Preparation and Applications of Fluorinated Graphenes
Abstract
:1. Introduction
2. The Conventional Direct Fluorination with Molecular Fluorine
3. Other Fluorinating Agents
4. Exfoliation of Graphite Fluoride
4.1. Mechanical
4.2. Thermal Exfoliation
4.3. In Liquid Media
4.4. Electrochemical Exfoliation
5. F-Diamane
6. Chemical Bonding and Related Properties
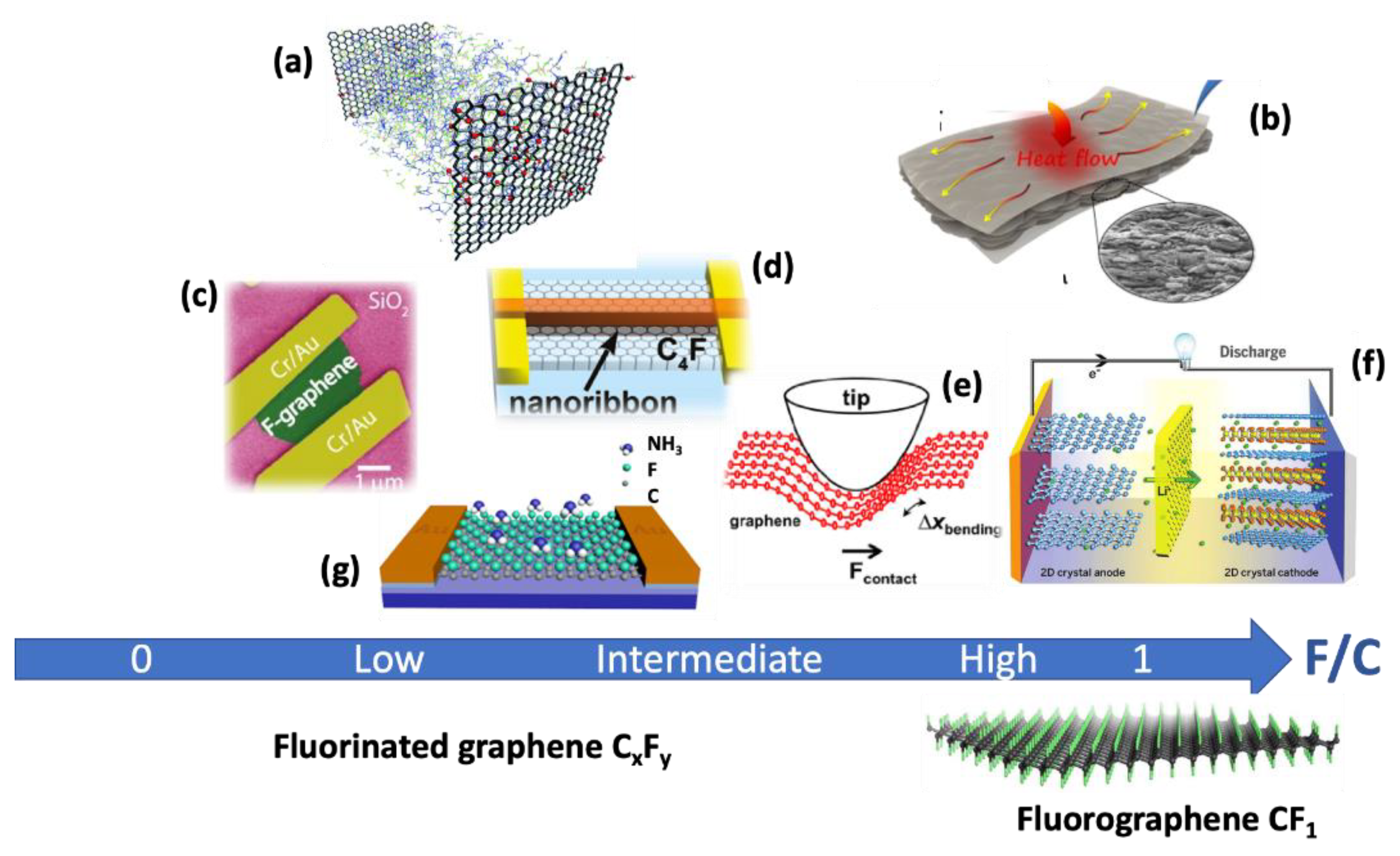
7. Applications
7.1. Tribological Performances
7.2. Energy Storage
7.3. Gas Sorption and Sensing
7.4. Nanocomposites and Coating
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pouzet, M.; Dubois, M.; Charlet, K.; Béakou, A. From hydrophilic to hydrophobic wood using direct fluorination: A localized treatment. C. R. Chim. 2018, 21, 800–807. [Google Scholar] [CrossRef]
- Pouzet, M.; Dubois, M.; Charlet, K.; Béakou, A.; Leban, J.M.; Baba, M. Fluorination renders the wood surface hydrophobic without any loss of physical and mechanical properties. Ind. Crops Prod. 2019, 133, 133–141. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [Green Version]
- Guérin, K.; Dubois, M.; Houdayer, A.; Hamwi, A. Applicative performances of fluorinated carbons through fluorination routes: A review. J. Fluor. Chem. 2012, 134, 11–17. [Google Scholar] [CrossRef]
- Lam, P.; Yazami, R. Physical characteristics and rate performance of (CFx)n (0.33 < x < 0.66) in lithium batteries. J. Power Sources 2006, 153, 354–359. [Google Scholar] [CrossRef]
- Zhong, G.; Chen, H.; Huang, X.; Yue, H.; Lu, C. High-Power-Density, High-Energy-Density Fluorinated Graphene for Primary Lithium Batteries. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Himmel, D.; Mansot, J.L.; Dubois, M.; Guérin, K.; Zhang, W.; Hamwi, A. Tribological Properties of Fluorinated Carbon Nanofibres. Tribol. Lett. 2009, 34, 49–59. [Google Scholar] [CrossRef]
- Lee, Y.-S. Syntheses and properties of fluorinated carbon materials. J. Fluor. Chem. 2007, 128, 392–403. [Google Scholar] [CrossRef]
- Sidorov, L.N.; Boltalina, O.V. Endohedral metal derivatives and exohedral fluorine derivatives of fullerenes. Russ. Chem. Rev. 2002, 71, 535–561. [Google Scholar] [CrossRef]
- Boltalina, O.V.; Galeva, N.A. Direct fluorination of fullerenes. Russ. Chem. Rev. 2000, 69, 609–621. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, L.; Wang, H.; Wang, H.; Jiao, W.; Chen, G.; Zhang, P.; Hui, D.; Jian, X. A brief review for fluorinated carbon: Synthesis, properties and applications. Nanotechnol. Rev. 2019, 8, 573–586. [Google Scholar] [CrossRef]
- Adamska, M.; Narkiewicz, U. Fluorination of Carbon Nanotubes—A Review. J. Fluor. Chem. 2017, 200, 179–189. [Google Scholar] [CrossRef]
- Nomède-Martyr, N.; Disa, E.; Thomas, P.; Romana, L.; Mansot, J.-L.; Dubois, M.; Guérin, K.; Zhang, W.; Hamwi, A. Tribological properties of fluorinated nanocarbons with different shape factors. J. Fluor. Chem. 2012, 144, 10–16. [Google Scholar] [CrossRef]
- Ahmad, Y.; Batisse, N.; Dubois, M.; Guérin, K.; Labbé, F.; Metkemeijer, R.; Berthon-Fabry, S.; Molina Concha, B.; Maillard, F.; Dubau, L.; et al. Fluorination of carbon based electrocatalysts for enhanced durability of PEMFC. In Proceedings of the SFEC Colloque de la Société Francophone d’Etude des Carbones, Carqueiranne, France, 17–20 May 2016. [Google Scholar]
- Claves, D. Spectroscopic study of fluorinated carbon nanostructures. New J. Chem. 2011, 35, 2477–2482. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craciun, M.F.; Russo, S.; Yamamoto, M.; Tarucha, S. Tuneable electronic properties in graphene. Nano Today 2011, 6, 42–60. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine Structure Constant Defines Visual Transparency of Graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.H.; Zou, K.; Okino, F.; Gutierrez, H.R.; Gupta, A.; Shen, N.; Eklund, P.C.; Sofo, J.O.; Zhu, J. Reversible fluorination of graphene: Evidence of a two-dimensional wide bandgap semiconductor. Phys. Rev. B 2010, 81, 205435. [Google Scholar] [CrossRef] [Green Version]
- Withers, F.; Dubois, M.; Savchenko, A.K. Electron properties of fluorinated single-layer graphene transistors. Phys. Rev. B 2010, 82, 073403. [Google Scholar] [CrossRef] [Green Version]
- Withers, F.; Russo, S.; Dubois, M.; Craciun, M.F. Tuning the electronic transport properties of graphene through functionalisation with fluorine. Nanoscale Res. Lett. 2011, 6, 526. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Narasaki, M.; Zhang, Z.; Takahashi, K.; Chen, J.; Zhang, X. Ultra-strong stability of double-sided fluorinated monolayer graphene and its electrical property characterization. Sci. Rep. 2020, 10, 17562. [Google Scholar] [CrossRef]
- Sofo, J.O.; Chaudhari, A.S.; Barber, G.D. Graphane: A two-dimensional hydrocarbon. Phys. Rev. B 2007, 75, 153401. [Google Scholar] [CrossRef] [Green Version]
- Boukhvalov, D.W.; Katsnelson, M.I. Chemical functionalization of graphene. J. Phys. Condens. Matter 2009, 21, 344205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.T.; Burgess, J.S.; Junkermeier, C.E.; Badescu, S.C.; Reinecke, T.L.; Perkins, F.K.; Zalalutdniov, M.K.; Baldwin, J.W.; Culbertson, J.C.; Sheehan, P.E.; et al. Properties of Fluorinated Graphene Films. Nano Lett. 2010, 10, 3001–3005. [Google Scholar] [CrossRef]
- Lee, W.-K.; Robinson, J.T.; Gunlycke, D.; Stine, R.R.; Tamanaha, C.R.; King, W.P.; Sheehan, P.E. Chemically Isolated Graphene Nanoribbons Reversibly Formed in Fluorographene Using Polymer Nanowire Masks. Nano Lett. 2011, 11, 5461–5464. [Google Scholar] [CrossRef]
- Feng, W.; Long, P.; Feng, Y.; Li, Y. Two-Dimensional Fluorinated Graphene: Synthesis, Structures, Properties and Applications. Adv. Sci. 2016, 3, 1500413. [Google Scholar] [CrossRef]
- Struzzi, C.; Scardamaglia, M.; Reckinger, N.; Sezen, H.; Amati, M.; Gregoratti, L.; Colomer, J.F.; Ewels, C.; Snyders, R.; Bittencourt, C. Probing plasma fluorinated graphene via spectromicroscopy. Phys. Chem. Chem. Phys. 2017, 19, 31418–31428. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Bakandritsos, A.; Pykal, M.; Zbořil, R.; Otyepka, M. Chemistry, properties, and applications of fluorographene. Appl. Mater. Today 2017, 9, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.S.; Sofer, Z.; Plutnar, J.; Pumera, M. Fluorographenes for Energy and Sensing Application: The Amount of Fluorine Matters. ACS Omega 2018, 3, 17700–17706. [Google Scholar] [CrossRef]
- Mazánek, V.; Jankovský, O.; Luxa, J.; Sedmidubský, D.; Janoušek, Z.; Šembera, F.; Mikulics, M.; Sofer, Z. Tuning of fluorine content in graphene: Towards large-scale production of stoichiometric fluorographene. Nanoscale 2015, 7, 13646–13655. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Wang, X.; Dong, C.; Li, B.; Liu, Y.; Chen, T.; Wu, P.; Cheng, Z.; Liu, X. Reduction and transformation of fluorinated graphene induced by ultraviolet irradiation. Phys. Chem. Chem. Phys. 2015, 17, 24056–24062. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Dai, Y.; Wang, W.; Ren, M.; Li, B.; Fan, C.; Liu, X. Fluorographene with High Fluorine/Carbon Ratio: A Nanofiller for Preparing Low-κ Polyimide Hybrid Films. ACS Appl. Mater. Interfaces 2014, 6, 16182–16188. [Google Scholar] [CrossRef]
- Fan, K.; Fu, J.; Liu, X.; Liu, Y.; Lai, W.; Liu, X.; Wang, X. Dependence of the fluorination intercalation of graphene toward high-quality fluorinated graphene formation. Chem. Sci. 2019, 10, 5546–5555. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.-G.; Zhao, G.; Liu, X.-H.; Ge, C.-W.; Wang, J.-T.; Li, B.-L.; Wang, Q.-G.; Li, W.-S.; Chen, Q.-Y. Fluorinated graphene: Facile solution preparation and tailorable properties by fluorine-content tuning. J. Mater. Chem. A 2014, 2, 8782–8789. [Google Scholar] [CrossRef]
- Urbanová, V.; Karlický, F.; Matěj, A.; Šembera, F.; Janoušek, Z.; Perman, J.A.; Ranc, V.; Čépe, K.; Michl, J.; Otyepka, M.; et al. Fluorinated graphenes as advanced biosensors—Effect of fluorine coverage on electron transfer properties and adsorption of biomolecules. Nanoscale 2016, 8, 12134–12142. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Tubon Usca, G.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Vacacela Gomez, C. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Mar, M.; Dubois, M.; Guérin, K.; Batisse, N.; Simon, B.; Bernard, P. Tuning fluorine and oxygen distribution in graphite oxifluorides for enhanced performances in primary lithium battery. Carbon 2019, 141, 6–15. [Google Scholar] [CrossRef]
- Jeon, K.-J.; Lee, Z.; Pollak, E.; Moreschini, L.; Bostwick, A.; Park, C.-M.; Mendelsberg, R.; Radmilovic, V.; Kostecki, R.; Richardson, T.J.; et al. Fluorographene: A Wide Bandgap Semiconductor with Ultraviolet Luminescence. ACS Nano 2011, 5, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Ko, J.-H.; Jeon, K.-J.; Kim, Y.-H.; Park, J.Y. Enhanced Nanoscale Friction on Fluorinated Graphene. Nano Lett. 2012, 12, 6043–6048. [Google Scholar] [CrossRef]
- Su, C.-Y.; Yang, C.-Y.; Jhang, B.-W.; Hsieh, Y.-L.; Sin, Y.-Y.; Huang, C.-C. Pool Boiling Heat Transfer Enhanced by Fluorinated Graphene as Atomic Layered Modifiers. ACS Appl. Mater. Interfaces 2020, 12, 10233–10239. [Google Scholar] [CrossRef] [PubMed]
- Stine, R.; Lee, W.-K.; Whitener, K.E.; Robinson, J.T.; Sheehan, P.E. Chemical Stability of Graphene Fluoride Produced by Exposure to XeF2. Nano Lett. 2013, 13, 4311–4316. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.L.; Sahin, H.; Jeon, K.-J.; Bostwick, A.; Horzum, S.; Koch, R.; Speck, F.; Ostler, M.; Nagel, P.; Merz, M.; et al. Luminescence, Patterned Metallic Regions, and Photon-Mediated Electronic Changes in Single-Sided Fluorinated Graphene Sheets. ACS Nano 2014, 8, 7801–7808. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.D.; Weis, J.E.; Frank, O.; Fridrichová, M.; Bastl, Z.; Kalbac, M. Do defects enhance fluorination of graphene? RSC Adv. 2016, 6, 81471–81476. [Google Scholar] [CrossRef]
- Xu, J.-Y.; Yu, J.-S.; Liao, J.-H.; Yang, X.-B.; Wu, C.-Y.; Wang, Y.; Wang, L.; Xie, C.; Luo, L.-B. Opening the Band Gap of Graphene via Fluorination for High-Performance Dual-Mode Photodetector Application. ACS Appl. Mater. Interfaces 2019, 11, 21702–21710. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Wang, T.; Cao, H.; Zhu, H.; Di, Z.; Liu, X. Antibacterial ability, cytocompatibility and hemocompatibility of fluorinated graphene. Colloids Surf. B. Biointerfaces 2019, 173, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Antonova, I.V.; Kurkina, I.I.; Gutakovskii, A.K.; Kotin, I.A.; Ivanov, A.I.; Nebogatikova, N.A.; Soots, R.A.; Smagulova, S.A. Fluorinated graphene suspension for flexible and printed electronics: Flakes, 2D films, and heterostructures. Mater. Des. 2019, 164, 107526. [Google Scholar] [CrossRef]
- Nebogatikova, N.A.; Antonova, I.V.; Prinz, V.Y.; Kurkina, I.I.; Vdovin, V.I.; Aleksandrov, G.N.; Timofeev, V.B.; Smagulova, S.A.; Zakirov, E.R.; Kesler, V.G. Fluorinated graphene dielectric films obtained from functionalized graphene suspension: Preparation and properties. Phys. Chem. Chem. Phys. 2015, 17, 13257–13266. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Li, Y.; Long, P.; Gao, Y.; Qin, C.; Cao, C.; Feng, Y.; Feng, W. Hydrothermal preparation of fluorinated graphene hydrogel for high-performance supercapacitors. J. Power Sources 2016, 312, 146–155. [Google Scholar] [CrossRef]
- Lee, M.G.; Lee, S.; Cho, J.; Bae, S.; Jho, J.Y. Effect of the Fluorination of Graphene Nanoflake on the Dispersion and Mechanical Properties of Polypropylene Nanocomposites. Nanomaterials 2020, 10, 1171. [Google Scholar] [CrossRef]
- Lee, W.H.; Suk, J.W.; Chou, H.; Lee, J.; Hao, Y.; Wu, Y.; Piner, R.; Akinwande, D.; Kim, K.S.; Ruoff, R.S. Selective-Area Fluorination of Graphene with Fluoropolymer and Laser Irradiation. Nano Lett. 2012, 12, 2374–2378. [Google Scholar] [CrossRef]
- Zhang, H.; Fan, L.; Dong, H.; Zhang, P.; Nie, K.; Zhong, J.; Li, Y.; Guo, J.; Sun, X. Spectroscopic Investigation of Plasma-Fluorinated Monolayer Graphene and Application for Gas Sensing. ACS Appl. Mater. Interfaces 2016, 8, 8652–8661. [Google Scholar] [CrossRef] [PubMed]
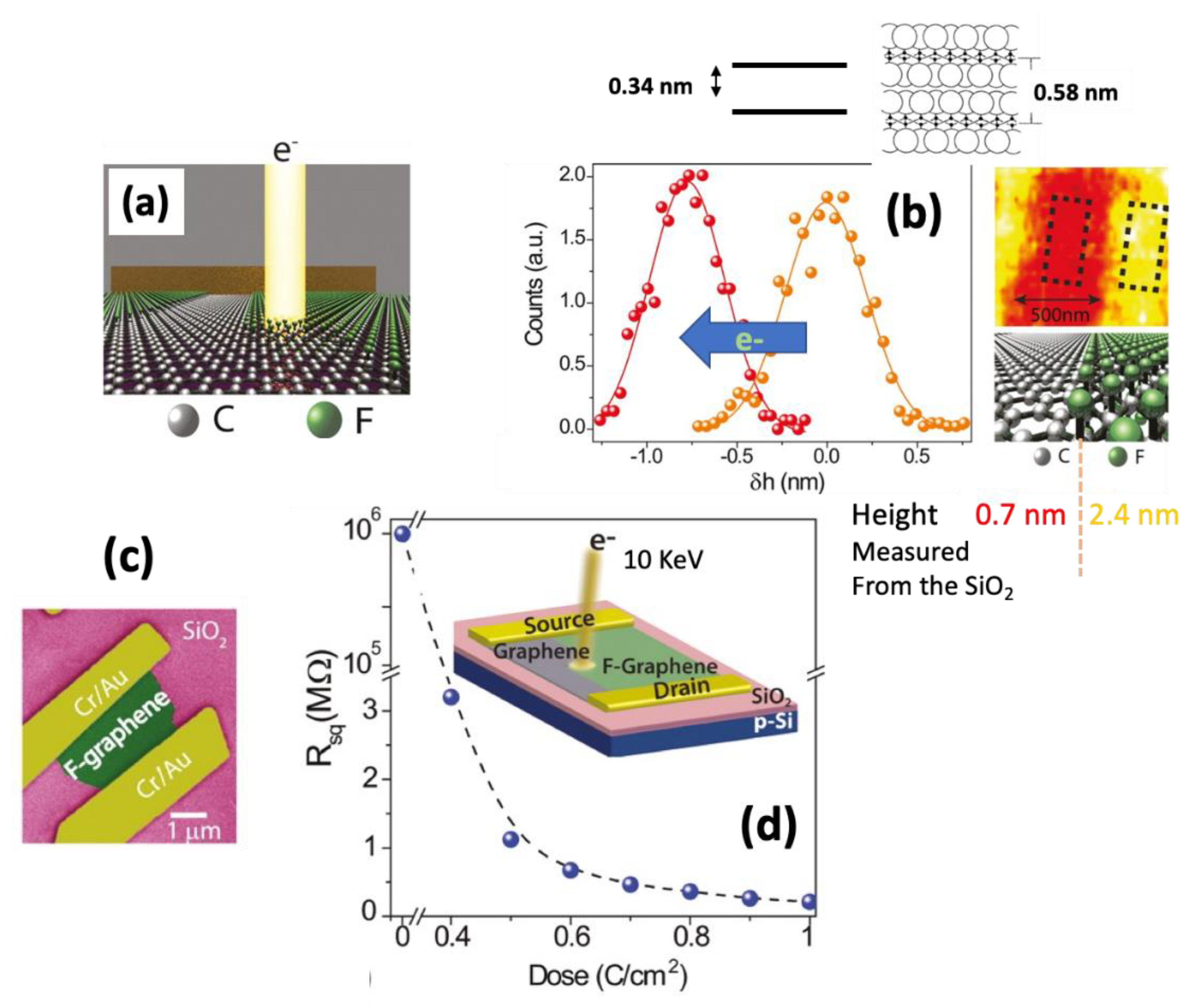
- Withers, F.; Bointon, T.H.; Dubois, M.; Russo, S.; Craciun, M.F. Nanopatterning of Fluorinated Graphene by Electron Beam Irradiation. Nano Lett. 2011, 11, 3912–3916. [Google Scholar] [CrossRef]
- Dubois, M.; Guérin, K.; Ahmad, Y.; Batisse, N.; Mar, M.; Frezet, L.; Hourani, W.; Bubendorff, J.-L.; Parmentier, J.; Hajjar-Garreau, S.; et al. Thermal exfoliation of fluorinated graphite. Carbon 2014, 77, 688–704. [Google Scholar] [CrossRef]
- Bulusheva, L.G.; Tur, V.A.; Fedorovskaya, E.O.; Asanov, I.P.; Pontiroli, D.; Riccò, M.; Okotrub, A.V. Structure and supercapacitor performance of graphene materials obtained from brominated and fluorinated graphites. Carbon 2014, 78, 137–146. [Google Scholar] [CrossRef]
- Poh, H.L.; Sofer, Z.; Klímová, K.; Pumera, M. Fluorographenes via thermal exfoliation of graphite oxide in SF6, SF4 and MoF6 atmospheres. J. Mater. Chem. C 2014, 2, 5198–5207. [Google Scholar] [CrossRef]
- Zbořil, R.; Karlický, F.; Bourlinos, A.B.; Steriotis, T.A.; Stubos, A.K.; Georgakilas, V.; Šafářová, K.; Jančík, D.; Trapalis, C.; Otyepka, M. Graphene Fluoride: A Stable Stoichiometric Graphene Derivative and its Chemical Conversion to Graphene. Small 2010, 6, 2885–2891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, L.; Yang, S.; Wang, Y.; Wang, Y.; Ling, L.; Feng, X. Fabrication of Fully Fluorinated Graphene Nanosheets Towards High-Performance Lithium Storage. Adv. Mater. Interfaces 2014, 1, 1300149. [Google Scholar] [CrossRef]
- Wang, X.; Wu, P. Highly Thermally Conductive Fluorinated Graphene Films with Superior Electrical Insulation and Mechanical Flexibility. ACS Appl. Mater. Interfaces 2019, 11, 21946–21954. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, Y.; Yu, M.; Lang, H.; Cao, X.a.; Zou, K. Dynamic Sliding Enhancement on the Friction and Adhesion of Graphene, Graphene Oxide, and Fluorinated Graphene. ACS Appl. Mater. Interfaces 2018, 10, 8214–8224. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Zhu, Y.; Che, J.; Xiao, Y. Two-dimensional transparent hydrophobic coating based on liquid-phase exfoliated graphene fluoride. Carbon 2013, 63, 149–156. [Google Scholar] [CrossRef]
- Chang, H.; Cheng, J.; Liu, X.; Gao, J.; Li, M.; Li, J.; Tao, X.; Ding, F.; Zheng, Z. Facile Synthesis of Wide-Bandgap Fluorinated Graphene Semiconductors. Chem. Eur. J. 2011, 17, 8896–8903. [Google Scholar] [CrossRef]
- Gong, P.; Wang, Z.; Wang, J.; Wang, H.; Li, Z.; Fan, Z.; Xu, Y.; Han, X.; Yang, S. One-pot sonochemical preparation of fluorographene and selective tuning of its fluorine coverage. J. Mater. Chem. 2012, 22, 16950–16956. [Google Scholar] [CrossRef]
- Gong, P.; Wang, J.; Sun, W.; Wu, D.; Wang, Z.; Fan, Z.; Wang, H.; Han, X.; Yang, S. Tunable photoluminescence and spectrum split from fluorinated to hydroxylated graphene. Nanoscale 2014, 6, 3316–3324. [Google Scholar] [CrossRef]
- Ye, X.; Ma, L.; Yang, Z.; Wang, J.; Wang, H.; Yang, S. Covalent Functionalization of Fluorinated Graphene and Subsequent Application as Water-based Lubricant Additive. ACS Appl. Mater. Interfaces 2016, 8, 7483–7488. [Google Scholar] [CrossRef]
- Hou, K.; Gong, P.; Wang, J.; Yang, Z.; Wang, Z.; Yang, S. Structural and tribological characterization of fluorinated graphene with various fluorine contents prepared by liquid-phase exfoliation. RSC Adv. 2014, 4, 56543–56551. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Sun, W.; Li, S.; Zhu, T.; Liu, W.; Liu, G. Superhydrophobic epoxy coating modified by fluorographene used for anti-corrosion and self-cleaning. Appl. Surf. Sci. 2017, 401, 146–155. [Google Scholar] [CrossRef]
- Vu, M.C.; Thi Thieu, N.A.; Lim, J.-H.; Choi, W.-K.; Chan Won, J.; Islam, M.A.; Kim, S.-R. Ultrathin thermally conductive yet electrically insulating exfoliated graphene fluoride film for high performance heat dissipation. Carbon 2020, 157, 741–749. [Google Scholar] [CrossRef]
- Yogapriya, R.; Kasibhatta, K.R.D. Hydrophobic-Superoleophilic Fluorinated Graphene Nanosheet Composites with Metal–Organic Framework HKUST-1 for Oil–Water Separation. ACS Appl. Nano Mater. 2020, 3, 5816–5825. [Google Scholar] [CrossRef]
- Lei, F.; Yang, M.; Jiang, F.; Zhang, H.; Zhang, Z.; Sun, D. Microwave-assisted liquid phase exfoliation of graphite fluoride into fluorographene. Chem. Eng. J. 2019, 360, 673–679. [Google Scholar] [CrossRef]
- Zhu, M.; Xie, X.; Guo, Y.; Chen, P.; Ou, X.; Yu, G.; Liu, M. Fluorographene nanosheets with broad solvent dispersibility and their applications as a modified layer in organic field-effect transistors. Phys. Chem. Chem. Phys. 2013, 15, 20992–21000. [Google Scholar] [CrossRef]
- Sun, C.; Feng, Y.; Li, Y.; Qin, C.; Zhang, Q.; Feng, W. Solvothermally exfoliated fluorographene for high-performance lithium primary batteries. Nanoscale 2014, 6, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, J.; Li, Z.; Gong, P.; Ren, J.; Wang, H.; Han, X.; Yang, S. Cooperatively exfoliated fluorinated graphene with full-color emission. RSC Adv. 2012, 2, 11681–11686. [Google Scholar] [CrossRef]
- Gong, P.; Du, J.; Wang, D.; Cao, B.; Tian, M.; Wang, Y.; Sun, L.; Ji, S.; Liu, Z. Fluorinated graphene as an anticancer nanocarrier: An experimental and DFT study. J. Mater. Chem. B 2018, 6, 2769–2777. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, H.; Heidarpour, A.; Ghasemi, S. Electrochemical synthesis of fluorinated graphene nanoplatelets in electrolytes containing hydrofluoric acid and TiO2 nanoparticles. FlatChem 2020, 22, 100172. [Google Scholar] [CrossRef]
- Ribas, M.A.; Singh, A.K.; Sorokin, P.B.; Yakobson, B.I. Patterning nanoroads and quantum dots on fluorinated graphene. Nano Res. 2011, 4, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Kvashnin, A.G.; Chernozatonskii, L.A.; Yakobson, B.I.; Sorokin, P.B. Phase Diagram of Quasi-Two-Dimensional Carbon, From Graphene to Diamond. Nano Lett. 2014, 14, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Antipina, L.Y.; Sorokin, P.B. Converting Chemically Functionalized Few-Layer Graphene to Diamond Films: A Computational Study. J. Phys. Chem. C 2015, 119, 2828–2836. [Google Scholar] [CrossRef]
- Leenaerts, O.; Peelaers, H.; Hernández-Nieves, A.D.; Partoens, B.; Peeters, F.M. First-principles investigation of graphene fluoride and graphane. Phys. Rev. B 2010, 82, 195436. [Google Scholar] [CrossRef] [Green Version]
- Odkhuu, D.; Shin, D.; Ruoff, R.S.; Park, N. Conversion of multilayer graphene into continuous ultrathin sp3-bonded carbon films on metal surfaces. Sci. Rep. 2013, 3, 3276. [Google Scholar] [CrossRef] [Green Version]
- Bakharev, P.V.; Huang, M.; Saxena, M.; Lee, S.W.; Joo, S.H.; Park, S.O.; Dong, J.; Camacho-Mojica, D.C.; Jin, S.; Kwon, Y.; et al. Chemically induced transformation of chemical vapour deposition grown bilayer graphene into fluorinated single-layer diamond. Nat. Nanotechnol. 2020, 15, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekaran, S.; Abild-Pedersen, F.; Ogasawara, H.; Nilsson, A.; Kaya, S. Interlayer Carbon Bond Formation Induced by Hydrogen Adsorption in Few-Layer Supported Graphene. Phys. Rev. Lett. 2013, 111, 085503. [Google Scholar] [CrossRef] [PubMed]
- Piazza, F.; Kelvin, C.; Monthioux, M.; Puech, P.; Gerber, I. Raman evidence for the successful synthesis of diamane. Carbon 2020, 169, 129–133. [Google Scholar] [CrossRef]
- Piazza, F.; Gough, K.; Monthioux, M.; Puech, P.; Gerber, I.; Wiens, R.; Paredes, G.; Ozoria, C. Low temperature, pressureless sp2 to sp3 transformation of ultrathin, crystalline carbon films. Carbon 2019, 145, 10–22. [Google Scholar] [CrossRef]
- Piazza, F.; Monthioux, M.; Puech, P.; Gerber, I.C. Towards a better understanding of the structure of diamanoïds and diamanoïd/graphene hybrids. Carbon 2020, 156, 234–241. [Google Scholar] [CrossRef]
- Mortazavi, B.; Shojaei, F.; Javvaji, B.; Azizi, M.; Zhan, H.; Rabczuk, T.; Zhuang, X. First-principles investigation of mechanical, electronic and optical properties of H-, F- and Cl-diamane. Appl. Surf. Sci. 2020, 528, 147035. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhan, H.; Nie, Y.; Xu, X.; Qi, D.; Gu, Y. Single layer diamond—A new ultrathin 2D carbon nanostructure for mechanical resonator. Carbon 2020, 161, 809–815. [Google Scholar] [CrossRef]
- Sato, Y.; Shiraishi, S.; Mazej, Z.; Hagiwara, R.; Ito, Y. Direct conversion mechanism of fluorine–GIC into poly(carbon monofluoride), (CF)n. Carbon 2003, 41, 1971–1977. [Google Scholar] [CrossRef]
- Sato, Y.; Itoh, K.; Hagiwara, R.; Fukunaga, T.; Ito, Y. On the so-called “semi-ionic” C–F bond character in fluorine–GIC. Carbon 2004, 42, 3243–3249. [Google Scholar] [CrossRef]
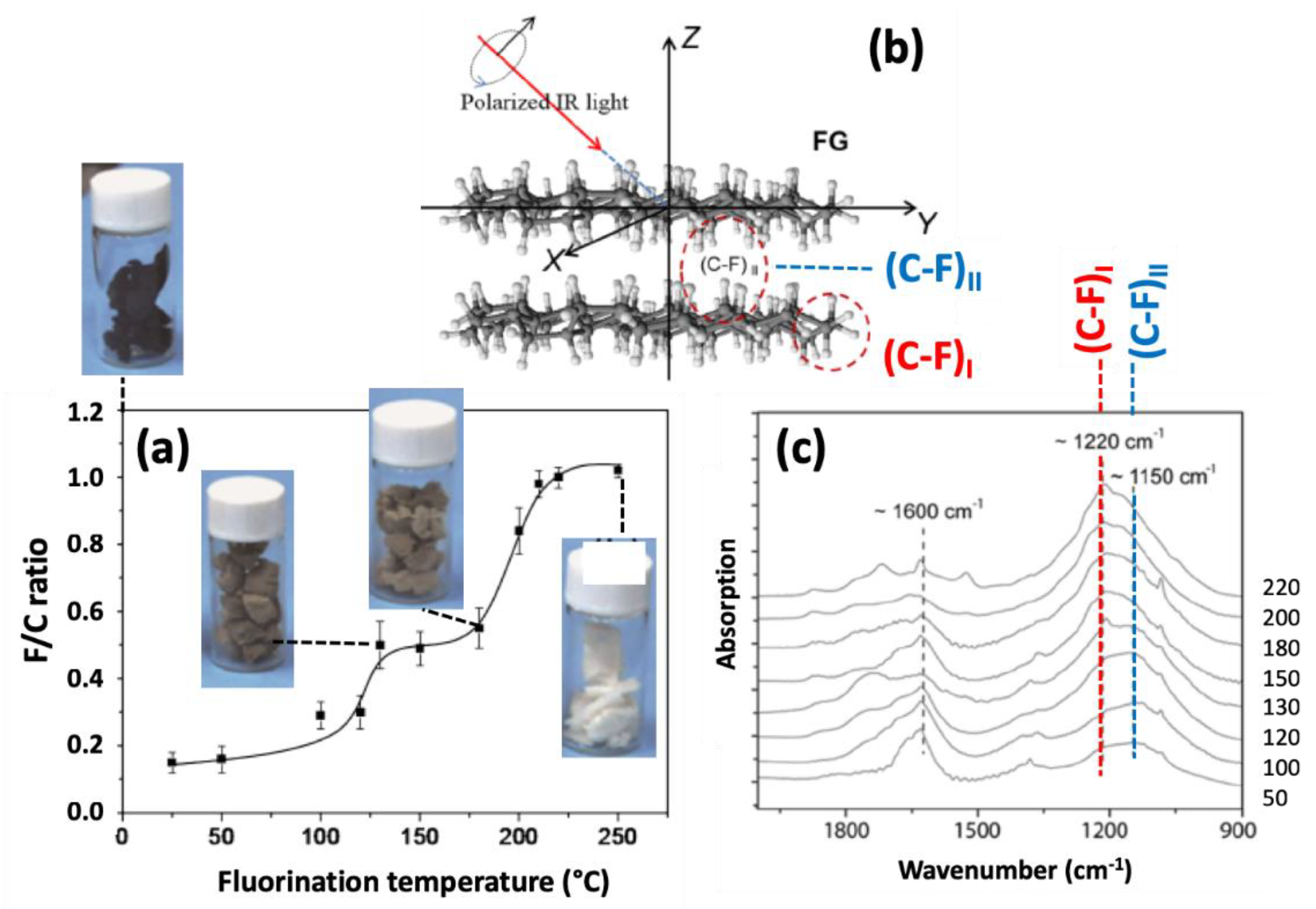
- Wang, X.; Wang, W.; Liu, Y.; Ren, M.; Xiao, H.; Liu, X. Characterization of Conformation and Locations of C–F Bonds in Graphene Derivative by Polarized ATR-FTIR. Anal. Chem. 2016, 88, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Koon, G.K.W.; Shin, D.W.; Fedorov, V.E.; Choi, J.-Y.; Yoo, J.-B.; Özyilmaz, B. Property Control of Graphene by Employing “Semi-Ionic” Liquid Fluorination. Adv. Funct. Mater. 2013, 23, 3329–3334. [Google Scholar] [CrossRef]
- Dubecký, M.; Otyepková, E.; Lazar, P.; Karlický, F.; Petr, M.; Čépe, K.; Banáš, P.; Zbořil, R.; Otyepka, M. Reactivity of Fluorographene: A Facile Way toward Graphene Derivatives. J. Phys. Chem. Lett. 2015, 6, 1430–1434. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Wang, W.; Qin, R.; Lai, W.; Ou, A.; Liu, Y.; Liu, X. Nitrogen-Doping Chemical Behavior of Graphene Materials with Assistance of Defluorination. J. Phys. Chem. C 2019, 123, 584–592. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Rasheed, H.E. The reduction of friction in axially non-cylindrical journal bearings using grooved bearing shells. In Tribology Series; Dowson, D., Taylor, C.M., Childs, T.H.C., Dalmaz, G., Berthier, Y., Flamand, L., Georges, J.M., Lubrecht, A.A., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 34, pp. 535–541. [Google Scholar]
- Barnes, A.M.; Bartle, K.D.; Thibon, V.R.A. A review of zinc dialkyldithiophosphates (ZDDPS): Characterisation and role in the lubricating oil. Tribol. Int. 2001, 34, 389–395. [Google Scholar] [CrossRef]
- Keresztes, R.; Odrobina, M.; Nagarajan, R.; Subramanian, K.; Kalacska, G.; Sukumaran, J. Tribological characteristics of cast polyamide 6 (PA6G) matrix and their composite (PA6G SL) under normal and overload conditions using dynamic pin-on-plate system. Compos. Part B Eng. 2019, 160, 119–130. [Google Scholar] [CrossRef]
- Ci, X.; Zhao, W.; Luo, J.; Wu, Y.; Ge, T.; Xue, Q.; Gao, X.; Fang, Z. How the fluorographene replaced graphene as nanoadditive for improving tribological performances of GTL-8 based lubricant oil. Friction 2020. [Google Scholar] [CrossRef]
- Spear, J.C.; Ewers, B.W.; Batteas, J.D. 2D-nanomaterials for controlling friction and wear at interfaces. Nano Today 2015, 10, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Liu, D.; Song, Z.; Dai, K. Isosteric design of friction-reduction and anti-wear lubricant additives with less sulfur content. Friction 2018, 6, 164–182. [Google Scholar] [CrossRef]
- Ky, D.L.C.; Tran Khac, B.-C.; Le, C.T.; Kim, Y.S.; Chung, K.-H. Friction characteristics of mechanically exfoliated and CVD-grown single-layer MoS2. Friction 2018, 6, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Kasar, A.K.; Menezes, P.L. Synthesis and recent advances in tribological applications of graphene. Int. J. Adv. Manuf. Technol. 2018, 97, 3999–4019. [Google Scholar] [CrossRef]
- Taghioskoui, M. Trends in graphene research. Mater. Today 2009, 12, 34–37. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Chen, X.; Luo, J. Fluorinated Graphene: A Promising Macroscale Solid Lubricant under Various Environments. ACS Appl. Mater. Interfaces 2019, 11, 40470–40480. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Sumant, A.V. Extraordinary Macroscale Wear Resistance of One Atom Thick Graphene Layer. Adv. Funct. Mater. 2014, 24, 6640–6646. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Jin, L.; Li, L.; Mo, Y.; Su, G.; Li, X.; Zhu, H.; Tian, Y. Recent advances in friction and lubrication of graphene and other 2D materials: Mechanisms and applications. Friction 2019, 7, 199–216. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Wang, J.; Liu, S.; Mu, B.; Ren, J.; Wang, H.; Yang, S. Tribology Study of Reduced Graphene Oxide Sheets on Silicon Substrate Synthesized via Covalent Assembly. Langmuir 2010, 26, 15830–15836. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Peng, H.; Liu, Z. Chemistry Makes Graphene beyond Graphene. J. Am. Chem. Soc. 2014, 136, 12194–12200. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Chen, X.; Wang, X.; Liu, X.; Liu, Y.; Lai, W.; Liu, X. Toward Excellent Tribological Performance as Oil-Based Lubricant Additive: Particular Tribological Behavior of Fluorinated Graphene. ACS Appl. Mater. Interfaces 2018, 10, 28828–28838. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, Y.; Gao, H.; Min, S.; Li, S.; Liu, H.K.; Guo, Z. Surface Engineering and Design Strategy for Surface-Amorphized TiO2@Graphene Hybrids for High Power Li-Ion Battery Electrodes. Adv. Sci. 2015, 2, 1500027. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Song, H.; Wang, Z.; Yang, J.; Jia, X.; Zhang, Z. Facile synthesis of copper/polydopamine functionalized graphene oxide nanocomposites with enhanced tribological performance. Chem. Eng. J. 2017, 324, 51–62. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Martini, A. Atomic roughness enhanced friction on hydrogenated graphene. Nanotechnology 2013, 24, 375701. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P.; et al. Tribological properties of oleic acid-modified graphene as lubricant oil additives. J. Phys. D Appl. Phys. 2011, 44, 205303. [Google Scholar] [CrossRef]
- Lee, W.-K.; Haydell, M.; Robinson, J.T.; Laracuente, A.R.; Cimpoiasu, E.; King, W.P.; Sheehan, P.E. Nanoscale Reduction of Graphene Fluoride via Thermochemical Nanolithography. ACS Nano 2013, 7, 6219–6224. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, Y.; Lang, H.; Liu, L. Controllable Nanotribological Properties of Graphene Nanosheets. Sci. Rep. 2017, 7, 41891. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, Q.; Qi, Y.; Cheng, Y.; Wang, H.; Li, Q.; Meng, Y. Wear evolution of monolayer graphene at the macroscale. Carbon 2017, 115, 600–607. [Google Scholar] [CrossRef]
- Bhowmick, S.; Banerji, A.; Alpas, A.T. Role of humidity in reducing sliding friction of multilayered graphene. Carbon 2015, 87, 374–384. [Google Scholar] [CrossRef]
- Arif, T.; Colas, G.; Filleter, T. Effect of Humidity and Water Intercalation on the Tribological Behavior of Graphene and Graphene Oxide. ACS Appl. Mater. Interfaces 2018, 10, 22537–22544. [Google Scholar] [CrossRef]
- Inagaki, M.; Kang, F. Graphene derivatives: Graphane, fluorographene, graphene oxide, graphyne and graphdiyne. J. Mater. Chem. A 2014, 2, 13193–13206. [Google Scholar] [CrossRef]
- Nair, R.R.; Ren, W.; Jalil, R.; Riaz, I.; Kravets, V.G.; Britnell, L.; Blake, P.; Schedin, F.; Mayorov, A.S.; Yuan, S.; et al. Fluorographene: A Two-Dimensional Counterpart of Teflon. Small 2010, 6, 2877–2884. [Google Scholar] [CrossRef]
- Huang, W.; Pei, Q.-X.; Liu, Z.; Zhang, Y.-W. Thermal conductivity of fluorinated graphene: A non-equilibrium molecular dynamics study. Chem. Phys. Lett. 2012, 552, 97–101. [Google Scholar] [CrossRef]
- Meduri, P.; Chen, H.; Xiao, J.; Martinez, J.J.; Carlson, T.; Zhang, J.-G.; Deng, Z.D. Tunable electrochemical properties of fluorinated graphene. J. Mater. Chem. A 2013, 1, 7866–7869. [Google Scholar] [CrossRef]
- Mori, T.; Kikuzawa, Y.; Takeuchi, H. N-type field-effect transistor based on a fluorinated-graphene. Org. Electron. 2008, 9, 328–332. [Google Scholar] [CrossRef]
- Samarakoon, D.K.; Chen, Z.; Nicolas, C.; Wang, X.-Q. Structural and Electronic Properties of Fluorographene. Small 2011, 7, 965–969. [Google Scholar] [CrossRef]
- Ho, K.-I.; Huang, C.-H.; Liao, J.-H.; Zhang, W.; Li, L.-J.; Lai, C.-S.; Su, C.-Y. Fluorinated Graphene as High Performance Dielectric Materials and the Applications for Graphene Nanoelectronics. Sci. Rep. 2014, 4, 5893. [Google Scholar] [CrossRef]
- Ko, J.-H.; Kwon, S.; Byun, I.-S.; Choi, J.S.; Park, B.H.; Kim, Y.-H.; Park, J.Y. Nanotribological Properties of Fluorinated, Hydrogenated, and Oxidized Graphenes. Tribol. Lett. 2013, 50, 137–144. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Zeng, Z.; Zhao, H.; Ge, X.; Wang, K.; Wang, L.; Xue, Q. PEGlated graphene as nanoadditive for enhancing the tribological properties of water-based lubricants. Carbon 2018, 137, 41–48. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Gao, J.; Huang, J.; Li, B.; Fan, C.; Yang, J.; Liu, X. High-Yield Production of Highly Fluorinated Graphene by Direct Heating Fluorination of Graphene-oxide. ACS Appl. Mater. Interfaces 2013, 5, 8294–8299. [Google Scholar] [CrossRef]
- Lai, W.; Xu, D.; Wang, X.; Wang, Z.; Liu, Y.; Zhang, X.; Li, Y.; Liu, X. Defluorination and covalent grafting of fluorinated graphene with TEMPO in a radical mechanism. Phys. Chem. Chem. Phys. 2017, 19, 24076–24081. [Google Scholar] [CrossRef]
- Ye, X.; Gong, P.; Wang, J.; Wang, H.; Ren, S.; Yang, S. Fluorinated graphene reinforced polyimide films with the improved thermal and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2015, 75, 96–103. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.-Z.; Kim, S.-P.; Shenoy, V.B.; Sheehan, P.E.; Robinson, J.T.; Carpick, R.W. Fluorination of Graphene Enhances Friction Due to Increased Corrugation. Nano Lett. 2014, 14, 5212–5217. [Google Scholar] [CrossRef]
- Fan, K.; Liu, X.; Liu, Y.; Li, Y.; Chen, Y.; Meng, Y.; Liu, X.; Feng, W.; Luo, L. Covalent functionalization of fluorinated graphene through activation of dormant radicals for water-based lubricants. Carbon 2020, 167, 826–834. [Google Scholar] [CrossRef]
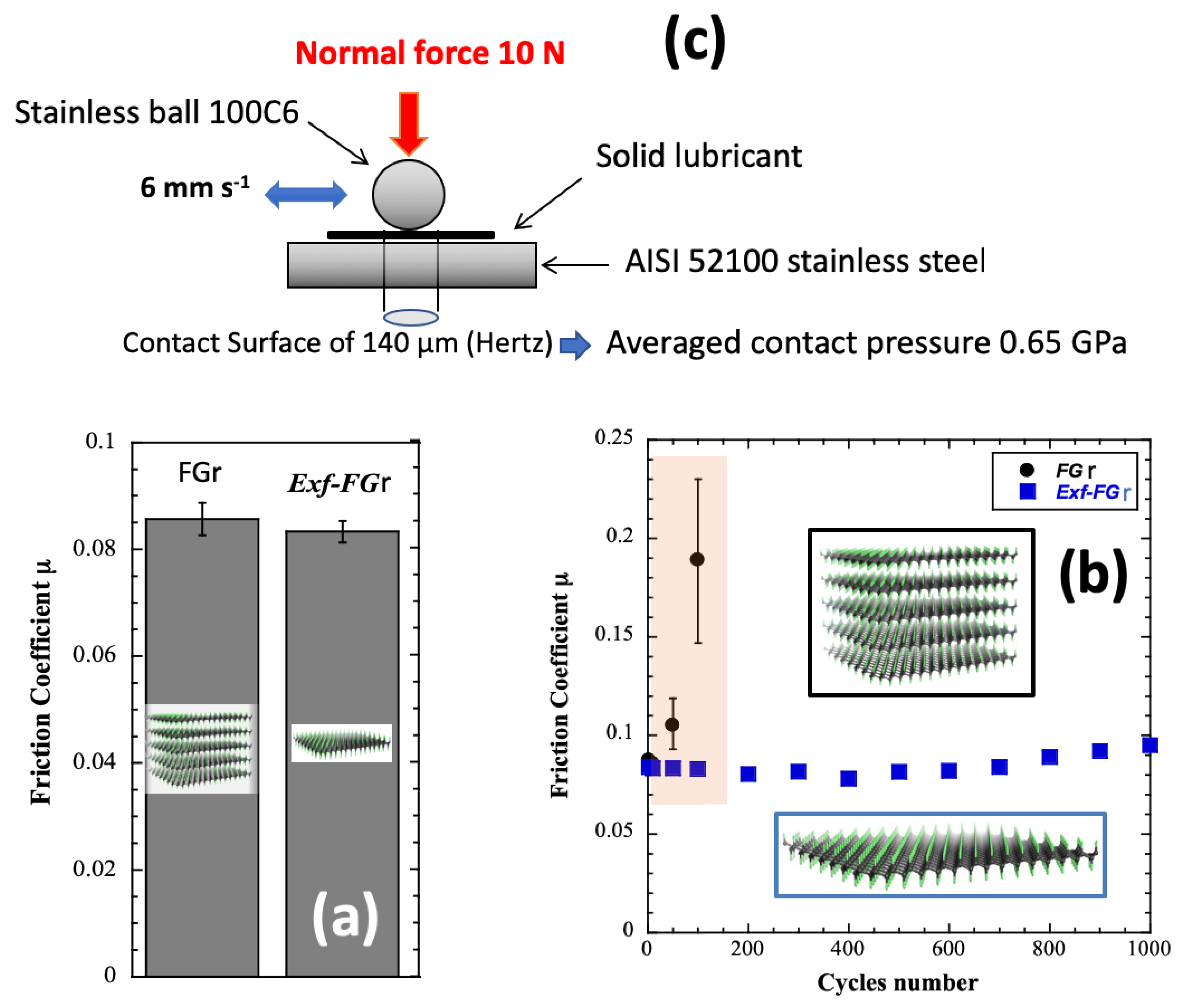
- Herraiz, M.; Dubois, M.; Batisse, N.; Petit, E.; Thomas, P. Exfoliated fluorinated carbons with a low and stable friction coefficient. RSC Adv. 2019, 9, 13615–13622. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.; Liu, Y.; Baek, J.-B.; Dai, L. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhao, X.; Chen, S.; Li, H.; Fu, X.; Wu, Q.; Li, S.; Li, Y.; Su, B.-L.; Ruoff, R.S. One-pot fabrication of FePt/reduced graphene oxide composites as highly active and stable electrocatalysts for the oxygen reduction reaction. Carbon 2014, 68, 755–762. [Google Scholar] [CrossRef]
- Lim, C.S.; Sofer, Z.; Toh, R.J.; Eng, A.Y.S.; Luxa, J.; Pumera, M. Iridium- and Osmium-decorated Reduced Graphenes as Promising Catalysts for Hydrogen Evolution. Chemphyschem 2015, 16, 1898–1905. [Google Scholar] [CrossRef]
- Damien, D.; Sudeep, P.M.; Narayanan, T.N.; Anantharaman, M.R.; Ajayan, P.M.; Shaijumon, M.M. Fluorinated graphene based electrodes for high performance primary lithium batteries. RSC Adv. 2013, 3, 25702–25706. [Google Scholar] [CrossRef]
- Cheng, H.; Mao, Y.; Xie, J.; Lu, Y.; Zhao, X. Dendrite-Free Fluorinated Graphene/Lithium Anodes Enabling in Situ LiF Formation for High-Performance Lithium–Oxygen Cells. ACS Appl. Mater. Interfaces 2019, 11, 39737–39745. [Google Scholar] [CrossRef]
- Zhang, K.; Lee, G.-H.; Park, M.; Li, W.; Kang, Y.-M. Recent Developments of the Lithium Metal Anode for Rechargeable Non-Aqueous Batteries. Adv. Energy Mater. 2016, 6, 1600811. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Wei, F.; Zhang, J.-G.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Park, C.-M.; Kim, J.-H.; Kim, H.; Sohn, H.-J. Li-alloy based anode materials for Li secondary batteries. Chem. Soc. Rev. 2010, 39, 3115–3141. [Google Scholar] [CrossRef]
- Peng, W.; Li, H.; Song, S. Synthesis of Fluorinated Graphene/CoAl-Layered Double Hydroxide Composites as Electrode Materials for Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 5204–5212. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xue, Q.; Ling, C.; Shan, M.; Liu, Z.; Tao, Y.; Li, X. Fluorine-Modified Porous Graphene as Membrane for CO2/N2 Separation: Molecular Dynamic and First-Principles Simulations. J. Phys. Chem. C 2014, 118, 7369–7376. [Google Scholar] [CrossRef]
- Kang, W.; Li, S. Preparation of fluorinated graphene to study its gas sensitivity. RSC Adv. 2018, 8, 23459–23467. [Google Scholar] [CrossRef] [Green Version]
- Park, M.-S.; Kim, K.H.; Kim, M.-J.; Lee, Y.-S. NH3 gas sensing properties of a gas sensor based on fluorinated graphene oxide. Colloids Surf. Phys. Eng. Asp. 2016, 490, 104–109. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, J.S.; Choi, Y.-R.; Park, S.Y.; Lee, S.Y.; Sohn, W.; Shim, Y.-S.; Lee, J.-H.; Park, C.R.; Choi, Y.S.; et al. Chemically fluorinated graphene oxide for room temperature ammonia detection at ppb levels. J. Mater. Chem. A 2017, 5, 19116–19125. [Google Scholar] [CrossRef]
- Katkov, M.V.; Sysoev, V.I.; Gusel’nikov, A.V.; Asanov, I.P.; Bulusheva, L.G.; Okotrub, A.V. A backside fluorine-functionalized graphene layer for ammonia detection. Phys. Chem. Chem. Phys. 2015, 17, 444–450. [Google Scholar] [CrossRef]
- Sysoev, V.I.; Okotrub, A.V.; Asanov, I.P.; Gevko, P.N.; Bulusheva, L.G. Advantage of graphene fluorination instead of oxygenation for restorable adsorption of gaseous ammonia and nitrogen dioxide. Carbon 2017, 118, 225–232. [Google Scholar] [CrossRef]
- Chia, X.; Ambrosi, A.; Otyepka, M.; Zbořil, R.; Pumera, M. Fluorographites (CFx)n Exhibit Improved Heterogeneous Electron-Transfer Rates with Increasing Level of Fluorination: Towards the Sensing of Biomolecules. Chem. A Eur. J. 2014, 20, 6665–6671. [Google Scholar] [CrossRef]
- Urbanová, V.; Holá, K.; Bourlinos, A.B.; Čépe, K.; Ambrosi, A.; Loo, A.H.; Pumera, M.; Karlický, F.; Otyepka, M.; Zbořil, R. Thiofluorographene–Hydrophilic Graphene Derivative with Semiconducting and Genosensing Properties. Adv. Mater. 2015, 27, 2305–2310. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, A.R.; Sidhureddy, B.; Keeler, W.; Chen, A. Facile one-pot synthesis of fluorinated graphene oxide for electrochemical sensing of heavy metal ions. Electrochem. Commun. 2017, 76, 42–46. [Google Scholar] [CrossRef]
- Manikandan, V.S.S.B.; Thiruppathi, A.R.; Chen, A. Sensitive Electrochemical Detection of Caffeic Acid in Wine Based on Fluorine-Doped Graphene Oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [Green Version]
- Hajian, S.; Zhang, X.; Khakbaz, P.; Tabatabaei, S.; Maddipatla, D.; Narakathu, B.B.; Blair, R.G.; Atashbar, M.Z. Development of a Fluorinated Graphene-Based Resistive Humidity Sensor. IEEE Sens. J. 2020, 20, 7517–7524. [Google Scholar] [CrossRef]
- DeYoung, A.D.; Park, S.-W.; Dhumal, N.R.; Shim, Y.; Jung, Y.; Kim, H.J. Graphene Oxide Supercapacitors: A Computer Simulation Study. J. Phys. Chem. C 2014, 118, 18472–18480. [Google Scholar] [CrossRef]
- Ji, L.; Meduri, P.; Agubra, V.; Xiao, X.; Alcoutlabi, M. Graphene-Based Nanocomposites for Energy Storage. Adv. Energy Mater. 2016, 6, 1502159. [Google Scholar] [CrossRef] [Green Version]


| Solvent | Boiling Point (°C) | Surface Tension at 20 °C (mN/m) | Relative Polarity | Exfoliation Method | Exfoliation Temperature (°C) | Post-Processing | Ref |
|---|---|---|---|---|---|---|---|
| Sulfolane | 285 | 46.0 (50 °C) | 0.41 | Sonication (B) | 50 | Separate supernatant | [64] |
| IPA | 82.5 | 23.0 | 0.55 | Sonication | - | - | [26] |
| IPA | 82.5 | 23.0 | 0.55 | Sonication | - | Centrifuge; Freeze drying | [65] |
| IPA | 82.5 | 23.0 | 0.55 | Sonication (B) | - | Centrifuge | [66] |
| Ethanol | 78.4 | 22.1 | 0.65 | Sonication | - | - | [67] |
| Ethanol | 78.4 | 22.1 | 0.65 | Sonication (B) | - | Remove sediment using separatory funnel | [68] |
| [bmim]Br | - | - | - | Sonication (B) | - | Centrifuge; Wash with ethanol | [69] |
| NMP | 202 | 40.8 | 0.36 | Sonication (B) | RT | - | [70] |
| NMP | 202 | 40.8 | 0.36 | Sonication (B) | RT | Wash with water; Freeze drying | [71] |
| NMP | 202 | 40.8 | 0.36 | Sonication (B) | RT | Filtration; Freeze drying | [72] |
| NMP | 202 | 40.8 | 0.36 | Sonication (B) | RT | Filtration; Freeze drying | [73] |
| NMP | 202 | 40.8 | 0.36 | Sonication (B) | - | Centrifuge; Washing; Freeze drying | [74] |
| NMP | 202 | 40.8 | 0.36 | Sonication (B) | RT | - | [111] |
| NMP | 202 | 40.8 | 0.36 | Ball milling | RT | Centrifuge; Washing; Freeze drying | [75] |
| DMF | 153 | 37.1 | 0.39 | Sonication | - | Separate supernatant | [76] |
| DMF | 153 | 37.1 | 0.39 | Sonication | - | Centrifuge | [77] |
| Chloroform | 61.2 | 29.9 (0 °C) | 0.26 | Sonication (P) | 0 | Centrifuge | [78] |
| Chloroform | 61.2 | 27.5 | 0.26 | Sonication | RT | Re-disperse in NMP; Centrifuge; Filtration | [79] |
| Acetonitrile | 82 | 28.9 | 0.46 | ||||
| Water (surfactant) | 100 | - | 1.00 | Sonication | RT | Filtration; Wash with water; Freeze drying | [80] |
| Water | 100 | 72.8 | 1.00 | Sonication | - | - | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, Y.; Batisse, N.; Chen, X.; Dubois, M. Preparation and Applications of Fluorinated Graphenes. C 2021, 7, 20. https://doi.org/10.3390/c7010020
Ahmad Y, Batisse N, Chen X, Dubois M. Preparation and Applications of Fluorinated Graphenes. C. 2021; 7(1):20. https://doi.org/10.3390/c7010020
Chicago/Turabian StyleAhmad, Yasser, Nicolas Batisse, Xianjue Chen, and Marc Dubois. 2021. "Preparation and Applications of Fluorinated Graphenes" C 7, no. 1: 20. https://doi.org/10.3390/c7010020
APA StyleAhmad, Y., Batisse, N., Chen, X., & Dubois, M. (2021). Preparation and Applications of Fluorinated Graphenes. C, 7(1), 20. https://doi.org/10.3390/c7010020







