Development of Pipeline Networks for Carbon Capture and Storage Schemes. Int. J. Greenh. Gas Control 2014, 30, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Chapoy, A.; Nazeri, M.; Kapateh, M.; Burgass, R.; Coquelet, C.; Tohidi, B. Effect of impurities on thermophysical properties and phase behaviour of a CO2-rich system in CCS. Int. J. Greenh. Gas Control 2013, 19, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenkamp, G. Final Publishable JRP Summary for ENG60 LNG II Metrological Support for LNG Custody Transfer and Transport Fuel Applications; Euramet: Braunschweig, Germany, 2017. [Google Scholar]
- Chromatotec. CO2 quality control—Gas chromatography solutions. In Gasworld Magazine; Gasworld: Truro, UK, 2018; p. 54. [Google Scholar]
- V&F Analyse- und Messtechnik GmbH, How Pure Is Your CO2? In Gasworld Magazine; Gasworld: Truro, UK, 2018.
- Hunter, N.G.L. Measurement Challenges for Carbon Capture and Storage. Meas. Control 2011, 44, 81–85. [Google Scholar]

| Post-Combustion/Steam Methane Reforming (cmol·mol−1) | Pre-Combustion (cmol·mol−1) | Oxy-Fuel (cmol·mol−1) | |
|---|---|---|---|
| CO2 | 99.8–99.9 | 95–99 | 95–97 |
| N2/O2/Ar | <0.1 | 0–1.5 | 1.6–4.1 |
| CO | <0.1 | 0.04 | <0.1 |
| H2S | - | 0–0.6 | - |
| SO2 | <0.1 | <0.1 | <0.5 |
| NO | <0.1 | - | 0–0.01 |
| H2O | <0.1 | 0–1.8 | - |
| CO2 | H2O | H2 | CO | N2 | O2 | CH4 | Ar | |
|---|---|---|---|---|---|---|---|---|
| Amine scrubber (post-combustion) | 94.4 | 5.6 | 0 | 0 | 0 | Trace level | 0 | 0 |
| Amine scrubber (pre-combustion) | 98.2 | 1.8 | 0 | 0 | 0 | 0 | 0 | 0 |
| Water cycle | 59.7 | 32.8 | 0 | 0.01 | 2.81 | 1 | 0 | 3.59 |
| S-Graz cycle | 61.7 | 30.9 | 0 | 0 | 2.91 | 0.85 | 0 | 3.69 |
| Oxyfuel carbon capture | 93.8 | 4.2 | 0 | 0 | 0.28 | 1.38 | 0 | 0.35 |
| Solid oxide fuel cell and gas turbine | 35.9 | 63.8 | 0 | 0 | 0.26 | 0 | 0 | 0 |
| Advanced zero emission power | 35.9 | 63.8 | 0 | 0 | 0.26 | 0 | 0 | 0 |
| Chemical looping combustion | 34.7 | 65.1 | 0 | 0 | 0.28 | 0 | 0 | 0 |
| Hydrogen membrane separation reactor | 62.4 | 35.5 | 0.92 | 0.57 | 0.45 | 0 | 0.12 | 0 |
| Appendix A: EIGA Limiting Characteristics For Carbon Dioxide to Be Used in Beverage for Source Specification | |
| Component | Amount Fraction |
| Assay | 99 cmol·mol−1 min |
| Moisture | 20 µmol·mol−1 max |
| Ammonia | 2.5 µmol·mol−1 max |
| Oxygen | 30 µmol·mol−1 max |
| Oxides of nitrogen (NO/NO2) | 2.5 µmol·mol−1 max each |
| Nonvolatile residues (particulates) | 10 µmol·mol−1 max |
| Nonvolatile organic residues (oil and grease) | 5 µmol·mol−1 max |
| Phosphine *** | 0.3 µmol·mol−1 max |
| Total volatile hydrocarbons (calculated as methane) | 50 µmol·mol−1 max of which 20 µmol·mol−1 max non-methane hydrocarbons |
| Acetaldehyde | 0.2 µmol·mol−1 max |
| Aromatic hydrocarbon | 0.02 µmol·mol−1 max |
| Carbon monoxide | 10 µmol·mol−1 max |
| Methanol | 10 µmol·mol−1 max |
| Hydrogen cyanide * | 0.5 µmol·mol−1 max |
| Total sulphur (as S) ** | 0.1 µmol·mol−1 max |
| Taste and odour in water | No foreign taste or odour |
| Appearance in water | No colour or turbidity |
| Odour and appearance of solid CO2 (snow) | No foreign odour or appearance |
| Author | Description | Year | Type of Document | Ref. |
|---|---|---|---|---|
| SNC Lavalin | This report was written by SNC for the International Energy Agency Greenhouse Gas (IEAGHG) research and development programme and is based on a study of two types of coal fired plants using conventional pulverised coal steam and an integrated gasification combined cycle plant. The report provides information on effects that some impurities may have to the process. | 2004 | Report | [25] |
| Anheden et al. | Discusses the required carbon dioxide purity specifications for a typical CCS process on the basis of transport, storage, safety, cost and environmental regulations. | 2005 | Paper | [26] |
| Metz et al. | The focus of the special report published by the Intergovernmental Panel on Climate Change is to assess current state of knowledge for important aspects of CCS which includes sources of impurities and impact on the overall process. | 2005 | Report | [12] |
| Dynamis | Written as part of the Dynamic project provides carbon dioxide purity specifications that were developed as guidance for the CCS stream of the HYPOGEN plant. | 2007 | Project (technical specification) | [4] |
| White et al. | The paper provides some guidance on possible impact of impurities in carbon dioxide for an oxyfuel process. | 2007 | Paper | [27] |
| World Resources Institute | Provides guidelines for CCS written by a group of over 80 stakeholders which includes information on effects of impurities. | 2008 | Report | [16] |
| Det Norske Veritas | Provides guidance and criteria (including recommended purity specifications) for carbon dioxide transport in steel pipelines. The guide was developed in partnership with key companies including British Petroleum, Petrobras, Shell, Statoil, Vattenfall, and Chevron. | 2010 | Best practice | [28] |
| IEAGHG | Written by the International Energy Agency, the report considers several storage scenarios including deep saline formations, depleted gas fields, and enhanced oil recovery, and provides impact assessments for impurities present in the carbon dioxide. | 2011 | Report | [18] |
| National Energy Technology Laboratory (USA) | The report provides a recommended purity specification for carbon dioxide used for general CCS processes including for transport through carbon steel pipelines, sequestration through saline aquifers, or enhanced oil recovery. | 2012 | Report | [29] |
| Wetenhall et al. | This paper studies how high-concentration inert compounds in the carbon dioxide can affect the hydraulic stage of the CCS process and overall cost. | 2014 | Paper | [3] |
| Harkin et al. | The CarbonNet Project developed a carbon dioxide purity specification to follow for its CCS hub which intended to receive carbon dioxide from various sources for storage. The specification considered pipeline, health and safety, and technical issues. | 2017 | Paper | [30] |
| The UK Health and Safety Executive | Guidance on workplace exposure limits of contaminants including flammables and toxic compounds | 2018 | Best practice | [31] |
| ISO TC 265 | This technical report written within ISO TC 265 (carbon dioxide capture, transportation, and geological storage), provides guidance on the key impurities that could be present in carbon dioxide and impact to the CCS process. The report does not provide threshold amount fraction levels that could be adhered to. | 2020 | ISO Technical Report | [32] |
| Pipeline | Storage | Ref. | |||
|---|---|---|---|---|---|
| Saline Reservoir Sequestration | Unmineable Coal Seams | Oil and Gas Recovery | |||
| H2O a | Use same maximum limits as storage method | 300 µmol·mol−1 | [29] | ||
| H2S b | 5 µmol·mol−1 | [31] | |||
| CO b | 20 µmol·mol−1 | [31] | |||
| O2 | 4 cmol·mol−1 | 10 µmol·mol−1 | [4,16,27,29] | ||
| CH4 c | 4 cmol·mol−1 | 1 cmol·mol−1 | [4,29] | ||
| N2 c | 4 cmol·mol−1 | 1 cmol·mol−1 | [4,29] | ||
| Ar c | 4 cmol·mol−1 | 1 cmol·mol−1 | [4,29] | ||
| H2 c | 4 cmol·mol−1 | 1 cmol·mol−1 | [4,29] | ||
| SOx b | 0.5 µmol·mol−1 | [31] | |||
| NOx b | 0.5 µmol·mol−1 | [31] | |||
| NH3 b | 25 µmol·mol−1 | [31] | |||
| C2H6 | 1 cmol·mol−1 | [29] | |||
| C3+ | 1 cmol·mol−1 | [29] | |||
| Particulates | 1 µmol·mol−1 | [29] | |||
| HCl b | 1 µmol·mol−1 | [31] | |||
| HF b | 1.8 µmol·mol−1 | [31] | |||
| HCN b | 0.9 µmol·mol−1 | [31] | |||
| Hg b | 0.02 mg·m−3 | [31] | |||
| Glycol | 46 nmol·mol−1 | [29] | |||
| MEA b | 1 µmol·mol−1 | [31] | |||
| Component | Upper Limit (µmol·mol−1) | Instrument |
|---|---|---|
| H2O | 300 | Cavity ringdown spectroscopy (CRDS) Quartz crystal microbalance |
| H2S | 5 | Gas chromatography with sulphur chemiluminescence |
| CO | 20 | Gas chromatography with methaniser and flame ionisation detector (GC-Meth-FID) Gas chromatography with thermal conductivity detector (GC-TCD) Nondispersive infrared (NDIR) Gas chromatography with pulsed discharge helium ionisation detector (GC-PDHID) |
| O2 | 100 | GC-PDHID |
| 40,000 | GC-TCD | |
| CH4 | 10,000 | GC-FID GC-TCD |
| 40,000 | ||
| N2 | 10,000 | GC-TCD |
| 40,000 | ||
| Ar | 10,000 | GC-TCD |
| 40,000 | ||
| H2 | 10,000 | GC-TCD |
| 40,000 | ||
| SOx | 0.5 | Ultraviolet fluorescence spectroscopy |
| NOx | 0.5 | Chemiluminescence analyser CRDS Cavity attenuated phase shift spectroscopy |
| NH3 | 25 | Fourier-transform infrared spectroscopy (FTIR) NDIR Selected-ion flow-tube mass spectrometry (SIFT-MS) Gas chromatography with mass spectrometry |
| C2H6 | 10,000 | GC-Meth-FID GC-FID GC-TCD |
| C3+ | 10,000 | GC-Meth-FID GC-FID GC-TCD |
| Particulates | 1 | Filter and mass weighing |
| HCl | 1 | CRDS |
| HF | 1.8 | FTIR NDIR |
| HCN | 0.9 | Gas chromatography with flame thermionic detector SIFT-MS |
| Hg | 0.002 | Atomic absorption spectrometry |
| Glycol | 0.046 | Scanning Mobility Particle Spectrometer GC-FID |
| Amines | 1 | Gas chromatography with nitrogen chemiluminescence detector |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugan, A.; Brown, R.J.C.; Wilmot, R.; Hussain, D.; Bartlett, S.; Brewer, P.J.; Worton, D.R.; Bacquart, T.; Gardiner, T.; Robinson, R.A.; et al. Performing Quality Assurance of Carbon Dioxide for Carbon Capture and Storage. C 2020, 6, 76. https://doi.org/10.3390/c6040076
Murugan A, Brown RJC, Wilmot R, Hussain D, Bartlett S, Brewer PJ, Worton DR, Bacquart T, Gardiner T, Robinson RA, et al. Performing Quality Assurance of Carbon Dioxide for Carbon Capture and Storage. C. 2020; 6(4):76. https://doi.org/10.3390/c6040076
Chicago/Turabian StyleMurugan, Arul, Richard J. C. Brown, Robbie Wilmot, Delwar Hussain, Sam Bartlett, Paul J. Brewer, David R. Worton, Thomas Bacquart, Tom Gardiner, Rod A. Robinson, and et al. 2020. "Performing Quality Assurance of Carbon Dioxide for Carbon Capture and Storage" C 6, no. 4: 76. https://doi.org/10.3390/c6040076
APA StyleMurugan, A., Brown, R. J. C., Wilmot, R., Hussain, D., Bartlett, S., Brewer, P. J., Worton, D. R., Bacquart, T., Gardiner, T., Robinson, R. A., & Finlayson, A. J. (2020). Performing Quality Assurance of Carbon Dioxide for Carbon Capture and Storage. C, 6(4), 76. https://doi.org/10.3390/c6040076