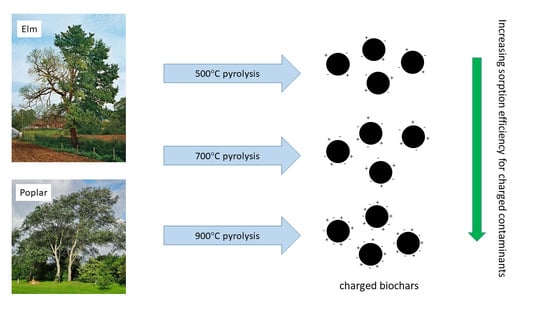
Surface Charge Effects on Adsorption of Solutes by Poplar and Elm Biochars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Chemical and Physical Properties
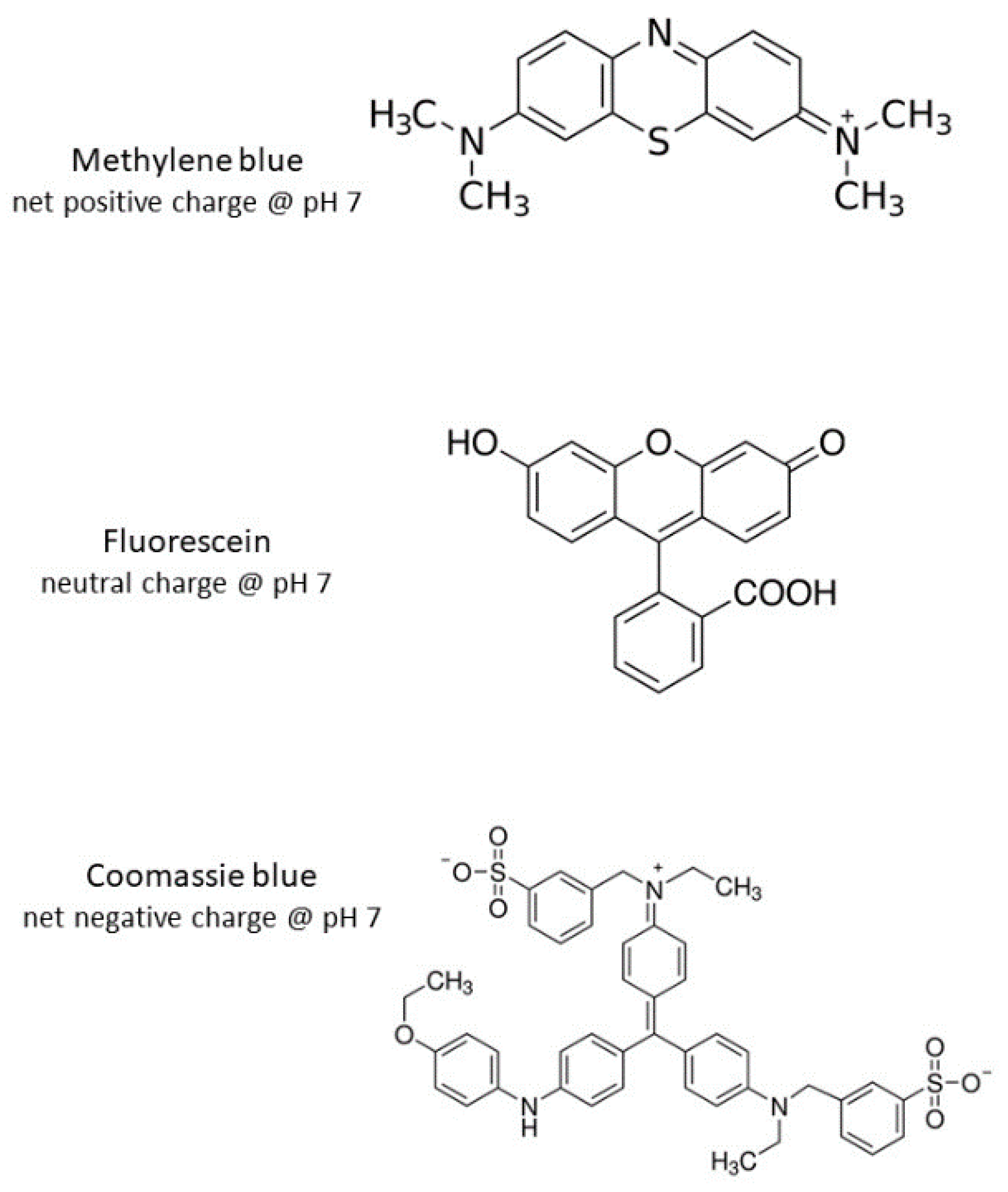
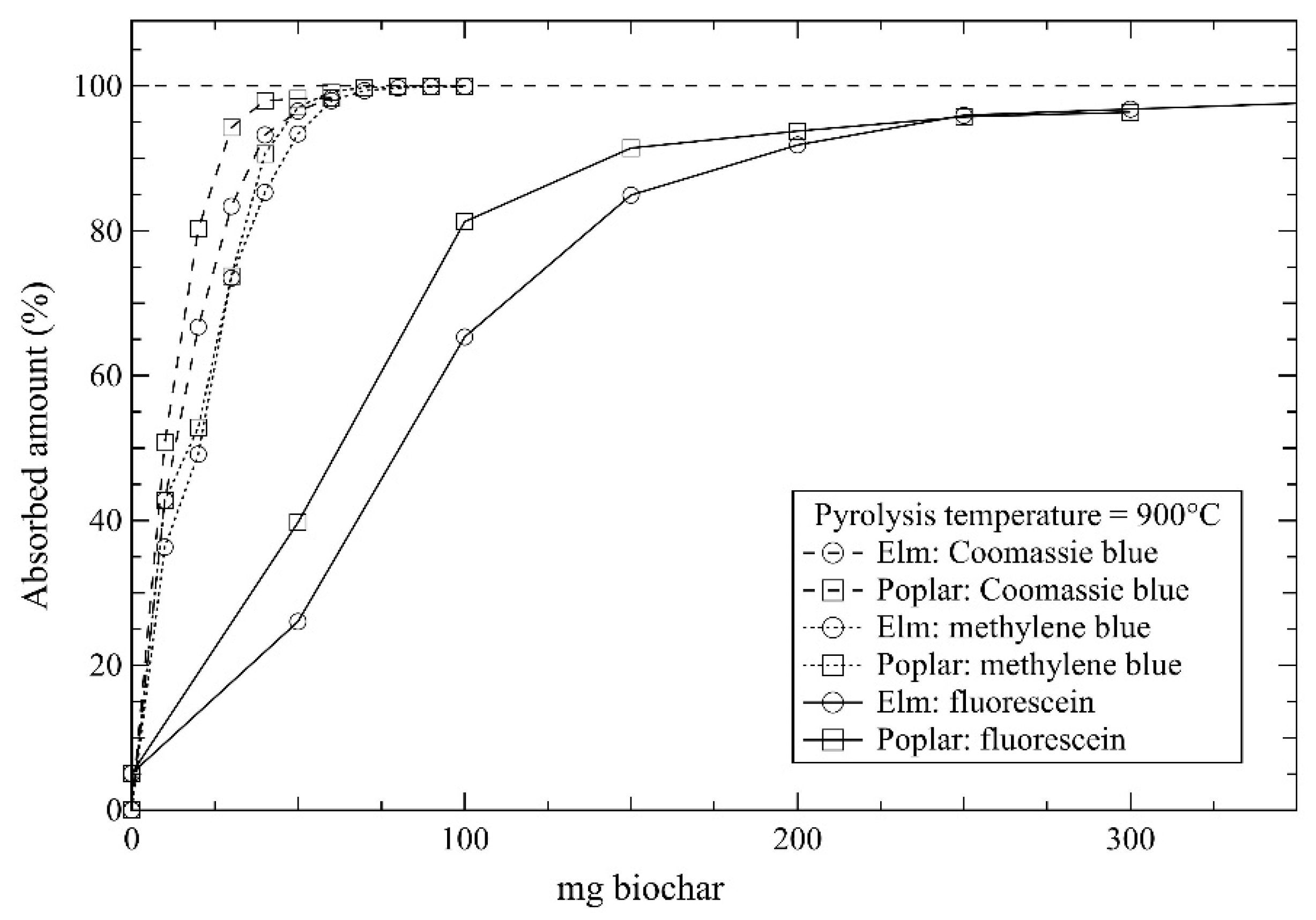
2.3. Dye Adsorption Studies
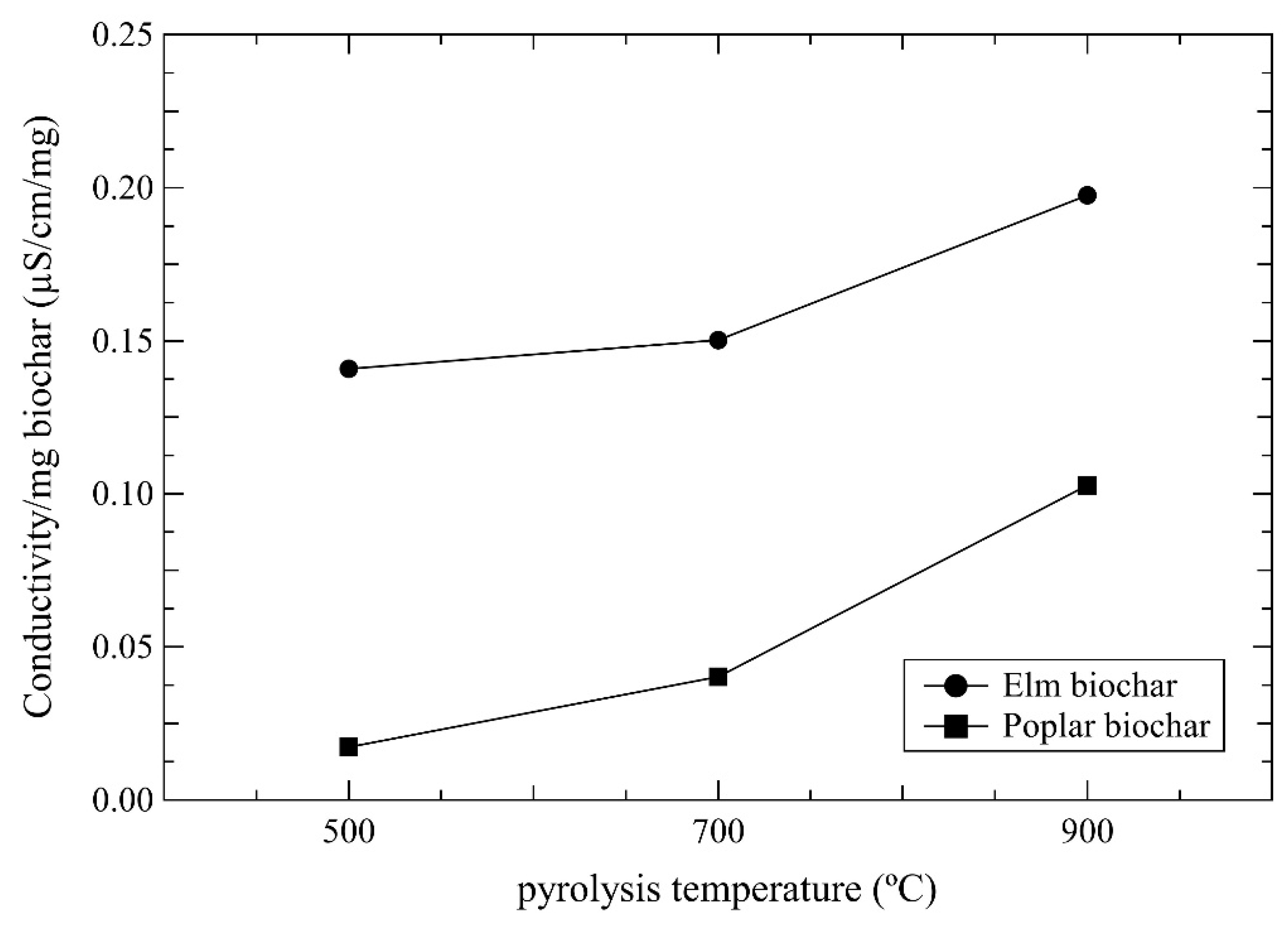
2.4. Conductivity Measurements
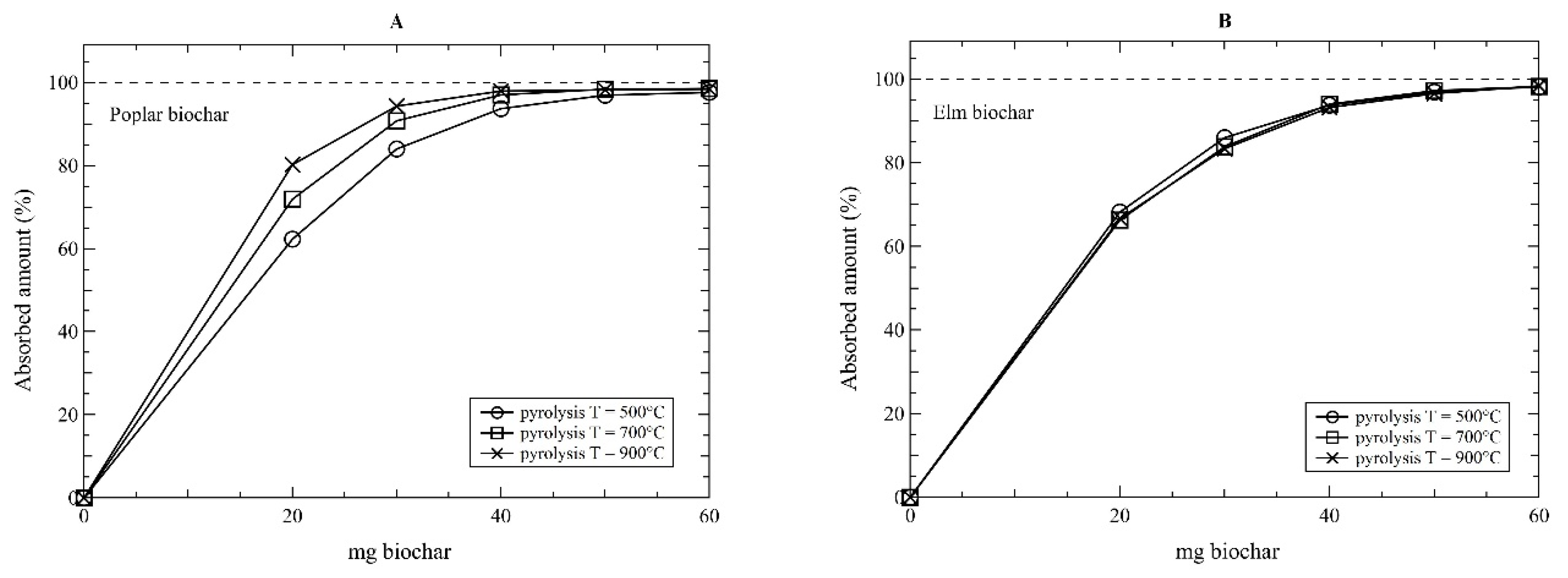
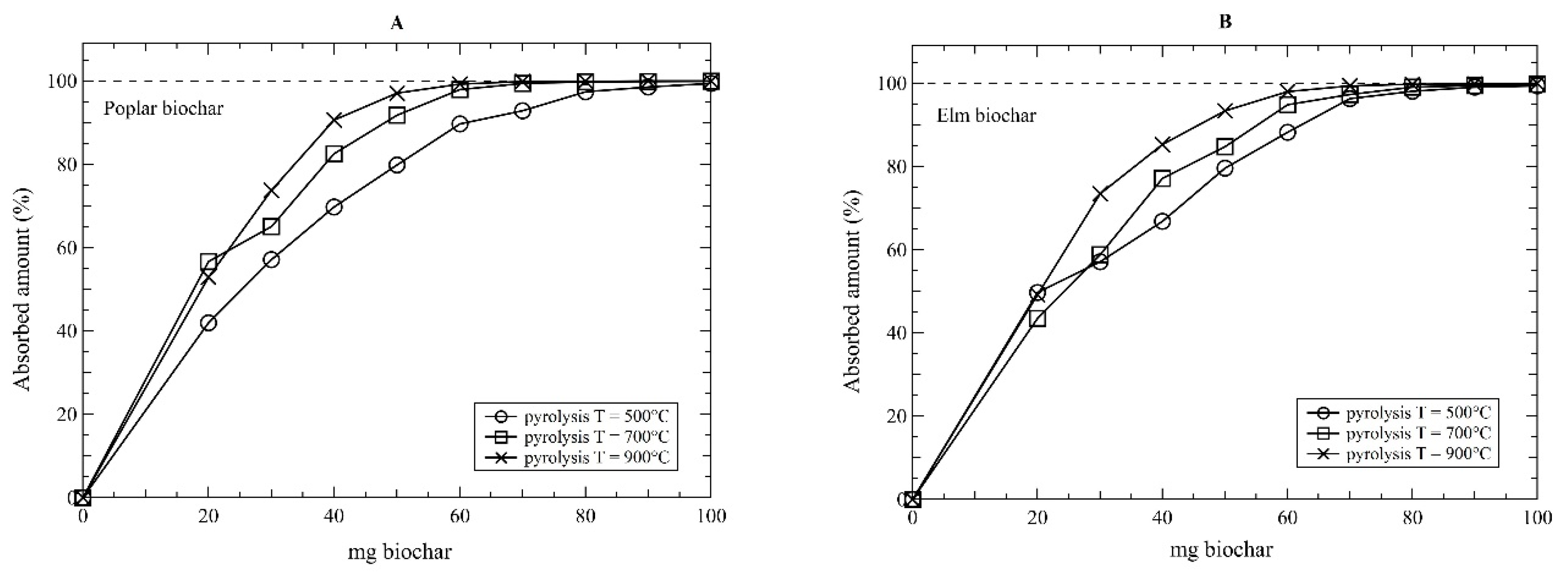
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 2nd ed.; Routledge: New York, NY, USA, 2015; 976p. [Google Scholar]
- Lee, Y.; Eum, P.-R.-B.; Ryu, C.; Park, Y.-K.; Jung, J.-H.; Hyun, S. Characteristics of biochar produced from slow pyrolysis of geodae-uksae 1. Bioresour. Technol. 2013, 130, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Liatsou, I.; Pashalidis, I.; Oezaslan, M.; Dosche, C. Surface characterization of oxidized biochar fibers derived from luffa cylindrica and lanthanide binding. J. Environ. Chem. Eng. 2017, 5, 4069–4074. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Dickenson, E. The potential role of biochar in the removal of organic and microbial contaminants from potable and reuse water: A review. Chemosphere 2015, 134, 232–240. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Wathukarage, A.; Herath, I.; Iqbal, M.C.M.; Vithanage, M. Mechanistic understanding of crystal violet dye sorption by woody biochar: Implications for wastewater treatment. Environ. Geochem. Health 2019, 41, 1647–1661. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Preparation and characterization of macroalgae biochar nanomaterials with highly efficient adsorption and photodegradation ability. Materials 2018, 11, 1709. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. High-performance porous biochar from the pyrolysis of natural and renewable seaweed (gelidiella acerosa) and its application for the adsorption of methylene blue. Bioresour. Technol. 2019, 278, 159–164. [Google Scholar] [CrossRef]
- Dai, L.; Zhu, W.; He, L.; Tan, F.; Zhu, N.; Zhou, Q.; He, M.; Hu, G. Calcium-rich biochar from crab shell: An unexpected super adsorbent for dye removal. Bioresour. Technol. 2018, 267, 510–516. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Huang, G.; Xu, C.; Lin, B. Hydrothermal carbonization synthesis of cassava slag biochar with excellent adsorption performance for rhodamine b. J.Clean. Prod. 2020, 251, 119717. [Google Scholar] [CrossRef]
- Santini, A.; Faccoli, M. Dutch elm disease and elm bark beetles: A century of association. iForest-Biogeosci. For. 2014, 8, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Lindroth, R.L.; Donaldson, J.R.; Stevens, M.T.; Gusse, A.C. Browse quality in quaking aspen (populus tremuloides): Effects of genotype, nutrients, defoliation, and coppicing. J. Chem. Ecol. 2007, 33, 1049–1064. [Google Scholar] [CrossRef] [PubMed]
- Afas, N.A.; Marron, N.; Van Dongen, S.; Laureysens, I.; Ceulemans, R. Dynamics of biomass production in a poplar coppice culture over three rotations (11 years). Forest Ecol. Manag. 2008, 255, 1883–1891. [Google Scholar] [CrossRef]
- Dillen, S.Y.; Djomo, S.N.; Al Afas, N.; Vanbeveren, S.; Ceulemans, R. Biomass yield and energy balance of a short-rotation poplar coppice with multiple clones on degraded land during 16 years. Biomass Bioenergy 2013, 56, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Nassi, O.; Di Nasso, N.; Guidi, W.; Ragaglini, G.; Tozzini, C.; Bonari, E. Biomass production and energy balance of a 12-year-old short-rotation coppice poplar stand under different cutting cycles. GCB Bioenergy 2010, 2, 89–97. [Google Scholar] [CrossRef]
- Kauter, D.; Lewandowski, I.; Claupein, W. Quantity and quality of harvestable biomass from populus short rotation coppice for solid fuel use—a review of the physiological basis and management influences. Biomass Bioenergy 2003, 24, 411–427. [Google Scholar] [CrossRef]
- Qiu, Y.; Zheng, Z.; Zhou, Z.; Sheng, G.D. Effectiveness and mechanisms of dye adsorption on a straw-based biochar. Bioresour. Technol. 2009, 100, 5348–5351. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Yang, Y.; Chun, Y.; Sheng, G.; Huang, M. Ph-dependence of pesticide adsorption by wheat-residue-derived black carbon. Langmuir 2004, 20, 6736–6741. [Google Scholar] [CrossRef]
- Peterson, S.C.; Chandrasekaran, S.R.; Sharma, B.K. Birchwood biochar as partial carbon black replacement in styrene–butadiene rubber composites. J. Elastomers Plast. 2015, 48, 305–316. [Google Scholar] [CrossRef]
- Vaughn, S.F.; Kenar, J.A.; Tisserat, B.; Jackson, M.A.; Joshee, N.; Vaidya, B.N.; Peterson, S.C. Chemical and physical properties of paulownia elongata biochar modified with oxidants for horticultural applications. Ind. Crops Prod. 2017, 97, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of pyrolysis temperature on biochar property and function as a heavy metal sorbent in soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Downie, A. Biochar Production and Use: Environmental Risks and Rewards. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2011. [Google Scholar]
- Lua, A.C.; Yang, T.; Guo, J. Effects of pyrolysis conditions on the properties of activated carbons prepared from pistachio-nut shells. J. Anal. Appl. Pyrol. 2004, 72, 279–287. [Google Scholar] [CrossRef]
- Peterson, S.C.; Jackson, M.A. Simplifying pyrolysis: Using gasification to produce corn stover and wheat straw biochar for sorptive and horticultural media. Ind. Crop. Prod. 2014, 53, 228–235. [Google Scholar] [CrossRef]
- Brown, R.A.; Kercher, A.K.; Nguyen, T.H.; Nagle, D.C.; Ball, W.P. Production and characterization of synthetic wood chars for use as surrogates for natural sorbents. Org. Geochem. 2006, 37, 321–333. [Google Scholar] [CrossRef]

| Feedstock/ Pyrolysis T (°C) | C (%) | H (%) | N (%) | O (%) a | Ash (%) | Density (g/cm3) | Surface Area (m2/g) |
|---|---|---|---|---|---|---|---|
| Elm 500 | 86.4 ± 2.7 | 1.85 ± 0.07 | 0.15 ± 0.03 | 9.1 | 2.54 | 1.58 | 8.4 |
| Elm 700 | 90.5 ± 0.7 | 1.12 ± 0.11 | 0.20 ± 0.01 | 5.6 | 2.56 | 1.66 | 318 |
| Elm 900 | 94.9 ± 0.7 | 0.55 ± 0.06 | 0.33 ± 0.01 | 1.4 | 2.83 | 1.90 | 176 |
| Poplar 500 | 89.3 ± 1.3 | 2.72 ± 0.21 | <0.05 b | 6.1 | 1.86 | 1.42 | 96 |
| Poplar 700 | 89.1 ± 4.4 | 1.52 ± 0.18 | <0.05 b | 7.4 | 1.97 | 1.66 | 373 |
| Poplar 900 | 96.5 ± 1.4 | 0.69 ± 0.09 | 0.07 ± 0.04 | 0.5 | 2.22 | 1.94 | 207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peterson, S.C.; Kim, S.; Adkins, J. Surface Charge Effects on Adsorption of Solutes by Poplar and Elm Biochars. C 2021, 7, 11. https://doi.org/10.3390/c7010011
Peterson SC, Kim S, Adkins J. Surface Charge Effects on Adsorption of Solutes by Poplar and Elm Biochars. C. 2021; 7(1):11. https://doi.org/10.3390/c7010011
Chicago/Turabian StylePeterson, Steven C., Sanghoon Kim, and Jason Adkins. 2021. "Surface Charge Effects on Adsorption of Solutes by Poplar and Elm Biochars" C 7, no. 1: 11. https://doi.org/10.3390/c7010011
APA StylePeterson, S. C., Kim, S., & Adkins, J. (2021). Surface Charge Effects on Adsorption of Solutes by Poplar and Elm Biochars. C, 7(1), 11. https://doi.org/10.3390/c7010011