Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications
Abstract
:1. Introduction
2. Properties
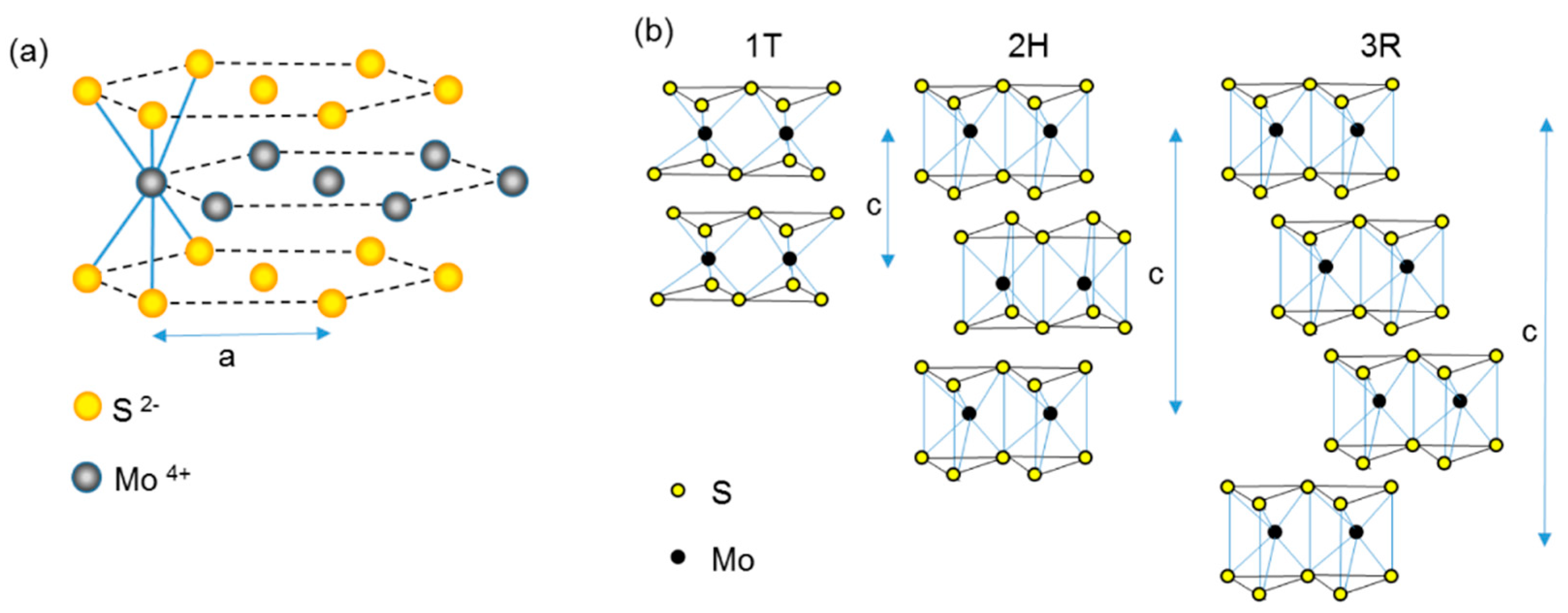
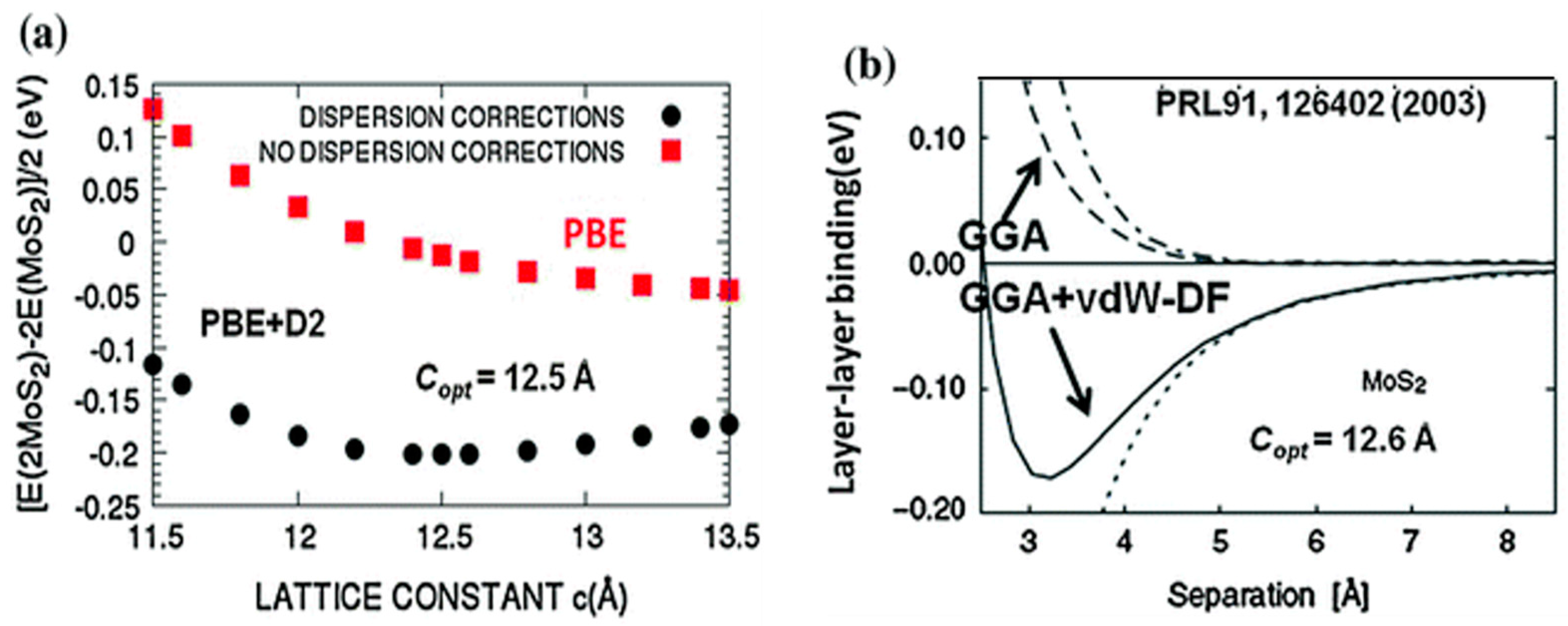
2.1. Structure
2.2. Electronic Properties
2.3. Optical Properties
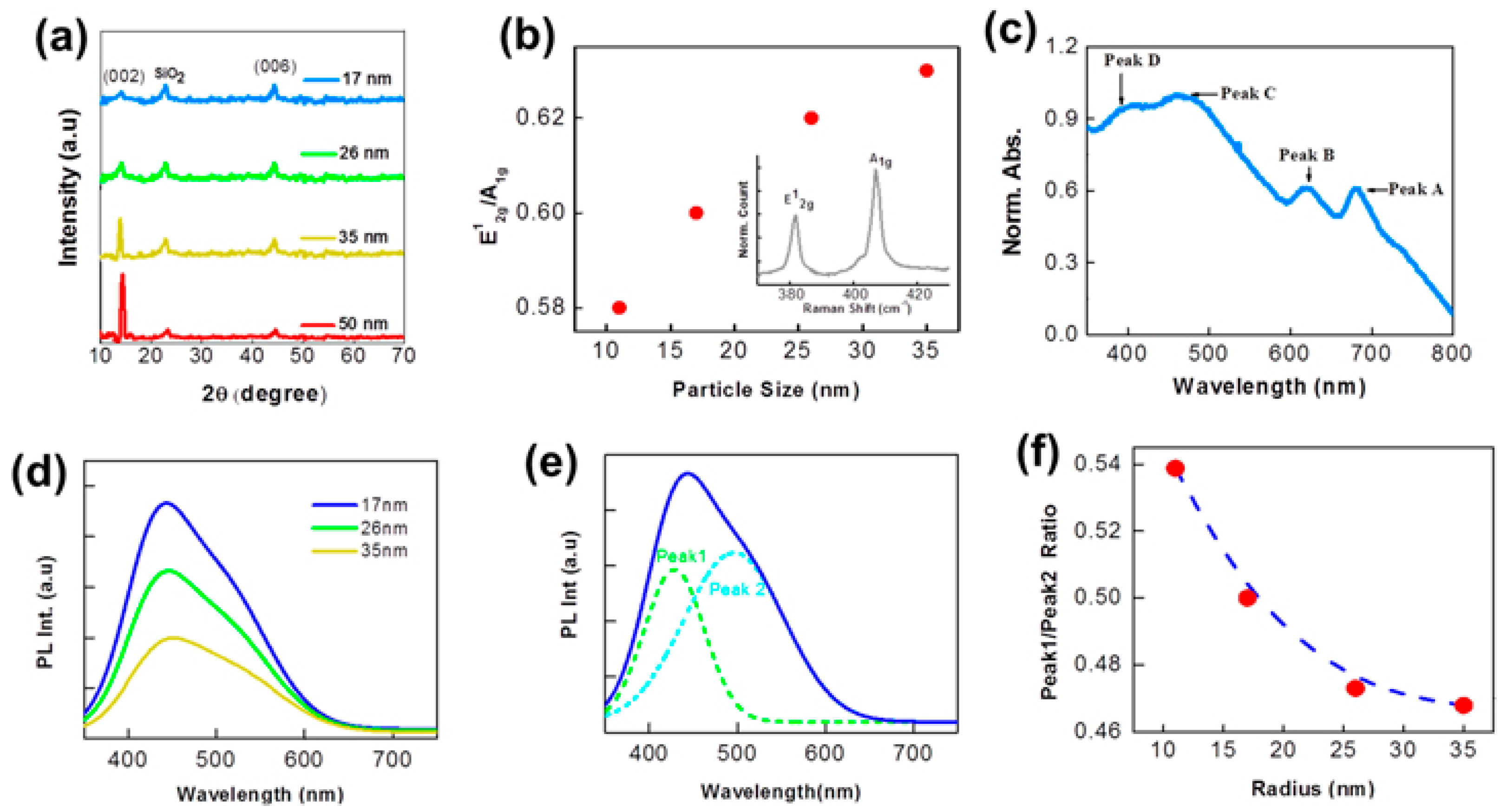
2.3.1. Size-Dependent Emission
2.3.2. Excitation-Dependent Emission
2.4. Electrocatalytic Properties
2.5. Biological Properties
2.5.1. Cytotoxicity
2.5.2. Fluorescence Stability
3. Synthesis Techniques
3.1. Top-Down
3.1.1. Sonication-Assisted Exfoliation
3.1.2. Ion Intercalation-Assisted Exfoliation

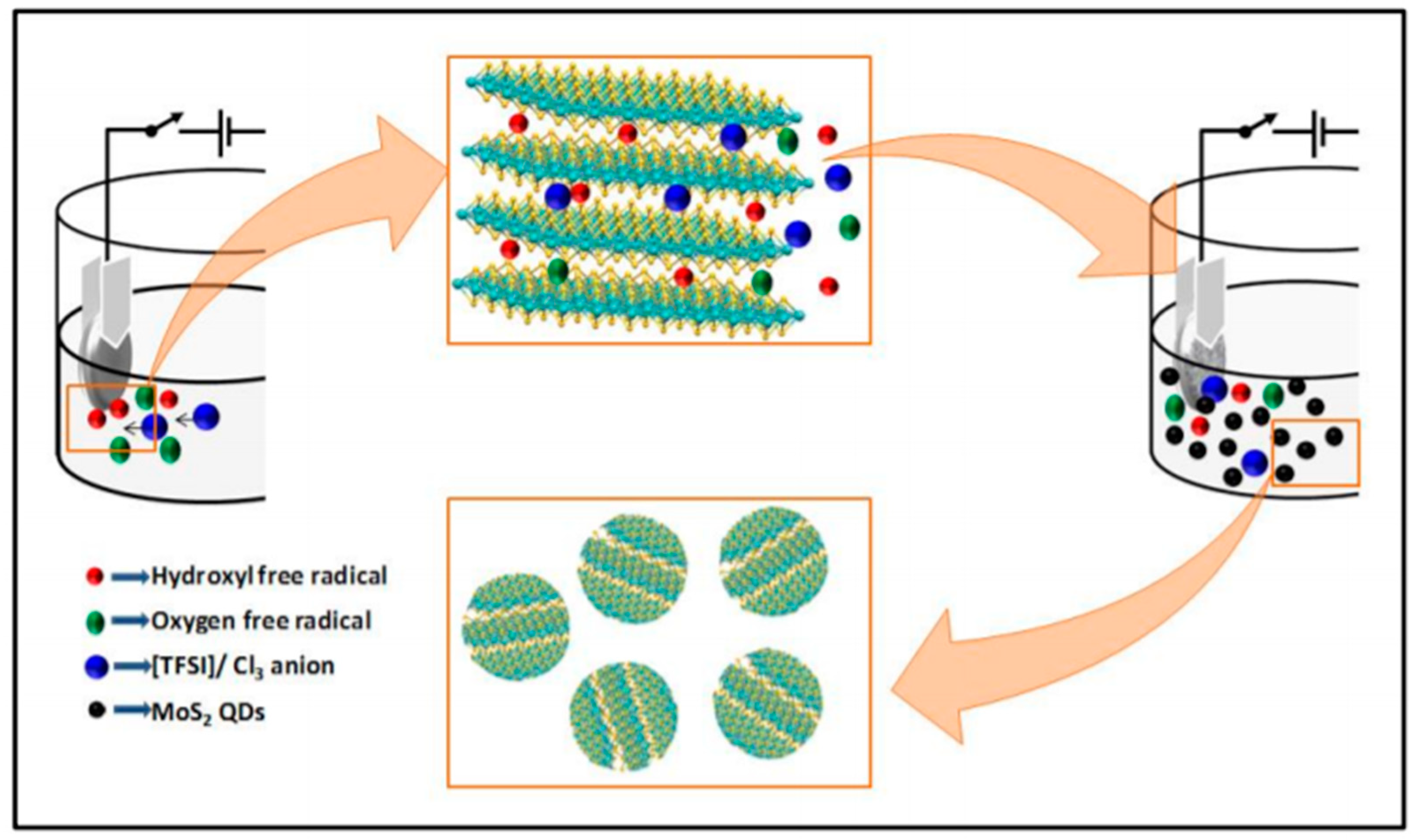
3.1.3. Electrochemical Synthesis
3.1.4. Thermal Ablation
3.1.5. Microwave Heating
3.2. Bottom-Up
3.2.1. Hydrothermal Synthesis
3.2.2. Chemical Bath Deposition
4. Applications
4.1. Energy
4.1.1. Electrocatalysis
4.1.2. Solar Cells
4.1.3. Energy Storage
4.2. Electronic and Optoelectronic Devices
4.2.1. FETs
4.2.2. Photodetectors and Phototransistors
4.2.3. LEDs
4.2.4. Resistive Switching/Memory Devices
4.3. Chemical Sensors
4.4. Biological Applications
4.4.1. Bioimaging
4.4.2. Photothermal Therapy (PTT) and Photodynamic Therapy (PDT) and Radiation Therapy (RT)
4.4.3. Biosensing
4.4.4. Other Theranostic Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Bertolazzi, S.; Samorì, P. A Universal Approach toward Light-Responsive Two-Dimensional Electronics: Chemically Tailored Hybrid van der Waals Heterostructures. ACS Nano 2019, 13, 4814–4825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Meng, X.; Deng, D.; Bao, X. Confinement Catalysis with 2D Materials for Energy Conversion. Adv. Mater. 2019, 31, e1901996. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Kostarelos, K.; Prato, M.; Bianco, A. Biomedical Uses for 2D Materials Beyond Graphene: Current Advances and Challenges Ahead. Adv. Mater. 2016, 28, 6052–6074. [Google Scholar] [CrossRef] [Green Version]
- McHugh, K.J.; Jing, L.; Severt, S.Y.; Cruz, M.; Sarmadi, M.; Jayawardena, H.S.N.; Perkinson, C.F.; Larusson, F.; Rose, S.; Tomasic, S.; et al. Biocompatible near-infrared quantum dots delivered to the skin by microneedle patches record vaccination. Sci. Transl. Med. 2019, 11, eaay7162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Lee, W.C.; Kong, X.Y.; Tan, L.-L.; Gui, M.M.; Sumathi, S.; Chai, S.-P. Molybdenum disulfide quantum dots decorated bismuth sulfide as a superior noble-metal-free photocatalyst for hydrogen evolution through harnessing a broad solar spectrum. Appl. Catal. B Environ. 2018, 232, 117–123. [Google Scholar] [CrossRef]
- Saha, A.; Sinhamahapatra, A.; Kang, T.-H.; Ghosh, S.C.; Yu, J.-S.; Panda, A.B. Hydrogenated MoS2 QD-TiO2 heterojunction mediated efficient solar hydrogen production. Nanoscale 2017, 9, 17029–17036. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, J.; Ruan, Y.; Ao, X.; Ostrikov, K.; Zhang, W.; Lu, J.; Li, Y.Y. Construction of MoO2 Quantum Dot–Graphene and MoS2 Nanoparticle–Graphene Nanoarchitectures toward Ultrahigh Lithium Storage Capability. ACS Appl. Mater. Interfaces 2017, 9, 28441–28450. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.; Cho, Y.; Hong, J.; Lee, J.; Lee, S.; Hou, B.; An, G.-H.; Lee, Y.-W.; Jang, J.E.; Im, H.; et al. Consecutive Junction-Induced Efficient Charge Separation Mechanisms for High-Performance MoS2/Quantum Dot Phototransistors. ACS Appl. Mater. Interfaces 2018, 10, 38264–38271. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, Z.; Zhang, Y.; Li, H.-Y.; Gao, N.; Tian, Z.; Zhou, L.; Zhang, B.; Tang, J.; Zhang, J.; et al. MoS2 Nanosheets Sensitized with Quantum Dots for Room-Temperature Gas Sensors. Nano-Micro Lett. 2020, 12, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Faramarzi, V.; Ahmadi, V.; Fotouhi, B.; Abasifard, M. A potential sensing mechanism for DNA nucleobases by optical properties of GO and MoS2 Nanopores. Sci. Rep. 2019, 9, 6230. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiu, W.; Sun, Y.; Zhu, D.; Zhang, Q.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. RGD-QD-MoS2 nanosheets for targeted fluorescent imaging and photothermal therapy of cancer. Nanoscale 2017, 9, 15835–15845. [Google Scholar] [CrossRef] [PubMed]
- DOE Awards $20 Million for Research on Rare Earth Elements. Department of Energy. Available online: https://www.energy.gov/articles/doe-awards-20-million-research-rare-earth-elements (accessed on 21 March 2021).
- Guo, Y.; Li, J. MoS2 quantum dots: Synthesis, properties and biological applications. Mater. Sci. Eng. C 2020, 109, 110511. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Setyawati, M.I.; Zou, H.L.; Dong, J.X.; Luo, H.Q.; Li, N.B.; Leong, D.T. Emerging 0D Transition-Metal Dichalcogenides for Sensors, Biomedicine, and Clean Energy. Small 2017, 13, 1700527. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, D.; Yap, Y.K. Recent Advances in Electronic and Optoelectronic Devices Based on Two-Dimensional Transition Metal Dichalcogenides. Electronics 2017, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Ye, M.; Winslow, D.; Zhang, D.; Pandey, R.; Yap, Y.K. Recent Advancement on the Optical Properties of Two-Dimensional Molybdenum Disulfide (MoS2) Thin Films. Photonics 2015, 2, 288–307. [Google Scholar] [CrossRef] [Green Version]
- Enyashin, A.N.; Yadgarov, L.; Houben, L.; Popov, I.; Weidenbach, M.; Tenne, R.; Bar-Sadan, M.; Seifert, G. New Route for Stabilization of 1T-WS2 and MoS2 Phases. J. Phys. Chem. C 2011, 115, 24586–24591. [Google Scholar] [CrossRef] [Green Version]
- Kadantsev, E. Electronic Structure of Exfoliated MoS2; Springer International Publishing Switzerland: London, UK, 2014; pp. 37–51. [Google Scholar] [CrossRef]
- Mattheiss, L.F. Band Structures of Transition-Metal-Dichalcogenide Layer Compounds. Phys. Rev. B 1973, 8, 3719–3740. [Google Scholar] [CrossRef]
- Coehoorn, R.; Haas, C.; Dijkstra, J.; Flipse, C.J.F.; de Groot, R.A.; Wold, A. Electronic structure of MoSe2, MoS2, and WSe2. I. Band-structure calculations and photoelectron spectroscopy. Phys. Rev. B 1987, 35, 6195–6202. [Google Scholar] [CrossRef] [Green Version]
- Coehoorn, R.; Haas, C.; de Groot, R.A. Electronic structure of MoSe2, MoS2, and WSe2. II. The nature of the optical band gaps. Phys. Rev. B 1987, 35, 6203–6206. [Google Scholar] [CrossRef] [Green Version]
- Böker, T.; Severin, R.; Müller, A.; Janowitz, C.; Manzke, R.; Voß, D.; Krüger, P.; Mazur, A.; Pollmann, J. Band structure of MoS2, MoSe2, and α−MoTe2:Angle-resolved photoelectron spectroscopy andab initiocalculations. Phys. Rev. B 2001, 64, 235305. [Google Scholar] [CrossRef] [Green Version]
- Jin, W.; Yeh, P.-C.; Zaki, N.; Zhang, D.; Sadowski, J.T.; Al-Mahboob, A.; van der Zande, A.M.; Chenet, D.A.; Dadap, J.I.; Herman, I.P.; et al. Direct Measurement of the Thickness-Dependent Electronic Band Structure of MoS2 Using Angle-Resolved Photoemission Spectroscopy. Phys. Rev. Lett. 2013, 111, 106801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuc, A.; Zibouche, N.; Heine, T. Influence of quantum confinement on the electronic structure of the transition metal sulfideTS2. Phys. Rev. B 2011, 83, 245213. [Google Scholar] [CrossRef] [Green Version]
- Consadori, F.; Frindt, R.F. Crystal Size Effects on the Exciton Absorption Spectrum of WSe2. Phys. Rev. B 1970, 2, 4893–4896. [Google Scholar] [CrossRef]
- Evans, B.L.; A Young, P. Exciton spectra in thin crystals: The diamagnetic effect. Proc. Phys. Soc. 1967, 91, 475–482. [Google Scholar] [CrossRef]
- Yoffe, A.D. Low-dimensional systems: Quantum size effects and electronic properties of semiconductor microcrystallites (zero-dimensional systems) and some quasi-two-dimensional systems. Adv. Phys. 1993, 42, 173–262. [Google Scholar] [CrossRef]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.Y.; Cheng, Y.C.; Schwingenschlögl, U. Giant spin-orbit-induced spin splitting in two-dimensional transition-metal dichalcogenide semiconductors. Phys. Rev. B 2011, 84, 153402. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-H.; Fan, X.; Lin, S.-H.; Kuo, J.-L. Orbital analysis of electronic structure and phonon dispersion in MoS2, MoSe2, WS2, and WSe2 monolayers under strain. Phys. Rev. B 2013, 88, 195420. [Google Scholar] [CrossRef]
- Reshak, A.H.; Auluck, S. Band structure and optical response of 2H−MoX2 compounds (X=S, Se, and Te). Phys. Rev. B 2005, 71, 155114. [Google Scholar] [CrossRef]
- Li, T.; Galli, G. Electronic Properties of MoS2 Nanoparticles. J. Phys. Chem. C 2007, 111, 16192–16196. [Google Scholar] [CrossRef]
- Lebègue, S.; Eriksson, O. Electronic structure of two-dimensional crystals fromab initiotheory. Phys. Rev. B 2009, 79, 115409. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wang, Y.; Ni, J.; Shi, L.; Shi, S.; Tang, W. First principles study of structural, vibrational and electronic properties of graphene-like MX2 (M=Mo, Nb, W, Ta; X=S, Se, Te) monolayers. Phys. B Condens. Matter 2011, 406, 2254–2260. [Google Scholar] [CrossRef]
- Kadantsev, E.S.; Hawrylak, P. Electronic structure of a single MoS2 monolayer. Solid State Commun. 2012, 152, 909–913. [Google Scholar] [CrossRef]
- Liu, G.-B.; Shan, W.-Y.; Yao, Y.; Yao, W.; Xiao, D. Three-band tight-binding model for monolayers of group-VIB transition metal dichalcogenides. Phys. Rev. B 2013, 88, 085433. [Google Scholar] [CrossRef] [Green Version]
- Vikraman, D.; Akbar, K.; Hussain, S.; Yoo, G.; Jang, J.-Y.; Chun, S.-H.; Jung, J.; Park, H.J. Direct synthesis of thickness-tunable MoS2 quantum dot thin layers: Optical, structural and electrical properties and their application to hydrogen evolution. Nano Energy 2017, 35, 101–114. [Google Scholar] [CrossRef]
- Mukherjee, S.; Maiti, R.; Midya, A.; Das, S.; Ray, S.K. Tunable Direct Bandgap Optical Transitions in MoS2 Nanocrystals for Photonic Devices. ACS Photonics 2015, 2, 760–768. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, D.; Mukherjee, S.; Mitra, R.K.; Ray, S.K. Size-dependent optical properties of MoS2 nanoparticles and their photo-catalytic applications. Nanotechnology 2020, 31, 145701. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, D.; Wu, P. One-Pot, Facile, and Versatile Synthesis of Monolayer MoS2/WS2 Quantum Dots as Bioimaging Probes and Efficient Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Damien, D.; Li, B.; Gullappalli, H.; Pillai, V.K.; Ajayan, P.M.; Shaijumon, M.M. Electrochemical synthesis of luminescent MoS2 quantum dots. Chem. Commun. 2015, 51, 6293–6296. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, L.; Zhu, Z.; Papakonstantinou, P.; Hu, J.; Liu, H.; Li, M. Enhanced electrocatalytic activity for hydrogen evolution reaction from self-assembled monodispersed molybdenum sulfidenanoparticles on an Au electrode. Energy Environ. Sci. 2013, 6, 625–633. [Google Scholar] [CrossRef]
- Chikan, V.; Kelley, D.F. Size-Dependent Spectroscopy of MoS2 Nanoclusters. J. Phys. Chem. B 2002, 106, 3794–3804. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of Monolayer MoS2 Quantum Dots using Temporally Shaped Femtosecond Laser Ablation of Bulk MoS2 Targets in Water. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Wang, Z.; Wang, W.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.; Tan, P.; Miao, F.; Wang, X.; et al. Strong Photoluminescence Enhancement of MoS2 through Defect Engineering and Oxygen Bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Sofer, Z.; Pumera, M. Will Any Crap We Put into Graphene Increase Its Electrocatalytic Effect? ACS Nano 2020, 14, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Zhu, H.; Zhuo, J.; Zhu, Z.; Papakonstantinou, P.; Lubarsky, G.; Lin, J.; Li, M. Biosensor Based on Ultrasmall MoS2 Nanoparticles for Electrochemical Detection of H2O2 Released by Cells at the Nanomolar Level. Anal. Chem. 2013, 85, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhang, Y.; Xia, Z.; Wei, W. As-prepared MoS2 quantum dot as a facile fluorescent probe for long-term tracing of live cells. Nanotechnology 2016, 27, 275101. [Google Scholar] [CrossRef] [PubMed]
- Sweet, C.; Pramanik, A.; Jones, S.; Ray, P.C. Two-Photon Fluorescent Molybdenum Disulfide Dots for Targeted Prostate Cancer Imaging in the Biological II Window. ACS Omega 2017, 2, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Lin, C.-Y.; Cheng, T.-L.; Tseng, W.-L. 6-Mercaptopurine-Induced Fluorescence Quenching of Monolayer MoS2 Nanodots: Applications to Glutathione Sensing, Cellular Imaging, and Glutathione-Stimulated Drug Delivery. Adv. Funct. Mater. 2017, 27, 1702452. [Google Scholar] [CrossRef]
- Dong, H.; Tang, S.; Hao, Y.; Yu, H.; Dai, W.; Zhao, G.; Cao, Y.; Lu, H.; Zhang, X.; Ju, H. Fluorescent MoS2 Quantum Dots: Ultrasonic Preparation, Up-Conversion and Down-Conversion Bioimaging, and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 3107–3114. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ke, S.; Tu, L.; Wang, Y.; Ye, S.; Kou, S.; Ren, L. Regulation of Autophagy Orchestrates Pyroptotic Cell Death in Molybdenum Disulfide Quantum Dot-Induced Microglial Toxicity. ACS Biomater. Sci. Eng. 2020, 6, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Sun, Y.; Fan, S.; Boudreau, M.D.; Chen, C.; Ge, C.; Yin, J.-J. Photogenerated Charge Carriers in Molybdenum Disulfide Quantum Dots with Enhanced Antibacterial Activity. ACS Appl. Mater. Interfaces 2019, 11, 4858–4866. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, L.; Lu, Q.; Yang, S.; Yang, L.; Cheng, Y.; Wang, Y.; Wang, S.; Song, Y.; Tan, F.; et al. Ultrasmall MoS2 Nanodots-Doped Biodegradable SiO2 Nanoparticles for Clearable FL/CT/MSOT Imaging-Guided PTT/PDT Combination Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 5771–5781. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Dong, H.; Fugetsu, B.; Cao, Y.; Lu, H.; Ma, X.; Zhang, X. Tunable Fabrication of Molybdenum Disulfide Quantum Dots for Intracellular MicroRNA Detection and Multiphoton Bioimaging. Small 2015, 11, 4158–4164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-D.; Zhang, J.; Wang, J.; Yang, J.; Chen, J.; Shen, X.; Deng, J.; Deng, D.; Changlong, L.; Sun, Y.-M.; et al. Highly Catalytic Nanodots with Renal Clearance for Radiation Protection. ACS Nano 2016, 10, 4511–4519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Lai, Z.; Liu, Z.; Tan, C.; Huang, Y.; Chaoliang, T.; Zhao, M.; Xie, L.; Huang, W.; Zhang, H. A Facile and Universal Top-Down Method for Preparation of Monodisperse Transition-Metal Dichalcogenide Nanodots. Angew. Chem. Int. Ed. 2015, 54, 5425–5428. [Google Scholar] [CrossRef]
- Muscuso, L.; Cravanzola, S.; Cesano, F.; Scarano, D.; Zecchina, A. Optical, Vibrational, and Structural Properties of MoS2 Nanoparticles Obtained by Exfoliation and Fragmentation via Ultrasound Cavitation in Isopropyl Alcohol. J. Phys. Chem. C 2015, 119, 3791–3801. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Sohn, W.; Oh, J.H.; Jang, H.W.; Kim, S.Y. Size-Dependent Properties of Two-Dimensional MoS2 and WS2. J. Phys. Chem. C 2016, 120, 10078–10085. [Google Scholar] [CrossRef]
- Štengl, V.; Henych, J. Strongly luminescent monolayered MoS2 prepared by effective ultrasound exfoliation. Nanoscale 2013, 5, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, D.; Damien, D.; Shaijumon, M.M. MoS2 Quantum Dot-Interspersed Exfoliated MoS2 Nanosheets. ACS Nano 2014, 8, 5297–5303. [Google Scholar] [CrossRef]
- Baby, M.; Kumar, K.R. Synthesis and characterisation of MoS2 quantum dots by liquid nitrogen quenching. Mater. Sci. Technol. 2019, 35, 1416–1427. [Google Scholar] [CrossRef]
- An, S.-J.; Park, D.Y.; Lee, C.; Bang, S.; Nguyen, D.A.; Kim, S.H.; Kim, H.Y.; Jeong, H.J.; Jeong, M.S.; Anh, N.D. Facile preparation of molybdenum disulfide quantum dots using a femtosecond laser. Appl. Surf. Sci. 2020, 511, 145507. [Google Scholar] [CrossRef]
- Huang, H.; Du, C.; Shi, H.; Feng, X.; Li, J.; Tan, Y.; Song, W. Water-Soluble Monolayer Molybdenum Disulfide Quantum Dots with Upconversion Fluorescence. Part. Part. Syst. Charact. 2015, 32, 72–79. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, S.; Song, X.; Zhang, X.; He, X.; Zhong, W.; Du, Y. Luminescent monolayer MoS2 quantum dots produced by multi-exfoliation based on lithium intercalation. Appl. Surf. Sci. 2015, 359, 130–136. [Google Scholar] [CrossRef]
- Li, B.L.; Chen, L.X.; Zou, H.L.; Lei, J.L.; Luo, H.Q.; Li, N.B. Electrochemically induced Fenton reaction of few-layer MoS2 nanosheets: Preparation of luminescent quantum dots via a transition of nanoporous morphology. Nanoscale 2014, 6, 9831–9838. [Google Scholar] [CrossRef]
- Park, S.J.; Pak, S.W.; Qiu, D.; Kang, J.H.; Song, D.Y.; Kim, E.K. Structural and optical characterization of MoS2 quantum dots defined by thermal annealing. J. Lumin. 2017, 183, 62–67. [Google Scholar] [CrossRef]
- Lu, G.; Wu, M.; Lin, T.; Chang, C.; Lin, W.; Chen, Y.T.; Hou, C.; Cheng, H.; Lin, T.; Shen, J.; et al. Electrically Pumped White-Light-Emitting Diodes Based on Histidine-Doped MoS2 Quantum Dots. Small 2019, 15, e1901908. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Jiang, K.; Wang, C.; Zhang, C. One-step synthesis of water-soluble and highly fluorescent MoS2 quantum dots for detection of hydrogen peroxide and glucose. Sens. Actuators B Chem. 2017, 252, 183–190. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, Y. Molybdenum Disulfide Quantum Dots as a Photoluminescence Sensing Platform for 2,4,6-Trinitrophenol Detection. Anal. Chem. 2014, 86, 7463–7470. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Pang, L.; Zhang, Y.; Ren, X.; Fan, H.; Liu, S. (Frank) One-step hydrothermal synthesis of monolayer MoS2 quantum dots for highly efficient electrocatalytic hydrogen evolution. J. Mater. Chem. A 2015, 3, 10693–10697. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Zhuang, Q.; Ni, Y. Label-Free Fluorescence Sensing of Lead(II) Ions and Sulfide Ions Based on Luminescent Molybdenum Disulfide Nanosheets. ACS Sustain. Chem. Eng. 2016, 4, 2535–2541. [Google Scholar] [CrossRef]
- Haldar, D.; Dinda, D.; Saha, S.K. High selectivity in water soluble MoS2 quantum dots for sensing nitro explosives. J. Mater. Chem. C 2016, 4, 6321–6326. [Google Scholar] [CrossRef]
- Xu, B.; Su, Y.; Li, L.; Liu, R.; Lv, Y. Thiol-functionalized single-layered MoS2 nanosheet as a photoluminescence sensing platform via charge transfer for dopamine detection. Sens. Actuators B Chem. 2017, 246, 380–388. [Google Scholar] [CrossRef]
- Gu, W.; Yan, Y.; Zhang, C.; Ding, C.; Xian, Y. One-Step Synthesis of Water-Soluble MoS2 Quantum Dots via a Hydrothermal Method as a Fluorescent Probe for Hyaluronidase Detection. ACS Appl. Mater. Interfaces 2016, 8, 11272–11279. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, C.; Wu, J.; Xu, Z.; Huang, Y.; Zhang, C. Colloidal synthesis of MoS2 quantum dots: Size-dependent tunable photoluminescence and bioimaging. N. J. Chem. 2015, 39, 8492–8497. [Google Scholar] [CrossRef]
- Najafi, L.; Taheri, B.; Martín-García, B.; Bellani, S.; Di Girolamo, D.; Agresti, A.; Oropesa-Nuñez, R.; Pescetelli, S.; Vesce, L.; Calabrò, E.; et al. MoS2 Quantum Dot/Graphene Hybrids for Advanced Interface Engineering of a CH3NH3PbI3 Perovskite Solar Cell with an Efficiency of over 20%. ACS Nano 2018, 12, 10736–10754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Wang, X.; Jiang, Y.; Sun, W.; Wang, C.; Yang, M.; Zhang, C. MoS2 -QD-Based Dual-Model Photoluminescence Sensing Platform for Effective Determination of Al3+ and Fe3+ Simultaneously in Various Environment. ChemistrySelect 2018, 3, 2326–2331. [Google Scholar] [CrossRef]
- Xie, M.-Y.; Su, K.-Y.; Peng, X.-Y.; Wu, R.-J.; Chavali, M.; Chang, W.-C. Hydrogen production by photocatalytic water-splitting on Pt-doped TiO2–ZnO under visible light. J. Taiwan Inst. Chem. Eng. 2017, 70, 161–167. [Google Scholar] [CrossRef]
- Zhu, Z.; Kao, C.-T.; Tang, B.-H.; Chang, W.-C.; Wu, R.-J. Efficient hydrogen production by photocatalytic water-splitting using Pt-doped TiO2 hollow spheres under visible light. Ceram. Int. 2016, 42, 6749–6754. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Kurenkova, A.Y.; Kolinko, P.A.; Saraev, A.A.; Gerasimov, E.Y.; Kozlov, D.V. Photocatalytic hydrogen production using Me/Cd0.3Zn0.7S (Me = Au, Pt, Pd) catalysts: Transformation of the metallic catalyst under the action of the reaction medium. Kinet. Catal. 2017, 58, 431–440. [Google Scholar] [CrossRef]
- Salgado, S.Y.A.; Zamora, R.M.R.; Zanella, R.; Peral, J.; Malato, S.; Maldonado, M.I. Photocatalytic hydrogen production in a solar pilot plant using a Au/TiO2 photo catalyst. Int. J. Hydrogen Energy 2016, 41, 11933–11940. [Google Scholar] [CrossRef]
- Qin, J.; Huo, J.; Zhang, P.; Zeng, J.; Wang, T.; Zeng, H. Improving the photocatalytic hydrogen production of Ag/g-C3N4 nanocomposites by dye-sensitization under visible light irradiation. Nanoscale 2016, 8, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-W.P.; Hsiao, C.-H.; Huang, J.-Y.; Peng, Y.-H.; Chang, C.-Y. Highly Efficient Hydrogen Evolution from Seawater by Biofunctionalized Exfoliated MoS2 Quantum Dot Aerogel Electrocatalysts that Is Superior to Pt. ACS Appl. Mater. Interfaces 2019, 11, 14159–14165. [Google Scholar] [CrossRef]
- Najafi, L.; Bellani, S.; Martín-García, B.; Oropesa-Nuñez, R.; Castillo, A.E.D.R.; Prato, M.; Moreels, I.; Bonaccorso, F. Solution-Processed Hybrid Graphene Flake/2H-MoS2 Quantum Dot Heterostructures for Efficient Electrochemical Hydrogen Evolution. Chem. Mater. 2017, 29, 5782–5786. [Google Scholar] [CrossRef] [Green Version]
- Tadi, K.K.; Palve, A.M.; Pal, S.; Sudeep, P.M.; Narayanan, T.N. Single step, bulk synthesis of engineered MoS2 quantum dots for multifunctional electrocatalysis. Nanotechnology 2016, 27, 275402. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, U.; Chatterjee, S.; Pal, A.J. Thin-film formation of 2D MoS2 and its application as a hole-transport layer in planar perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 172, 353–360. [Google Scholar] [CrossRef]
- Quy, V.H.V.; Vijayakumar, E.; Ho, P.; Park, J.-H.; Rajesh, J.A.; Kwon, J.; Chae, J.; Kim, J.-H.; Kang, S.-H.; Ahn, K.-S. Electrodeposited MoS2 as electrocatalytic counter electrode for quantum dot- and dye-sensitized solar cells. Electrochim. Acta 2018, 260, 716–725. [Google Scholar] [CrossRef]
- Ulaganathan, R.K.; Yadav, K.; Sankar, R.; Chou, F.C.; Chen, Y.-T. Hybrid InSe Nanosheets and MoS2 Quantum Dots for High-Performance Broadband Photodetectors and Photovoltaic Cells. Adv. Mater. Interfaces 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Xing, W.; Chen, Y.; Wang, X.; Lv, L.; Ouyang, X.; Ge, Z.; Huang, H. MoS2 Quantum Dots with a Tunable Work Function for High-Performance Organic Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 26916–26923. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef]
- Nie, W.; Tsai, H.; Asadpour, R.; Blancon, J.-C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Bi, D.; Yi, C.; Décoppet, J.-D.; Luo, J.; Zakeeruddin, S.M.; Hagfeldt, A.; Grätzel, M. A vacuum flash–assisted solution process for high-efficiency large-area perovskite solar cells. Science 2016, 353, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, A.; Haur, L.J.; Murray, P.; Fu, D.; Kulkarni, S.; Xing, G.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G. A large area (70 cm2) monolithic perovskite solar module with a high efficiency and stability. Energy Environ. Sci. 2016, 9, 3687–3692. [Google Scholar] [CrossRef]
- Hwang, K.; Jung, Y.-S.; Heo, Y.-J.; Scholes, F.H.; Watkins, S.E.; Subbiah, J.; Jones, D.J.; Kim, D.-Y.; Vak, D. Toward Large Scale Roll-to-Roll Production of Fully Printed Perovskite Solar Cells. Adv. Mater. 2015, 27, 1241–1247. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M.; et al. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Sha, W.E.I.; Ren, X.; Chen, L.; Choy, W.C.H. The efficiency limit of CH3NH3PbI3 perovskite solar cells. Appl. Phys. Lett. 2015, 106, 221104. [Google Scholar] [CrossRef] [Green Version]
- Baloch, A.A.B.; Hossain, M.I.; Tabet, N.; Alharbi, F.H. Practical Efficiency Limit of Methylammonium Lead Iodide Perovskite (CH3NH3PbI3) Solar Cells. J. Phys. Chem. Lett. 2018, 9, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.W.; Yip, H.L.; Luo, Y.; Wong, K.Y.; Lau, W.M.; Löw, K.H.; Chow, H.F.; Gao, Z.Q.; Yeung, W.L.; Chang, C.C. Blocking reactions between indium-tin oxide and poly (3,4-ethylene dioxythiophene):poly(styrene sulphonate) with a self-assembly monolayer. Appl. Phys. Lett. 2002, 80, 2788–2790. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Chen, S.; Yu, X.; Zhang, M.; Cao, J.; Li, Y.; Ding, L.; Shi, G. A graphene oxide/oxygen deficient molybdenum oxide nanosheet bilayer as a hole transport layer for efficient polymer solar cells. J. Mater. Chem. A 2015, 3, 18380–18383. [Google Scholar] [CrossRef]
- Li, S.-S.; Tu, K.-H.; Lin, C.-C.; Chen, C.-W.; Chhowalla, M. Solution-Processable Graphene Oxide as an Efficient Hole Transport Layer in Polymer Solar Cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Yang, X.; Zhang, Y.; Xu, M.; Chen, H. Ultra-stable two-dimensional MoS2 solution for highly efficient organic solar cells. RSC Adv. 2014, 4, 32744–32748. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Wu, Y.; Min, C.; Fang, J. Solution-Processed MoSxas an Efficient Anode Buffer Layer in Organic Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 8823–8827. [Google Scholar] [CrossRef]
- Ibrahem, M.A.; Lan, T.-W.; Huang, J.K.; Chen, Y.-Y.; Wei, K.-H.; Li, L.-J.; Chu, C.W. High quantity and quality few-layers transition metal disulfide nanosheets from wet-milling exfoliation. RSC Adv. 2013, 3, 13193–13202. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Y.; Gao, Y.; Yu, D.; Durstock, M.; Dai, L. Hole and Electron Extraction Layers Based on Graphene Oxide Derivatives for High-Performance Bulk Heterojunction Solar Cells. Adv. Mater. 2012, 24, 2228–2233. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Cui, W.; Li, H.; Wu, Z.; Zeng, Z.; Lee, S.-T.; Zhang, H.; Sun, B. A Solution-Processed Hole Extraction Layer Made from Ultrathin MoS2 Nanosheets for Efficient Organic Solar Cells. Adv. Energy Mater. 2013, 3, 1262–1268. [Google Scholar] [CrossRef]
- Kwon, K.C.; Kim, C.; van Le, Q.; Gim, S.; Jeon, J.-M.; Ham, J.Y.; Lee, J.-L.; Jang, H.W.; Kim, S.Y. Synthesis of Atomically Thin Transition Metal Disulfides for Charge Transport Layers in Optoelectronic Devices. ACS Nano 2015, 9, 4146–4155. [Google Scholar] [CrossRef]
- van Le, Q.; Nguyen, T.P.; Jang, H.W.; Kim, S.Y. The use of UV/ozone-treated MoS2 nanosheets for extended air stability in organic photovoltaic cells. Phys. Chem. Chem. Phys. 2014, 16, 13123–13128. [Google Scholar] [CrossRef]
- Niu, L.; Li, K.; Zhen, H.; Chui, Y.-S.; Zhang, W.; Yan, F.; Zheng, Z. Salt-Assisted High-Throughput Synthesis of Single- and Few-Layer Transition Metal Dichalcogenides and Their Application in Organic Solar Cells. Small 2014, 10, 4651–4657. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fu, W.; Liu, W.; Hong, J.; Cai, Y.; Jin, C.; Xu, M.; Wang, H.; Yang, D.; Chen, H. Engineering crystalline structures of two-dimensional MoS2 sheets for high-performance organic solar cells. J. Mater. Chem. A 2014, 2, 7727–7733. [Google Scholar] [CrossRef]
- Yun, J.-M.; Noh, Y.-J.; Lee, C.-H.; Na, S.-I.; Lee, S.; Jo, S.M.; Joh, H.-I.; Kim, D.-Y. Exfoliated and Partially Oxidized MoS2 Nanosheets by One-Pot Reaction for Efficient and Stable Organic Solar Cells. Small 2014, 10, 2319–2324. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Fang, G.; Ke, W.; Cheng, F.; Zheng, Q.; Wan, J.; Lei, H.; Zhao, X. In situ growth of double-layer MoO3/MoS2 film from MoS2 for hole-transport layers in organic solar cell. J. Mater. Chem. A 2014, 2, 2742–2756. [Google Scholar] [CrossRef]
- Xu, S.; Hessel, C.M.; Ren, H.; Yu, R.; Jin, Q.; Yang, M.; Zhao, H.; Wang, D. α-Fe2O3 multi-shelled hollow microspheres for lithium ion battery anodes with superior capacity and charge retention. Energy Environ. Sci. 2014, 7, 632–637. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Lou, X.W. (David) Synthesis of Highly Uniform Molybdenum-Glycerate Spheres and Their Conversion into Hierarchical MoS2 Hollow Nanospheres for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2016, 55, 7423–7426. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Q.; Niu, L.; Wang, D.; Yan, C.; She, Y.; Zheng, Z. Waterproof, Ultrahigh Areal-Capacitance, Wearable Supercapacitor Fabrics. Adv. Mater. 2017, 29, 1606679. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kady, M.F.; Kaner, R.B. Scalable fabrication of high-power graphene micro-supercapacitors for flexible and on-chip energy storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, C.; Xie, B.; Lin, Z.; Zhang, Z.; Liu, J.; Li, B.; Kang, F.; Wong, C.P. Scalable fabrication of MnO2 nanostructure deposited on free-standing Ni nanocone arrays for ultrathin, flexible, high-performance micro-supercapacitor. Energy Environ. Sci. 2014, 7, 2652–2659. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors Based on Flexible Graphene/Polyaniline Nanofiber Composite Films. ACS Nano 2010, 4, 1963–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Dai, L. Flexible supercapacitors based on carbon nanomaterials. J. Mater. Chem. A 2014, 2, 10756–10775. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Li, J.; Zhang, H.; Dong, H.; Zhang, M.; et al. A flexible all-solid-state asymmetric supercapacitors based on hierarchical carbon cloth@CoMoO4@NiCo layered double hydroxide core-shell heterostructures. Chem. Eng. J. 2018, 352, 29–38. [Google Scholar] [CrossRef]
- Tian, H.; Zhu, S.; Xu, F.; Mao, W.; Wei, H.; Mai, Y.; Feng, X. Growth of 2D Mesoporous Polyaniline with Controlled Pore Structures on Ultrathin MoS2 Nanosheets by Block Copolymer Self-Assembly in Solution. ACS Appl. Mater. Interfaces 2017, 9, 43975–43982. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, W.; Yang, D.; Zhang, Y.; Hoon, H.H.; Zhang, H.; Yan, Q. Multifunctional Architectures Constructing of PANI Nanoneedle Arrays on MoS2 Thin Nanosheets for High-Energy Supercapacitors. Small 2015, 11, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Sha, C.; Lu, B.; Mao, H.; Cheng, J.; Pan, X.; Lu, J.; Ye, Z. 3D ternary nanocomposites of molybdenum disulfide/polyaniline/reduced graphene oxide aerogel for high performance supercapacitors. Carbon 2016, 99, 26–34. [Google Scholar] [CrossRef]
- Palsaniya, S.; Nemade, H.B.; Dasmahapatra, A.K. Synthesis of polyaniline/graphene/MoS2 nanocomposite for high performance supercapacitor electrode. Polym. 2018, 150, 150–158. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Yan, Z.; Kang, L.; Xu, H.; Shi, F.; Lei, Z.; Liu, Z.-H. Three-Dimensional Tubular MoS2/PANI Hybrid Electrode for High Rate Performance Supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 28294–28302. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ghosh, R.; Mandal, D.; Nandi, A.K. Self-Assembled Nanostructured MoS2 Quantum Dot Polyaniline Hybrid Gels for High Performance Solid State Flexible Supercapacitors. ACS Appl. Energy Mater. 2019, 2, 6642–6654. [Google Scholar] [CrossRef]
- Hussain, S.; Shehzad, M.A.; Vikraman, D.; Khan, M.F.; Singh, J.; Choi, D.-C.; Seo, Y.; Eom, J.; Lee, W.-G.; Jung, J. Synthesis and characterization of large-area and continuous MoS2 atomic layers by RF magnetron sputtering. Nanoscale 2016, 8, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Shehzad, M.A.; Vikraman, D.; Iqbal, M.Z.; Singh, J.; Khan, M.F.; Eom, J.; Seo, Y.; Jung, J. Controlled synthesis and optical properties of polycrystalline molybdenum disulfide atomic layers grown by chemical vapor deposition. J. Alloys Compd. 2015, 653, 369–378. [Google Scholar] [CrossRef]
- Ganatra, R.; Zhang, Q. Few-Layer MoS2: A Promising Layered Semiconductor. ACS Nano 2014, 8, 4074–4099. [Google Scholar] [CrossRef]
- Sanne, A.; Ghosh, R.N.; Rai, A.; Movva, H.C.P.; Sharma, A.K.; Rao, R.; Mathew, L.; Banerjee, S.K. Top-gated chemical vapor deposited MoS2 field-effect transistors on Si3N4 substrates. Appl. Phys. Lett. 2015, 106, 062101. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Kim, E.; Yook, J.-G.; Jung, J. Intrinsic characteristics of transmission line of graphenes at microwave frequencies. Appl. Phys. Lett. 2012, 100, 223102. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Whitwick, M.B.; Kis, A. Integrated Circuits and Logic Operations Based on Single-Layer MoS2. ACS Nano 2011, 5, 9934–9938. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, J.; Bark, H.; Oh, I.-K.; Ryu, G.H.; Lee, Z.; Kim, H.; Cho, J.H.; Ahn, J.-H.; Lee, C. Synthesis of wafer-scale uniform molybdenum disulfide films with control over the layer number using a gas phase sulfur precursor. Nanoscale 2014, 6, 2821–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.-H.; Zhang, X.-Q.; Zhang, W.; Chang, M.-T.; Lin, C.-T.; Chang, K.-D.; Yu, Y.-C.; Wang, J.T.-W.; Chang, C.-S.; Li, L.-J.; et al. Synthesis of Large-Area MoS2 Atomic Layers with Chemical Vapor Deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, Y.; Liu, Z.; Najmaei, S.; Ajayan, P.M.; Lou, J. Large-Area Vapor-Phase Growth and Characterization of MoS2 Atomic Layers on a SiO2 Substrate. Small 2012, 8, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-C.; Zhang, W.; Huang, J.-K.; Liu, K.-K.; Lee, Y.-H.; Liang, C.-T.; Chu, C.-W.; Li, L.-J. Wafer-scale MoS2 thin layers prepared by MoO3 sulfurization. Nanoscale 2012, 4, 6637–6641. [Google Scholar] [CrossRef] [PubMed]
- Tamalampudi, S.R.; Lu, Y.-Y.; U., R.K.; Sankar, R.; Liao, C.-D.; B., K.M.; Cheng, C.-H.; Chou, F.C.; Chen, Y.-T. High Performance and Bendable Few-Layered InSe Photodetectors with Broad Spectral Response. Nano Lett. 2014, 14, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-B.; Xu, Z.-X.; Chen, X.; Tian, W.; Han, S.-T.; Zhou, Y.; Xu, J.-J.; Yang, X.-B.; Roy, V.A.L.; Vellaisamy, A.L.R. Poly(3-hexylthiophene) Nanotubes with Tunable Aspect Ratios and Charge Transport Properties. ACS Appl. Mater. Interfaces 2014, 6, 11874–11881. [Google Scholar] [CrossRef]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.; Gao, X.; Xing, R.; Zheng, L.; Wu, S.; Geng, Y.; Han, Y. Oriented Poly(3-hexylthiophene) Nanofibril with the π−π Stacking Growth Direction by Solvent Directional Evaporation. Langmuir 2011, 27, 4212–4219. [Google Scholar] [CrossRef] [PubMed]
- Tremel, K.; Ludwigs, S. Morphology of P3HT in Thin Films in Relation to Optical and Electrical Properties. Adv. Polym. Sci. 2014, 265, 39–82. [Google Scholar] [CrossRef]
- Sun, Y.; Xiao, G.; Lin, Y.; Su, Z.; Wang, Q. Self-assembly of large-scale P3HT patterns by confined evaporation in the capillary tube. RSC Adv. 2015, 5, 20491–20497. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.; Gopinathan, S.P.; Kumar, A. Solvent free chemical oxidative polymerization as a universal method for the synthesis of ultra high molecular weight conjugated polymers based on 3,4-propylenedioxythiophenes. Chem. Commun. 2012, 48, 4905. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Kumar, A.; Subramaniam, C. Exceptional photoconductivity of poly(3-hexylthiophene) fibers through in situ encapsulation of molybdenum disulfide quantum dots. Nanoscale 2018, 10, 10395–10402. [Google Scholar] [CrossRef]
- Choi, M.K.; Yang, J.; Kang, K.; Kim, D.C.; Choi, C.; Park, C.; Kim, S.J.; Chae, S.I.; Kim, T.-H.; Kim, J.H.; et al. Wearable red–green–blue quantum dot light-emitting diode array using high-resolution intaglio transfer printing. Nat. Commun. 2015, 6, 7149. [Google Scholar] [CrossRef]
- Chen, H.-S.; Hsu, C.-K.; Hong, H.-Y. InGaN-CdSe-ZnSe quantum dots white LEDs. IEEE Photonics Technol. Lett. 2005, 18, 193–195. [Google Scholar] [CrossRef]
- Li, Y.Q.; Rizzo, A.; Cingolani, R.; Gigli, G. Bright White-Light-Emitting Device from Ternary Nanocrystal Composites. Adv. Mater. 2006, 18, 2545–2548. [Google Scholar] [CrossRef]
- Pandey, P.K.; Ulla, H.; Satyanarayan, M.N.; Rawat, K.; Gaur, A.; Gawali, S.; Hassan, P.A.; Bohidar, H.B. Fluorescent MoS2 Quantum Dot–DNA Nanocomposite Hydrogels for Organic Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 1289–1297. [Google Scholar] [CrossRef]
- Gomez, E.F.; Steckl, A.J. Improved Performance of OLEDs on Cellulose/Epoxy Substrate Using Adenine as a Hole Injection Layer. ACS Photonics 2015, 2, 439–445. [Google Scholar] [CrossRef]
- Lian, L.; Wang, H.; Dong, D.; He, G. Highly robust and ultrasmooth copper nanowire electrode by one-step coating for organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 9158–9165. [Google Scholar] [CrossRef]
- Pavesi, L. Silicon-Based Light Sources for Silicon Integrated Circuits. Adv. Opt. Technol. 2008, 2008, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, M.; Kumari, R.; Parra, M.R.; Pandey, P.; Siddiqui, H.; Haque, F.Z. Electrochemical synthesis of MoS2 quantum dots embedded nanostructured porous silicon with enhanced electroluminescence property. Opt. Mater. 2017, 73, 763–771. [Google Scholar] [CrossRef]
- Qiu, D.Y.; Da Jornada, F.H.; Louie, S.G. Optical Spectrum of MoS2: Many-Body Effects and Diversity of Exciton States. Phys. Rev. Lett. 2013, 111, 216805. [Google Scholar] [CrossRef] [Green Version]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Leng, K.; Chen, Z.; Zhao, X.; Tang, W.; Tian, B.; Nai, C.T.; Zhou, W.; Loh, K.P. Phase Restructuring in Transition Metal Dichalcogenides for Highly Stable Energy Storage. ACS Nano 2016, 10, 9208–9215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.; Jin, S. Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS2 Nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nam, G.-H.; He, Q.; Wu, X.-J.; Zhang, K.; Yang, Z.; Chen, J.; Ma, Q.; Zhao, M.; Liu, Z.; et al. High phase-purity 1T′-MoS2- and 1T′-MoSe2-layered crystals. Nat. Chem. 2018, 10, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Sun, K.; Hu, Y.H. Memristive Behavior and Ideal Memristor of 1T Phase MoS2 Nanosheets. Nano Lett. 2016, 16, 572–576. [Google Scholar] [CrossRef]
- Zhu, X.; Li, D.; Liang, X.; Lu, W.D. Ionic modulation and ionic coupling effects in MoS2 devices for neuromorphic computing. Nat. Mater. 2019, 18, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Najmaei, S.; Liu, Z.; Bao, Y.; Wang, Y.; Zhu, X.; Halas, N.J.; Nordlander, P.; Ajayan, P.M.; Lou, J.; et al. Plasmonic Hot Electron Induced Structural Phase Transition in a MoS2 Monolayer. Adv. Mater. 2014, 26, 6467–6471. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, D.; Ouyang, B.; Raja, A.; Song, J.; Heinz, T.F.; Brus, L.E. Probing the Dynamics of the Metallic-to-Semiconducting Structural Phase Transformation in MoS2 Crystals. Nano Lett. 2015, 15, 5081–5088. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, L.; Cho, H.D.; Kang, T.W.; Fu, D.; Lee, D.; Lee, S.W.; Li, L.; Qi, T.; Chan, A.S.; et al. Molybdenum Disulfide Nanosheet/Quantum Dot Dynamic Memristive Structure Driven by Photoinduced Phase Transition. Small 2019, 15, e1903809. [Google Scholar] [CrossRef]
- Li, D.; Wu, B.; Zhu, X.; Wang, J.; Ryu, B.; Lu, W.D.; Liang, X. MoS2 Memristors Exhibiting Variable Switching Characteristics toward Biorealistic Synaptic Emulation. ACS Nano 2018, 12, 9240–9252. [Google Scholar] [CrossRef]
- Sangwan, V.K.; Lee, H.-S.; Bergeron, H.; Balla, I.; Beck, M.E.; Chen, K.-S.; Hersam, M.C. Multi-terminal memtransistors from polycrystalline monolayer molybdenum disulfide. Nat. Cell Biol. 2018, 554, 500–504. [Google Scholar] [CrossRef]
- Wang, M.; Cai, S.; Pan, C.; Wang, C.; Lian, X.; Zhuo, Y.; Xu, K.; Cao, T.; Pan, X.; Wang, B.; et al. Robust memristors based on layered two-dimensional materials. Nat. Electron. 2018, 1, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Jang, H.; Lee, M.-H.; Amanov, D.; Cho, Y.; Kim, H.; Park, S.; Shin, H.-J.; Ham, D. Vertical MoS2 Double-Layer Memristor with Electrochemical Metallization as an Atomic-Scale Synapse with Switching Thresholds Approaching 100 mV. Nano Lett. 2019, 19, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinda, D.; Gupta, A.; Shaw, B.K.; Sadhu, S.; Saha, S.K. Highly Selective Detection of Trinitrophenol by Luminescent Functionalized Reduced Graphene Oxide through FRET Mechanism. ACS Appl. Mater. Interfaces 2014, 6, 10722–10728. [Google Scholar] [CrossRef]
- Albers, A.E.; Okreglak, V.S.; Chang, C.J. A FRET-Based Approach to Ratiometric Fluorescence Detection of Hydrogen Peroxide. J. Am. Chem. Soc. 2006, 128, 9640–9641. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Min, Y.; Zeng, F.; Yu, C.; Wu, S. A Targeted and FRET-Based Ratiometric Fluorescent Nanoprobe for Imaging Mitochondrial Hydrogen Peroxide in Living Cells. Small 2014, 10, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Liu, Z.; Tian, Y.; Wu, M.; Niu, Z. Multifunctional self-assembled polymeric nanoprobes for FRET-based ratiometric detection of mitochondrial H2O2 in living cells. Chem. Commun. 2015, 51, 3641–3644. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, A.-J.; Dong, M.; Wang, Y.-W. A colorimetric and fluorescent chemosensor for the detection of an explosive—2,4,6-trinitrophenol (TNP). Chem. Commun. 2011, 47, 4505–4507. [Google Scholar] [CrossRef]
- Xu, Y.; Li, B.; Li, W.; Zhao, J.; Sun, S.; Pang, Y. “ICT-not-quenching” near infrared ratiometric fluorescent detection of picric acid in aqueous media. Chem. Commun. 2013, 49, 4764. [Google Scholar] [CrossRef]
- Roy, B.; Bar, A.K.; Gole, B.; Mukherjee, P.S. Fluorescent Tris-Imidazolium Sensors for Picric Acid Explosive. J. Org. Chem. 2013, 78, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wu, X.; Li, H.; Tong, H.; Wang, L. Selective Detection of TNT and Picric Acid by Conjugated Polymer Film Sensors with Donor–Acceptor Architecture. Macromolecules 2011, 44, 5089–5092. [Google Scholar] [CrossRef]
- Tu, R.; Liu, B.; Wang, Z.; Gao, D.; Wang, F.; Fang, A.Q.; Zhang, Z. Amine-Capped ZnS−Mn2+ Nanocrystals for Fluorescence Detection of Trace TNT Explosive. Anal. Chem. 2008, 80, 3458–3465. [Google Scholar] [CrossRef] [PubMed]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2003, 4, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.F.; Osborne, M.A. Photodynamics of a Single Quantum Dot: Fluorescence Activation, Enhancement, Intermittency, and Decay. J. Am. Chem. Soc. 2007, 129, 8936–8937. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Dong, L.; Zheng, S.; Hou, P.; Cai, L.; Zhao, M.; Zhang, X.; Wang, Q.; Li, J.; Xu, K. “Bottom-up” preparation of MoS2 quantum dots for tumor imaging and their in vivo behavior study. Biochem. Biophys. Res. Commun. 2019, 516, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, C.; Wang, X. A facile one-step method to produce MoS2 quantum dots as promising bio-imaging materials. RSC Adv. 2016, 6, 25605–25610. [Google Scholar] [CrossRef]
- Dong, X.; Yin, W.; Zhang, X.; Zhu, S.; He, X.; Yu, J.; Xie, J.; Guo, Z.; Yan, L.; Liu, X.; et al. Intelligent MoS2 Nanotheranostic for Targeted and Enzyme-/pH-/NIR-Responsive Drug Delivery To Overcome Cancer Chemotherapy Resistance Guided by PET Imaging. ACS Appl. Mater. Interfaces 2018, 10, 4271–4284. [Google Scholar] [CrossRef] [PubMed]
- Vadivelmurugan, A.; Anbazhagan, R.; Tsai, H.-C. Preparation of fluorescent MoS2 quantum dots conjugated with various ligands, and its fluorescence imaging. Mater. Lett. 2018, 218, 285–289. [Google Scholar] [CrossRef]
- Wang, J.; Tan, X.; Pang, X.; Liu, L.; Tan, F.; Li, N. MoS2 Quantum Dot@Polyaniline Inorganic–Organic Nanohybrids for In Vivo Dual-Modal Imaging Guided Synergistic Photothermal/Radiation Therapy. ACS Appl. Mater. Interfaces 2016, 8, 24331–24338. [Google Scholar] [CrossRef]
- Swaminathan, H.; Balasubramanian, K. Fӧrster resonance energy transfer between MoS2 quantum dots and polyaniline for turn-on bovine serum albumin sensing. Sens. Actuators B Chem. 2018, 264, 337–343. [Google Scholar] [CrossRef]
- Ha, H.D.; Han, D.J.; Choi, J.S.; Park, M.; Seo, T.S. Dual Role of Blue Luminescent MoS2 Quantum Dots in Fluorescence Resonance Energy Transfer Phenomenon. Small 2014, 10, 3858–3862. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Luo, X. Theoretical Studies of MoS2 and Phosphorene Drug Delivery for Antituberculosis Drugs. J. Phys. Chem. C 2020, 124, 8279–8287. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Dong, J.; Zhang, W.; Dang, G.; Yang, M.; Li, Y.; Chen, H.; Ji, H.; Dong, L. PEGylated MoS2 quantum dots for traceable and pH-responsive chemotherapeutic drug delivery. Colloids Surf. B Biointerfaces 2020, 185, 110590. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Ha, S.; Yeom, D.-I.; Ahn, Y.H.; Lee, S.; Park, J.-Y. Large-scale chemical vapor deposition growth of highly crystalline MoS2 thin films on various substrates and their optoelectronic properties. Curr. Appl. Phys. 2019, 19, 1127–1131. [Google Scholar] [CrossRef]






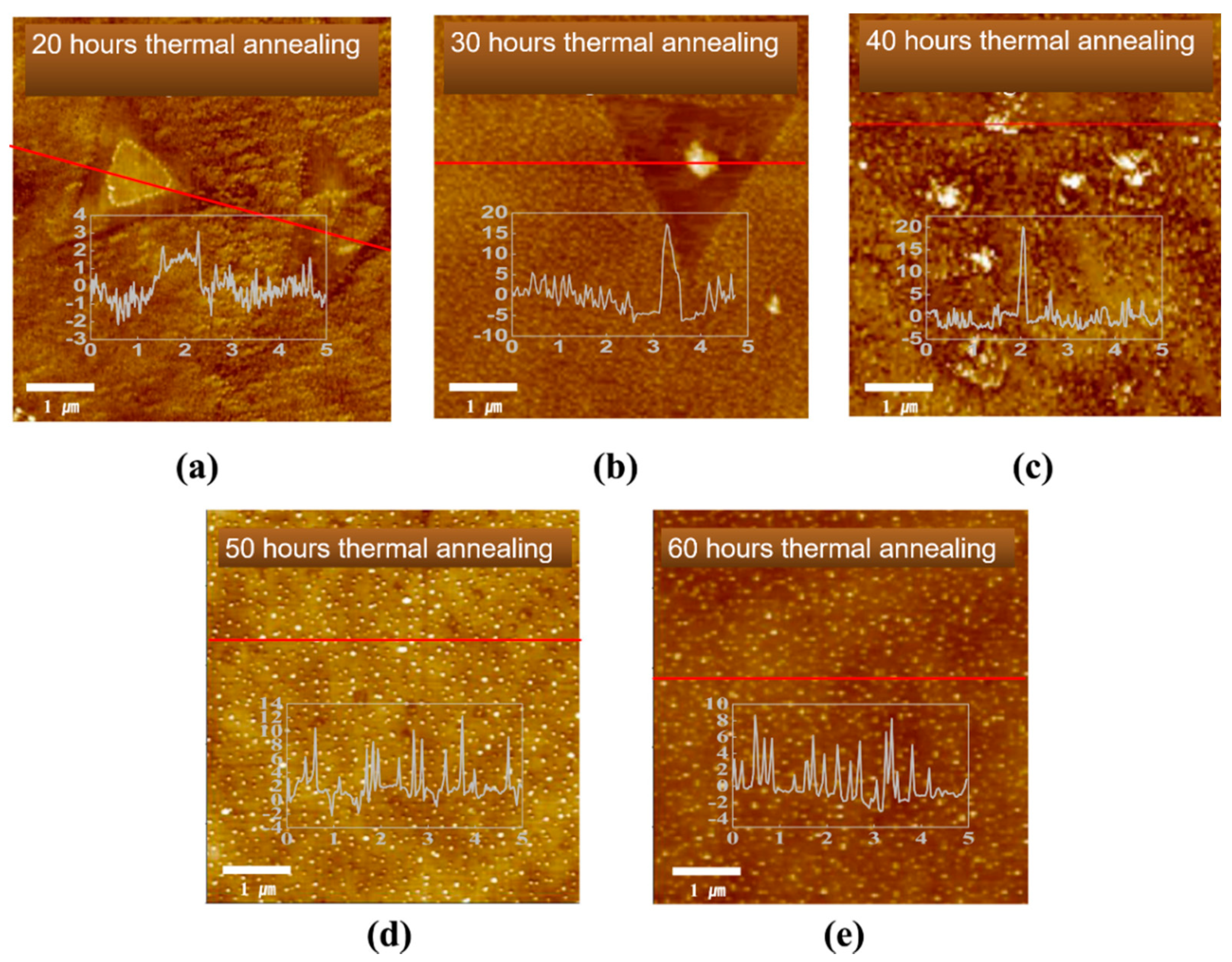

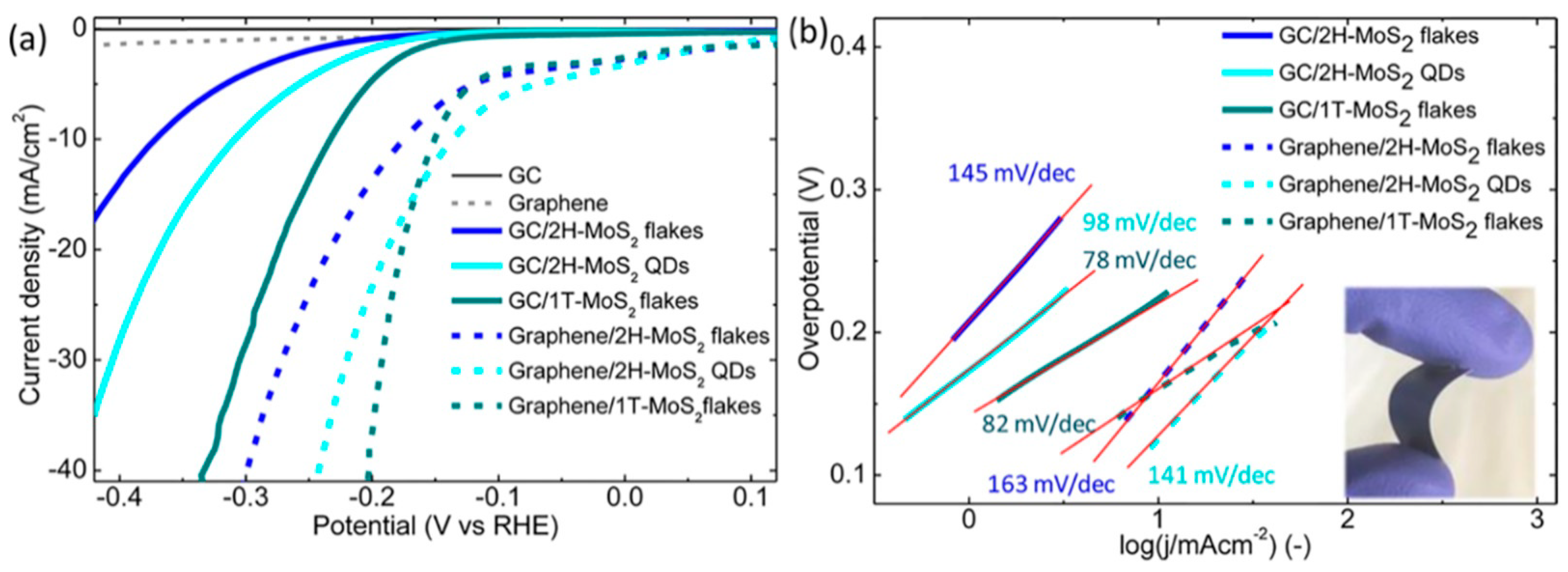
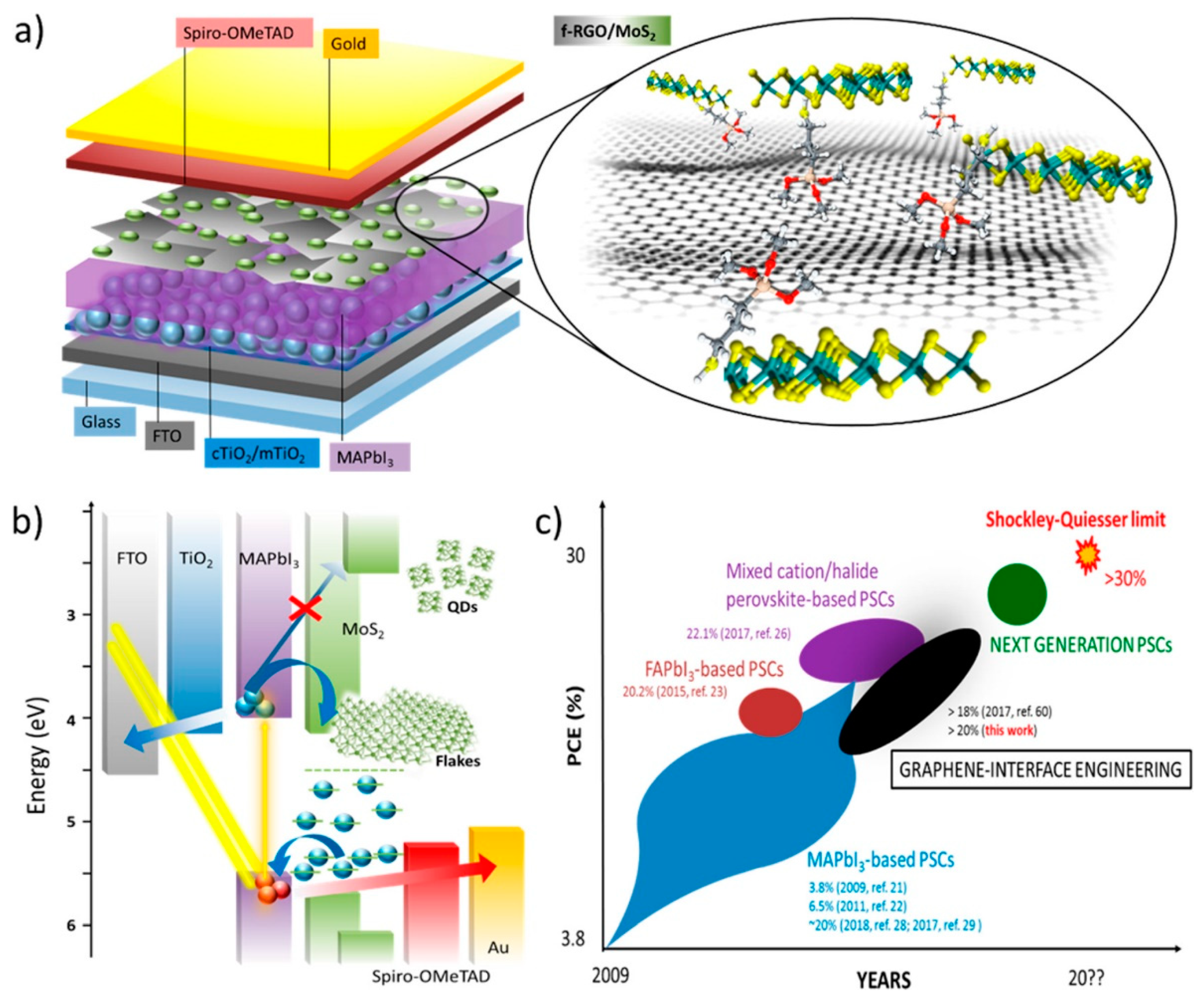
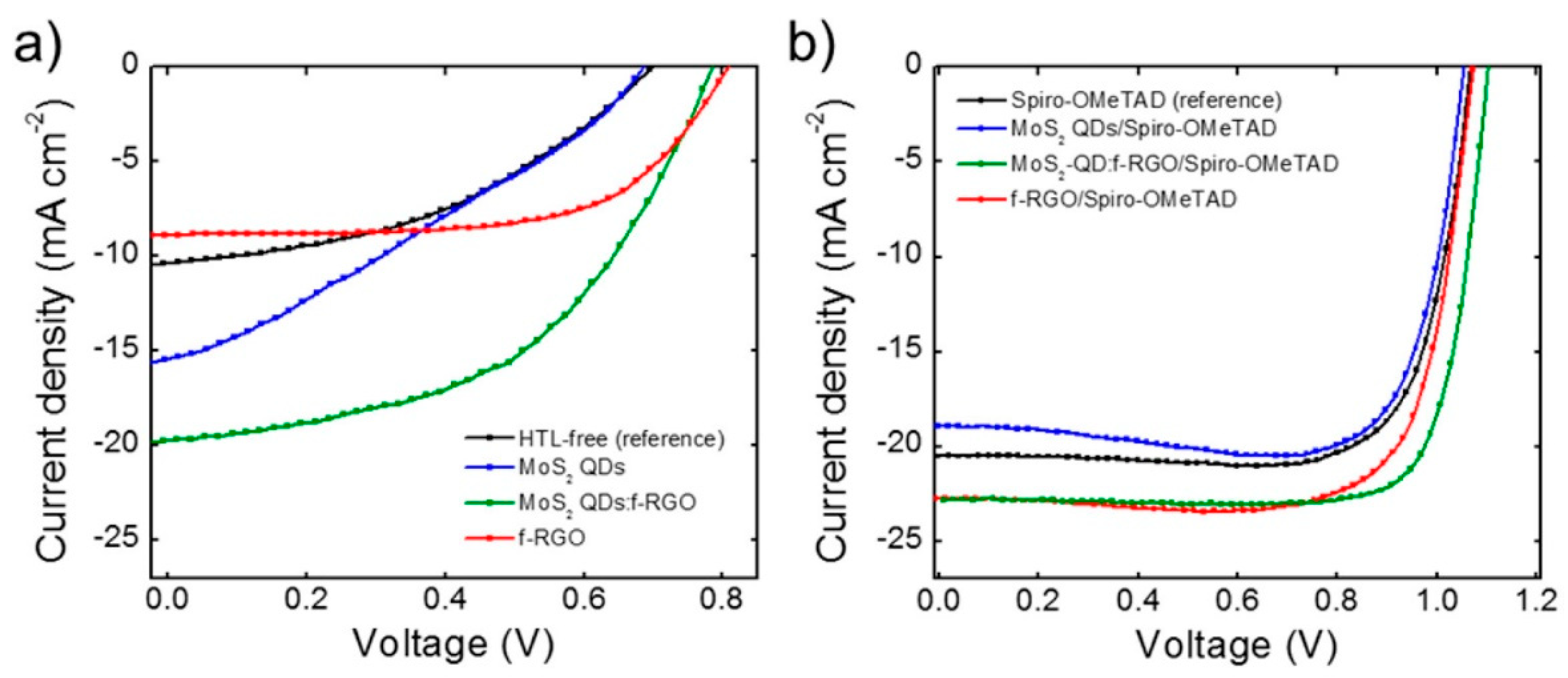
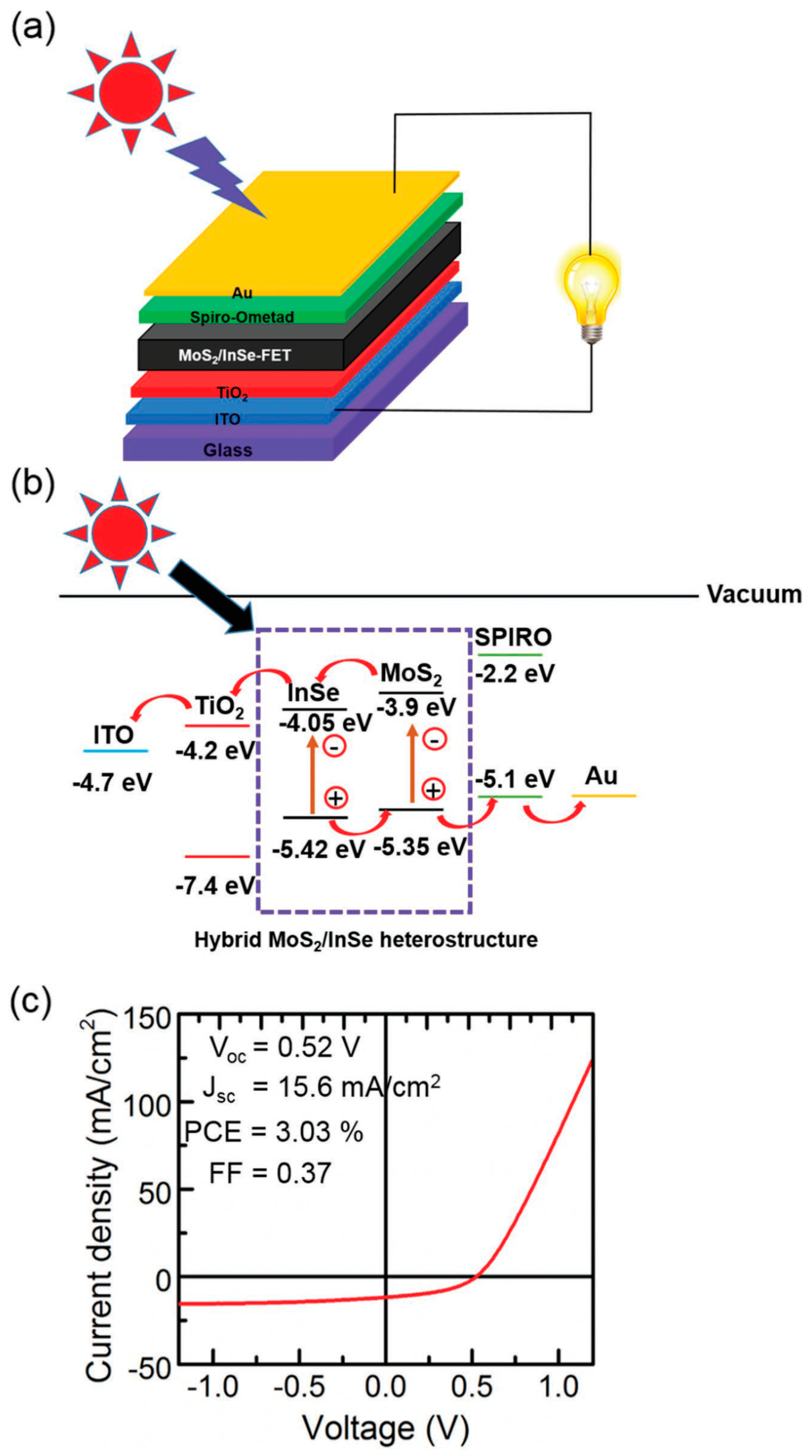
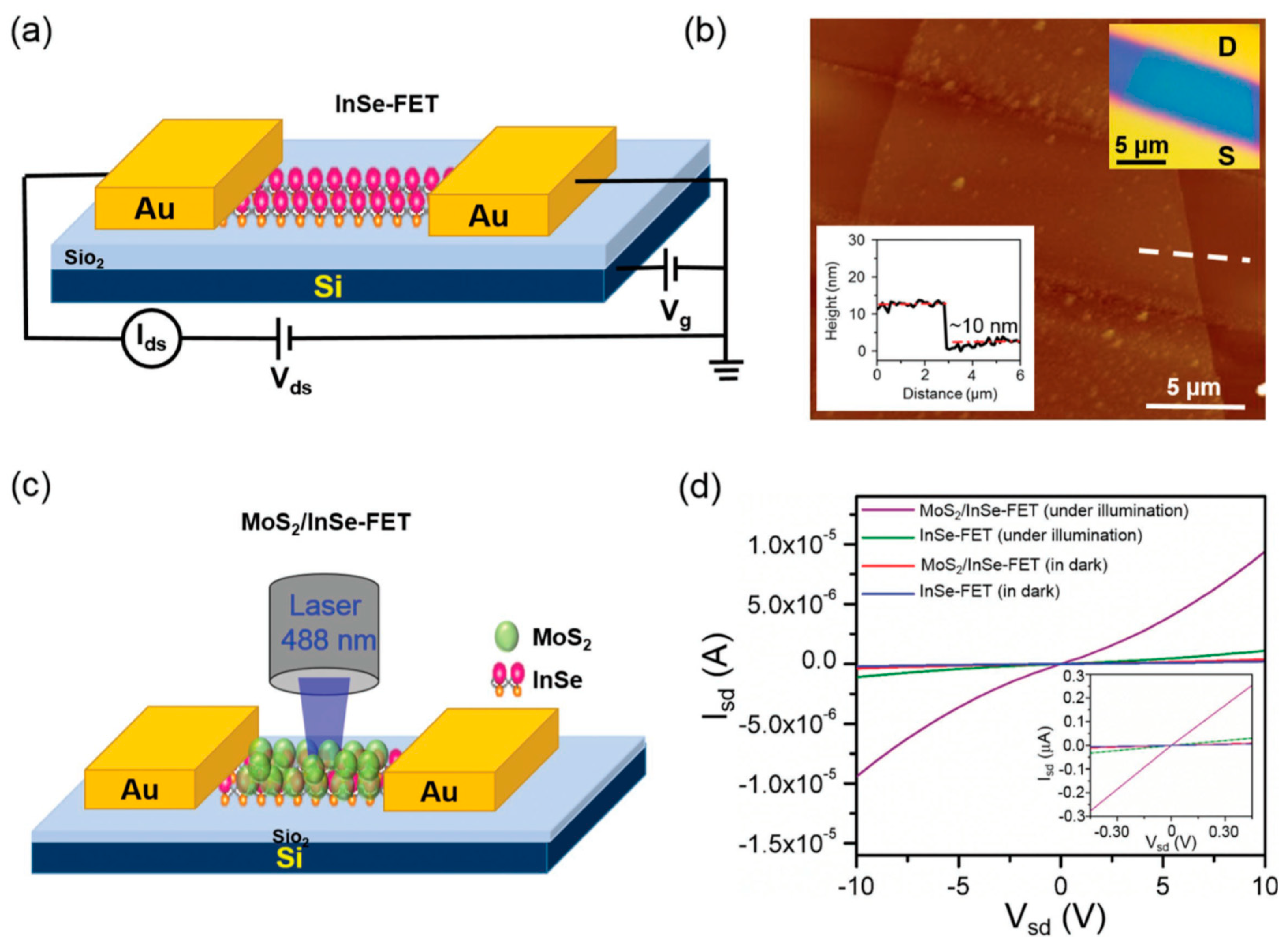
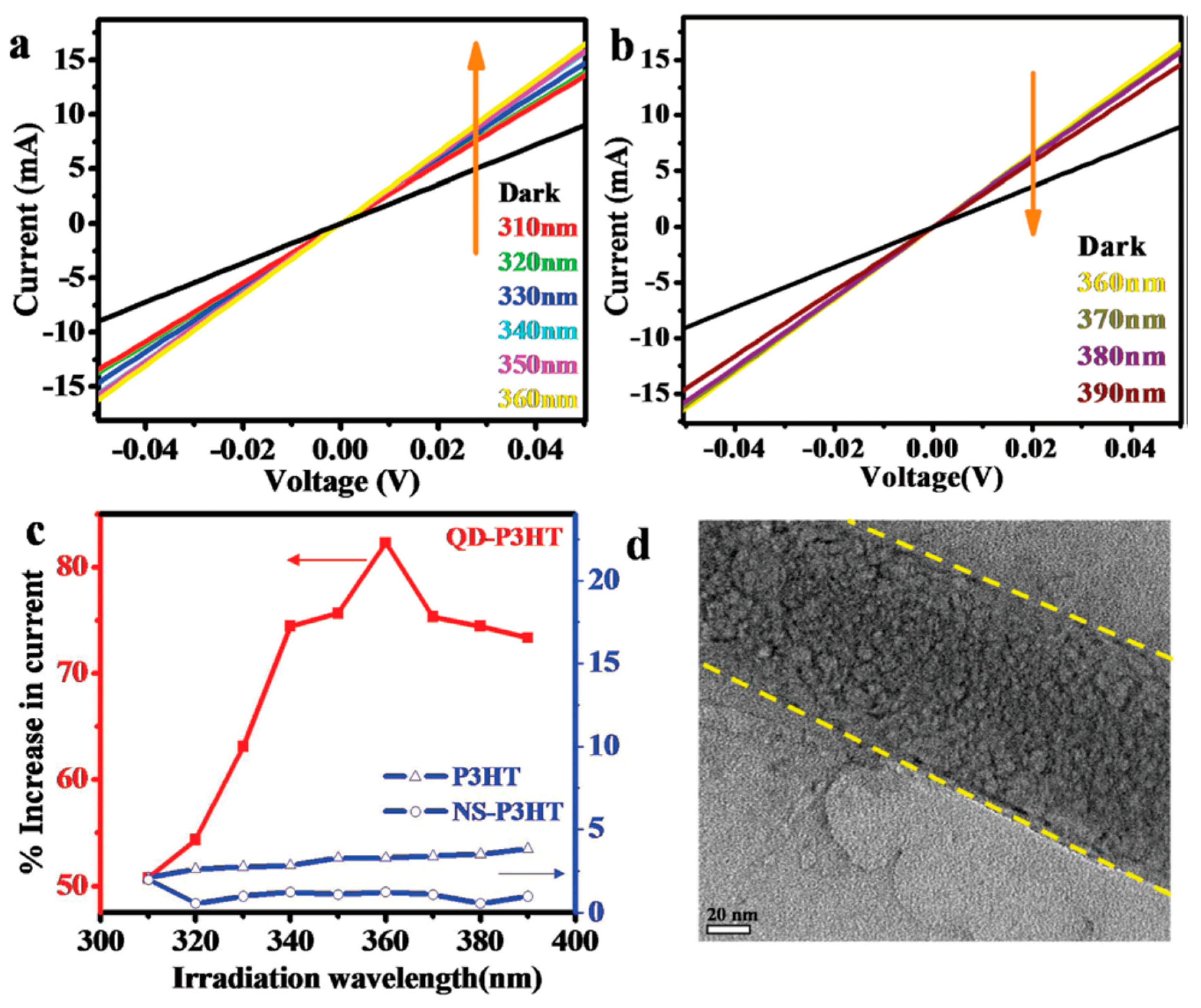

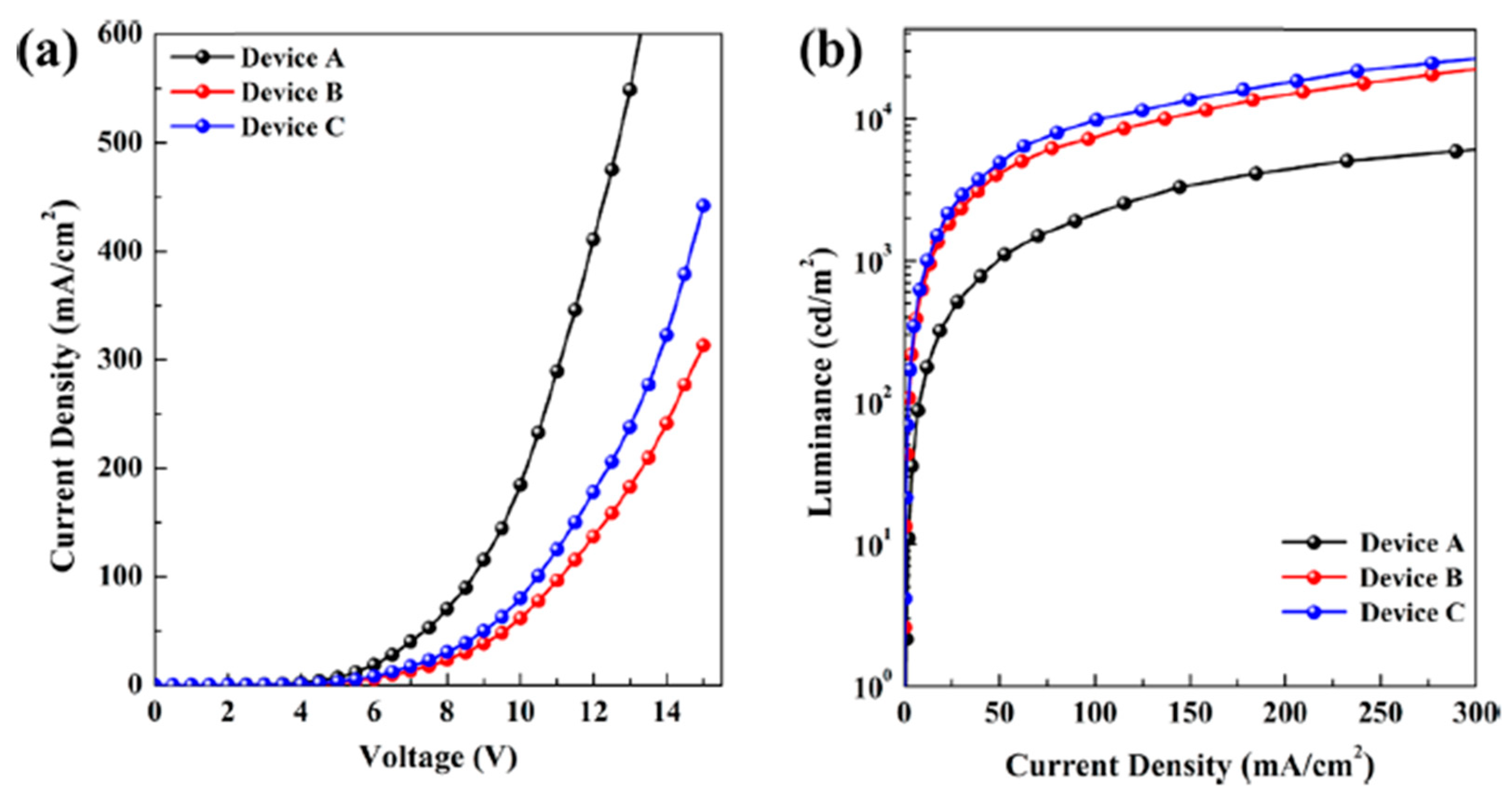



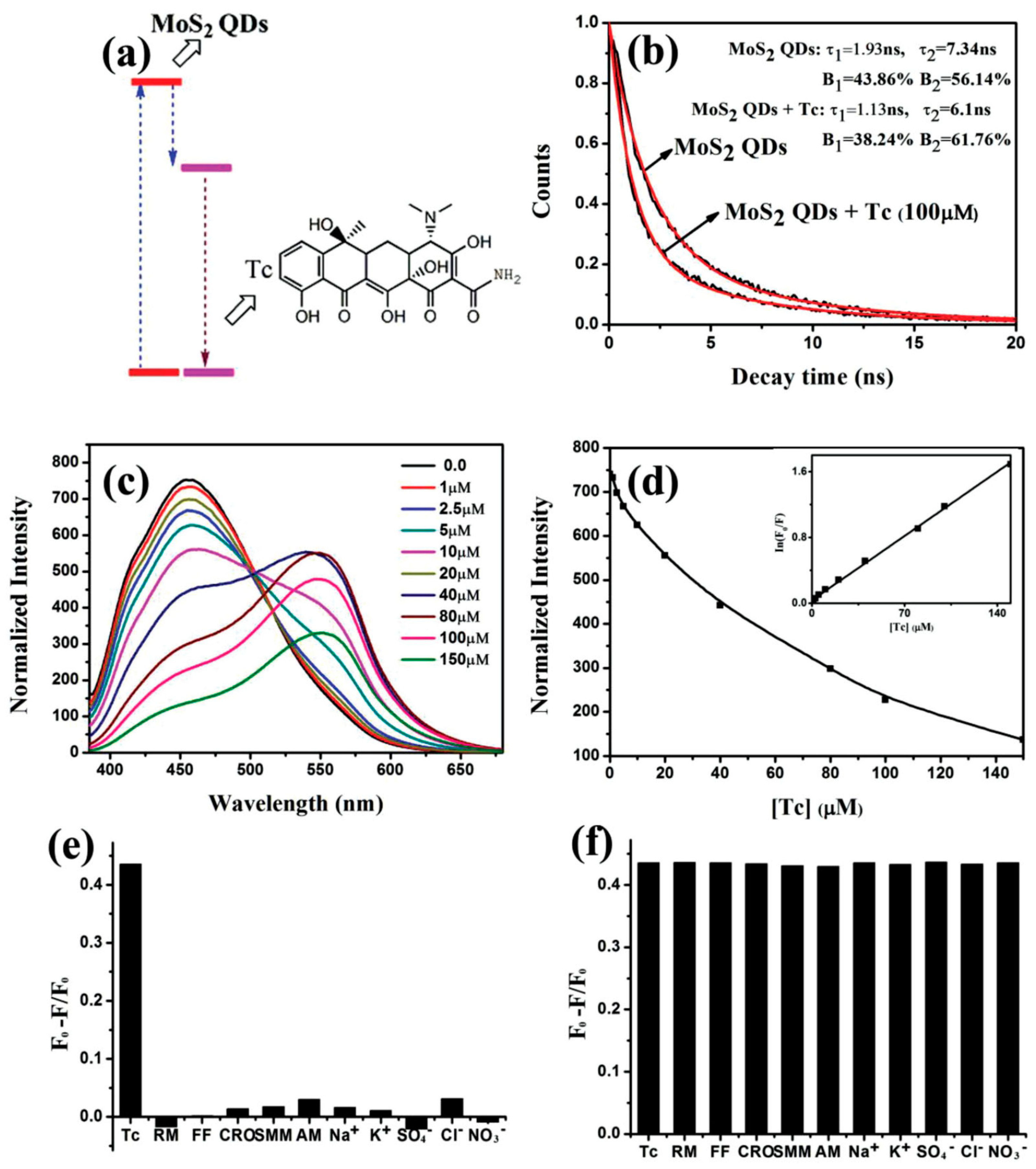


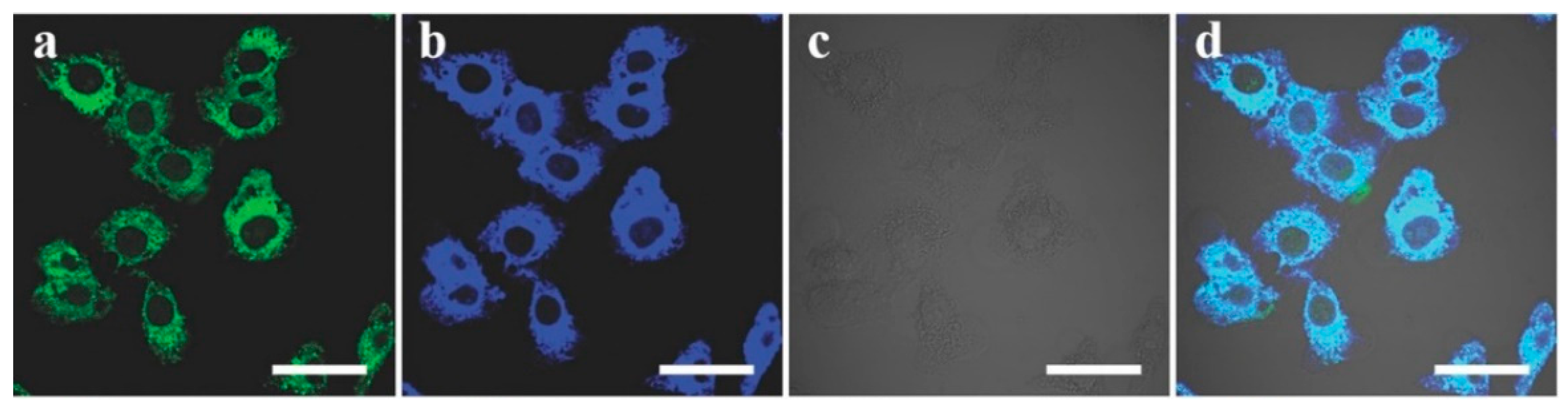
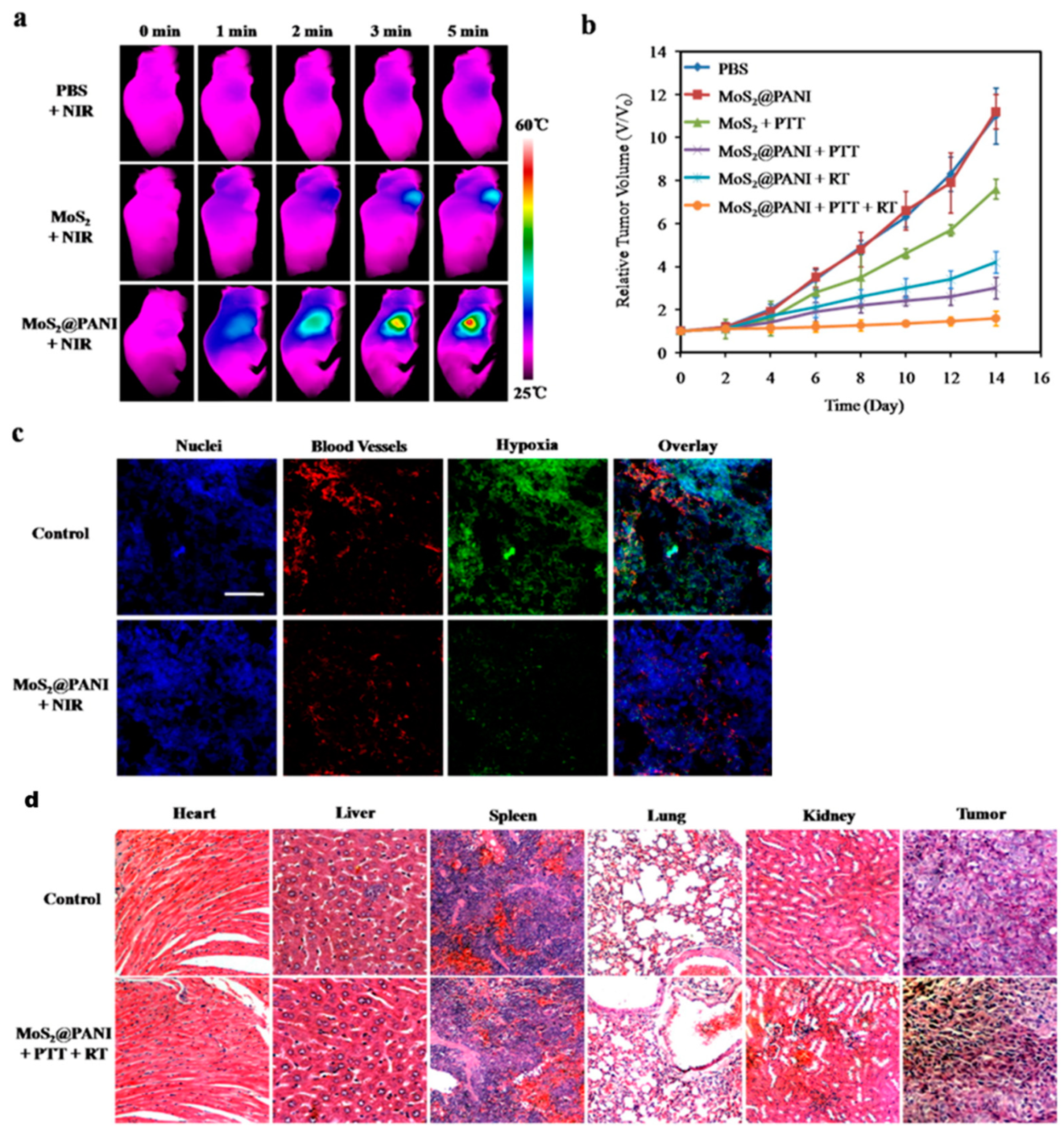
| Solvents | Notes | Emission (Excitation) | Size | Reference |
|---|---|---|---|---|
| Ethylene Glycol | Natural molybdenite suspended in the solvent and sonicated in a pressurized ultrasound reactor for 20 min, and then refluxed at 193 °C for 24 h. Dispersion filtered using Pragopor membrane. | 440 nm (350 nm) | 4–70 nm, 1 nm thick | [65] |
| Sulfuric Acid | MoS2 powder suspended in sulfuric acid and sonicated at 65 °C for 20 h. The solution was centrifuged for 30 min, dialyzed, and filtered. | 425 nm (300 nm) | 3.5 nm, 1–1.5 thick | [60] |
| DMF, NMP, DMEU, DI water, ethanol, acetone | Six solvents were tested. MoS2 powder suspended in a solvent and sonicated for 3 h. The top two-thirds were decanted and refluxed for 6 h below the solvent’s boiling point. The solution was allowed to sit for several hours, then centrifuged for 5 min. The supernatant was evaporated under vacuum, and the QDs were resuspended in DI water. | DMF: 465 nm (390 nm) NMP: 455 nm (380 nm) | DMF: 3.3 nm (avg.), 1.2 nm thick NMP: 3.4 nm DMEU: 3–4 nm | [43] |
| NMP | MoS2 powder dispersed in the solvent and sonicated it continuously in an ice bath for 3.5 h before tip sonicating it for another 3.5 h. The dispersion was left undisturbed overnight and then centrifuged for 90 min. | 575 nm (400 nm) | 0.5–4.0 nm (2.5 nm avg.) | [66] |
| Liquid Nitrogen + IPA | Heated MoS2 powder in a quartz boat to 340 °C under ambient air and maintained it for 3 min. Immediately after, it was quenched in a Dewar flask of liquid nitrogen. Once the liquid nitrogen was fully evaporated, quenched MoS2 was dispersed in IPA and sonicated for 30 min using a cycle of 7 s “on” and 3 s “off”. The solution was centrifuged for 30 min. | 440 nm (360 nm) | 1.41 nm (avg.), 1.5 nm thick | [67] |
| Precursors | Emission (Excitation) | Size | Reference |
|---|---|---|---|
| Sodium molybdate + Glutathione | 425 nm (340 nm) | 2.7 ± 0.3 nm (avg.) spherical | [74] |
| Sodium molybdate + L-cysteine | 402 nm (308 nm) | 3.5 nm, 1–1.5 thick | [75] |
| Sodium molybdate + Dibenzyl-disulfides | 280 nm (205 nm) | DMF: 3.3 nm (avg.), 1.2 nm thick NMP: 3.4 nm DMEU: 3–4 nm | [75] |
| Sodium molybdate + Thiourea | 406 nm (250 nm) | 0.5–4.0 nm (2.5 nm avg.) | [77] |
| [(NH4)6Mo7O24 • 4H2O] + Sodium Sulfide | 1.41 nm (avg.), 1.5 nm thick | [78] | |
| Molybdenyl acetylacetonate + Thioglycolic Acid + Sodium Sulfide | [79] | ||
| (NH4)6Mo7O24 + Thiourea + N-acetyl- l-cysteine | 480 nm (380 nm) | 2.1 nm (avg.) < 0.9 nm thick | [69] |
| (NH4)2MoS + N2H4 | 400 nm (330 nm) | 2.8 nm (avg.) 1.4–2.5 nm thick | [80] |
| (NH4)2MoS + Oleylamine | 575 nm (500 nm) | 4.5 ± 0.5 nm (avg.) 3 nm thick | [81] |
| Material | Overpotential (mV) | Tafel Slope (mV/dec) | Reference |
|---|---|---|---|
| MoS2 QDs in Aerogel | 53 | 41 | [89] |
| MoS2 QDs between MoS2 Nanosheets | 190 | 74 | [66] |
| MoS2 QDs on Glassy Carbon | 210 | 60 | [44] |
| MoS2 QDs | 140 | 66 | [48] |
| MoS2 QDs on Graphene Flakes | 136 | 141 | [90] |
| MoS2 QDs | 160 | 59 | [76] |
| Fe-doped MoS2 QDs | 121 | [91] | |
| Li-doped MoS2 QDs | 109 | [91] | |
| Mg-doped MoS2 QDs | 91 | [91] | |
| MoS2 QDs on Au | 130 | 94 | [39] |
| MoS2 QDs | 120 | 115 | [44] |
| Design | PCE (%) | Reference |
|---|---|---|
| MoS2 on F-doped SnO2 as a Counter Electrode | 3.69 | [93] |
| MoS2 QDs/InSe–FET | 3.03 | [94] |
| MoS2 QDs with UV–ozone on MoS2 Nanosheets | 8.66 | [95] |
| MoS2 QDs on Graphene Flakes | 20.12 | [82] |
| Chemical | Selectivity | Concentration | Reference |
|---|---|---|---|
| 2,4,6–trinitrophenol | F0/F = 1.42 | 10.0 μm | [75] |
| 2,4,6–trinitrophenol | F/F0 = 0.95 | 1.0 mM | [78] |
| Pb | (F- F0)/F0 > 0.9 | 5.0 μm | [77] |
| S | (F- F0)/F0 > 0.3 | 10.0 μm | [77] |
| Tetracycline hydrochloride | (F- F0)/F0 > 0.4 | 0.05 mM | [79] |
| Glucose | (F- F0)/F0 = 0.17 | 0.5 mM | [74] |
| Al | (F- F0)/F0 > 2.7 | 1.0 mM | [83] |
| Fe | (F- F0)/F0 > 0.5 | 1.0 mM | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabel, J.; Sharma, S.; Acharya, A.; Zhang, D.; Yap, Y.K. Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications. C 2021, 7, 45. https://doi.org/10.3390/c7020045
Kabel J, Sharma S, Acharya A, Zhang D, Yap YK. Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications. C. 2021; 7(2):45. https://doi.org/10.3390/c7020045
Chicago/Turabian StyleKabel, Jeff, Sambhawana Sharma, Amit Acharya, Dongyan Zhang, and Yoke Khin Yap. 2021. "Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications" C 7, no. 2: 45. https://doi.org/10.3390/c7020045
APA StyleKabel, J., Sharma, S., Acharya, A., Zhang, D., & Yap, Y. K. (2021). Molybdenum Disulfide Quantum Dots: Properties, Synthesis, and Applications. C, 7(2), 45. https://doi.org/10.3390/c7020045







